Summary
In recent years, significant advancements have been made in the synthesis and application of 1,3-dienes. This specific structural motif has garnered significant attention from researchers in materials science and biology due to its unique aggregation-induced emission (AIE) properties and extensive conjugation systems. The luminescent characteristics of these compounds are notably influenced by the geometry of the two double bonds. Therefore, it is essential to consolidate stereoselective synthetic strategies for 1,3-dienes. This comprehensive review seeks to elucidate the diverse techniques employed to attain stereo-control in the synthesis of 1,3-diene-based AIE luminogens (AIEgens). Particular emphasis is placed on comprehending the determinants of stereoselectivity and exploring the array of substrates amenable to these methods. Furthermore, the review underscores the AIE properties exhibited by these compounds and their extensive utility in organic light-emitting diodes (OLEDs), stimuli-responsive materials, sensors, bioimaging, and photodynamic therapy (PDT).
Subject areas: Classification description, Organic chemistry, Physical chemistry
Graphical abstract

Classification description: Organic chemistry; Physical chemistry
Introduction
1,3-Dienes represent a noteworthy category of functional molecules with diverse applications in fields such as materials science, natural products, and drug molecules, among others.1,2,3,4 The creation of these compounds has intrigued chemists for a long time due to the remarkable reactivity exhibited by their C–C double bonds when interacting with various electrophilic or nucleophilic substances.5,6,7 Specifically, the controlled synthesis methods and uses of 1,3-diene-based AIE luminogens (AIEgens) have garnered considerable attention in the domains of optoelectronics, chemical sensing, and bioimaging research.8 The presence of double bonds has spurred the development of numerous synthetic techniques, including stoichiometric reactions and transition metal-catalyzed transformations. While these approaches offer practical routes for diene synthesis, the selective formation of 1,3-dienes with specific stereochemistry remains a persistent challenge in organic chemistry. Both double bonds are susceptible to isomerization when exposed to light, heat, bases, or catalysts, highlighting the necessity of a comprehensive summary of approaches for achieving stereoselective synthesis of 1,3-dienes.
The utilization of luminescent materials has garnered significant interest in academia. Nevertheless, the existence of intermolecular π-π stacking interactions among fluorogens has given rise to the occurrence of aggregation-caused quenching (ACQ) in fluorescent materials. This leads to diminished emission in solid states and constrains their prospective applications.9,10,11 Fortunately, Tang et al. introduced a captivating and divergent phenomenon known as AIE in 2001, presenting a hopeful resolution to this constraint.12 This phenomenon sheds light on the photophysical traits of luminescent materials, wherein they manifest non-emissive properties at the molecular scale but exhibit robust emission upon aggregation. The comprehension of this phenomenon has been attained by integrating the notion of restricted intramolecular motion (RIM). This notion includes both restricted intramolecular vibration (RIV) and restricted intramolecular rotation (RIR).
The occurrence of AIE is attributed to the unique molecular structure and properties of 1,3-dienes. However, there is currently a dearth of comprehensive literature, including both the synthesis methods and practical applications of these compounds. Therefore, this paper aims to offer insights into stereo-selective synthetic methodologies and explore the wide array of applications associated with these substances.
The current review investigates the investigation of stereoselectivity in the synthesis of 1,3-dienes. Various strategies for achieving stereoselective synthesis are discussed, including (1) alkene reacting with alkenes, (2) alkene reacting with alkynes, (3) diene/alkyne addition, (4) isomerization, and (5) derivatization of lower dienes. It is worth mentioning that our selected cases exclusively encompass strategies pertaining to 1,3-diene stereoselective synthesis, while omitting methods lacking effective control over the geometry of double bonds. Furthermore, the highlighted strategies are already extensively employed by materials chemists, thereby excluding sporadic instances of dienes constructed with specialized skeletal substrates in our review. Additionally, this review primarily focuses on the recent advancements concerning the utilization of diene derivatives in organic OLEDs, stimuli-responsive materials, sensors, bioimaging, and photodynamic therapy (PDT).
Stereo-synthesis of 1,3-diene based AIEgens
Alkenes reacting with alkenes
The synthesis of 1,3-dienes via the reaction between alkenes and alkenes represents the most common approach. This strategy includes various methodologies, including the Heck reaction,13 oxidative coupling,14 and reductive coupling.15 Three main strategies are used to regulate the geometry of double bonds: (1) The selective use of alkene substrates with specific configurations, (2) the control of β-H elimination through steric effects, and (3) the selective activation of vinyl C–H bonds.
Heck-type reactions involving stereo-defined alkenes derivatives
Heck reactions
The Heck reaction, a cross-coupling process, utilizes alkenes and organic halides as starting materials in the presence of a base and palladium catalyst. This reaction follows a well-documented three-step mechanism, which includes oxidative addition, migratory insertion, and β-H elimination (Figure 1A).16,17
Figure 1.
Alkenes reacting with alkenes
(A) The mechanism of Heck coupling.
(B) Palladium-catalyzed Heck reactions of vinyl bromides and alkenes.
(C) Regioselective synthesis of branched disubstituted 1,3-dienes. (A) Synthesis of symmetrical (E,E)-1,4-diaryl-1,3-butadienes by one-pot method. (B) Synthesis of 1,3-dienes by Suzuki coupling. (C) Synthesis of 2-alkoxy-3-alkyl(aryl)thiobuta-1,3-dienes by Negishi coupling.
(D) Nickel-catalyzed Kumada vinylation for the preparation of 2-substituted 1,3-dienes.
(E) Microwave-assisted Stille coupling for construction of 1,3-dienes.
(F) Hiyama cross-coupling reactions for construction of trans aryl 1,3-dienes.
(G) Hiyama reactions for construction of highly functionalized 1,3-dienes.
(H) Direct cross-coupling of alkenyllithium.
(I) Ligand-free FeCl3-catalyzed procedures.
(J) Ni-Catalyzed reductive coupling to form highly substituted dienes.
(K) Chemoselective Ni-catalyzed reductive coupling reactions.
(L) Ligand-free FeCl3-catalyzed procedures.
(M) Ni-Catalyzed reductive coupling to form highly substituted dienes.
(N) Chemoselective Ni-catalyzed reductive coupling reactions.
Palladium-catalyzed Heck cross-coupling reactions provide an effective means of forming C–C bonds.13,18 Expanding upon this method, Doucet and Santelli et al. proposed a more efficient and stereoselective strategy for constructing 1,3-dienes.19 They employed the [Pd(η3-C3H5)Cl]2/Tedicyp system to generate a wide range of (E)- and (E,E)-1,3-dienes at low catalyst loadings. This method is applicable to brominated olefins with both aromatic and alkyl substituents, displaying excellent compatibility with various functional groups, unaffected by electronic or steric factors (Figure 1B).
Oxidative heck reactions
The Heck reaction provides numerous viable strategies for converting C–H bonds into C–C bonds.14,20 Utilizing the Heck reaction to synthesize branched 1,3-dienes with differing regioselectivity is considered an innovative approach. Stahl et al. reported a PdⅡ-catalyzed aerobic oxidative coupling of vinyl boronic acids 4 and electronically unbiased alkenes 5 to access regioselective branched 1,3-disubstituted dienes 6 (Figure 1C).21 Noticeable enhancements in both yields and the inclination toward a branched isomer were detected when utilizing chelating ligands L, which show substituents positioned in proximity to the nitrogen atoms. The use of this ligand enables the selective generation of products in internal positions, with steric effects rather than electronic effects driving this selectivity. Electron-neutral and electron-rich vinyl-boronic acid compounds display commendable reactivity within the reaction. Conversely, compounds bearing electron-withdrawing groups demonstrate an inability to attain the corresponding product formation.
Additionally, stereo-defined 1,3-dienes can be obtained via the oxidative coupling of two vinyl-boranes. Recently, Wen et al. developed a one-pot method cascade reaction for the synthesis of (E,E)-1,4-diaryl-1,3-butadienes using terminal alkynes 7 and bis(pinacolato)-diboron 8 (Figure 1D).22 The generation of alkenyl borates 9 during the initial stage eliminates the requirement for separation and purification. Consequently, it facilitates the direct synthesis of the desired products via a palladium-catalyzed homocoupling reaction. This streamlined methodology takes advantage of readily accessible starting materials, resulting in the efficient production of valuable compounds. The protocol simultaneously demonstrates remarkable efficacy in the synthesis of symmetrical 1,4-diaryl-1,3-dienes 10 while also showing great compatibility with various functional groups. The presence of oxygen is essential in this system to facilitate the regeneration of PdⅡ.
