Abstract
Background
In interstitial pneumonia (IP)-associated lung cancer, immune checkpoint inhibitor pneumonitis (ICIP) is common with immune checkpoint inhibitor (ICI) treatment. The purpose of the present study was to clarify the safety and efficacy of ICI treatment for patients with lung cancer with IP.
Methods
This multicentre retrospective observational study was conducted from June 2016 to December 2020 in patients with primary lung cancer with IP who received ICI treatment.
Results
A total of 200 patients (median age 70 years; male/female, 176/24) were enrolled from 27 institutions. ICIP occurred in 61 patients (30.5%), pneumonitis grades 3–5 in 32 patients (15.5%) and death in nine patients (4.5%). The common computed tomography pattern of ICIP was organising pneumonia in 29 patients (47.5%). Subsequently, diffuse alveolar damage (DAD) pattern was observed in 19 patients (31.1%) who had a significantly worse prognosis than those with a non-DAD pattern (median progression-free survival (PFS) 115 days versus 226 days, p=0.042; median overall survival (OS) 334 days versus 1316 days, p<0.001). Immune-related adverse events (irAEs) occurred in approximately 50% of patients. Patients with irAEs (n=100) had a better prognosis than those without irAEs (n=100) (median PFS 200 days versus 77 days, p<0.001; median OS 597 days versus 390 days p=0.0074). The objective response rate and disease control rate were 41.3% and 68.5%, respectively.
Conclusions
Although ICI treatment was effective for patients with lung cancer with IP, ICIP developed in approximately 30% of patients. Patients with irAEs had a significantly better PFS and OS than those without irAEs.
Shareable abstract
ICIs were an effective treatment for patients with lung cancer with IP and ICIP grades 3–5, with death occurring in 32 (16.0%) and nine (4.5%) patients. Patients with irAEs had significantly better PFS and OS than those without irAEs. https://bit.ly/3vuctoa
Introduction
A high lung cancer incidence (2.7–48.0%) has been found in patients with interstitial pneumonia (IP), particularly for those with idiopathic pulmonary fibrosis (IPF). The prevalence in Asian and European cohorts was 15.3% and 11.6%, respectively [1–3]. Acute exacerbation of IP (AE-IP) occurs in approximately 5–15% of affected patients. It can occur at any time even in the absence of lung cancer and may be fatal [4–6]. Treatments of patients with IP who developed lung cancer include surgery, radiotherapy and pharmacological therapy. However, anticancer drug therapy may trigger AE-IP. More often, it is difficult to distinguish AE-IP from anticancer drug-induced pneumonia based on clinical findings, computed tomography (CT) imaging and laboratory findings [4]. Thus, immune checkpoint inhibitor (ICI)-induced adverse events have been reported as immune checkpoint inhibitor pneumonitis (ICIP) [7]. Additionally, checkpoint inhibitor pneumonitis and immune checkpoint inhibitor-related pneumonitis have been reported to have the same meaning. Some reports in recent years have also defined them as ICIP [7, 8].
The first-line chemotherapy for patients with nonsmall cell lung cancer (NSCLC) with IP uses platinum doublet including nab-paclitaxel or S-1 [9, 10]. The Diffuse Lung Disease Research Group in Japan conducted a multicentre retrospective study, and found that the 1-year survival rate from first-line cytotoxic chemotherapy was 61% and the 1-year survival rate from second-line chemotherapy was 32% in patients with lung cancer with IP treated using cytotoxic anticancer agents [11]. The J-SONIC trial, the first randomised-controlled phase III study for patients with IPF and lung cancer, did not reveal a significantly prolonged exacerbation-free survival with nintedanib plus chemotherapy compared with chemotherapy alone, but confirmed the safety profile of the combined treatment using carboplatin plus nab-paclitaxel with nintedanib [12].
Currently, the treatment strategies for treatment-naïve advanced NSCLC without IP include ICI alone or ICI plus chemotherapy if the driver mutation/translocation is negative [13]. As a second-line therapy, ICIs are a therapeutic option when cytotoxic anticancer drugs are administered as initial therapy [14–17]. Furthermore, the addition of ICI to platinum combination therapy for extensive-stage small cell lung cancer prolonged overall survival (OS) and progression-free survival (PFS) [18, 19]. However, large-scale phase III trials validating the efficacy of ICIs usually exclude patients with pre-existing IP. Hence, ICI safety and efficacy in lung cancer with IP have not been established. In a Japanese pilot study for patients with NSCLC with mild IP (n=6) treated with nivolumab, the objective response rate (ORR) was 50% and the disease control rate (DCR) was 100% [20]. A phase II trial (n=18) using nivolumab for patients with NSCLC with mild IP had a response rate of 39% and a DCR of 72% [21]. Thus, prospective studies of second-line or later ICI therapy for lung cancer with mild IP suggests high efficacy. The TORG1936/AMBITIOUS trial of atezolizumab in previously treated patients with NSCLC with IP was discontinued due to frequent episodes of ICIP; the ICIP incidence was 29.4% (n=5) for all grades, 23.5% (n=4) for grade 3 or higher and 6% (n=1) for grade 5 [22, 23]. Therefore, the safety of immunotherapy for patients with IP and lung cancer remains unknown.
The previously reported studies have many limitations, including: 1) small sample sizes; 2) ICIP in patients with lung cancer with IP which has not been fully investigated in real-world settings; 3) evaluating ICI treatment on its own; and 4) the limited literature available on the safety and efficacy of cytotoxic anticancer drugs plus ICIs. If the safety and efficacy of ICIs are elucidated, this will provide guidance for the use of ICIs for patients with lung cancer with IP. Thus, this study retrospectively examined the safety and efficacy of ICIs for patients with lung cancer with IP.
