Abstract
The novel variant IBDV (nVarIBDV, genotype A2dB1), characterized by bursal atrophy of fabricius and decreased lymphocytes, has been emerging on a large scale in Asia (including China) since late 2018. nVarIBDV is a new threat to the poultry industry, yet the currently licensed commercial vaccines, including the live viral vector vaccine, IBDV immune complex vaccine or VP2 subunit vaccine, are ineffective against nVarIBDV infection. In this study, specific-pathogen-free (SPF) chickens and broilers divided into 3 groups were vaccinated with the live viral vector vaccine, the VP2 subunit vaccine or the IBDV immune complex vaccine at 1 day-old, respectively. The SPF chickens received a secondary vaccination with the live B87 strain vaccine at 11-day-old. The bursa/body weight ratio, histopathology lesion of the bursa, and the differentiation between infected and vaccinated animals (DIVA) by qRT-PCR confirmed that the live viral vector vaccine or immune complex vaccine plus live B87 strain booster could provide at least 80% protection against the FJ2019-01 strain of nVarIBDV in SPF chickens. The broilers also received a secondary vaccination using a live W2512 G-61 strain vaccine at 14-day-old, and analyses showed that the VP2 subunit vaccine or immune complex vaccine plus the live W2512 G-61 strain booster also provided more than 80% protection against the FJ2019-01 strain of nVarIBDV. Unfortunately, the live viral vector vaccine plus live W2512 G-61 strain booster provided poor to moderate protection against FJ2019-01 in broilers. These findings suggest that combining commercial vaccines with rational booster immunization can effectively protect chickens against an nVarIBDV challenge.
Key words: infectious bursal disease virus, novel variant IBDV, booster immunization, efficacy
INTRODUCTION
Infectious bursal disease virus (IBDV), a member of the Avibirnavirus genus, family Birnaviridae, was first reported in the Gumboro region of the USA in 1957 and is distributed worldwide now, leading to immunosuppression in chickens (Eterradossi and Saif, 2020). Virus neutralization and cross-protection studies identified 2 IBDV serotypes, but only the serotypes 1 strains are pathogenic to chickens. These strains are classified into avirulent, classical (also known as standard), variant of USA, very virulent (vv) (Jackwood et al., 1982; Müller et al., 2003), and novel variant IBDV (nVarIBDV) (Fan et al., 2019; Hou et al., 2022; Zhang et al., 2022) following their pathogenicity. The IBDV is nonenveloped with A and B genome segments. The VP2 and VP1 genes from A and B segments, respectively are important for virus pathogenicity and antigenicity (Eterradossi and Saif, 2020). Especially the VP2 protein is a major host-protective immunogen for inducing neutralizing antibodies, and the vp2 gene possesses a hypervariable region (HVR, nt 616–1050) and is involved in antigenic variation and virulence (Letzel et al., 2007; Mato et al., 2020).
Various nVarIBDV strains have recently emerged in large-scale poultry farms in Asia (including China), a new threat to the poultry industry (Zhang et al., 2022). For instance, nVarIBDV strains (FJ2019-01 and SHG19) are novel pathogenic viruses significantly different from the previously known American IBDV variants. These nVarIBDV variants are characterized by high pathogenicity leading to bursal atrophy, decreased lymphocyte levels in the bursa, macrophage infiltration of the follicle, fibrous tissue proliferation around the follicle, and severe follicle atrophy in chickens (Fan et al., 2019; Hou et al., 2022). The nVarIBDV variant causes subclinical infections that increase the susceptibility of chickens to other pathogens and induces a poor immune response to vaccines (Fan et al., 2019). However, no gross clinical symptoms or mortality are observed in the chicken infected with SHG19 or FJ2019-01 IBDV variants (Fan et al., 2019; Hou et al., 2022).
Vaccination is the optimal strategy for preventing and controlling IBD (Jackwood, 2017). However, the currently used commercial IBDV vaccines for vvIBDV, including the live viral vector vaccine (recombinant vaccine generated by inserting IBDV VP2 gene into the HVT genome), the IBDV immune complex vaccine and the VP2 subunit vaccine, are ineffective against these nVarIBDV variants (Fan et al., 2020a; Hou et al., 2022). Immunosuppression and nVarIBDV infection are very common in China today (Zhang et al., 2022). Fortunately, the virus-like particle (VLP) from the VP2 protein of nVarIBDV (a representative strain of SHG19 or FJ-1812) elicits neutralization antibodies and provides 100% protection against nVarIBDV (genotype A2dB1). This VLP vaccine also protects against lethal vvIBDV (Li et al., 2020; Wang et al., 2021). Furthermore, a reassortment virus strain, rGtVarVP2, expresses the main protective VP2 antigen of the nVarIBDV based on the skeleton of an attenuated vaccine Gt strain. The reassortment rGtVarVP2 also induces specific neutralizing antibodies against nVarIBDV and provides complete protection against nVarIBDV (genotype A2dB1) (Fan et al., 2020b). However, all novel vaccines for poultry must be licensed and approved by the government for commercialization, which is a very difficult and costly procedure. Therefore, this study evaluated licensed commercial IBDV vaccines (VP2 subunit vaccine, IBDV immune complex vaccine, live viral vector vaccine, and attenuated live vaccine) using a novel booster immunization strategy to ascertain their effectiveness against nVarIBDV in SPF chickens or broilers. This report provides important technical support for preventing and controlling nVarIBDV in the future.