Cross-coupling involving two stereo-defined alkene derivatives
Transition-metal catalyzed cross-coupling reactions are widely used for constructing conjugated dienes, and the motifs have been used in the synthesis of functional materials, natural products.23 Using two single geometries of alkenes as substrates presents an alternative pathway for the stereoselective synthesis of 1,3-dienes. By selecting different substrates, corresponding products with a single geometry can be obtained individually.
Suzuki-Miyaura reaction
In 2005, Molander et al. successfully achieved stereoselective synthesis of multi-substituted 1,3-diene scaffolds via Suzuki reaction (Figure 1E).24 This was accomplished using stereo-defined alkenyl potassium fluoroborate and alkenyl bromine reagents. The geometry of the two double bonds is dictated by the substrate, and interestingly, even the Z-type structure remains unaffected by isomerization during the reaction process. By utilizing substrates with varying geometry, the stereo-divergent synthesis of 1,3-dienes can be achieved.
Negishi coupling reaction
Negishi coupling is an efficient way to construct multi-substituted dienes.25 Jin et al. demonstrated that the stereoselective synthesis of the 2-alkoxy-3-alkyl(aryl)thiobuta-1,3-dienes 16 could be achieved by Negishi reaction using vinyl bromides and vinyl zinc chloride as substrates (Figure 1F).26 In addition, this stereoselectivity of the products comes from the substrates. This strategy further broadens the range of both substrates and products, making it more practical.
Kumada coupling reaction
The Pd-catalyzed Kumada cross-coupling reactions using Grignard reagents buta-1,3-dien-2-ylmagnesium chloride 18 is an attractive way to prepare 2-substituted 1,3-dienes (Figure 1G), but the poor stability of these dienyl Grignard and the low functional group tolerance limited the scope of reaction.27,28 Hence, Mazet et al. illustrated a NiⅡ-catalyzed Kumada vinylation of vinyl magnesium bromide with vinyl phosphates to provide 2-substituted 1,3-dienes 23 featuring simple operations, low catalyst loadings and cheap cost that use of stoichiometric quantities of each coupling partner (Figure 1G).29 In some cases, isomerization appears at low temperatures implies that to adjust the temperature of reaction could access a variety of different olefin isomers with high levels of stereoselectivity by capitalizing on a competing isomerization. The expansion of this protocol to synthesize more conjugated 1,3-dienes has been also demonstrated starting from alkenyl Grignard reagents and/or alkenyl enol phosphates.
Stille coupling reaction
Stille coupling has shown great practical value in the field of constructing dienes by combining vinylstannanes with vinyl halides.30 Maleczka et al. first used substituted terminal alkynes 24 and organotin reagent Bu3SnCl under microwave to produce vinyltin reagents 25, which were then coupled with vinyl halides to obtain 1,3-dienes (Figure 1H).31 While it has been proven highly effective, the greater toxicity of tin reagents is still plaguing.
Hiyama cross-coupling reactions
Ranu et al. established a straightforward and effective procedure using a Pd-catalyzed Hiyama cross-coupling reaction to synthesize 1,3-dienes from vinyl bromides 28 and triethoxyvinylsilane 29 (Figure 1I).32 The presence of both electron-withdrawing and electron-donating groups on the benzene ring does not compromise the yields under these conditions. The protocol presented here also enables access to phenyl bis-1,3-diene 30aa, thereby demonstrating the significant potential for its application.
Remarkably, a notable level of stereoselectivity was attained, yielding exclusively trans-1,3-dienes, irrespective of whether cis- or trans-vinyl bromides were employed in the reaction. The sole restriction pertains to the limited selection of organosilicon compounds available, which imposes restrictions on the production of a wide variety of functionalized coupling products.
Subsequently, Cook et al. utilized propargylic alcohols 31 to create a broad spectrum of structurally varied vinylsilanes 32 (Figure 1J).33 These vinylsilanes were subsequently subjected to a palladium-catalyzed coupling reaction with β-iodostyrene 33, resulting in the generation of the corresponding dienes. This approach partially addressed the previous limitation; however, further enhancement of the universality of the substrate is required.
Alkene bromides reacting with alkenyllithiums
Alkenyllithium reagents exhibit high activity and facile preparation, offering significant advantages in cross-coupling reactions to provide stereo-defined 1,3-dienes. Feringa et al. initially disclosed a direct Pd-catalyzed olefination cross-coupling between organic halides 35 and alkenyllithium reagents 36 to produce disubstituted 1,3-dienes (Figure 1K).34 The results showed effective coupling for both (E)- and (Z)-alkenyl bromides, leading to dienes with complete retention of stereochemistry. Additionally, the products maintained their original (Z)-configuration, originating from the corresponding (Z)-alkenyllithium reagents obtained through the treatment of (Z)-1-bromoolefins with elemental lithium. However, it is important to note that the product structure in this study is relatively simple and does not address the compatibility of functional groups with the reaction.
Furthermore, Peng and Wong et al. have introduced an innovative and effective methodology. In this approach, organolithium reagents 39 serve as cross-coupling partners in ligand-free FeCl3-catalyzed procedures, combining with vinyl halides 38 to create new C(sp2)–C(sp2) bonds (Figure 1L).35 This process successfully produces products from both electron-rich and electron-deficient aryl vinyl iodides, with the steric impact having a relatively minimal influence. A stereochemistry investigation reveals that (E)-β-(hetero)aryl vinyl iodides react with (E)-propenyllithium, maintaining the geometry of both substrates, resulting in 1,3-dienes. However, isomerization occurs when employing (Z)-propenyllithium, attributable to thermodynamic considerations. Additionally, the authors have demonstrated the suitability of trimethylsilyl vinyl lithium reagents as viable substrates in this reaction, thereby enabling the synthesis of conjugated polyenes.
Reductive coupling of two electrophiles
The Ni-catalyzed reductive coupling of two electrophiles has seen significant advancements recently.36,37 The reaction model presented here offers a novel synthetic pathway for the formation of 1,3-dienes. Weix et al. have developed a reductive approach to obtain highly substituted 1,3-dienes with exceptional functional group tolerance and have demonstrated the tunability of the Ni and Pd co-catalytic system for selectively coupling electrophilic partners (Figure 1M).38 Nonetheless, their investigation exclusively presented instances involving cyclic vinyl bromides 41 and vinyl triflates 42.
Wu and Gong et al. subsequently broadened the applicability of this reaction by incorporating terminal alkenyl bromides 44 and branched alkenyl bromides 45 (Figure 1N).39 The chemical selectivity observed in this protocol is attributed to distinct steric influences between the two coupling partners. It is noteworthy to emphasize that the inclusion of 3 equivalents of terminal alkenyl bromides is indispensable, as their omission leads to homo-dimerization, underscoring the significance of maintaining statistical control over cross-selectivity.
Vinyl C–H bond alkenylation
Vinyl C–H bond alkenylation provides an efficient way to form the corresponding conjugated dienes by coupling two inactivated alkenes.40 In this approach, the substrates are readily available, and no pre-functionalized precursor is required, making it relatively more atom- and step-economical.41 It is necessary to install directing groups nearby the double bond for controlling the regioselectivity due to the similar reactivity of C–H bonds. To date, two commonly used strategies are directing groups (DGs)42,43,44 and traceless directing groups (TDGs).45,46
Directing group assisted C–H bond alkenylation
In this case, the lone-pair electrons on a heteroatom are capable of coordinating to transition metals, enabling the activation of C–H bonds by forming stable cyclometalated species.47,48 These species can be captured by the migratory insertion of the other alkene to afford the corresponding diene products.
