Abstract
Objective:
The aim of our study was to assess postoperative lower limbs muscle strength (MS) as a predictor of late surgical success (36 months).
Methods:
Body composition analyses and isokinetic dynamometry evaluation were performed before (T0: n=123), six months (T1: n=123) and 36 months (T2: n=79) after Roux-en-y gastric bypass (RYGB). Surgical success (SS) was defined as ≥ 50% excess weight loss (EWL) 36 months after surgery or ≤ 50% surgical failure (SF).
Results:
There was no difference between relative MS extension (Ext) and flexion (Flex) in T1 and T2. There was also, no difference between relative MS Ext and Flex in T1 and T2 between patients with SS and SF. There was a difference in relative MS Ext (144.9 ± 39.8 Nm/kg x 125.5 ± 29.2 Nm/kg; p=0.04) and Flex (73.6 ± 21.8 Nm/kg x 60.4 ± 15.8 Nm/kg; p=0.02) between SS and SF patients only in T2. Patients with an increment in Ext and Flex MS ≥4 Nm/kg at T1 had approximately 76% of SS at 36 months.
Conclusion:
An increase of lower limbs MS ≥4 Nm/kg 6 months after RYGB predicts SS at 36 months. ClinicalTrials.gov ID: NCT04129801
Keywords: Bariatric Surgery, Biolectric Impedance, Body Composition, Morbid Obesity, Muscle Strength
Introduction
Bariatric surgery is the most effective treatment for patients with severe obesity resulting in more than 60% of excess weight loss (EWL)[1]. Patients with severe obesity present a compromise of functionality due to overload mechanics[2]. Despite these important limitations, there is no consensus about physical habilitation regarding optimal perioperative care in patients with severe obesity[3]. Pre habilitation comprises preoperative physical conditioning to improve functional and physiological capacity[3,4]. After surgery, the intense weight loss in the first months may determine a reduction in fat-free mass (FFM)[2] that ideally should not exceed 22% of EWL[5]. An excessive FFM loss may determine muscle weakness[6], decrease functional capacity, reduce skeletal muscle strength (MS) and endurance[7,8]. Previous studies showed a 30% to 35% loss in FFM 6 months after surgery associated with a 15% to 40% reduction in absolute MS of lower limbs 1 year after bariatric surgery[9,10]. Absolute strength[11] is important for the execution of normal daily activities. Relative strength[11] (an individual’s force relative to body mass) is particularly useful for comparing individuals with different body dimensions. After surgery there is a decrease of absolute MS and an increase of relative MS due to a reduction in body weight. Nevertheless, in a recent meta-analysis[12], the initial improvement in relative muscle strength observed 6 months after bariatric surgery was not maintained on long term follow up (36 months). There are no studies that assess surgical success (SS) and MS[13] on long term follow-up. We consider weight loss alone inadequate to determine (SS) since resolution of comorbidities and improvement of functionality are also very important[14]. The aim of our study was to assess MS of lower limbs as a predictor of late surgical success (36 months).
Materials and Methods
We consecutively evaluated 123 patients with severe obesity undergoing Roux-en-Y gastric bypass (RYGB) in a Terciary University Hospital between Juny 2017 to December 2020. The inclusion criteria were age between 18 and 60 years, a body mass index (BMI) between 40 and 60 kg/m2 and a Timed Up and Go (TUG) ≤10 seconds. Patients with functional disability (TUG >10)[15], treatment with steroid medication for any reason or the use of artificial devices such as an orthosis or a prosthesis were excluded. Patients were subject to anthropometric measurements, body composition analysis and dynamometry evaluation before (T0), six months (T1) and 36 months (T2) after RYGB.
Anthropometric and body composition evaluation
Body composition was determined by bioelectrical impedance analysis (BIA) under constant conditions (with subjects appropriately hydrated and at the same time of day). The body composition analyzer (InBody230, Biospace Co., Gangnam-gu, Seoul, South Korea) determined in Kg and percentages (%) fat mass (FM), fat free mass (FFM), fat mass of lower limbs (FMLL) and fat free mass of lower limbs (FFMLL).
Dynamometry
The dynamometer was calibrated before each test. Participants remained seated on the dynamometer chair, with the hip and knee joints at 90° flexion, and performed four submaximal contractions involving MS extension (Ext) and flexion (Flex) of the knees during a warm-up period to familiarize themselves with their maximum voluntary contractions (MVCs) and produce consistent results. Participants then executed two series of four uninterrupted repetitions of both legs, first with the dominant member and subsequently with the non-dominant member, at an angular velocity of 60°/s, with a 60-second interval between series[16]. The MVC variables evaluated included absolute Ext and Flex torques (Nm) and Ext and Flex torques relative to the body weight (Nm/Kg)[17].