MATERIALS AND METHODS
Virus, Vaccines, and Animals
The nVarIBDV FJ2019-01 strain (GenBank: MZ736578 or MZ044944) was locally available in our laboratory (Hou et al., 2022). The live viral vector vaccine (VAXXITEK, recombinant vaccine generated by inserting the IBDV VP2 gene into the HVT genome; Boehringer Ingelheim, China), the IBDV immune complex vaccine (TRANSMUNE, W2512 G-61 strain, CEVA, China), and VP2 subunit vaccine (YEBIO, Qingdao, China), the attenuated live vaccine (B87 strain, Liaoning Yikang, Liaoyang, China), and the attenuated live vaccine (W2512 G-61 strain; CEVA, China) were commercially licensed by the Chinese Ministry of Agriculture and used to against vvIBDV and/or classical IBDV in China.
The SPF chickens (White Leghorn) were purchased from Jinan SPAFAS poultry co., LTD (Shandong, China). The broilers were purchased from Fujian Sunner Development Co., LTD (Fujian, China). The FAAS's Institutional Animal Care and Use Committee guided all the animal experiments, performed following the animal ethics guidelines and approved protocols. Furthermore, all husbandry procedures followed the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. All chickens were euthanized with Tiletamine Hydrochloride and Zolazepam Hydrochloride injections (Virbac, Carros, France), minimizing suffering.
Animal Experiments
Experiment 1: Briefly, 50 SPF chickens (1-day-old) were randomly divided into 5 groups (n = 10 per group). Chickens in groups 1 and 2 were not vaccinated, but those in groups 3, 4, and 5 were vaccinated with the live viral vector vaccine (group 3), the VP2 subunit vaccine (group 4), and the IBDV immune complex vaccine (W2512 G-61 strain) (group 5) at 1-day-old with one dose following the manufacturer's instructions, respectively. At 11 d, each chicken in groups 3, 4, and 5 was boosted with the attenuated live vaccine (B87 strain) using one dose following the manufacturer's instructions. Each chicken in groups 2, 3, 4, and 5 was challenged (ocular) with 0.1 mL (100 bird infectious dose (BID)) of the nVarIBDV FJ2019-01 strain at 21 d, and the non-infected control (group 1) was given 0.1 mL PBS. Serum was collected from chickens of all groups for detecting IBDV antibodies at 11 d (before booster immunization) and 21 d (before challenge).
Experiment 2: Seventy broilers (1-day-old) were randomly divided into 5 groups (n = 10 per group), and the remaining 20 chickens (group 0) were used for serum sampling (at 1 d). Chickens in groups 1 and 2 were not vaccinated, but each chicken in groups 3, 4, and 5 was vaccinated with the live viral vector vaccine (group 3), the VP2 subunit vaccine (group 4), and the IBDV immune complex vaccine (group 5) at hatching, with one dose following the manufacturer's instructions, respectively. At 14 d after vaccination, serum was collected from chickens of all groups for detecting IBDV antibodies, and each chicken in groups 3, 4, and 5 was booster-immunized using the attenuated live vaccine (W2512 G-61strain) with one dose following the manufacturer's instructions. Ten d after the booster immunization, serum was collected from chickens of all groups for detecting IBDV antibodies. Each chicken in groups 2, 3, 4, and 5 was challenged (ocular) with 0.1 mL (100 BID) of the nVarIBDV FJ2019-01 strain at 24 d. The non-infected control birds in group 1 were given 0.1 mL PBS.
All chickens were euthanized after 10 d of the ocular challenge. The bursa and body weight were measured in euthanized birds. These bursa samples were fixed in 10% neutral buffed formalin for further histopathological examination and simultaneously stored at -70°C for viral load detection by qRT-PCR.