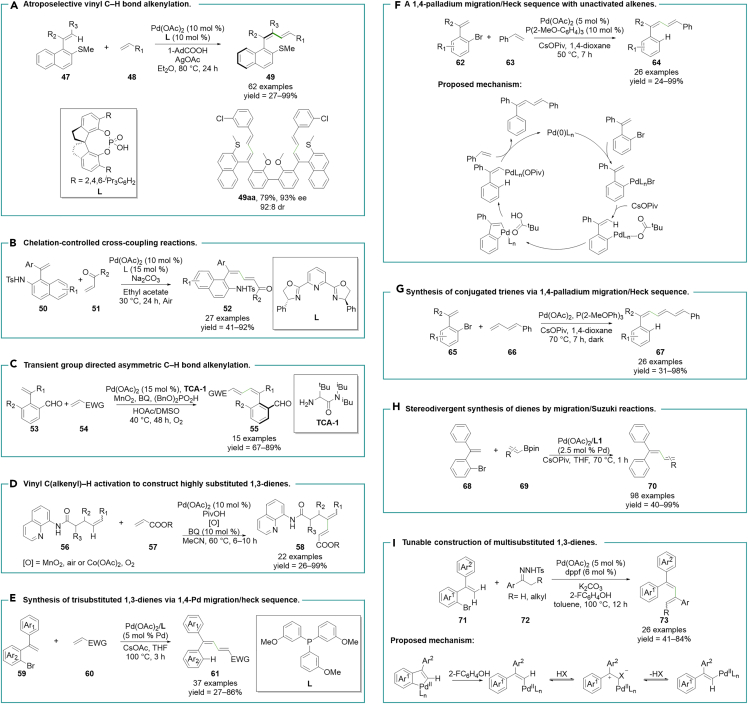
Using thioether as a directing group, Shi et al. reported a palladium-catalyzed atroposelective vinyl C–H bond alkenylation in the presence of chiral spiro phosphoric acid L (Figure 2A).49 Both terminal and internal alkenes 47 participated in this C–H bond activation procedure smoothly, and electron-neutral alkenes can also capture the alkenyl palladium intermediates as efficient Heck coupling partners, yielding satisfactory yields. Complete (Z)-selectivity was observed in the corresponding products due to the cis C–H bond activation process. Furthermore, rational substrate design allows the construction of functional molecules featuring multi-1,3-diene skeletons, with several potential applications in chiral AIE materials.
Figure 2.
Vinyl C–H bond alkenylation
(A) Atroposelective vinyl C–H bond alkenylation.
(B) Chelation-controlled cross-coupling reactions.
(C) Transient group directed asymmetric C–H bond alkenylation.
(D) Vinyl C(alkenyl)–H activation to construct highly substituted 1,3-dienes.
(E) Stereoselective Synthesis of trisubstituted 1,3-dienes via 1,4-palladium migration/heck sequence.
(F) A 1,4-palladium migration/Heck sequence with unactivated alkenes.
(G) Stereoselective synthesis of conjugated trienes via 1,4-palladium migration/Heck sequence.
(H) Stereodivergent synthesis of dienes by migration/Suzuki reactions.
(I) Tunable construction of multisubstituted 1,3-dienes.
Nitrogen-containing functional groups were also valid directing groups to initiate this protocol. Xu et al. constructed axially chiral conjugated dienes via the tosylamine group-assisted C–H bond activation strategy under mild reaction conditions (Figure 2B).50 The C–H bond activation sequence can only be initiated by 1,1-diaryl substituted alkene 50, and only activated olefins 51 can participate in the following Heck insertion process.
The aldehyde group, serving as an electron-withdrawing group, plays an essential role in the construction of donor–π–acceptor (D–π–A) structures. Zhong and Zhang et al. introduced a transient guide group approach, enabling aldehyde-directed C–H bond alkenylation (Figure 2C).51 They employed an amino acid-derived transient chiral auxiliary (TCA) that reacted with the aldehyde group, forming imine intermediates. These intermediates effectively coordinated with a palladium center, facilitating the subsequent C–H bond activation. After the reaction was completed, the imine intermediates underwent hydrolysis, regenerating the aldehyde group. Both electron-deficient and electron-neutral olefins 53 were compatible with the following Heck process, delivering 1,1,4-triaryl dienes 55. The yields and enantiomeric excess (ee) values dramatically decreased when using a carboxyl group as a directing group, and internal alkenes require further exploration.
Liu and Engle et al. reported a palladium-mediated vinyl C–H activation induced by 8-aminoquinoline to construct highly substituted dienes 58 (Figure 2D).52 This process exhibited full selectivity at the γ-site for C–H alkenylation, forming a stable six-membered palladacycle species instead of β- or δ-site selectivity. The stereoselectivities of the two double bonds were governed by the substrates and the Heck reaction. Additionally, the compatibility of internal alkynes offers an opportunity to create 1,2,4-trisubstituted alkenes.
Traceless directing group assisted C–H bond alkenylation
While traditional DG-assisted C–H bond alkenylation methods have seen significant advancements, the utilization of directing groups has constrained their applicability in the materials domain.53,54 The removal of directing groups diminishes reaction efficiency.55 To address this limitation, traceless directing groups have garnered attention as a viable alternative.56 These directing groups spontaneously eliminate themselves at the conclusion of the reaction, making them a promising choice.
Feng and Lin et al. first applied the traceless directing group strategy to the synthesis of trisubstituted 1,3-dienes via aryl to vinyl 1,4-palladium migration/Heck sequence (Figure 2E).57 Electron-deficient alkenes 60 can rapidly capture the vinyl palladium intermediates to produce aryl-containing electron-deficient dienes 61. The stereoselectivity of the above two double bonds resulted from the steric hindrance effect of the Heck reaction and the cis C–H bond activation, respectively. While this approach offers a convenient method for the highly stereoselective synthesis of 1,3-dienes, it is important to note that electron-neutral alkenes, like styrene, are unable to engage in these reactions due to their limited reactivity.
To solve the above problem, the same group made inert alkenes feasible by modulating various factors, including temperature and base (Figure 2F).58 Temperature was proven to play a crucial role in controlling the reaction of two alkene substrates 62 and 63, rather than the dimerization of the one olefin. Stereodefined 1,1,4-triaryl-substituted 1,3-diene 64 can be efficiently synthesized with high yields ranging from good to excellent. In this work, high stereoselectivity was achieved even when the substrates bore two similar aryl groups at terminal positions of the alkene, which is often difficult to control by steric hindrance or electronic effects. This protocol involved several key steps, including oxidative addition, C–H activation, regioselective protonation of palladacycle, and concluded with a Heck coupling reaction. The stereoselectivity of the two double bonds was regulated by the cis aryl to vinyl 1,4-palladium migration process and the subsequent trans β-H elimination.
Nevertheless, diene substrates 66 were also suitable for this reaction, which could provide 1,3,5-trienes 67 with high stereoselectivity (Figure 2G).59 This laid the foundation for the synthesis of nonlinear optical materials.
Although the migration/Heck sequence is crucial for achieving stereoselective synthesis of 1,3-dienes, it is important to note that a stereodivergent synthesis of these compounds has not yet been accomplished. This is attributed to the fact that Heck reactions are difficult to provide large hindrance products. Given these considerations, the migration/Suzuki reaction emerged as a viable alternative (Figure 2H).60 The configuration of the double bond could be precisely controlled by employing various configurations of alkenyl boron substrates 69. This approach yielded 1,1,4-triaryl and 1,1,4,4-tetra-aryl dienes 70 with high yields and stereoselectivity. When employing the less stable (Z)-alkenyl boronate as the substrate, it led to the formation of the corresponding (Z)-product instead of the Z/E isomerization product. This method enabled the separate synthesis of four different tetra-aryl 1,3-dienes with distinct stereoselectivity, which is a challenging feat to achieve using traditional methods.
In a recent development, Zhou and Yu et al. utilized carbene compounds 72 to trap palladium migration intermediates, thereby enabling the construction of diene structures (Figure 2I).61 The process involved 1,4-palladium migration, carbene insertion, and β-H elimination steps, establishing a viable pathway for diene synthesis. Although the presence of 2-F-C6H4-OH may accelerate aryl to vinyl 1,4-palladium migration process, avoiding the in situ migratory insertion of carbene compounds, the use of this additive attributed to causing the isomerization of the vinyl palladium species, which can only provide the Z/E mixture of 1,3-dienes. It provides a new approach for the synthesis of 1,1,3-trisubstituted dienes, whereas this study is not satisfactory in the stereoselectivity of the double bond.