Surgical procedure
The surgical procedure performed was Roux-en-Y gastric bypass (RYGB), with a biliopancreatic loop of 60-75 cm and food loop of 100-120 cm, with 30 ml pouch volume.
Surgical success
Surgical success (SS) was defined as ≥50% EWL 36 months after surgery. EWL of less than 50% was considered surgical failure (SF)[18-19]. The excess weight loss was calculated using the following formula[20]:
% Loss of Excess Weight = [(Preoperative weight - Current weight) / (Preoperative weight − Ideal weight)] x100
Statistical analysis
Continuous variables were expressed as mean + standard deviation and categorical variables were expressed as frequency and percentages. The Kolmogorov-Smirnov test was used to assess the distribution of quantitative variables. Spearman’s coefficient was used to assess the correlation between the variables. The paired T test and the paired nonparametric Mann-Whitney test were used for two different time points in continuous variables. We consider a p<0.05 as significant with a 95% confidence interval. R software version 4.0.2 was used to perform all analyses.
Results
Hundred twenty-three patients with severe obesity were evaluated in T0 and 6 months after bariatric surgery (T1). Seventy-nine patients were reevaluated in the late postoperative period (T2). Forty-four patients were excluded: thirty-one did not respond to contact attempts, twelve did not attend the evaluation and one patient died of unknown cause.
The results of anthropometric measurements, body composition analysis and muscle strength evaluation are in Table 1. There was a significant difference (p<0.01) between all parameters of body composition after bariatric surgery (T1 and T2) in relation to T0. There was no difference in body composition between the two postoperative evaluation periods (T1 x T2).
Table 1.
Results of anthropometric measurements, total and segmental body composition, muscle strength of patients with severe obesity before and after RYGB.
| Patients | T 0 (n=123) | T 1 (n=123) | T 2 (n=79) |
|---|---|---|---|
| Age (years) | 39 ± 10 | 41 ± 9.7* | 45 ± 10 ** *** |
| Height (cm) | 163.6 ± 10 | 163,0 ± 9,9 | 161.8 ± 9.5 |
| Weight (kg) | 128.0 ± 20.8 | 91.6 ± 15.7* | 87 ± 16.4** *** |
| BMI (kg/m2) | 47.6 ± 4.7 | 34.3 ± 4.4* | 33.1 ± 4.8** |
| FFM (%) | 49.5 ± 4.2 | 61.7 ± 8.2* | 61.1 ± 8.0** |
| FM (%) | 50.5 ± 4.2 | 38.5 ± 8.0* | 38.9 ± 8.0** |
| FFM (kg) | 63.4 ± 12.3 | 56.3 ± 11.9* | 52.8 ± 10.6** |
| FM (Kg) | 64.6 ± 11.2 | 35.3 ± 10.3* | 34.2 ± 11.0** |
| FFMLL (Kg) | 18.6 ± 4.4 | 16.3 ± 4.7* | 15.5 ± 3.6** |
| FMLL (Kg) | 17.7 ± 4.3 | 10.8 ± 6.2* | 10.1 ± 3.4** |
| FFMLL (%) | 51.3 ± 5.6 | 60.8 ± 9.0* | 60.8 ± 8.4** |
| FMLL (%) | 48.7 ± 5.6 | 39.2 ± 9.0* | 39.2 ± 8.4** |
| EXT (Nm) | 160.7 ± 46 | 140.2 ± 40.7* | 124.6 ± 42.7 ** *** |
| EXT (Nm/kg) | 125.3 ± 31.2 | 141.9 ± 35.0* | 140.0 ± 38.6** |
| FLEX (Nm) | 73.7 ± 24.0 | 63.0 ± 22.7* | 62.2 ± 23.1** |
| FLEX (Nm/kg) | 57.4 ± 16.8 | 67.9 ± 20.9* | 70.2 ± 21.4** |
where: T0 = preoperative; T1 = 6 months after surgery; T2 = 36 months after surgery; BMI: body mass index; FFM: fat free mass; FFMLL: fat free mass of lower limbs; FM: fat mass; FMLL: fat mass of lower limbs; Ext: extension; Flex: flexion; Nm: newton-meter; Nm/kg: newton-meter/kilograms
T0 x T1- p<0.01;
T0 x T2- p<0.01;
T1 x T2- p<0.01.