Histopathology
All bursas of fabricius were fixed with 10% formalin, dehydrated for transparency, wax embedded, sectioned, and stained by standard H&E methods. The histopathological lesions of the tissues were evaluated under a microscope. Histopathological analysis of the bursa included scoring lesions as described previously (Hou et al., 2022): 0 for no lesions, 1 for slightly decreased number of lymphocytes, atrophy of the follicle and broadening of the mesenchyme, 2 for slightly decreased number of lymphocytes, atrophy of the follicle and broadening of the mesenchyme, 3 for moderately decreased number of lymphocytes, atrophy of the follicle and broadening of the mesenchyme, and 4 for severely decreased number of lymphocytes, atrophy of the follicle and expansion of the mesenchyme. The mean lesion scores of the respective tissues were calculated for each group.
Bursal Viral Loads
Viral RNA extraction was performed using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. For specific detection of the nVarIBDV FJ2019-01 or vaccine strain, the one-step real-time TaqMan RT-PCR assay was performed on a LightCycler96 (Roche, Basel, Switzerland) as described previously (Wang et al., 2023). Briefly, the 20 μL reaction contained 5 μL Fast 1-Step Mix (4 ×) (ThermoFisher, Lithuania, Vilnius), 0.5 μL forward or reverse primer (10 μM; v-F [5′-CCT CCT TCT AYA RYG CTR TCA T-3′], and v-R [5′-CGT ATG AAC GGA ACA ATC TG-3′]), 0.25 μL probe (10 μM; vac-P [5′-FAM-AGT AGA GAT CAG ACA AA-MGB-3′] and nVar-P [5′-VIC-TAG AGA TCA GAC GAA CG-MGB-3′]), 5 μL template RNA or plasmid DNA, and 8.5 μL deionized distilled water. The reaction involved: 50°C for 10 min; 95°C for 20 s; 40 cycles of 95°C for 3 s, and 60°C for 30 s. The standard curve was generated by plotting the Cq values vs log10 of 10-fold serial dilutions (108 to 101) of plasmid DNA. All reactions and amplifications were analyzed using the LightCycler® 96 SW 1.1 software.
Serological Detection and Analysis
The IBDV antibodies in sera of SPF chickens and broilers were evaluated using an IBDV antibody test kit (ProFLOCKTM IBD PLUS Ab, Zoetis, Parsippany, USA) following the manufacturer's instructions. The sample-to-positive (S/P) ratios were calculated based on the optical density (OD) values at 405 nm. Sera with S/P ratios ≥ 0.3 (titers ≥ 1002) were considered having anti-IBDV antibodies, and ratios < 0.3 (titers < 1002) were considered negative. The box plot was used to evaluate the antibody level in each group.
RESULTS
Protection of Booster Immunization Against nVarIBDV FJ2019-01 Challenge in SPF Chickens
To evaluate the protection efficiency of booster immunization using commercial vaccines against nVarIBDV FJ2019-01 strain, we vaccinated the SPF chickens with live viral vector vaccine, VP2 subunit vaccine or IBDV immune complex vaccine at the 1-day-old and secondarily inoculated with the attenuated live vaccine (B87 strain) at 11-day-old. The vaccinated chickens had higher seroprevalence and titers of IBDV antibodies at 21 d than 11-day-old chickens (Figures 1A and 1B). Before the booster immunization at 11 d, the chickens of group 5 had higher seroprevalence and average titers of IBDV antibodies compared to groups 3 and 4 (Figure 1A). All immunized chickens were positive for IBDV antibodies, and the IBDV antibody titers in groups 3, 4, and 5 were similar after 10 d of booster immunization with the live B87 strain vaccine (Figure 1B). The serum of negative or positive control chickens (groups 1 or 2) tested negative for IBDV antibodies at 21-days-old (Figure 1B).
Figure 1.
Efficacy of commercial vaccines and booster immunization against the nVarIBDV FJ2019-01 strain in SPF chickens. (A, B) ELISA detection of IBDV antibodies at 11 or 21-day-old (cutoff titer = 1002), respectively. (C) Bursa: body weight index of chickens challenged with the FJ2019-01 strain after 10 d. (D) Histopathology lesions in the bursa at 10 d after chickens were challenged with the IBDV FJ2019-01 strain. (E) The bursal viral load in FJ2019-01-challenged chickens. Samples were quantified using the standard curve, and the average viral genome copy number was calculated. The results were shown as the viral genome copy number of the log10 (viral copies). G1: negative control; G2: challenged control; G3: vaccinated with live viral vector vaccine at 1-day-old and live B87 strain vaccine at 11-day-old; G4: vaccinated with VP2 subunit vaccine at 1-day-old and live B87 strain vaccine at 11-day-old; G5: vaccinated with IBDV immune complex vaccine at 1-day-old and live B87 strain vaccine at 11-day-old.