Alkenes reacting with alkynes
Ene-yne cross-metathesis process
Ene-yne metathesis (EYM) is a notably efficient approach for coupling alkynes and alkenes in almost equal proportions, yielding 1,3-dienes with exceptional atom efficiency. This strategy frequently encounters challenges in controlling the stereoselectivity of double bonds. It typically yields terminal 1,3-dienes to circumvent stereoselectivity or produces Z/E mixture. While there are currently no instances of highly stereoselective synthesis of 1,3-dienes using this strategy, the presentation of some non-stereoselective synthesis cases can provide valuable heuristic insights for researchers. We introduce two representative examples for discussion. In 2017, Bruneau and Fischmeister et al. proposed a ruthenium-catalyzed process that effectively utilized asymmetric 1,2-diarylacetylenes 74 and ethylene 75 as starting materials to produce asymmetric 2,3-diaryl-1,3-dienes 76 (Figure 3B).62 This transformation displayed excellent compatibility with various functional groups. Nonetheless, specific substrates featuring lone pair electrons, like nitrogen and oxygen atoms, as well as compounds prone to creating cyclic intermediates with the metal center, were observed to be unresponsive in this particular process. Additionally, these reactions typically employ a 1,3-enyne substrate to construct cyclic 1,3-dienes.
Figure 3.
Alkenes reacting with alkynes
(A) Alkenes react with alkynes.
(B) Ruthenium-catalyzed process for preparation of 2,3-diaryl-1,3-dienes.
(C) Ene−yne metathesis for synthesis of phosphorus-containing 1,3-dienes.
(D) Regio- and stereoselective codimerization leading to dienes.
(E) Regio- and stereoselective intermolecular enyne coupling.
(F) Nickel-catalyzed dimerization of acrylates and alkynes.
(G) Ruthenium-catalyzed regio- and stereoselective synthesis of 2-amino-1,3-diene derivatives.
(H) Room temperature alkene–alkyne coupling for 1,3-diene synthesis.
(I) Palladium-catalyzed intermolecular ene–yne coupling.
(J) Sequential three-component cross-coupling to 1,3-dienes.
(K) Amide-directed alkenylation of sp2 C–H bonds.
(L) Regio- and stereoselective approach to substituted (2Z,4Z)-dienamides.
(M) Ruthenium-catalyzed hydrovinylation to highly substituted 1,3-dienes.
(N) Controlled synthesis of stereodefined dienes.
Diver et al. used a special Grubbs catalyst containing cyclic alkyl amino carbene (CAAC) ligands to selectively construct 1,3-dienes by EYM.63 In their study, a series of 1,3-disubstituted conjugated dienes were obtained by the reaction of phosphorus-containing alkenes 77 with terminal alkynes 78, and the stereoselectivity of this reaction was elucidated in Figure 3C. Notably, when the R group is –COOMe, unprecedented Z-selective product generation is achieved. In summary, this study further broadens the selectivity of EYM and the range of substrates to obtain conjugated olefin products containing phosphate groups.
Cyclometallation progress
The cyclometallation reaction of alkenes and alkynes represents a pivotal approach for synthesizing multiply substituted 1,3-conjugated alkenes, holding significant importance in organic chemistry. Transition metals, including Rh,64 Co,65,66 Ni,67 and Ru68,69 have demonstrated their catalytic proficiency in facilitating this transformative process. In this reaction context, significant emphasis has been placed on exploring the reactivity between terminal alkenes and internal alkynes. The stereoselectivity of the resulting products is controlled by the cyclometallation process, which dictates the orientation of the two double bonds.
Tanaka et al. reported a Rh-catalyzed codimerization of alkenes and alkynes, yielding 1,2,4-trisubstituted dienes (Figure 3D).64 The creation of the new carbon–carbon bond occurs with regioselectivity at the terminal carbon atom of the alkene 80, proximate to the alkyl-bearing carbon of the alkyne 81. When R2 represents an alkyl group, predominantly (E)-configured products are obtained. Conversely, when R2 is an aryl or ester group, mainly (Z)-configured products are formed. This observed regioselectivity is attributed to the formation of cyclic metal intermediates. It is noteworthy that poor regioselectivity is observed when both carbons of the alkyne display similar electronic effects. However, it is important to mention that the substrates for this reaction are restricted to alkenes lacking α-hydrogen and electron-deficient alkynes.
Cheng et al. proposed a highly regioselective and stereoselective method for synthesizing 1,3-dienes, employing a cobalt catalyst in conjunction with 1,2-disubstituted acetylenes 83 and terminal olefins 84 as substrates (Figure 3E).66 Most of the reactions are assisted by electron-deficient ligands coordinating with the metal center. This coordination results in the creation of a five-membered metallacycle with the substrate, ultimately leading to product formation through β-H elimination. Nevertheless, there are a few substrates that depend on electron-donating ligands. The resulting products predominantly exhibit E-selectivity, wherein the regioselectivity favors the formation of new carbon–carbon bonds between the terminal carbon atom of the olefin and the alkyne carbon one away from the aryl. The regioselectivity observed can be attributed to steric hindrance effects. However, this method is not applicable to terminal alkynes due to their potential initiation of polymerization reactions.
Kurahashi and Matsubara et al. utilized electron-deficient alkenes as starting materials and performed a nickel-catalyzed dimerization reaction with 1,2-disubstituted acetylenes 87, yielding 1,3-dienes (Figure 3A).67 When compared to phosphine ligands, the use of nitrogen-containing ligands exhibits improved regioselectivity to some extent, primarily influenced by the steric effects of the intermediate. However, employing electron-deficient alkynes as reactants could potentially lead to decreased yields. Moreover, this transformation is not suitable for terminal alkynes.
A ruthenium catalyst enables the synthesis of amino-substituted 1,3-dienes 92 to be rendered feasible. Saito and Sato et al. accomplished this procedure by coupling ynamides 90 with ethylene 91 (Figure 3G).69 This advancement not only broadens the prospects for additional product modifications but also holds substantial significance in terms of its potential applications. The reaction displays distinctive regioselectivity and stereoselectivity, attributed to the intermediates formed. Nonetheless, practical challenges remain concerning terminal alkynes.
Following the diligent efforts of Zhang and Zhao et al., they successfully broadened the range of olefins to include acrylic esters or amides (Figure 3H).70 These olefins were then subjected to ruthenium complex-catalyzed reactions with internal alkynes, resulting in the formation of multisubstituted 1,3-dienes. This transformation exhibits excellent tolerance for various functional groups, regioselectivity, and high catalytic efficiency. The protocol predominantly yields products with the (2E,4Z)-configuration, highlighting impressive stereoselectivity. This observation also suggests the possible involvement of oxidative cyclization of alkenes with alkynes in the mechanistic pathway. Furthermore, by adjusting the ligands employed in the reaction system, terminal phenylacetylene can be incorporated into this process, yielding 1,4-disubstituted-1,3-dienes 95aa.
Heck-type cross-coupling reaction
Heck-type reaction initiated by protonation of alkynes
The Heck-type reaction involving alkenes and alkynes offers an alternative pathway for the synthesis of 1,3-dienes. Two primary mechanisms govern the coupling of alkenes and alkynes in the Heck reaction. The initial mechanism involves an addition reaction between alkynes and metal-hydride species, resulting in the formation of vinyl metal-hydride species. These species subsequently undergo the Heck reaction with alkenes and conclude via β-Hydrogen elimination. The second mechanism commences with the activation of C–H bonds in alkenes, followed by migration insertion with alkynes, ultimately concluding through protonation. In contrast to the cyclometallation mechanism, this reaction initiates with the migratory insertion of a metal hydrogen species into an alkyne.