There was a reduction (p<0.01) of absolute MS Ext and Flex after surgery. There was no difference (p>0.05) between relative MS Ext at T2 (141.9 ± 35 Nm/kg) in relation to T1 (140 ± 38.6 Nm/kg) but a significant increase (p<0.01) between T1 and T2 to T0 (125.3 ± 31.2 Nm/Kg). There was no difference (p>0.05) between relative MS Flex at T2 (70.2 ± 21.4 Nm/kg) and T1 (67.9 ± 20.9 Nm/kg) but a significant increase (p<0.01) between T1 and T2 in relation to T0 (57.4 ± 16.8 Nm/kg).
Correlation of extension and flexion MS with body composition
The results of the correlations between Ext and Flex MS with total and appendicular body composition are in Table 2. A positive correlation (p<0.01) was observed between absolute MS Ext and FFM (kg) (r=0.65; r=0.71; r=0.66) and FFMLL (kg) (r=0.61; r=0.70; r=0.63) in T0, T1 and T2 respectively. There was also a positive correlation (p<0.01) between relative MS Ext and FFM% (r=0.49; r=0.59; r=0.46) and FFMLL % (r=0.51; r=0.63; r=0.49) in the three analyzed times. A positive correlation (p<0.01) was observed between the absolute MS Flex and FFM (kg) (r=0.54; r=0.59; r=0.63) and FFMLL (kg) (r=0.54; r=0.59; r=0.59) in all analyzed times. There was also a positive correlation (p<0.01) between relative MS Flex, FFM% (r=0.49; r=0.54; r=0.50) and FFMLL % (r= 0.42; r=0.57; r=0.53) in the three analyzed times.
Table 2.
Correlation of absolute and relative extension MS to body weight with the total and segmental body composition in patients with severe obesity before and after RYGB.
| T 0 (n=123) | T 1 (n=123) | T 2 (n=79) | |
|---|---|---|---|
| Ext (Nm) | |||
| FFM (kg) | 0.65** | 0.71** | 0.66** |
| FFMLL (kg) | 0.61** | 0.70** | 0.63** |
| Ext (Nm/Kg) | |||
| FFM (%) | 0.49** | 0.59** | 0.46** |
| FFMLL (%) | 0.51** | 0.63** | 0.49** |
| Flex (Nm) | |||
| FFM (kg) | 0.54** | 0.59** | 0.63** |
| FFMLL (kg) | 0.54** | 0.59** | 0.59** |
| Flex (Nm/Kg) | |||
| FFM (%) | 0.49** | 0.54** | 0.50** |
| FFMLL (%) | 0.42** | 0.57** | 0.53** |
where: T0 = preoperative; T1 = 6 months after surgery; T2 = 36 months after surgery; FFM: fat free mass; FFMLL: fat free mass of lower limbs; FM: fat mass; FMLL: fat mass of lower limbs; Ext: extension; Flex: flexion; Nm: newton-meter; Nm/kg: newton-meter/kilograms
p<0.01;
* p<0.05.
Anthropometric and muscle strength results according to surgical results
The anthropometric characteristics and absolute and relative Ext and Flex MS according to late surgical results are in Table 3. 59 (74.6%) patients achieved SS and 20 (25.4%) patients were considered SF. There were significant differences between both groups with respect to age, BMI, FMLL, FFMLL, Ext and Flex MS before surgery.
Table 3.
Anthropometric characteristics and absolute and relative extension and flexion muscle strength according to late surgical results.