Material and methods
Study design and patients
From 1 January 2016 to 31 December 2020, patients who satisfied all the following criteria were included in the study (information was collated from charts of 27 participating institutions from the Diffuse Pulmonary Disorders Research Group, organised by the Japanese Ministry of Health, Labour and Welfare): 1) patients with pathologically confirmed primary lung cancer; 2) with IP; and 3) in whom ICIs were administered, including single ICIs (nivolumab, pembrolizumab, atezolizumab and durvalumab), or cytotoxic anticancer drug+ICI (+ICI) combination therapy, or ICI+ICI. The primary end-points were ICIP occurrence as an immune-related adverse event (irAE) and the development of severe irAEs. Secondary end-points included the response rate to ICI monotherapy or combination therapy, PFS and OS. The Institutional Review Board of Toho University Medical Centre Omori Hospital approved this multicentre retrospective study (M21240, 2164, 20306, 20274, 20184). The University Hospital Medical Information Network number is ID000043974.
irAE
irAE is defined as a side-effect of ICI administration. It is thought to be a side-effect of an excessive autoimmune reaction and affects various organs, including the lungs, skin, digestive system, thyroid and pituitary gland. The severity of symptoms associated with irAE was determined using the Common Terminology Criteria for Adverse Events [24], which provides defined standards for grading the severity of organ toxicity.
IPF diagnosis
When high-resolution computed tomography (HRCT) findings show the usual interstitial pneumonia (UIP) pattern, a surgical lung biopsy is not required to establish IPF diagnosis; however, a combination of HRCT and bronchoscopy or surgical lung biopsy are required for a non-UIP pattern. Diagnoses are established by a respiratory physician, radiologist and pathologist with a multi-disciplinary discussion to make a comprehensive judgement [25]. The criteria of the official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society clinical practice guidelines were used to define the UIP pattern on HRCT [26].
Definition of ICIP
ICIP was defined as lung injury during ICI treatment of patients with primary lung cancer. CT patterns of CIP were classified into diffuse alveolar damage (DAD), organising pneumonia (OP), hypersensitivity pneumonitis (HP) and nonspecific IP (NSIP) patterns [27–30]. The OP pattern is characterised by areas of consolidation that are predominantly peripheral or peribronchovascular in distribution. The DAD pattern shows extensive bilateral area of ground-glass opacity and depends on airspace consolidation with traction bronchiectasis. The HP pattern shows small, poorly defined centrilobular nodules with or without vast areas of ground-glass opacity or lobular areas of decreased attenuation and vascularity. The NSIP pattern consists of patchy or diffuse areas of ground-glass opacity, predominately for the peripheral and lower lung zones [27–30].
Statistical analysis
We performed statistical analysis using data from 200 registered patients. RECIST ver.1.1 was used to determine stable disease (SD), partial response (PR), complete response (CR), progressive disease (PD) or not evaluable (NE). The best response means the best objective evaluation of tumour regression from the initiation to termination of ICI therapy. The ORR is the proportion of CR and PR at the best response. The DCR is the proportion of patients with CR, PR and SD at the best response. The PFS was defined as the time to disease progression or death due to any cause according to the RECIST criteria, from the start of each treatment. The OS was defined as the time from treatment initiation to death from any cause. Patients who were lost to follow-up were censored at the date of the last confirmation of survival.
Statistical analysis was performed using IBM Statistics software (version 24.0). To draw survival curves, the Kaplan–Meier method was used, and the log-rank test was used for statistical analysis. We analysed correlations of ICIP grades 3–5 with clinical variables before ICI treatment, such as sex, age, performance status (PS), smoking index, history of collagen disease, number of metastatic sites, programmed death-ligand 1 (PD-L1) expression, IPF, UIP pattern on HRCT, combined pulmonary fibrosis and emphysema (CPFE), PaO2/FIO2 (arterial oxygen tension/ inspiratory oxygen fraction) ratio, peripheral oxygen saturation (SpO2), interstitial lung disease (ILD) global alignment and proportion score, Krebs von den Lungen-6 (KL-6), surfactant protein D (SP-D), forced expiratory volume in 1 s (FEV1) percentage, percentage vital capacity, percentage forced vital capacity, percentage diffusing capacity of the lung for carbon monoxide (DLCO), history of administration of steroid, pirfenidone and immunosuppressive drug, home oxygen therapy (HOT), history of surgery and radiation therapy, white blood cell count, neutrocyte count, lymph before treatment, eosinocyte count, neutrophil–lymphocyte ratio, total bilirubin (T-Bil), serum creatinine (Cr), C-reactive protein (CRP) and HbA1c. As explanatory variables, multivariate logistic regression analysis was conducted using the clinical variables, such as IPF (yes versus no), UIP pattern on HRCT (yes versus no), CPFE (yes versus no), SpO2 (%), KL-6 (U·mL−1), SP-D (ng·mL−1), HOT (yes versus no), lymphocyte count (%), T-Bil (mg·dL−1), Cr (mg·dL−1) and CRP (mg·dL−1). Statistical significance was set at p-values of <5%.