Prior reports considered < 0.70 BBIX (bursa: body weight index (BBIX) [BBIX= (bursa: body weight ratios)/(bursa: body weight ratios in the negative group)]) as bursal atrophy (Lucio and Hitchner, 1979). Surprisingly, chickens in group 3 displayed the same BBIX values as chickens in group 1 (negative control), 30% (3/10) of the vaccinated chickens in group 4 had >0.70 BBIX, and all chickens of group 5 showed a significantly atrophied bursa with a BBIX below 0.70 compared to group 1 and 3 after the challenge (Figure 1C). Severe bursal atrophy was found in all chickens in group 2 after 10 d of the challenge with nVarIBDV FJ2019-01 strain (Figure 1C). Additionally, the bursas showed slight or light pathological lesions in the chickens from all 3 vaccinated groups after a challenge with the FJ2019-01 strain compared to group 2 (Figure 1D and Figure 2). Especially, 60% (6/10) of the chickens in group 3 showed no lesions in the bursa similar to the negative control, while all the other chickens had slight lesions in the bursa. The chickens of group 2 were characterized by severe lesions in the bursa (Figure 1D and Figure 2). Hence, the histopathology results and BBIX index indicated that the live vector vaccine plus the live B87 strain vaccine could provide effective protection against IBDV strain FJ2019-01.
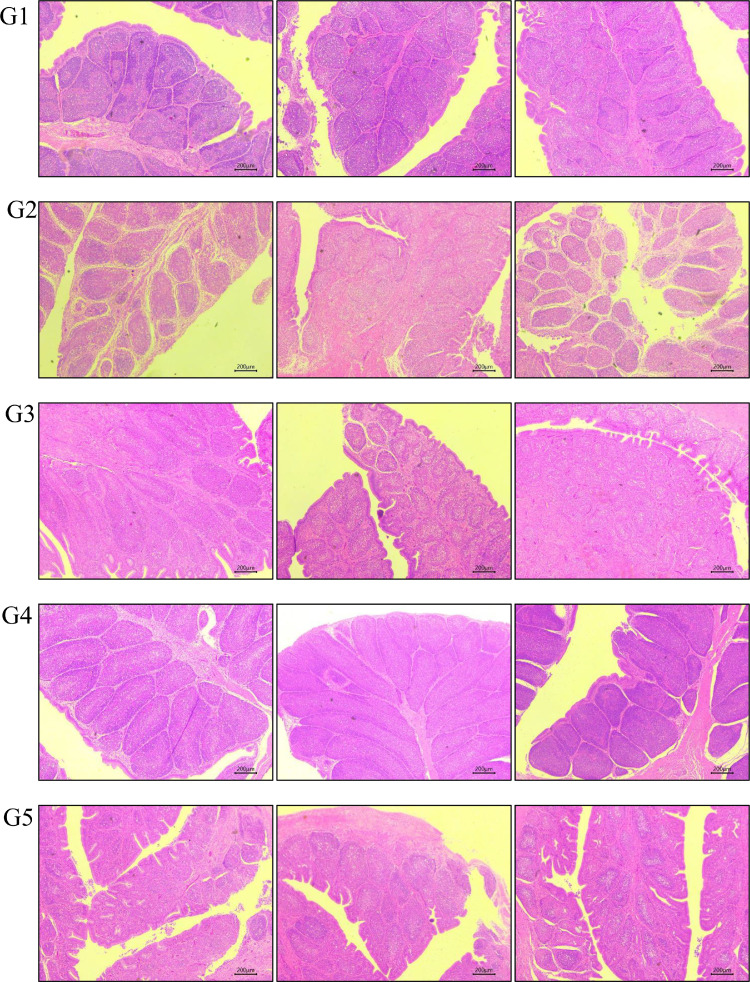
Figure 2.
Pathological sections of bursa in different groups of SPF chickens challenged with the nVarIBDV FJ2019-01 strain. Three typical images were selected from each group.
Thus, a one-step real-time TaqMan RT-PCR assay was performed to detect viral loads of the nVarIBDV FJ2019-01 or vaccine strain in the bursa because the W2512 G-61strain was causing bursa atrophy and lesions. The bursa of all chickens in group 3 and group 5 had moderate levels of B87 and/or W2512 G-61 viral RNA (3.0 - 5.0 log10 copies/mL), but not copies of the nVarIBDV FJ2019-01 strain (Figure 1E). Unfortunately, 40% (4/10) chickens of group 4 had approximate 5.0 log10 copies/mL nVarIBDV FJ2019-01 in the bursa, and the control challenged chickens of group 2 had > 6.0 log10 copies/ mL IBDV FJ2019-01 at 10 d post-infection (dpi) (Figure 1E). Interestingly, despite the severe bursal atrophy in vaccinated chickens of group 5, the bursal viral loads results suggested that the immune complex vaccine plus the live B87 vaccine could prevent infection by nVarIBDV FJ2019-01. This data showed that commercial vaccines for rational booster immunization could effectively protect against nVarIBDV, strain FJ2019-01.