| SS (n=59) | SF (n=20) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | |
| Age (years) | 40.2 ± 10.2 | 42.4 ± 10.3* | 45.5 ± 10.4** *** | 39.5 ± 8.9 | 41.5 ± 9.2* | 44.5 ± 9.1** *** |
| BMI (kg/m2) | 47 ± 4.4 | 33.5 ± 3.4* | 31.2 ± 3.3** *** | 47.6 ± 5.2 | 36.4 ± 5.0* | 38.8 ± 3.7** *** |
| FMLL (kg) | 17.0 ± 3.9 | 10 ± 2.5* | 8.7 ± 2.2** *** | 17.3 ± 3.1 | 11.6 ± 3.2* | 14.2 ± 2.9** *** |
| FFMLL (kg) | 17.8 ± 3.7 | 15.6 ± 3.4* | 15.0 ± 3.4** | 18.0 ± 3.9 | 16.9 ± 3.9 | 16.9 ± 3.7 |
| Ext. (Nm) | 155.5 ± 40.7 | 122.6 ± 32.3* | 120.6 ± 39.4** | 161.2 ± 51.5 | 130.6 ± 57.7* | 136.4 ± 48.3** |
| Flex (Nm) | 71.6 ± 21.1 | 59.0 ± 17.7* | 60.9 ± 21.2** | 74.3 ± 24.2 | 64.3 ± 33.4* | 66 ± 27.1** |
| Ext (Nm/kg) | 125.4 ± 28.4 | 140.5 ± 32.3* | 144.9 ± 39.8** | 125.3 ± 32.6 | 133.7 ± 45.2 | 125.5 ± 29.2 |
| Flex (Nm/kg) | 57.9 ± 15.7 | 67.7 ± 17.8* | 73.6 ± 21.8** | 57.8 ± 16.7 | 62.6 ± 30.6 | 60.4 ± 15.8 |
where: T0 = preoperative; T1 = 6 months; T2 = 36 months; BMI: body mass index; Ext: extension; Flex: flexion; Nm: newton-meter; Nm/kg: newton-meter/kilogram; FMLL: fat mass of lower limbs; FFMLL: fat free mass of lower limbs. Differences between times
T0 x T1- p<0.05;
T0 x T2- p<0.05;
T1 x T2- p<0.05.
In the SS group there was a significant decrease (p<0.05) in FMLL (T0 = 17.0 ± 3.9 kg x T1 = 10 ± 2.5 kg x T2 = 8.7 ± 2.2 kg) between three evaluation periods and in FFMLL between T1(15.6 ± 3.4 kg) and T2 (15 ± 3.4 kg) to T0 (17.8 ± 3.7 kg). There was a significant decrease (p<0.05) in absolute MS Ext and Flex between T1 and T2 to T0. There was no difference (p=0.5) in relative MS Ext between T2 (144.9 ± 39.8 Nm/Kg) and T1 (140.5 ± 32.3 Nm/Kg), but a significant increase (p<0.05) between T1 and T2 to T0 (125.4 ± 28.4 Nm/Kg). There was also no difference (p=0.25) in relative MS Flex between T2 (73.6 ± 21.8 Nm/Kg) and T1 (67.7 ± 17.8 Nm/Kg) but a significant increase (p<0.05) between T1 and T2 to T0 (57.9 ± 15.7 Nm/Kg).
In the SF group there was a significant decrease (p<0.05) in FMLL (T0 = 17.3 ± 3.1 kg x T1 = 11.6 ± 3.2 kg x T2 = 14.2 ± 2.9 kg) between three evaluation periods. There was no difference (p>0.05) in FFMLL (T0 = 18.0 ± 3.9 kg x T1 = 16.9 ± 3.9 kg x T2 =16.9 ± 3.7kg) between three evaluation periods. There was a significant decrease (p<0.05) in absolute MS Ext and Flex between T2 and T1 to T0. There was no difference in relative MS Ext between three evaluation periods (T0 = 125.3 ± 32.6 Nm/Kg x T1 =133.7 ± 45.2 Nm/Kg x T2 = 125.5 ± 29.2 Nm/Kg; p>0.05). There was no difference (p>0.05) in relative MS Flex (T0 = 57.8 ± 16.7 Nm/Kg x T1 = 62.6 ± 30.6 Nm/Kg x T2 = 60.4 ± 15.8 Nm/Kg) between three evaluation periods.
The absolute MS Ext and Flex according to surgical results
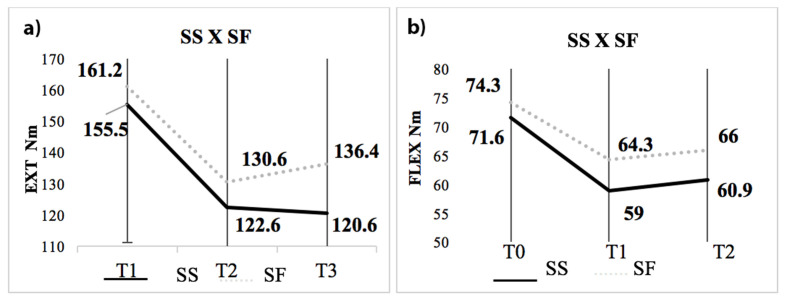
There was no difference between the absolute MS Ext between SS and SF at T0 (155.5 ± 40.7 Nm x 161.2 ± 51.5 Nm; p=0.61), T1 (122.6 ± 32.3 Nm x 130.6 ± 57.7 Nm; p=0.45) and T2 (120.6 ± 39.4 Nm x 136.4 ± 48.3; p=0.15). There was also no difference in absolute MS Flex between SS and SF at T0 (71.6 ± 21.1 Nm x 74.3 ± 24.2 Nm; p = 0.63), T1 (59.0 ± 17.7 Nm x 64.3 ± 33.4 Nm; p = 0.74) and T2 (73.6 ± 21.8 Nm x 66.0 ± 27.1 Nm; p=0.39), respectively (Figure 1).