Results
Basic characteristics
A total of 200 patients were enrolled from 27 participating centres. The mean age was 69 years, and 176 patients were male. PS 0–1 accounted for 95% of patients. Most patients had a history of smoking. Approximately 92% of patients were diagnosed with NSCLC and nine patients had small cell lung cancer. Most patients were clinical stage III or IV. PD-L1 expression of 50% or higher was present in 33.5% of patients (table 1).
TABLE 1.
Basic characteristics of patients (n=200)
| Age (years) range | 49–90 |
| Mean±sd | 69±6.9 |
| Sex | |
| Male | 176 (88.0%) |
| Female | 24 (12.0%) |
| Performance status | |
| 0 | 90 (45.0%) |
| 1 | 100 (50.0%) |
| 2–3 | 8 (4.0%) |
| Unknown | 2 (1.0%) |
| Histology | |
| Ad | 89 (44.5%) |
| Sq | 81 (40.5%) |
| NSCLC-NOS | 14 (7.0%) |
| SCLC | 9 (4.5%) |
| Others | 7 (3.5%) |
| Clinical stage | |
| I | 7 (3.5%) |
| II | 10 (5.0%) |
| III | 55 (27.5%) |
| IV | 100 (50.0%) |
| Rec | 28 (14.0%) |
| Smoking history | |
| Current | 73 (36.5%) |
| Former | 124 (62.0%) |
| Never | 3 (1.5%) |
| Pack-years (mean±sd) | 53.3±29.0 |
| PD-L1 expression (TPS%) | |
| 50–100 | 67 (33.5%) |
| 1–49 | 55 (27.5%) |
| 0 | 33 (16.5%) |
| Unknown | 50 (25.0%) |
| EGFR mutation | |
| Positive | 3 (1.5%) |
| Negative | 144 (72.0%) |
| Unknown | 53 (26.5%) |
| ALK rearrangement | |
| Positive | 0 (0.0%) |
| Negative | 133 (66.5%) |
| Unknown | 67 (33.5%) |
Ad: adenocarcinoma; Sq: squamous cell carcinoma; NSCLC: nonsmall cell lung cancer; NOS: not otherwise specified; SCLC: small cell lung cancer; Rec: recurrence after surgical resection; PD-L1: programmed death-ligand 1; TPS: tumour proportion score; EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase receptor.
Characteristics of patients with pre-existing IP
The characteristics of patients with pre-existing IP are shown in table 2. Of these, 92 were patients with IPF. In non-IPF patients, unclassifiable ILD accounted for most patients (32.5%). The most common HRCT findings were an indeterminate UIP pattern (33%), followed by a UIP pattern (25.5%) and probable UIP pattern (25.5%). CPFE was found in 53.5% of patients. Background IP treatment was extremely rare (14.0% of patients). Corticosteroids were administered in 9% of patients, pirfenidone in 3.5%, nintedanib in 4% and nonsteroid immunosuppressive drugs in 1.5% of patients. Pre-treatment pulmonary function testing results revealed slightly reduced average % DLCO for all patients.
TABLE 2.
Characteristics of patients with pre-existing IP (n=200)
| IPF or non-IPF | |
| IPF | 92 (46.0%) |
| Non-IPF | 108 (54.0%) |
| Non-IPF classification | |
| CHP | 5 (2.5%) |
| COP | 2 (1.0%) |
| DIP | 2 (1.0%) |
| NSIP | 10 (5.0%) |
| CVD-IP | 10 (5.0%) |
| Unclassifiable ILD | 65 (32.5%) |
| Others | 14 (7.0%) |
| HRCT pattern | |
| UIP | 51 (25.5%) |
| Probable UIP | 51 (25.5%) |
| Indeterminate for UIP | 66 (33.0%) |
| Alternative diagnosis | 19 (9.5%) |
| Others | 13 (6.5%) |
| CPFE | |
| Yes | 107 (53.5%) |
| No | 93 (46.5%) |
| Steroids | |
| Yes | 18 (9.0%) |
| No | 182 (91.0%) |
| Antifibrotic drugs | |
| Pirfenidone | 7 (3.5%) |
| Nintedanib | 8 (4.0%) |
| Nonsteroid immunosuppressive drugs | |
| Yes | 3 (1.5%) |
| No | 197 (98.5%) |
| Serum marker of IP | |
| LDH (IU·mL−1) | 221.9±73.6 |
| KL-6 (U·mL−1) | 686.4±467.9 |
| SP-D (ng·mL−1) | 126.9±101.5 |
| Pulmonary function testing | |
| % VC (%) | 93.0±21.1 |
| % FVC (%) | 93.2±20.0 |
| FEV1% (%) | 74.9±10.7 |
| % DLCO (%) | 74.2±25.1 |
IP: interstitial pneumonia; IPF: idiopathic pulmonary fibrosis; CHP: chronic hypersensitivity pneumonia; COP: cryptogenic organising pneumonia; DIP: desquamative interstitial pneumonia; NSIP: nonspecific interstitial pneumonia; CVD: collagen vascular diseases; ILD: interstitial lung disease; UIP: usual interstitial pneumonia; CPFE: combined pulmonary fibrosis and emphysema; LDH: lactate dehydrogenase; KL-6: Krebs von den Lungen-6; SP-D: surfactant protein D; VC: % vital capacity; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide.