Protection of Booster Immunization Against nVarIBDV FJ2019-01 Challenge in Broilers
The broilers with high maternal antibodies against IBDV at hatching were vaccinated with the live viral vector vaccine, the VP2 subunit vaccine, and the IBDV immune complex vaccine, respectively (Figure 3A). At 1-day-old, the broilers had not been vaccinated yet, so IBD antibody titers were represented in all the broiler groups. However, maternal IBD antibody titers gradually decreased as broilers aged, as evidenced by groups 1 and 2 at 14 d and 24 d (Figures 3A and 3B). Before the booster immunization with the live W2512 G-61 strain vaccine (at 14 d), antibody titers in groups 1 and 2 were similar to the other vaccinated groups (3, 4, and 5) (Figure 3A). Furthermore, the vaccinated chickens of group 4 displayed the highest anti-IBDV antibody titers after 10 d with booster immunization (on day 24) (Figure 3B). Moreover, the anti-IBDV antibody titer of groups 3 and 5 was significantly higher than the maternal antibody titers (groups 1 and 2) on day 24 (Figure 3B). These results demonstrated that anti-IBDV antibody titers were higher in SPF chickens than in broilers before the challenge. Ten d after the nVarIBDV FJ2019-01 challenge (at 34 -day-old), a necropsy showed bursal atrophy in vaccinated (groups 3, 4, and 5) and challenged chickens (group 2) (Figure 3C). Nonetheless, BBIX was significantly reduced in the challenged than the negative control group (Figure 3C). Additionally, pathology showed slight lesions in the bursas in chickens from group 4 after a challenge with the FJ2019-01 strain compared with group 2 (Figure 4). The chickens in group 2 were characterized by severe lesions in the bursa (Figure 4), but the chickens in groups 3 and 5 were characterized by light to moderate lesions in the bursa. Hence, the histopathology results suggestted that the VP2 subunit vaccine plus the live W2512 G-61 strain vaccine could provide effective protection against IBDV strain FJ2019-01. The one-step real-time TaqMan RT-PCR assay was performed to differentiate the nVarIBDV FJ2019-01 or W2512 G-61 strain in the bursa. On day 34 (10 dpi), group 1 (no-challenge) had undetectable virus levels, but all the vaccinated and challenged chickens had moderate to high viral loads in the bursa. The results indicated that the bursa of all vaccinated and challenged chickens contained the genomes of the vaccine, or FJ2019-01 viruses. The bursa of the challenged control (group 2) had high levels of FJ2019-01 (> 6.0 log10 copies/mL), but not the vaccine strain (W2512 G-61) (Figure 3D). Surprisingly, 8/10 bursas in group 4 and 8/10 bursas in group 5 had moderate levels of W2512 G-61 RNA (3.0∼5.0 log10 copies/mL), but not RNA of the FJ2019-01 strain. The remaining 2 birds from groups 4 and 5 had a high viral load of FJ2019-01 in the bursa (Figure 3D). The 6/10 chickens in group 3 (vaccinated using live viral vector and W2512 G-61 vaccines) had a high viral load with FJ2019-01 in the bursa (Figure 3D), but other 4/10 chickens had moderate W2512 G-61 viral load (Figure 3D). The histopathology and TaqMan RT-PCR results indicated that the immune complex vaccine and the VP2 subunit vaccine, plus the live W2512 G-61 booster could prevent nVarIBDV FJ2019-01 replication in the bursa. Hence, rational booster immunization using commercial vaccines could effectively protect broilers with high maternal antibodies against nVarIBDV (strain FJ2019-01) infection.
Figure 3.
Efficacy of commercial vaccine and booster immunization against the nVarIBDV FJ2019-01 strain in broilers. (A, B) ELISA detection of IBDV antibodies at 14- and 24-day-old (cutoff titer = 1002). G0 was maternal IBD antibody titers in broilers at 1-day-old. (C) Bursa: body weight index of chickens challenged with the FJ2019-01 strain after 10 d. (D) The bursal viral load in FJ2019-01-challenged chickens. Samples were quantified using the standard curve, and the average viral genome copy number was calculated. The results were shown as the viral genome copy number of the log10 (viral copies). G1: negative control; G2: challenged control; G3: vaccinated with live viral vector vaccine at 1-day-old and live W2512 G-61 strain vaccine at 14-day-old; G4: vaccinated with VP2 subunit vaccine at 1-day-old and live W2512 G-61 strain vaccine at 14-day-old; G5: vaccinated with IBDV immune complex vaccine at 1 day-old and live W2512 G-61 strain vaccine at 14-day-old.
Figure 4.