Skrydstrup et al. applied a palladium-catalyzed Heck-type cross-coupling approach, using disubstituted alkynes 96 and electron-deficient alkenes 97 as starting materials, to stereoselectively generate trisubstituted conjugated compounds (Figure 3I).71 The regioselectivity of the resulting products is primarily determined by steric factors. Stereoselectivity is achieved through the syn-addition of alkynes to metal-hydride species and the subsequent anti-elimination of β-H. It is worth noting that phenylacetylene exhibited limited reactivity under the given conditions, and further investigation of monosubstituted alkyne substrates remains warranted.
Later, Wu et al. discovered a Pd-catalyzed three-component cross-coupling that utilizes terminal alkenes as both hydride and alkenyl donors to generate highly stereoselective (Z,E)-conjugated dienes (Figure 3J).72 In this research, the influence of electronic effects and the position of functional groups on product formation is minimal. Additionally, when this method is combined with cross-coupling reactions, it allows for the synthesis of tetraphenylethenes (TPE), demonstrating significant promise in the field of material science.
Heck-type reaction initiated by C–H bond activation
Although the Heck reaction, initiated by the addition of alkynes and metal-hydride species, enables coupling between terminal alkenes and alkynes, its efficacy is limited for internal alkynes. Therefore, the activation mode of the C–H bond has emerged as a valuable adjunct. Tanaka et al. introduced a method that utilizes the 1-pyrrolidinecarbonyl group as a directing group to synthesize highly substituted conjugated dienes through internal alkene 103 and internal alkyne 104 reactions (Figure 3K).73 The presence of the 1-pyrrolidinecarbonyl group plays a crucial role in this reaction, likely due to its electron density. The stereoselectivity of the reaction is linked to the syn C–H activation of the alkene, followed by the syn-insertion of metal-hydride species into the alkyne.
In 2017, Zhang and Zhong et al. introduced an appealing methodology involving ruthenium-catalyzed and amide-directed hydrovinylation of internal alkynes with internal alkenes (Figure 3L).74 This approach enabled the synthesis of multisubstituted (Z,Z)-dienamides 108 with exceptional stereo- and regioselectivity. Competition experiments showed that alkynes with dialkyl substitution exhibited higher reactivity than those with diaryl substitution. In the case of asymmetric alkynes, the products displayed distinct regioselectivity. Notably, while this system exhibited reduced yields with strong electron-donating groups, it demonstrated favorable compatibility with various functional groups. Additionally, it reduced the need for excessive oxidants, thereby minimizing waste generation and resource pollution.
Plietker et al. proposed another ruthenium-catalyzed hydrovinylation of internal alkynes, unlike previous investigations, with an expanded substrate scope, including both terminal alkynes and internal alkynes (Figure 3M).75 Furthermore, this catalytic system functioned effectively in the presence of coordinating functional groups. The addition reactions of alkynes showed a preference for syn-addition, resulting in the predominant formation of the (Z)-isomer. Regioselectivity in this reaction was influenced by both electronic and steric factors.
Dong et al. introduced a substrate-controlled strategy to enhance product diversity by modulating reaction conditions (Figure 3N).76 They employed 7-azaindoles 112 and internal alkynes 113 for the stereoselective synthesis of dienes via trans-addition of the alkene moiety. The use of asymmetric alkynes led to the formation of isomeric mixtures.
Diene/alkyne addition
Hydrogenation of diynes
The hydrogenation of 1,3-diyne and 1,3-enyne represents a significant methodology for the synthesis of 1,3-diene (Figure 4A).
Figure 4.
Diene/alkyne addition
(A) Diene/alkyne addition.
(B) Copper-catalyzed stereoselective hydrogenation of 1,3-diynes.
(C) Z-selective semihydrogenation of internal alkynes.
(D) Pd-catalyzed carbonylation of 1,3-diynes.
(E) Allylic alkylation of pronucleophiles with unactivated enynes.
(F) Iron-catalyzed regio- and stereoselective hydrosilylation.
(G) Selective trans-semihydrogenation of 1,3-enynes with ethanol.
(H) Trans-hydroalkynylation of internal 1,3-enynes.
The Lindlar catalyst is known to effectively produce (Z,Z)-dienes but with the drawback of occasional overreduction and isomerization. This method is limited to exclusively providing disubstituted dienes. Teichert et al. introduced a copper complex as a catalyst and H2 as a hydrogen donor to stereoselectively produce dienes with different configurations depending on the substitution pattern of the diynes substrates (Figure 4B).77 Diaryl-diynes yield fully (E,E)-dienes, while dialkyl-diynes prefer (Z,Z)-products. Experiments suggest the existence of (Z)-enynes intermediates and subsequent isomerization. The use of THF as a solvent may be the only way to broaden the substrate scope. It is speculated that dialkyl-substituted diynes cannot participate in isomerization because only the aromatic ring can stabilize the allene intermediates.
Kirchner et al. expanded the substrate scope to include 1,3-diynes 119 and 1,3-enynes 120 using their own strategy, achieving the construction of (Z)-alkenes through Fe-catalyzed semi-hydrogenation. Outstanding stereoselectivity is observed in the (Z,Z)-isomer products 121 (Figure 4C).78 Both electron-rich and electron-deficient groups in the para-position of benzene are well-tolerated. Deuteration experiments confirm that this stereoselectivity arises from the syn-addition of iron-hydride species.
Common methods typically yield 1,4-disubstituted dienes as products. Beller et al. proposed a Pd-catalyzed strategy to address this limitation and construct highly substituted conjugated dienes (Figure 4D).79 This method not only produces previously inaccessible trisubstituted products 123 superior to other methods but also offers excellent control over stereoselectivity. Additionally, it utilizes the exclusive ligand 1,1′-ferrocenediyl-bis(tert-butyl(pyridin-2-yl)phosphine) and operates at a gentle room temperature. The approach demonstrates excellent compatibility with functional groups in both aromatic and aliphatic diynes, even with unsymmetrical substrates.
Enynes addition
To establish a novel method for the construction of 1,3-dienes, Yao and Lin et al. described a Pd-catalyzed allylic alkylation in which inactivated 1,4-enynes 124 and pronucleophiles 125 serve as substrates, eliminating the need for sophisticated pro-functionalization and oxidants (Figure 4E).80 While steric effects have a slight impact on the yield, substituents with different electronic effects are well-tolerated by both enynes and pronucleophiles. Moreover, using various acids or nitrogen heterocyclics enables the construction of C–O bonds and C–N bonds. The process likely involves Pd-H species insertion,81 β-Hydrogen elimination, and π–σ–π isomerization. However, this method does not provide a means to control stereoselectivity, yielding mostly a mixture of (E)-configurations.
Liu and Huang et al. have introduced a method for the construction of 1,3-dienylsilanes with better regio- and stereoselectivity using eco-friendly ionic catalysis (Figure 4F).82 Linear and branched alkyl substitutions at the alkyne position of 1,3-enynes 127 demonstrate good tolerance for functional groups, and even inactivated terminal alkenes can be involved. However, overcoming steric effects around the alkyne group remains challenging. The hydrosilylation involves the syn-addition of an Si–H bond into the alkyne group of 1,3-enynes, with the silicon groups preferentially locating themselves adjacent to the olefin. These dienylsilanes serve as versatile building blocks for further transformations.
The same group has developed another Ir-catalyzed system for the generation of (E,E)-1,4-diaryldienes 131 using aryl-containing enynes 130 under cooperative catalyst ethanol (Figure 4G).83 Interestingly, the less active Ir monohydride species is more effective and leads to no detection of overreduction products. The electronic and steric effects of the enynes have little impact on the reaction process and the formation of the products. Additionally, 1,3,5-trienes can be obtained with extraordinary selectivity. GC analysis suggests the possibility of cis-semihydrogenation and sequential isomerization from initial (E,Z)-dienes to the ultimate (E,E)-products.
Another strategy involves trans-hydrogenation of 1,3-enynes. Liu et al. introduced a trans-hydroalkynylation of enynes 132 with terminal alkynes 133 under a palladium-dominated multi-catalyst system, which highlights the promoting effect of Lewis acid (Figure 4H).84 While electron-withdrawing groups in the terminal alkynes may lead to dimerization byproducts and reduced yields, functional groups positioned near the remote alkenes carbon atom of enynes exhibit excellent tolerance, even accommodating a hydrogen atom. The resulting products serve as versatile building blocks for further transformations across various fields.