ICIP
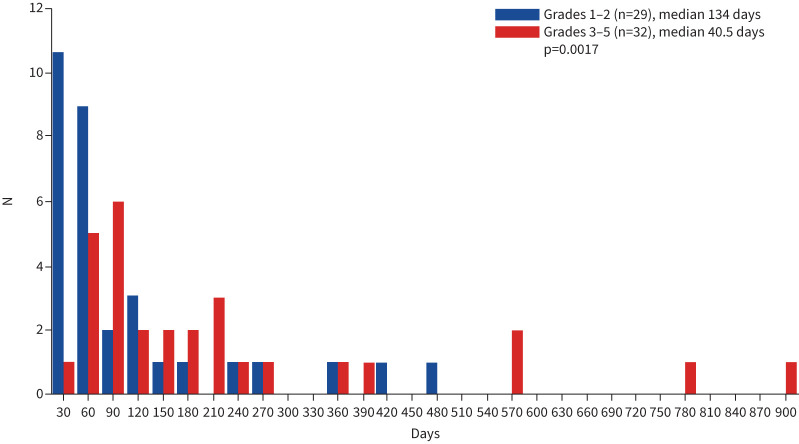
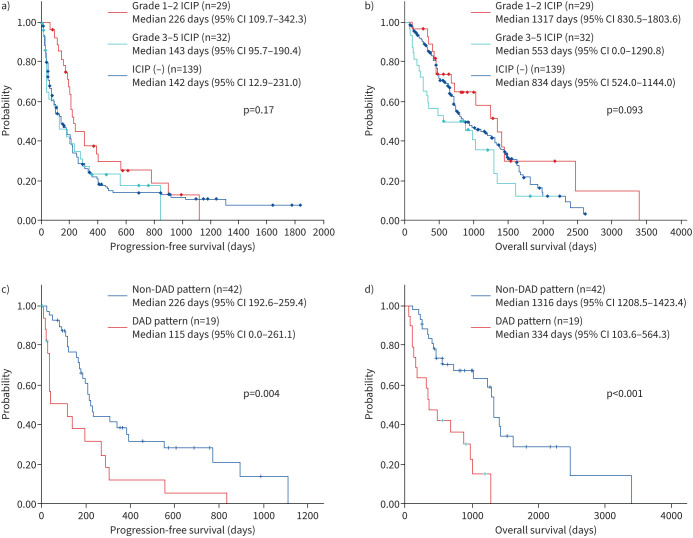
ICIP was noted in 61 out of 200 patients (30.5%) (table 3). Of these, 32 out of 200 patients (16.0%) were ≥ grade 3. The median time to start the ICI treatment was 134 days in patients with grades 1–2 and 40.5 days in those with grades 3–5 (p=0.0017) (figure 1). Grade 5 ICIP occurred in nine patients (4.5%). The group with grades 1–2 ICIP had a better prognosis than the group with grades 3–5 and the one without ICIP (median PFS 226 days versus 143 days versus 142 days, p=0.17; median OS 1317 days versus 553 days versus 834 days, p=0.093) (figure 2). HRCT analysis showed that approximately half of the patients had an OP pattern (29/61 patients). Steroid therapy was used in 36 (59.0%) patients, steroid pulse therapy in 17 (27.9%) patients and ICI discontinuation in eight patients. Steroid therapy and steroid pulse therapy were used successfully in 86.9% of patients (table 3).
TABLE 3.
Characteristics of patients with immune checkpoint inhibitor pneumonitis (n=200)
| Grade | |
| 0 | 139 (69.5%) |
| 1 | 10 (5.0%) |
| 2 | 19 (9.5%) |
| 3 | 23 (11.5%) |
| 4 | 0 (0.0%) |
| 5 | 9 (4.5%) |
| CT pattern (n=61) | |
| OP pattern | 29 (47.5%) |
| DAD pattern | 19 (31.1%) |
| NSIP pattern | 6 (9.8%) |
| HP pattern | 3 (4.9%) |
| Others | 4 (3.3%) |
| Medication (n=61) | |
| Steroid | 36 (59.0%) |
| Steroid pulse | 17 (27.9%) |
| Misadministration of ICI | 8 (13.1%) |
CT: computed tomography; OP: organising pneumonia; DAD: diffuse alveolar damage; NSIP: nonspecific interstitial pneumonia; HP: hypersensitivity pneumonitis; ICI: immune checkpoint inhibitors.
FIGURE 1.
Immune checkpoint inhibitor pneumonitis in patients with interstitial pneumonia as the underlying disease and primary lung cancer (n=61). The median time of onset was 134 days in all patients and 40.5 days in patients with ≥ grade 3.
FIGURE 2.
Prognosis of immune checkpoint inhibitor pneumonitis in primary lung cancer patients with underlying interstitial pneumonia (n=200). a, b) Grades 1–2 have a better prognosis than grades 3–5 and patients without ICIP (median progression-free survival 226 days versus 143 days versus 142 days, p=0.17; median overall survival 1317 days versus 553 days versus 834 days, p=0.093). c, d) Patients with non-DAD (N=42) had a significantly better prognosis than patients with DAD (N=19) (median PFS 226 days versus 115 days p=0.042; median overall survival 1316 days versus 334 days, p<0.001). DAD: diffuse alveolar damage; ICIP: immune checkpoint inhibitor pneumonitis.
DAD pattern of ICIP
Patients with a non-DAD pattern (N=42) had a significantly better prognosis than DAD patients (N=19) (median PFS 226 days versus 115 days p=0.042; median OS 1316 days versus 334 days, p=0.035) (figure 2).