Pathological sections of bursa in different groups of broilers challenged with the nVarIBDV FJ2019-01 strain. Three typical images were selected from each group.
DISCUSSION
A combination of the molecular, antigenic, and pathogenicity characteristics of IBDV classifies this strain into classic, variant from the USA, very virulent, attenuated, and novel variant IBDV(nVarIBDV) strains (van den Berg et al., 2004; Jackwood et al., 2018; Fan et al., 2019; Hou et al., 2022). In 2018, nVarIBDV was first reported in eastern China, a variant different from the American IBDV variants at the molecular level (Fan et al., 2019; Hou et al., 2022). The nVarIBDV variants cause bursal atrophy, follicle atrophy, and broadening of bursal mesenchyme and significantly decreases the number of lymphocytes but do not lead to mortality (Fan et al., 2019; Hou et al., 2022). To date, nVarIBDV has been emerging on a large scale in Asia, including Japan, and is becoming a new threat to the poultry industry (Zhang et al., 2022).
Vaccination remains the optimal strategy for preventing and controlling IBD (Jackwood, 2017). Commercialized vaccines, including the live viral vector vaccine (a recombinant vaccine generated by inserting the IBDV VP2 gene into the HVT genome), the IBDV immune complex vaccine, and the VP2 subunit vaccine, as well as other attenuated, intermediate, and intermediate-plus vaccines were successfully used to control the classic or vv IBDV strain (Le Gros et al., 2009; Müller et al., 2012; Jackwood, 2017). For instance, VAXXITEK® (live viral vector vaccine) effectively protects against various IBDVs, including very virulent, classical, and USA variants (Bublot et al., 2007; Perozo et al., 2009). Unfortunately, these commercial vaccines do not prevent bursal atrophy and histopathological lesions and are ineffective against nVarIBDV strains (Fan et al., 2020a; Hou et al., 2022). Hence, nVarIBDV has been spreading in immunized chicken flocks. Therefore, developing a vaccine or devising new immunization strategies for broilers against nVarIBDV is urgently needed.
This study assessed the commercialized vaccines with the novel booster immunization strategy in SPF chickens. The results showed that all vaccinated chickens had high titers of IBDV antibodies, but some immunized and challenged control groups showed severe bursal atrophy after the challenge. The mean BBIX of these groups was < 0.70, while the bursas of non-challenged groups showed non-observable atrophy. Previous reports showed that < 0.70 BBIX is considered bursal atrophy (Lucio and Hitchner, 1979). Moreover, immune-complex and live vaccines, especially intermediate and intermediate plus vaccines, may cause atrophied bursa and severe bursal lesions (Müller et al., 2012; Okura et al., 2021). Hence, bursal viral loads and differentiation between infected and vaccinated animals (DIVA) served to evaluate the safety and effectiveness of each vaccine. The results demonstrated that most chickens of group 3 and 5 had moderate W2512 G-61 or B87 levels (3.0-5.0 log10 copies/mL), but not IBDV FJ2019-01 in the bursa of SPF chickens. Thus, this immunization schedule could prevent localization and replication of the nVarIBDV strain in the bursa. However, 40% chickens of group 4 had moderate nVarIBDV FJ2019-01 viral loads (104∼106 copies) in the bursa, while the other 60% birds had B87 viral loads in their bursa. These results demonstrated that the atrophied bursas of vaccinated chickens from group 5 were due to the vaccine, not the nVarIBDV FJ2019-01 challenge strain. Additionally, the histopathology results showed slight to light lesions in the bursas, suggesting that the vaccines used in these groups could provide effective protection against the IBDV strain FJ2019-01, especially in group 3. Hence, a novel booster immunization strategy using these commercial vaccines could effectively protect against nVarIBDV in SPF chickens and prevent nVarIBDV localization and replication in the bursa.