Figure 1.
Results of absolute MS according to surgical results. a) Ext and b) Flex. T0 = preoperative; T1 = 6 months; T2 = 36 months; Ext: extension; Flex: flexion; Nm: newton-meter; Differences between times * p<0.05.
The relative MS Ext and Flex according to surgical results
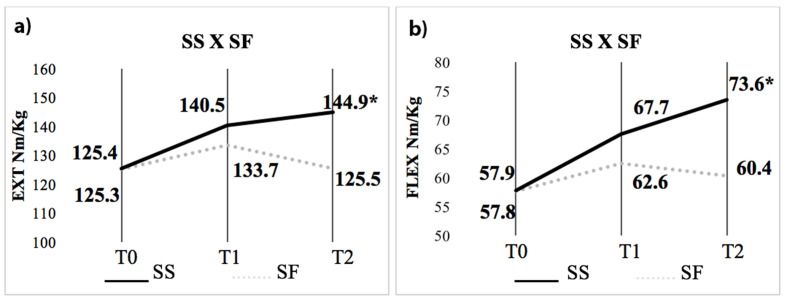
There was no difference in relative MS Ext between SS and SF at T0 (125.4 ± 28.4 Nm/kg x 125.3 ± 32.6 Nm/kg; p=0.98) and T1 (140.5 ± 32.3 Nm/kg x 133.7 ± 45.2 Nm/kg; p=0.42), respectively. There was also no difference in relative MS Flex between SS and SF in T0 (58 ± 15.7 Nm/kg x 57.8 ± 16.7 Nm/kg; p=0.96) and T1 (67.7 ± 17.8 Nm/Kg x 62.6 ± 30.6 Nm/kg; p=0.74) respectively. There was a significant difference in relative MS Ext between SS and SF in T2 (144.9 ± 39.8 Nm/kg x 125.5 ± 29.2 Nm/kg; p=0.04) and Flex/kg (73.6 ± 21.8 Nm/kg x 60.4 ± 15.8 Nm/kg; p=0.02), respectively (Figure 3).
Figure 2.
Results of relative MS according to surgical results. a) Ext and b) Flex. T0 = preoperative; T1 = 6 months; T2 = 36 months; Ext: extension; Flex: flexion; Nm: newton-meter/Kilograms; Differences between times * p<0.05.
Muscle Strength as a predictor of surgical success
Fifty-nine patients (75%) achieved success with surgical treatment at 36 months. 45 (76%) and 43 (73%) of these patients had an increase in Ext and Flex MS ≥4 Nm/kg at 6 months, respectively. In females, 46 achieved surgical success in 36 months, and 32 (70%) had an increase in both Ext and Flex MS ≥4 Nm/kg in 6 months. In males, 13 (100%) achieved successful surgical treatment and presented an increase in MS Ext and Flex ≥4 Nm/kg in 6 months. 45 patients with BMI >40 kg/m2 achieved successful surgical treatment in 36 months, and 34 (75%) and 31 (69%) had MS increase in Ext and Flex ≥4 Nm/kg at 6 months, respectively. 14 patients with BMI >50 kg/m2 achieved surgical success in T2, and 11 (79%) and 12 (86%) respectively, had an increase in Ext and Flex MS ≥4 Nm/kg in 6 months.
Discussion
Skeletal muscle, one of the most dynamic tissues involved in voluntary contraction, comprises approximately 40% of total body weight[21]. Slight changes in muscle mass and MS of the upper and lower limbs are associated with undesirable effects on physical function and the occurrence of disorders of the musculoskeletal system, especially after massive weight loss[22]. A large part of the weight loss is due to FM but also includes a decrease in lean mass and muscle strength occurring in the first 6 months postoperatively[22,23]. Alba et al verified that those who lost little lean mass after RYGB would maintain absolute strength and/or have an increase or stabilization of relative muscle strength[23].