ICI therapy and ICIP
Table 4 shows ICIP incidence in patients having ICI treatments. Single ICIs were administered to 163 patients (81.5%) and they were primarily first-line and second-line therapies. ICIP occurred in five out of nine (55.6%) durvalumab-treated patients across all ICIP grades. Furthermore, grade 3–5 were observed in 9 of 28 patients (32.1%) treated with atezolizumab and two of eight patients (25.0%) treated with carboplatin (CBDCA)+ pemetrexed (PEM)+pembrolizumab. All grades of ICIP were observed in 52 of 163 patients (31.9%), and ICIP grades 3–5 were observed in 28 of 163 patients (17.2%) who received ICI monotherapy. Conversely, ICIP of all grades was seen in 9 of 35 patients (25.7%), and ICIP grades 3–5 was seen in 4 of 35 patients (11.4%) with cytotoxic drug plus ICI combination therapy. No significant difference was observed between patients receiving ICI monotherapy and patients receiving a cytotoxic drug plus ICI therapy.
TABLE 4.
Patients with ICIP treated with ICI therapies (n=200)
| Regimen | n | All grades | ≥ grade 3 |
| ICI monotherapy | 163 | 52 (31.9%) | 28 (17.2%) |
| Pembrolizumab | 81 | 18 (40.0%) | 7 (15.5%) |
| Nivolumab | 45 | 19 (22.1%) | 10 (11.6%) |
| Atezolizumab | 28 | 10 (35.7%) | 9 (32.1%) |
| Durvalumab | 9 | 5 (55.6%) | 2 (22.2%) |
| ICI (+ICI) +platinum doublet | 35 | 9 (25.7%) | 4 (11.4%) |
| CBDCA+PEM+pembrolizumab | 10 | 4 (50.0%) | 2 (25.0%) |
| CBDCA+PAC+pembrolizumab | 8 | 0 (0.0%) | 0 (0.0%) |
| CDDP+PEM+pembrolizumab | 1 | 1 (100%) | 1 (100%) |
| CBDCA+nabPAC+pembrolizumab | 1 | 1 (10%) | 0 (0.0%) |
| CBDCA+PEM+atezolizumab | 7 | 0 (0.0%) | 0 (0.0%) |
| CBDCA+PAC+atezolizumab+BEV | 5 | 1 (20%) | 1 (20.0%) |
| CBDCA+VP-16+atezolizumab | 1 | 2 (28.6%) | 0 (0.0%) |
| CBDCA+VP-16+durvalumab | 1 | 0 (0.0%) | 0 (0.0%) |
| CBDCA+PEM+ipilimumab+nivolumab | 1 | 0 (0.0%) | 0 (0.0%) |
| ICI+ICI | 2 | 0 (0.0%) | 0 (0.0%) |
| Ipilimumab+nivolumab | 2 | 0 (0.0%) | 0 (0.0%) |
ICIP: immune checkpoint inhibitor pneumonitis; ICI: immune checkpoint inhibitor; CBDCA: carboplatin; PEM: pemetrexed; PAC: paclitaxel; CDDP: cisplatin; nabPAC: nab-paclitaxel; BEV: bevacizumab; VP-16: etoposide.
Efficacy of ICI therapy
CR was found in four patients, PR in 78 patients, SD in 49 patients, PD in 57 patients and NE in 12 patients, with an ORR of 41.0% and DCR of 65.5% (Supplementary table 1). The median PFS was 172 days and the median OS after ICI treatment was 933 days (Supplementary figure 1). No significant difference was found in the PFS between ICI monotherapy and cytotoxic chemotherapy plus ICI combination therapy (median PFS 180 days (95% CI 103.9–216.0) versus 160 days (95% CI 114.4–245.6, p=0.73)); however, a significant difference was found for OS (median OS 655 days (95% CI 390.4–919.6) versus 1062 days (95% CI 626.5–1425.5, p=0.0091)) (Supplementary figure 2). Patients with high PD-L1 expression (≥50%) showed significantly longer PFS and OS than patients with low PD-L1 expression (1–49%) or no PD-L1 expression (Supplementary figure 3).
Risk factors for developing serious ICIP
Multivariate analysis was used to determine the predictors of ICIP grades 3–5 using the pre-ICI clinical variables. IPF (odds ratio (OR) 2.87, 95% CI 1.699–7.69, p=0.036) and pre-treatment CRP (OR 1.11, 95% CI 1.014–1.22, p=0.023) were significant risk factors for ICIP grades 3–5 (table 5).
TABLE 5.
Risk factors for developing severe immune checkpoint inhibitor pneumonitis (n=200)
| Risk factor | Odds ratio | 95% CI | p-value |
| IPF (+ versus −) | 2.87 | 1.699–7.69 | 0.036 |
| CPFE (+ versus −) | 1.34 | 0.49–3.62 | 0.56 |
| UIP pattern (+ versus −) | 1.84 | 0.59–5.68 | 0.29 |
| SpO2 | 0.91 | 0.73–1.14 | 0.43 |
| KL-6 (U·mL−1) | 1.0002 | 0.9993–1.0011 | 0.62 |
| SP-D (ng·mL−1) | 1.0052 | 0.9995–1.011 | 0.072 |
| HOT (+ versus −) | 1.2116 | 0.35–4.15 | 0.75 |
| Lymphocyte count (%) | 0.98 | 0.92–1.035 | 0.43 |
| T-bil (mg·dl−1) | 2.31 | 0.20–26.02 | 0.50 |
| Cr (mg·dl−1) | 0.32 | 0.032–3.15 | 0.32 |
| CRP (mg·dl−1) | 1.11 | 1.014–1.22 | 0.023 |
IPF: idiopathic pulmonary fibrosis; CPFE: combined pulmonary fibrosis and emphysema; UIP: usual interstitial pneumonia; SpO2: oxygen saturation of peripheral artery; KL-6: Krebs von den Lungen-6; SP-D: surfactant protein D; HOT: home oxygen therapy; T-bil: total bilirubin; Cr: serum creatinine; CRP: C-reactive protein.