Breeder vaccination generates progeny with high maternal antibodies, protecting young chicks whose B-lymphocytes are most vulnerable to IBDV infection. However, maternal antibodies can also neutralize commercial live vaccines at vaccination. In this study, the maternal antibody titers were high in 1-day-old broilers and moderate in 14-day-old broilers. So the live W2512 G-61 strain vaccine, which is an intermediate strain compared with the attenuated B87 strain, was used as a booster shot in 14-day-old broilers, and reduces the interference of maternal antibodies. The live W2512 G-61 strain vaccine was successfully colonized and replicated in the bursa of chickens vaccinated using the immune complex vaccine or the VP2 subunit vaccine plus live the W2512 G-61 strain vaccine booster, despite the moderate levels of maternal antibodies before the booster immunization at 14 d. Moreover, the nVarIBDV FJ2019-01 strain did not replicate in the bursa of chickens vaccinated with the immune complex vaccine or the VP2 subunit vaccine plus the live W2512 G-61 booster after 10 d in the challenge. Unfortunately, the live viral vector vaccine plus the live W2512 G-61 booster could not reliably prevent the FJ2019-01 nVarIBDV localization and replication in the bursa of vaccinated chickens; the exact cause is unknown. Attenuated IBDV live vaccines might cause the bursal atrophy of fabricius when they replicate. The interference of maternal antibodies with the replication of vaccine viruses implies that the vaccines might not fully protect the chickens from virulent or very virulent IBDV (vvIBDV) strains in the field (Okura et al., 2021). Hence, the W2512 G-61 strain vaccine was hardly localized or replicated in the bursa of chickens from group 3 vaccinated by the live viral vector vaccine plus the live W2512 G-61 strain booster. An efficient vaccination program depends on the time of vaccination, which can be affected by residual maternal antibodies (Jackwood, 2017). Although all the live vaccines caused marked microscopic bursal lesions, they effectively protected chickens against classical IBDV challenges and moderately protected against the USA IBDV variant (Delaware E strain) (Ashash et al., 2019). In this study, the chickens in groups 4 and 5 had bursal atrophy but successfully obstructed nVarIBDV FJ2019-01 localization and replication in their bursa of broilers. The limitation of these findings is that the mechanisms of booster immunization and the preventive strategies using the immune complex vaccine, the VP2 subunit vaccine, and the live W2512 G-61 strain vaccine against FJ2019-01 nVarIBDV localization and replication in the bursa remain unexplained.
CONCLUSIONS
These findings showed that rational booster immunization strategy can effectively protect against nVarIBDV in SPF chickens and broilers. Moreover, the W2512 G-61 strain can successfully localize and replicate in the bursa to prevent nVarIBDV localization and replication in the broilers. These results may reveal an alternative IBDV immunization strategy after hatching and in the field against nVarIBDV. Nonetheless, the mode of action and mechanisms for booster immunization requires elucidation before its application against nVarIBDV in China.
Acknowledgments
This study was supported by the Sci-Tech Innovation Team of Fujian Academy of Agricultural Sciences (Grant No. CXTD2021014-3) and 5511 Collaborative Innovation Project (Grant No. XTCXGC2021008).
DISCLOSURES
The authors declare that they have no competing interests.
REFERENCES
- Ashash U., Noach C., Perelman B., Costello C., Sansalone P., Brazil T., Raviv Z. In Ovo and Day of Hatch Application of a Live Infectious Bursal Disease Virus Vaccine to Commercial Broilers. Avian Dis. 2019;63:713–720. doi: 10.1637/aviandiseases-D-19-00087. [DOI] [PubMed] [Google Scholar]
- Bublot M., Pritchard N., Le Gros F.X., Goutebroze S. Use of a vectored vaccine against infectious bursal disease of chickens in the face of high-titred maternally derived antibody. J. Comp. Pathol. 2007;137(Suppl 1):S81–S84. doi: 10.1016/j.jcpa.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Eterradossi N., Saif Y.M. In: Diseases of Poultry. Swayne D.E., Boulianne M., Logue C.M., McDouglad L.R., Nair V., et al., editors. Wiley-Blackwell Publishing; Hoboken, NJ, USA: 2020. Infectious bursal disease; pp. 257–283. [Google Scholar]
- Fan L., Wu T., Hussain A., Gao Y., Zeng X., Wang Y., Gao L., Li K., Wang Y., Liu C., Cui H., Pan Q., Zhang Y., Liu Y., He H., Wang X., Qi X. Novel variant strains of infectious bursal disease virus isolated in China. Vet. Microbiol. 2019;230:212–220. doi: 10.1016/j.vetmic.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Fan L., Wu T., Wang Y., Hussain A., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., Pan Q., Zhang Y., Wang X., Qi X. Novel variants of infectious bursal disease virus can severely damage the bursa of fabricius of immunized chickens. Vet. Microbiol. 2020;240 doi: 10.1016/j.vetmic.2019.108507. [DOI] [PubMed] [Google Scholar]