Our results also demonstrated reductions significant reduction in lower limbs muscle absolute muscle strength 6 and 36 months after surgery, consistent with previous studies[23,24]. Interestingly, there was also no difference in absolute muscle strength between SS and SF patients. These results also reinforce the relevance of the association of lower limb MS with FFM before and after severe weight loss[24-28]. Studies have shown that these associations are moderate to weak in women before surgery[27,28]. Our results demonstrate positive correlations between absolute and relative MS with FFM and FFMLL maintained on long-term follow-up, a finding that had not been previously reported after surgery[24,29]. Despite the significant postoperative decline in lean mass and absolute muscle strength, there was an increase in relative muscle strength (“muscle quality”) 6 and 36 months after surgery. Muscle strength expressed in relation to body weight is an interesting way of estimating the functional performance of an individual[29]. Relative muscle strength significantly increased between 6 and 12 months postoperatively in some studies, which suggests that the initial gain in relative strength paralleled the weight loss after bariatric surgery[24,29,30]. Nevertheless, in our series we also observed a significant increase after surgery but without difference between 6 and 36 months.
The maintenance of relative MS between 6 and 36 months may be due to the heterogeneity of our patients. The decrease in relative strength seen after sustained weight loss from RYGB may be associated with a decline in physical function, which may be a concern, especially in elderly patients[24,30]. However, patients with an increase in relative strength at 6 months were the same patients who achieved surgical success at 36 months. The strength increase associated with SS was ≥4 Nm/kg in both Ext and Flex MS. We hypothesize that SS patients could increase muscle strength of lower limbs even without difference in FFMLL due to a great weight loss. We must consider that weight regain in SF patients may contribute to maintain FFMLL but without muscle quality since there was also an increase in FMLL that compromises muscle fibers.
The most important observation in our study is that an increase in relative MS 6 months after surgery may identify individuals with surgical success on long term follow up. In fact, 15-35% of individuals who did not achieve surgical success, regained the weight lost in less than one year[31]. Then, we consider important to include an exercise program with preset goals after surgical treatment to potentiate clinically relevant changes in physical capabilities[30,31]. In addition to improving functionality, resistance training exercises improve MS and may minimize FFM loss closely related to sarcopenic obesity[32]. We consider physical training essential as an adjuvant therapy in postoperative care to improve physical fitness and prevent weight regain[33]. Current literature emphasizes that the pre-operative period can be effectively evaluated to start exercise and learn appropriate physical activities[3,4]. Future studies should compare lower extremity muscle strength values in individuals with and without pre-post-operative period exercise interventions.
Also, the balance ability of this individuals, considering changes in muscle strength, BMI and body composition should be evaluate in future studies on long term follow up after bariatric surgery.
The main limitation of our study was not to include physical training to improve muscle mass and possible long-term benefits in postoperative care.
Our findings demonstrate that an increase ≥4 Nm/kg in both Ext and Flex MS at 6 months can predict surgical success in 36 months.
Ethics approval
The study protocol was performed according to the ethical recommendations of the Declaration of Helsinki and was approved by the Ethical Committee of the University of São Paulo Medical School (protocol number 01038912.6.0000.0068).
Consent to participate
Signed informed consent was obtained from all individual participants included in the study.
Funding
We gratefully acknowledge grant support from Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES).