irAEs other than ICIP
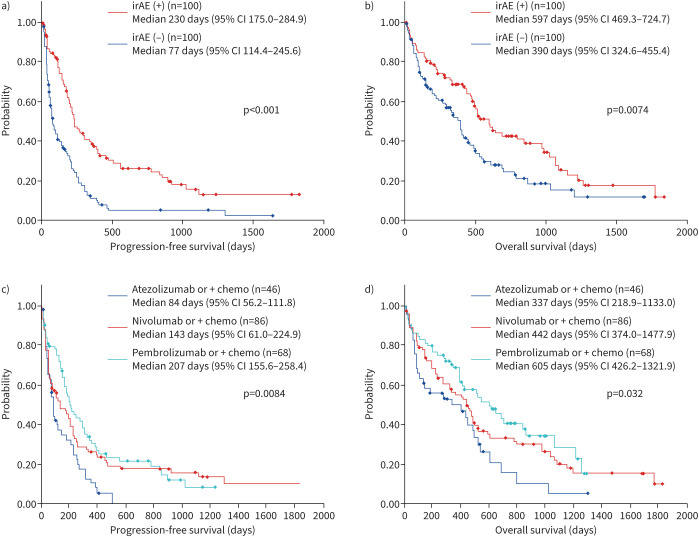
irAEs occurred in approximately 50% of patients: serious irAEs other than ICIP (grade 3 or higher) included skin eruption in two patients, liver injury in one patient, fever and pituitary injury in two patients, encephalitis in two patients, diarrhoea in three patients and myocarditis and renal injury in three patients each, and neutropenia and pancreatitis were observed in one patient each. The PFS and OS were significantly better in the group with irAEs (n=100) compared with those without irAEs (n=100) (median PFS 230 days versus 77 days, p<0.001; median OS after ICI treatment 597 days versus 390 days, p<0.0074) (figure 3).
FIGURE 3.
Efficacy of immune checkpoint inhibitor therapy in primary lung cancer patients with underlying interstitial pneumonia (n=200). a, b) Prognosis was significantly better in patients with irAEs (n=100) compared with patients without irAEs (n=100) (post-ICI treatment, median PFS 230 days versus 77 days, median OS 597 days versus 390 days). c, d) A significant difference was observed in prognosis of patients treated with different ICI agents (PFS, p=0.0084; OS, p=0.032): pembrolizumab monotherapy or combination therapy (median PFS 207 days; median OS 605 days), nivolumab monotherapy or combination therapy (median PFS 143 days; median OS 442 days) and atezolizumab monotherapy or combination therapy (median PFS 87 days; median OS 337 days). ICI: immune checkpoint inhibitor; irAE: immune-related adverse event; OS: overall survival; PFS: progression-free survival.
Comparison of prognosis by ICI agent in lung cancer with IP
We also compared the prognosis using the ICI agent: pembrolizumab with or without chemotherapy (median PFS 207 days; median OS 605 days), nivolumab with or without chemotherapy (median PFS 143 days; median OS 442 days) and atezolizumab with or without chemotherapy (median PFS 64 days; median OS 337 days). A significant difference was observed between the ICI agents used (PFS, p=0.0084; OS, p=0.032) (figure 3).
Discussion
To investigate the efficacy and safety of ICI treatments, we used real-world data from a nationwide survey in a cohort of 200 lung cancer patients with IP. To our knowledge, this is the largest study dealing with immunotherapy for such patients.
The frequency of ICIP was 30.5% in our study and was 0–10% in the two prospective clinical trials for patients with lung cancer with mild IP run by Fujimoto et al. [20, 21]. Our findings are similar to of Ikeda et al.’s [22] phase II trial and the studies of Kanai et al. [31] and Shibaki et al. [32], including patients with UIP. Previous studies have reported the frequencies of grade 3 or higher and grade 5 ICIP to be 6.0–23.5% and 0.0–6.5%, respectively. The frequencies of grade 3 or higher and grade 5 ICIP in present study are consistent with previously reported ones. Interestingly, ICIP onset was earlier in patients with ICIP grades 3–5. An integrated analysis of phase III trials, including IMpower130, IMpower132 and IMpower150, showed a significantly prolonged OS in grades 1–2 group compared with those with no irAE and grades 3–5 groups [33]. Moreover, Sugano et al. [34] reported that the ORR and PFS were significantly improved in patients with ICIP compared with non-ICIP patients in those with lung cancer and IP. This suggested that mild ICIP could have beneficial effects on the prognosis in patients with lung cancer and IP.
The rationale for the high frequency of durvalumab associated ICIP may be related to its use as maintenance therapy after radiation therapy. The Pacific trial reported that ICIP had a higher frequency of 33.9% for all grades [35]. The KEYNOTE 189 study reported a low incidence of ICIP (4.4% for all grades and 2.7% for ≥ grade 3) [36]. However, a retrospective study of patients with lung cancer and IP reported an IP-AE rate of 28.6% for pemetrexed used as a second-line therapy [11]; therefore, pemetrexed-containing regimens should be used with caution. Atezolizumab was reported to be associated with a low incidence of ICIP [33]. In our study, the reason for the high incidence of ICIP grades 3–5 with atezolizumab monotherapy is unknown.