Isomerization
Isomerization is a strategic approach that facilitates the stereoselective synthesis of 1,3-dienes, categorized into three types based on substrate classification: dienes isomerization, alkynes isomerization, and allenes isomerization (Figure 5A).
Figure 5.
Isomerization
(A) Isomerization mode.
(B) Cobalt-catalyzed Z to E geometrical isomerization of 1,3-dienes.
(C) Dienyl isomerization to access highly substituted 1,3-dienes.
(D) Selective E to Z isomerization of 1,3-dienes.
(E) Stereo-convergent synthesis of Z-1,3-disubstituted-1,3dienes.
(F) Stereoselective access to branched 1,3-dienes by remote conjugative isomerization.
(G) Cobalt-catalyzed migration isomerization of dienes.
(H) Synthesis of conjugated dienals from terminal acetylenes and acrolein.
(I) Au-catalyzed isomerization of alkynes to 1,3-dienes.
(J) Gold-catalyzed isomerization of unactivated allenes into 1,3-dienes.
(K) A palladium-mediated Suzuki−Miyaura coupling allene isomerization sequence.
Isomerization of dienes
Classical approaches like Wittig olefination,85 Julia-Kocienski method,86 Peterson elimination87,88 and ene–yne cross-metathesis (EYCM)89 are efficient at producing functional 1,3-dienes. These reactions can access stereo-defined 1,3-diene compounds under substrate dependent process, yet they frequently encounter challenges in terms of atom economy. Isomerization of dienes presents a solution to this issue and can be classified into two types: configuration isomerization and double bond position isomerization.
Z/E isomerization
Dienes obtained through conventional methods often consist of a mixture of isomers. To address this, Chen and Xia et al. proposed stereoconvergent conversion from mixed diene isomers 135, synthesized via Wittig olefination, into exclusively (E)-configured 1,3-dienes 136 (Figure 5B).90 They achieved this using low-cost and environmentally friendly cobalt catalysts and an amido-diphosphine-oxazoline ligand. This method is compatible with both electron-donating and electron-withdrawing groups, including strong electron-deficient trifluoromethyl substituents, and demonstrates excellent yields and stereoselectivity. Mechanistic experiments ruled out a radical pathway and indicated that Z/E isomerization can occur, ultimately transforming into advantageous (E)-products under thermodynamic control.
(E)-1,3-dienes are typically more thermodynamically stable, while the isomerization from E to Z usually requires additional chemical energy. Diver et al. introduced Z-selective stereoconvergent isomerization of 1,3-dienes under Uphill photocatalysis (Figure 5C).91 Using catalytic ene–yne metathesis to generate a variety of functional 1,3-substituted dienes 139, they obtained a mixture of stereoisomeric dienes, primarily new compounds and E-isomers. They subsequently developed a straightforward tandem reaction that directly produced the corresponding Z-1,3-dienes 140 without the need for excessive purification and separation of intermediate mixtures. The method tolerated various oxygen-containing groups with excellent regio- and stereoselectivity, providing 1,3-disubstituted dienes distinct from traditional methods.
Murahashi et al. introduced a new concept for E-to-Z isomerization, relying on a conjugated reaction instead of photoirradiation or other external stimuli (Figure 5D).92 They used electron-deficient dienes 141 as substrates under a dinuclear PdI–PdI bonded complex [Pd2(CH3CN)6][BF4]2 and the ligand 1,3,5,7-cyclooctatetraene (COT), achieving near-perfect Z-selectivity in the resulting dienes.
Double bonds position isomerization
In this phase of the research, the most frequently observed reaction involves the isomerization of terminal alkenes into internal ones. This transformation is primarily governed by kinetic factors. The only limitation lies in the consistent presence of a methyl group within the resulting products.
The ene-yne reaction can use simple alkynes and alkenes to afford 1,3-disubstituted dienes, but it is challenging to obtain dienes with 1,1,4-trisubstitution and 1,1,4,4-tetrasubstitution. To address this limitation, Diver et al. provided access to highly substituted dienes based on the above method (Figure 5E).93 Previous studies have indicated that a metal hydride may accelerate the isomerization of the dienyl position. In this reaction, they utilized alcohol as an additive, which was perceived to promote the generation of ruthenium hydrides, decomposing from the Grubbs ruthenium catalysis, and achieving the construction of (E)-dienes with good regioselectivity and moderate tolerance for functional groups. Simultaneously, it shows isomerization that is subject to the thermodynamic stability of the linear products.
Mazet et al. have devised strategies for creating unusual branched 1,3-dienes through a kinetic process-controlled remote functionalization approach (Figure 5F).94 This method involves a chemoselective conjugative isomerization of two distant alkenes using a ruthenium hydride complex, yielding branched products 146 with high yields, precise regioselectivity, and stereocontrol. The substituents at the terminal position of vinylarene 145 exhibit broad compatibility with various electronic effects. Notably, this approach is also applicable to substrates containing alkyl groups, highlighting its practical utility. This process likely proceeds through a metal-hydride mechanism, involving repeated migration insertion/β-H elimination steps. The strict spatial requirements of the substrates restrict the competitive pathway, favoring chemically selective insertion at the terminal site.
Hong and Lu et al. have elucidated a novel cobalt-catalyzed isomerization process. This process involves the transformation of mixed isomers of terminally conjugated dienes 147 into stereoselectively internal 1,4-disubstituted dienes 148, with a majority of them being 1-aryl products (Figure 5G).95 The catalyst employed is an 8-oxazoline iminoquinoline ligand. This transformation demonstrates remarkable tolerance toward a variety of electron-donating and electron-withdrawing groups, exerting minimal influence on both the conversion and (E,E)-selectivity. Additionally, the length of the alkyl chain does not significantly impact the reaction outcome.
The proposed mechanism suggests that both carbon–carbon double bonds might simultaneously undergo migration, involving the successive steps of cobalt hydride migratory insertion and further migration until one of the double bonds reaches the benzyl site. This process culminates in the final step of β-H elimination, leading to the formation of the desired compounds. It is worth noting that the presence of this migration step may be related to the existence of stable thermodynamic intermediates.
Isomerization of alkynes
The benefits of using alkynes as substrates in isomerization processes include their ease of availability and commendable atom economy. Li et al. provided a palladium-catalyzed straightforward and diastereoselective construction of conjugated (E,E)-dienes 150 using readily available substrate 4-alkynals 149, through the synthesis of terminal acetylenes and acrolein under another Pd catalysis(Figure 5H).96 Substrates containing aryl groups display great compatibility with both electron-poor and electron-rich groups, with poor regioselectivity, as well as a few side products, observed in the inactivated aliphatic substrates. The probable mechanism may include a palladium-hydride-assisted pathway and an acetate-assisted one.
The isomerization of alkynyl compounds under transition metal catalysis is another method for the synthesis of (E,E)-1,3-dienes. In 2014, Zhang et al. reported a ligand-regulated method for propargylic deprotonation under gold catalysis, enabling the isomerization of alkynes 151 and providing a new synthetic method for the preparation of multisubstituted 1,3-dienes 152 (Figure 5I).97
Isomerization of allenes
Allenes have also been identified as suitable starting materials for diene generation. However, only those equipped with functional groups can participate in the reaction. In 2012, Liu et al. published a study on the Au-catalyzed isomerization of inert inactivated allenes 153 into internal or external 1,3-dienes 132 using nitrosobenzene as an additive (Figure 5J).98 The mechanism involves the intermediate formation of a gold allylic cation, which can be regulated by a Brønsted acid and undergo intramolecular proton transfer. This process accommodates a wide range of highly tri- and tetrasubstituted allenes, resulting in good yields under mild conditions. It is worth noting that when producing internal dienes, the stereoselectivity is relatively lower.
In 2016, Kimber et al. described a sequential reaction involving Pd-catalyzed Suzuki–Miyaura coupling/isomerization without the need for a base, utilizing propargyl alcohols 155 and boronic acids 156 (Figure 5K).99 The process involves two steps, as demonstrated by the isolation of intermediate allenes 157 with high yields. The mechanism relies on a Pd species H-PdⅡ-OB(OH)2, facilitating rearrangement and in situ generation of 1,3-dienes through dehydropalladation reactions.