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Buchwald H, Buchwald JN. Metabolic (Bariatric and Nonbariatric) Surgery for Type 2 Diabetes:A Personal Perspective Review. Diabetes Care. 2019;42(2):331–40. doi: 10.2337/dc17-2654. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson DJ, Erskine RM, Morse CI, Winwood K, Onambélé-Pearson G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. 2016;17(3):467–83. doi: 10.1007/s10522-015-9626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenberg E, Dos Reis Falcão LF, O'Kane M, Liem R, Pournaras DJ, Salminen P, et al. Guidelines for Perioperative Care in Bariatric Surgery:Enhanced Recovery After Surgery (ERAS) Society Recommendations:A 2021 Update. World J Surg. 2022;46(4):729–751. doi: 10.1007/s00268-021-06394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorell A, MacCormick AD, Awad S, Reynolds N, Roulin D, Demartines N, et al. Guidelines for Perioperative Care in Bariatric Surgery:Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016;40(9):2065–2083. doi: 10.1007/s00268-016-3492-3. [DOI] [PubMed] [Google Scholar]
- 5.Campanha-Versiani L, Pereira DAG, Ribeiro-Samora GA, Ramos AV, de Sander Diniz MFH, De Marco LA, et al. The Effect of a Muscle Weight-Bearing and Aerobic Exercise Program on the Body Composition, Muscular Strength, Biochemical Markers, and Bone Mass of Obese Patients Who Have Undergone Gastric Bypass Surgery. Obes Surg. 2017;27(8):2129–37. doi: 10.1007/s11695-017-2618-5. [DOI] [PubMed] [Google Scholar]
- 6.Anandacoomarasamy A, Fransen M, March L. Obesity and the musculoskeletal system. Current Opinion in Rheumatology. 2009;21(1):71–7. doi: 10.1097/bor.0b013e32831bc0d7. [DOI] [PubMed] [Google Scholar]
- 7.Paolillo FR, Milan JC, Bueno P, de G, Paolillo AR, Borghi-Silva A, Parizotto NA, et al. Effects of excess body mass on strength and fatigability of quadriceps in postmenopausal women. Menopause. 2012;19(5):556–61. doi: 10.1097/gme.0b013e3182364e80. [DOI] [PubMed] [Google Scholar]
- 8.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321(4):249–79. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hassannejad A, Khalaj A, Mansournia MA, Rajabian Tabesh M, Alizadeh Z. The Effect of Aerobic or Aerobic-Strength Exercise on Body Composition and Functional Capacity in Patients with BMI >/=35 after Bariatric Surgery:a Randomized Control Trial. Obes Surg. 2017;27(11):2792–801. doi: 10.1007/s11695-017-2717-3. [DOI] [PubMed] [Google Scholar]
- 10.Stegen S, Derave W, Calders P, Van Laethem C, Pattyn P. Physical fitness in morbidly obese patients:effect of gastric bypass surgery and exercise training. Obes Surg. 2011;21(1):61–70. doi: 10.1007/s11695-009-0045-y. [DOI] [PubMed] [Google Scholar]
- 11.Hue O, Berrigan F, Simoneau M, Marcotte J, Marceau P, Marceau S, et al. Muscle force and force control after weight loss in obese and morbidly obese men. Obes Surg. 2008;18(9):1112–8. doi: 10.1007/s11695-008-9597-5. [DOI] [PubMed] [Google Scholar]
- 12.Adil MT, Jain V, Rashid F, Al-Taan O, Whitelaw D, Jambulingam P. Meta-analysis of the effect of bariatric surgery on physical function. Br J Surg. 2018;105(9):1107–18. doi: 10.1002/bjs.10880. [DOI] [PubMed] [Google Scholar]
- 13.Herring LY, Stevinson C, Davies MJ, Biddle SJ, Sutton C, Bowrey D, et al. Changes in physical activity behavior and physical function after bariatric surgery:a systematic review and meta-analysis. Obes Rev. 2016;17(3):250–61. doi: 10.1111/obr.12361. [DOI] [PubMed] [Google Scholar]
- 14.Gil S, Goessler K, Dantas WS, Murai IH, Merege-Filho CAA, Pereira RMR, et al. Constraints of Weight Loss as a Marker of Bariatric Surgery Success:An Exploratory Study. Front. Physiol. 2021;11(12):640191. doi: 10.3389/fphys.2021.640191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vakula MN, Fisher KL, Garcia SA, Holmes SC, Post BK, Costa PB, et al. Quadriceps Impairment Is Associated with Gait Mechanics in Young Adults with Obesity. Med Sci Sports Exerc. 2019;51(5):951–61. doi: 10.1249/MSS.0000000000001891. [DOI] [PubMed] [Google Scholar]
- 16.Calmels PM, Nellen M, van der Borne I, Jourdin P, Minaire P. Concentric and eccentric isokinetic assessment of flexor-extensor torque ratios at the hip, knee, and ankle in a sample population of healthy subjects. Arch Phys Med Rehabil. 1997;78(11):1224–30. doi: 10.1016/s0003-9993(97)90336-1. [DOI] [PubMed] [Google Scholar]