- Fan L., Wang Y., Jiang N., Gao L., Li K., Gao Y., Cui H., Pan Q., Liu C., Zhang Y., Wang X., Qi X. A reassortment vaccine candidate of the novel variant infectious bursal disease virus. Vet. Microbiol. 2020;251 doi: 10.1016/j.vetmic.2020.108905. [DOI] [PubMed] [Google Scholar]
- Hou B., Wang C.Y., Luo Z.B., Shao G.Q. Commercial vaccines used in China do not protect against a novel infectious bursal disease virus variant isolated in Fujian. Vet. Rec. 2022;191:e1840. doi: 10.1002/vetr.1840. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J. Advances in vaccine research against economically important viral diseases of food animals: Infectious bursal disease virus. Vet. Microbiol. 2017;206:121–125. doi: 10.1016/j.vetmic.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J., Schat K.A., Michel L.O., de Wit S. A proposed nomenclature for infectious bursal disease virus isolates. Avian Pathol. 2018;47:576–584. doi: 10.1080/03079457.2018.1506092. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J., Saif Y.M., Hughes J.H. Characteristics and serologic studies of two serotypes of infectious bursal disease virus in turkeys. Avian Dis. 1982;26:871–882. [PubMed] [Google Scholar]
- Le Gros F.X., Dancer A., Giacomini C., Pizzoni L., Bublot M., Graziani M., Prandini F. Field efficacy trial of a novel HVT-IBD vector vaccine for 1-day-old broilers. Vaccine. 2009;27:592–596. doi: 10.1016/j.vaccine.2008.10.094. [DOI] [PubMed] [Google Scholar]
- Letzel T., Coulibaly F., Rey F.A., Delmas B., Jagt E., van Loon A.A., Mundt E. Molecular and structural bases for the antigenicity of VP2 of infectious bursal disease virus. J. Virol. 2007;81:12827–12835. doi: 10.1128/JVI.01501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Kuang H., Guo H., Cai L., Chu D., Wang X., Hu J., Rong J. Development of a recombinant VP2 vaccine for the prevention of novel variant strains of infectious bursal disease virus. Avian Pathol. 2020;49:557–571. doi: 10.1080/03079457.2020.1791314. [DOI] [PubMed] [Google Scholar]
- Lucio B., Hitchner S.B. Response of susceptible versus immune chicks to killed, live-modified, and wild infectious bursal disease virus vaccines. Avian Dis. 1979;23:1037–1050. [PubMed] [Google Scholar]
- Mato T., Tatar-Kis T., Felfoldi B., Jansson D.S., Homonnay Z., Banyai K., Palya V. Occurrence and spread of a reassortant very virulent genotype of infectious bursal disease virus with altered VP2 amino acid profile and pathogenicity in some European countries. Vet. Microbiol. 2020;245 doi: 10.1016/j.vetmic.2020.108663. [DOI] [PubMed] [Google Scholar]
- Müller H., Mundt E., Eterradossi N., Islam M.R. Current status of vaccines against infectious bursal disease. Avian Pathol. 2012;41:133–139. doi: 10.1080/03079457.2012.661403. [DOI] [PubMed] [Google Scholar]
- Müller H., Islam M.R., Raue R. Research on infectious bursal disease—the past, the present and the future. Vet. Microbiol. 2003;97:153–165. doi: 10.1016/j.vetmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Okura T., Otomo H., Suzuki S., Ono Y., Taneno A., Oishi E. Efficacy of a novel in ovo-attenuated live vaccine and recombinant vaccine against a very virulent infectious bursal disease virus in chickens. J. Vet. Med. Sci. 2021;83:1686–1693. doi: 10.1292/jvms.21-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo F., Villegas A.P., Fernandez R., Cruz J., Pritchard N. Efficacy of single dose recombinant herpesvirus of turkey infectious bursal disease virus (IBDV) vaccination against a variant IBDV strain. Avian Dis. 2009;53:624–628. doi: 10.1637/8687-31009RESNOTE.1. [DOI] [PubMed] [Google Scholar]
- van den Berg T.P., Morales D., Eterradossi N., Rivallan G., Toquin D., Raue R., Zierenberg K., Zhang M.F., Zhu Y.P., Wang C.Q., Zheng H.J., Wang X., Chen G.C., Lim B.L., Müller H. Assessment of genetic, antigenic and pathotypic criteria for the characterization of IBDV strains. Avian Pathol. 2004;33:470–476. doi: 10.1080/03079450400003650. [DOI] [PubMed] [Google Scholar]
- Wang C., Hou B., Shao G., Wan C. Development of a one-step real-time taqman reverse transcription polymerase chain reaction (RT-PCR) assay for the detection of the novel variant infectious bursal disease virus (nVarIBDV) circulating in China. Viruses. 2023;15:1453. doi: 10.3390/v15071453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jiang N., Fan L., Gao L., Li K., Gao Y., Niu X., Zhang W., Cui H., Liu A., Pan Q., Liu C., Zhang Y., Wang X., Qi X. Development of a viral-like particle candidate vaccine against novel variant infectious bursal disease virus. Vaccines. (Basel) 2021;9:142. doi: 10.3390/vaccines9020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Wang X., Gao Y., Qi X. The over-40-years-epidemic of infectious bursal disease virus in China. Viruses. 2022;14:2253. doi: 10.3390/v14102253. [DOI] [PMC free article] [PubMed] [Google Scholar]