Derivatization of 1,3-diene and equivalent dienylation reagents
Using dienes as substrates
Suzuki reactions
In 2007, Farinola et al. utilized 1,4-diiodine-1,3-butadiene 159 as the substrate to execute Suzuki reactions with aryl boron 160 (Figure 6A).100 This approach led to the synthesis of all-(E) symmetrical 1,4-diaryl dienes 161, demonstrating a high level of stereoselectivity. Importantly, this reaction retained the geometry of the double bond, which was governed by the nature of the substrate.
Figure 6.
Derivatization of 1,3-diene and equivalent dienylation reagents
(A) Stereoselectivity synthesis of (E)-symmetrical 1,4-diaryl dienes.
(B) Nickel-catalyzed divergent Mizoroki–Heck reaction.
(C) Stereoconvergent synthesis of polyenes by Heck-type alkylation.
(D) Regioselective C–H pyridination of 1,3-dienes.
(E) Using olefin cross-metathesis for chemoselective construction of substituted dienes.
(F) Z-Selective cross-metathesis for synthesis of 1,3-dienes.
(G) Selective synthesis of 1,3-dienes through C−C bond scission of cyclobutene.
(H) Regio- and stereoselective construction of dienes by using sulfolene as diene synton.
Heck reactions
Chen et al. proposed a nickel-catalyzed stereo-divergent reaction for crafting structurally diverse 1,3-dienes 164 (Figure 6B).101 Under these conditions, the nickel catalyst effectively facilitated the formation of linear 1,3-dienes 164a and enabled the construction of branched products 164b by introducing bulky ligands. This method provided an efficient pathway for diverse diene synthesis, demonstrating excellent compatibility with functional groups and stereoselectivity. While strong electron-withdrawing groups in the substrate slightly reduced the yield of linear products, they did not hinder the formation of (E,E)-1,3-dienes. However, electron-withdrawing groups tended to result in minor formation of 1,4-addition byproducts in branched product synthesis. When alkyl dienes were employed as substrates, a mixture of isomeric branched products was observed. Notably, through the sequential use of two sets of conditions, it became feasible to construct polyenes bearing both linear and branched diene motifs.
Radical addition process
Zhu et al. proposed a radical-mediated alkylation method to apply easily accessible isomer-mixed conjugated dienes, yielding single isomers of corresponding products with perfect stereo-convergence (Figure 6C).102 They selected aryl-containing diene substrates and found that electron-withdrawing and -donating groups were compatible with the reaction conditions. Moreover, 1,3,5-trienes 167aa could also serve as feasible substrates in this transformation. For alkylated reagents, it was illustrated that an ester group was essential, while a cyano group was not. Both alkyl and aryl groups could be incorporated into the conjugated dienes.
Electrophilic reaction
Electrophilic addition reactions can also be employed to synthesize multi-substituted 1,3-dienes. However, most of this functionalization primarily occurs at the C-1 position, as electrophilic reagents attack, indicating limited regioselectivity and types of functional groups. Zhao et al. demonstrated an alternative approach involving pyridination at the C-2 position using electrophilic organoselenium catalysis and nucleophilic fluoropyridinium salt to react with monosubstituted dienes (Figure 6D).103 Aromatic and aliphatic dienes with different substitution patterns were able to participate in the reaction and yield moderate to good yields. For most diene products, stereoselectivity depended on the substrates, generating a mixture of isomers when 1,4-desubstituted dienes underwent the transformation. Furthermore, the complete regioselectivity depended on the Se catalyst, as an electrophilic reagent, which occupied the C-1 position initially, resulting in the pyridination reagent being located at the C-2 position.
Diene cross-metathesis
Using olefin cross-metathesis is an alternative strategy to access to highly substituted conjugated dienes from relatively simple terminal alkenes and 1,3-diene building blocks. However, to control the chemo- and stereoselectivity is still challenging. Grubbs et al. found that the selectivity of this cross-metathesis could be controlled by a Ru catalyst (Figure 6E). In this system, new 1,3-dienes could be formed with excellent chemo- and stereoselectivity by using high steric resistance substituted dienes171a and 171b as substrates, and both electron-deficient and -rich alkenes have good reactivity and do not affect the E-selective formation of products.104
To achieve the generation of Z-selective dienes, Houk and Grubbs et al. proposed another protocol (Figure 6F). Under optimized conditions, the terminal alkenes 174 and only 1.5 equivalents of diene substrates 175 could smoothly complete the transformation which is scarcely bothered by the functional groups. Moreover, the new double bonds formed in conjugated dienes are mainly Z-configuration. This strategy is also applicable to the construction of multi-substituted 1,3-dienes.105
Using dienes equivalent reagents as substrates
Using cyclobutene as diene synton
Carrow et al. reported a Pd-catalyzed aerobic coupling using cyclobutene 177 as a diene precursor to regio- and stereoselectively construct substituted 1,3-dienes 179 with (hetero)arylboronic acids 178.106 The mechanism involved the Heck process, where a 3-substituted cyclobutene was initially generated, followed by the opening of the pericycle through C–C bond scission to yield the final diene products. This mechanism allowed control over the geometry of the olefins, as the cleavage of the C–C bond was stereospecific through the pericycle pathway. High regioselectivity was also achieved, as Pd-catalyzed reactions were formed exclusively through stereospecific syn-migratory insertion and syn-β-H elimination in the absence of chain walking (Figure 6G).
Using sulfolene as diene synton
Sulfolenes, stable compounds resistant to air and moisture, could also participate in the reaction as equivalent reagents to 1,3-butadiene for synthesizing 1,3-diene compounds. To address dienylsulfinate salt isomerization and control product geometry, Larionov et al. described a practical regio- and stereoselective dienylation reaction using readily accessible sulfolenes.107 They demonstrated that regioselectivity was structure-dependent. Potassium methoxide, acting as a base, mediated the ring opening of sulfolenes, leading to the regioselective formation of conjugated dienes in subsequent Pd-catalyzed coupling reactions with haloarenes. When using non-substituted, 2-substituted, and 3,4-disubstituted sulfolenes, the resulting products were 1-E selective dienes. Conversely, 3-substituted or 2,4-disubstituted sulfolenes tended to yield products with 1-Z stereoselectivity, suggesting kinetic preferences in catalytic steps involving E-selective intermediates. Furthermore, this reaction could be employed to incorporate two dienyl moieties, valuable in constructing complex intermediate compounds (Figure 6H).
Subsequently, the research group expanded the substrates to include aryl sulfonates and aryl iodides, enabling efficient and highly stereoselective conversion to obtain substituted dienes and polyenes with good to excellent yields.108 Furthermore, a stereo-complementary Pd/Xantphos-catalyzed dienylation was developed to synthesize Z-dienes, relying on ligand-driven stereodisparity. Unlike previous studies, the high Z-selectivity of the Pd/Xantphos-catalyzed system arose from the inhibition of Z to E isomerization. The stereo-divergence between the Xantphos- and dppbz-based reactions proved useful in generating the required isomers of target compounds109 (Figure 6H).
Applications of 1,3-diene-based AIEgens
In the early part of 2001, Tang et al. conducted an investigation using the dye 1-methyl-1,2,3,4,5-pentaphenylsilole.12 They observed a notable increase in emission intensity when the dye aggregated. This phenomenon is commonly seen in dyes with a twisted molecular structure and propeller-like substituents. Since they introduced the concept of AIE, AIE luminogens (AIEgens) have gained popularity for their ability to overcome limitations associated with ACQ observed in traditional fluorophores. In recent years, the field of aggregology has garnered significant attention owing to its emphasis on investigating the physicochemical properties exhibited by molecular aggregates.110,111 The AIE effect112 is ascribed to the confinement of RIM, which restricts molecular rotation and vibration.113 However, recent investigations have proposed that the restriction of access to the dark state (RADS) also constitutes a crucial factor in elucidating the AIE mechanism.114 The study of AIE phenomena encompasses not only a comprehensive exploration of the underlying mechanisms but also an in-depth discussion on its wide-ranging applications.115 AIEgens find practical applications across various domains, including light-emitting diodes, bioimaging, chemo-sensing, anti-counterfeiting, and more.116 Among the well-established AIEgens, multiphenyl-substituted 1,3-butadiene (MPB) derivatives have generated significant interest due to their simple molecular structures and fundamental applications. In comparison to the ‘star molecule’ TPE, MPBs display a more expansive conjugated system, which results in a redshifted emission wavelength. Introducing the D-π-A unit enables the construction of fluorescent materials with full wavelength emission. However, due to the extended conjugated skeleton, the aryl substituents within the molecule are relatively distant from each other, leading to a loose structure in aggregated state. Upon excitation by light, these molecules can undergo vibration and rotation to some extent, thereby resulting in a relatively low quantum yield. This section provides a comprehensive review of significant advancements made over the past several years using these compounds in optoelectronic devices, stimuli-responsive materials, sensors, therapeutics, and other fields.