- 17.Gaines JM, Talbot LA. Isokinetic strength testing in research and practice. Biol Res Nurs. 1999;1(1):57–64. doi: 10.1177/109980049900100108. [DOI] [PubMed] [Google Scholar]
- 18.Van de Laar AW, Acherman YI. Weight loss percentile charts of large representative series:a benchmark defining sufficient weight loss challenging current criteria for success of bariatric surgery. Obes Surg. 2014;24(5):727–34. doi: 10.1007/s11695-013-1130-9. [DOI] [PubMed] [Google Scholar]
- 19.Tamboli RA, Hossain HA, Marks PA, et al. Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2010;18(9):1718–1724. doi: 10.1038/oby.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbajo MA, Jimenez JM, Luque-de-Leon E, Cao MJ, Lopez M, Garcia S, et al. Evaluation of Weight Loss Indicators and Laparoscopic One-Anastomosis Gastric Bypass Outcomes. Sci Rep. 2018;8(1):1961. doi: 10.1038/s41598-018-20303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frontera WR, Ochala J. Skeletal muscle:a brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–95. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 22.Kim B, Tsujimoto T, So R, Zhao X, Oh S, Tanaka K. Changes in muscle strength after diet-induced weight reduction in adult men with obesity:A prospective study. Diabetes Metab Syndr Obes. 2017;10:187–194. doi: 10.2147/DMSO.S132707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alba DL, Wu L, Cawthon PM, Mulligan K, Lang T, Patel S, et al. Changes in Lean Mass, Absolute and Relative Muscle Strength, and Physical Performance After Gastric Bypass Surgery. J Clin Endocrinol Metab. 2019;104:711–720. doi: 10.1210/jc.2018-00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira FT, de Oliveira GS, Gonçalves VSS, Neri SGR, de Carvalho KMB, Dutra ES. Effect of physical exercise on muscle strength in adults following bariatric surgery:A systematic review and meta-analysis of different muscle strength assessment tests. PLoS One. 2022;17(6):e0269699. doi: 10.1371/journal.pone.0269699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E, Brumagne S. Study of differences in peripheral muscle strength of lean versus obese women:an allometric approach. Int J Obes Relat Metab Disord. 2001;25(5):676–81. doi: 10.1038/sj.ijo.0801560. [DOI] [PubMed] [Google Scholar]
- 26.Miller GD, Nicklas BJ, You T, Fernandez A. Physical function improvements after laparoscopic Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2009;5(5):530–7. doi: 10.1016/j.soard.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinmann A, Gafner SC, Hilfiker R, Bruyneel AV, Pataky Z, Allet L. Bariatric Surgery:Consequences on Functional Capacities in Patients with Obesity. Frontiers in Endocrinology. 2021:12. doi: 10.3389/fendo.2021.646283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadducci AV, de Cleva R, de Faria Santarem GC, Silva PRS, Greve JMD, Santo MA. Muscle strength and body composition in severe obesity. Clinics (São Paulo) 2017;72(5):272–5. doi: 10.6061/clinics/2017(05)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyytinen T, Liikavainio T, Pääkkönen M, Gylling H, Arokoski JP. Physical function and properties of quadriceps femoris muscle after bariatric surgery and subsequent weight loss. J Musculoskelet Neuronal Interact. 2013;13(3):291–300. [PubMed] [Google Scholar]
- 30.Bellicha A, Ciangura C, Roda C, Torcivia A, Aron-Wisnewsky J, Poitou C, et al. Effect of exercise training after bariatric surgery:A 5-year follow-up study of a randomized controlled trial. PLoS One. 2022;15(17(7)):e0271561. doi: 10.1371/journal.pone.0271561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coen PM, Carnero EA, Goodpaster BH. Exercise and Bariatric Surgery:An Effective Therapeutic Strategy. Exerc Sport Sci Rev. 2018;46(4):262–70. doi: 10.1249/JES.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oppert JM, Bellicha A, Roda C, Bouillot JL, Torcivia A, Clement K, et al. Resistance Training and Protein Supplementation Increase Strength After Bariatric Surgery:A Randomized Controlled Trial. Obesity. 2018;26(11):1709–1720. doi: 10.1002/oby.22317. [DOI] [PubMed] [Google Scholar]
- 33.Bellicha A, van Baak MA, Battista F, Beaulieu K, Blundell JE, Busetto L, Carraça EV, Dicker D, Encantado J, Ermolao A, Farpour-Lambert N, Pramono A, Woodward E, Oppert JM. Effect of exercise training before and after bariatric surgery:a systematic review and meta-analysis. Obes Rev. 2021;22(S4):e13296. doi: 10.1111/obr.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]