Some prospective clinical trials on patients with lung cancer and mild IP in Japan reported a high efficacy with an ORR of 39–50% and DCR of 72–100% [20, 21]. A meta-analysis reported an ORR of 34% and DCR of 66% [8] that was similar to our study. Interestingly, when comparing patients with and without irAEs, including ICIP, the PFS and OS were significantly better in patients with irAEs. A similar result was reported for lung cancer without IP, suggesting that ICIP-induced irAEs could be different from AE triggered by cytotoxic anticancer agents [37].
When comparing prognosis by ICI agent, pembrolizumab showed the highest median PFS and OS, with significant differences between all ICI agents. This could be related to the high levels of PD-L1 expression in patients who received pembrolizumab in this study: 63.2% (43/68) of patients who received pembrolizumab had a PD-L1 expression of ≥50%. Yamaguchi et al. [38] reported no significant difference in the prognosis between the presence or absence of pre-existing IP in patients with NSCLC with PD-L1 expression ≥50%. Therefore, patients with a high PD-L1 expression in lung cancer and IP could be favourable candidates for pembrolizumab monotherapy.
The identification of risk factors for serious ICIP is warranted. Ikeda et al. [23]. reported that a honeycomb lung on HRCT may be a risk factor for ICIP through a logistic regression analysis. They found that ICIP grades 3–5 occurred in 57% (4/7) of patients with a honeycomb lung, whereas grade 1 ICIP occurred in 10% (1/10) without it. However, their analysis was based on a limited sample of only 17 patients. Yamaguchi et al. [39] reported that the fibrosis score on CT was a risk factor for ICIP through multivariate logistic regression analysis (p=0.0008). A meta-analysis of three prospective trials and seven retrospective trials did not identify the UIP pattern on HRCT as a ICIP risk (hazard ratio 0.88, 95% CI 0.31–2.50) [8]. In our study, IPF was a risk factor for ICIP grades 3–5, but an UIP pattern on HRCT was not. Among patients with CIP grades 3–5, 9 out of 22 (40.9%) had a non-UIP pattern (probable UIP pattern: n=5, indeterminate UIP pattern: n=4). However, we believe that IPF with a non-UIP pattern should be considered as a risk factor for developing ICIP.
Our current study had several limitations. First, this was a retrospective, multicentre study; thus, a selection bias may exist. Second, a central review of HRCT findings for pre-existing IP, IPF diagnoses and ICIP classifications was not performed; however, each institution had experienced interstitial lung disease specialists.
In conclusion, the incidence of ICIP in patients with cancer with IP in a real-world setting was approximately 30%. However, a non-DAD pattern showed favourable clinical outcomes. Patients with irAEs had a better prognosis than those without irAEs. Moreover, IPF and CRP were identified as risk factors for ICIP grades 3–5. Immunotherapy is considered a therapeutic option for selected patients with lung cancer and IP, especially those with non-IPF and preserved lung function.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Table S1 00981-2023.SUPPLEMENT (7.9KB, pdf)
Figure S1 00981-2023.SUPPLEMENT (658.1KB, tif)
Figure S2 00981-2023.SUPPLEMENT2 (902.7KB, tif)
Figure S3 00981-2023.SUPPLEMENT3 (997.6KB, tif)
Acknowledgements
We thank Chiaki Nishimura of CN Medical Research Inc. (Tokyo Japan) and Nao Utsunomiya, the Clinical Research Coordinator in Toho University Omori Medical Centre (Tokyo Japan).
Provenance: Submitted article, peer reviewed.
Conflict of interest: K. Isobe, Y. Nakamura, S. Sakamoto have nothing to disclose.
Conflict of interest: K. Tomii discloses consultation fees from Eli Lilly Japan K.K., and honoraria from Boehringer Ingelheim Japan Inc., Shionogi & Co., Ltd, AstraZeneca K.K., Ono Pharmaceutical Co., Ltd, Bristol Myers Squibb K.K., Chugai Pharmaceutical Co. and Taiho Pharmaceutical Co., Ltd.
Conflict of interest: T. Takimoto has nothing to disclose.
Conflict of interest: Y. Miyazaki has received funding from Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co. and Bristol Myers Squibb K.K. and honoraria from Boehringer Ingelheim Japan Inc. and AstraZeneca K.K.
Conflict of interest: M. Matsumoto discloses honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., Taiho Pharmaceutical Co., Kyowa Hakko Kirin Co. and Ono Pharmaceutical Co., Ltd.
Conflict of interest: K. Sugino, K. Ichikado and S. Morigichi have nothing to disclose.
Conflict of interest: K. Yamaguchi discloses honoraria from Ono Pharmaceutical Co., Ltd and Chugai Pharmaceutical Co.
Conflict of interest: T. Baba has nothing to disclose.
Conflict of interest: H. Ozasa discloses honoraria from MSD K.K., Ono Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co. and AstraZeneca K.K.
Conflict of interest: F. Igata, K. Anabuki and S. Homma have nothing to disclose.
Conflict of interest: H. Date discloses honoraria from Johnson and Johnson.
Conflict of interest: T. Suda has nothing to disclose.
Conflict of interest: K. Kishi has received funding from the Ministry of Health, Labour and Welfare, Japan, during the conduct of the study and honoraria from Chugai Pharmaceutical Co., Boehringer Ingelheim Japan Inc. and Shionogi & Co., Ltd.
Support statement: This study was partially supported by a grant from the Ministry of Health, Labour and Welfare of Japan awarded to the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on Intractable Disease.
Ethics statement: The Institutional Review Board of Toho University Medical Centre Omori Hospital approved this multicentre retrospective study (M21240, 2164, 20306, 20274 and 20184).
References
- 1.Ballester B, Milara J, Cortijo J. Idiopathic pulmonary fibrosis and lung cancer: mechanisms and molecular targets. Int J Mol Sci 2019; 20: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown SW, Dobelle M, Padilla MA, et al. Idiopathic pulmonary fibrosis and lung cancer. A systematic review and meta-analysis. Ann Am Thorac Soc 2019; 16: 1041–1051. [DOI] [PubMed] [Google Scholar]
- 3.Kewalramani N, Machahua C, Poletti V, et al. Lung cancer in patients with fibrosing interstitial lung diseases: an overview of current knowledge and challenges. ERJ Open Res 2022; 8: 00115-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. [DOI] [PubMed] [Google Scholar]
- 5.Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011; 37: 356–363. [DOI] [PubMed] [Google Scholar]
- 6.Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2010; 27: 103–110. [PubMed] [Google Scholar]
- 7.Cheng M, Lin R, Bai N, et al. Deep learning for predicting the risk of immune checkpoint inhibitor-related pneumonitis in lung cancer. Clin Radiol 2023; 78: e377–e385. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Fan Y, Nie L, et al. Clinical outcomes of immune checkpoint inhibitor therapy in patients with advanced non-small cell lung cancer and preexisting interstitial lung diseases: a systematic review and meta-analysis. Chest 2022; 161: 1675–1686. [DOI] [PubMed] [Google Scholar]
- 9.Minegishi Y, Sudoh J, Kuribayasi H, et al. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer 2011; 71: 70–74. [DOI] [PubMed] [Google Scholar]
- 10.Sekine A, Satoh H, Baba T, et al. Safety and efficacy of S-1 in combination with carboplatin in non-small cell lung cancer patients with interstitial lung disease: a pilot study. Cancer Chemother Pharmacol 2016; 77: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 11.Minegishi Y, Gemma A, Homma S, et al. Acute exacerbation of idiopathic interstitial pneumonias related to chemotherapy for lung cancer: nationwide surveillance in Japan. ERJ Open Res 2020; 6: 00184-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otsubo K, Kishimoto J, Ando M, et al. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: a randomised phase 3 trial. Eur Respir J 2022; 60: 2200380. [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 14.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 17.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220–2229. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (Caspian): a randomised, controlled, open-label, phase 3 trial. Lancet 2019; 394: 1929–1939. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto D, Morimoto T, Ito J, et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer 2017; 111: 1–5. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto D, Yomota M, Sekine A, et al. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: a multicenter, open-label single-arm phase II trial. Lung Cancer 2019; 134: 274–278. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda S, Kato T, Kenmotsu H, et al. A Phase 2 study of atezolizumab for pretreated NSCLC with idiopathic interstitial pneumonitis. J Thorac Oncol 2020; 15: 1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda S, Kato T, Kenmotsu H, et al. Atezolizumab for pretreated non-small cell lung cancer with idiopathic interstitial pneumonia: final analysis of Phase II AMBITIOUS study. Oncologist 2022; 27: 720–e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. Bethseda, US Department of Health and Human Services, 2017. [Google Scholar]
- 25.Sverzellati N. Highlights of HRCT imaging in IPF. Respir Res 2013; 14: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. [DOI] [PubMed] [Google Scholar]
- 27.Erasmus JJ, McAdams HP, Rossi SE. High-resolution CT of drug-induced lung disease. Radiol Clin North Am 2002; 40: 61–72. [DOI] [PubMed] [Google Scholar]
- 28.Nishino M, Hayakawa K, Kawamata K, et al. MRI of early unruptured ectopic pregnancy: detection of gestational sac. J Comput Assist Tomogr 2002; 26: 134–137. [DOI] [PubMed] [Google Scholar]
- 29.Cleverley JR, Screaton NJ, Hiorns MP, et al. Drug-induced lung disease: high-resolution CT and histological findings. Clin Radiol 2002; 57: 292–299. [DOI] [PubMed] [Google Scholar]
- 30.Johkoh T, Lee KS, Nishino M, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Chest 2021; 159: 1107–1125. [DOI] [PubMed] [Google Scholar]
- 31.Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 2018; 9: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibaki R, Murakami S, Matsumoto Y, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother 2020; 69: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Socinski MA, Jotte RM, Cappuzzo F, et al. Association of immune-related adverse events with efficacy of atezolizumab in patients with non-small cell lung cancer: pooled analyses of the Phase 3 IMpower130, IMpower132, and IMpower150 randomized clinical trials. JAMA Oncol 2023; 9: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugano T, Seike M, Saito Y, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac Cancer 2020; 11: 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in Stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 36.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 37.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi O, Kaira K, Shinomiya S, et al. Pre-existing interstitial lung disease does not affect prognosis in non-small cell lung cancer patients with PD-L1 expression ≥50% on first-line pembrolizumab. Thorac Cancer 2021; 12: 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer 2018; 125: 212–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Table S1 00981-2023.SUPPLEMENT (7.9KB, pdf)
Figure S1 00981-2023.SUPPLEMENT (658.1KB, tif)
Figure S2 00981-2023.SUPPLEMENT2 (902.7KB, tif)
Figure S3 00981-2023.SUPPLEMENT3 (997.6KB, tif)