Structure and photophysical properties
Generally, even minor structural modifications can result in substantial changes to material properties. Therefore, a comprehensive understanding of the structure and photophysical characteristics of MPBs is crucial for the design and synthesis of innovative 1,3-diene based materials.
Structure of MPBs
The MPBs that exhibit AIE properties primarily include 1,1,4-triphenyl-1,3-butadiene (1,1,4-TriPB), 1,1,2,4-tetraphenyl-1,3-butadiene (1,1,2,4-TPB), 1,1,4,4-tetraphenyl-1,3-butadiene (1,1,4,4-TPB), and hexaphenyl-13 butadiene (HPB) (Figure 7A). The simplest luminous unit in this type of skeleton is 1,4-diaryl-1,3-butadiene (1,4-DPB). Several studies have suggested that the crystal structure and luminescent properties of these compounds are governed by the number and position of aromatic rings. The pronounced torsion angles in their structures provide a structural foundation for their AIE properties (Figure 7B).117 Compound stability is a crucial parameter; generally, as the conjugated system enlarges, the energy difference between HOMO and LUMO progressively diminishes, facilitating photoisomerization and thermally induced isomerization. Compared to TPEs, MPBs tend to be less stable. It has been observed that more conjugated 1,3,5-triene compounds often necessitate protection from light exposure. Furthermore, in certain unique structures, the double bond in a 1,3-diene can function as a dienophile reacting with another molecular, leading to side reactions that cause its metamorphism.
Figure 7.
Structure and photophysical properties of MPBs
(A) The structure of MPBs.
(B) The crystal structure of 1,2-DPB. Reproduced with permission from ref.117 Copyright 2014 Royal Society of Chemistry.
(C) The structure of 1,4-DPB-based AIEgens. Reproduced with permission from ref.118 Copyright 2008 American Chemical Society.
(D) Plots of relative PL intensity versus the composition of THF/water mixtures of TPBs. Reproduced with permission from ref.119 Copyright 2019 Springer Nature.
(E) The AIE property of 1,1,4-TriPB-F. Reproduced with permission from ref.60 Copyright 2020 Elsevier.
(F) The structure of TMT and TMJ. Reproduced with permission from ref.120 Copyright 2020 Royal Society of Chemistry.
(G) Electrostatic potential maps of TMT (top) and TMJ (bottom). Reproduced with permission from ref.120 Copyright 2020 Royal Society of Chemistry.
(H) Variations in the emission intensity of TMJ and TMT, considering absorption weighting, were observed when PEG/PAR content increased (top) and viscosity increased (bottom) in both ACN–PEG mixtures and Tol–PAR mixtures. Reproduced with permission from ref.120 Copyright 2020 Royal Society of Chemistry.
Photophysical properties of MPBs
The MPB skeleton presents a wide array of potential molecules suitable for AIE and AEE materials. These compounds demonstrate limited or absent luminescence in diluted solutions, but exhibit strong emission when aggregated. MPBs satisfy the prerequisites of multiple rotors and RIM constraints, thereby exemplifying typical AIE attributes. In general, the MPB skeleton possesses a substantial torsion angle that facilitates the rotation of the benzene ring. MPBs with rotors experience high-frequency motion in diluted solutions, which leads to prompt non-radiative decay of the excited state, resulting in feeble or absent emission in solution. The close packing between molecules impedes the aggregation of these motions, thereby enabling subsequent radiation pathways.
Das et al. conducted a comparative analysis of the quantum yields of 1,4-DPBs in acetonitrile solution and their solid-state forms to characterize their photophysical properties (Figure 7C).118 The results revealed that certain compounds exhibited AEE properties; however, this phenomenon was not prominently observed due to the limited number of substituents in the diene skeleton and insufficient molecular distortion. In 2019, Dong et al. successfully confirmed the AIE character of 1,1,2,4-TPB (Figure 7D).119 The fluorescence intensity of TPBs in the water fraction (fw), which is below 70%, was found to be significantly diminished due to nonradiative decay resulting from the unrestricted intramolecular rotation of single bonds between phenyl and alkenyl groups. However, when fw reached 99%, the fluorescence intensity of TPB-1 exhibited an approximate 29-fold increase, while TPB-2 and TPB-3 showed approximately 5-fold and 0.5-fold increases, respectively. In 2020, Feng and Lin et al. underscored the notable AIE effect exhibited by 1,1,4-TriPB-F (Figure 7E).60 The fluorescence intensity of this compound was found to be approximately 5-fold greater in its aggregated state compared to its pure THF state.
To explore the impact of different substitution patterns on the fluorescence characteristics in solvents with varying viscosities and in dye aggregates, they introduced a propeller-like triphenylamine unit into 1,3-diene skeleton and analyzed their AIE property (Figures 7F–7H). Studies found that the degree of emission enhancement due to viscosity directly correlated with the number of twisted triphenylamine donors. The presence of this propeller-like triphenylamine moiety, with its twisted structure, effectively mitigated the well-known ACQ phenomenon. As a result, triphenylamine derivatives have emerged as a highly promising choice for developing dyes with AIE properties. This improvement in emission is attributed to the specific connection of the central nitrogen atom, which enhances the interaction between the nitrogen electron lone pair and the atoms of the three carbon phenyl rings. Molecular structures with donor-acceptor (D-A) configurations are known for their ability to form twisted intramolecular charge transfer (TICT) systems. These systems exhibit optical properties that are strongly influenced by various factors in their microenvironment, including solvent polarity, viscosity, and temperature. Researchers examined the characteristics of triphenylamine-allylidenemalononitrile-julolidine (TMJ) and triphenylamine-allylidenemalononitrile-triphenylamine (TMT), employing the donor-acceptor (D-A) structures. This led to the creation of nanoparticles loaded with a vibrant dye. The use of an excitation laser power of 25% for TMT and 65% for TMJ demonstrated that the solid-state emission of TMT significantly surpassed that of TMJ. The occurrence of dye aggregates in both dyes coincided with a red shift in absorption, indicating increased polarity in the dye microenvironment due to higher water content, with this shift being more pronounced in TMT.120
Effects of double bond geometry on photophysical properties
In contrast to isolated alkenes, 1,3-dienes are characterized by two double bonds and theoretically present four unique stereoisomers. A comprehensive exploration of the impact of double bond geometry on photophysical properties is crucial for understanding the underlying mechanism of the AIE effect and for the development of innovative AIE small molecules. The unique properties exhibited by the E/Z isomers of AIE compounds are widely accepted, however, the synthesis and purification of these isomers pose significant challenges. As a result, investigating the potential involvement of E/Z isomerization in the AIE process has proven to be a formidable undertaking, with only a limited number of examples available to illustrate this phenomenon.
In 2003, Das et al. conducted a study on the photo-induced isomerization behavior of 1,4-DPB liquid crystals (Figure 8A).121 The researchers observed that upon exposure to light, the (E,E)-isomers of these compounds undergo photoisomerization, resulting in the formation of heat-stable (E,Z) and (Z,E)-isomers. The original (E,E)-isomers can be restored by utilizing different wavelengths of light. Moreover, upon photoisomerization, the derivatives of the (E,E)-isomers experience an isotropic transition as a result of the appearance of non-liquid crystalline (E,Z) and (Z,E)-isomers. The thermal stability exhibited by these substances allows for reversible manipulation of their optical characteristics via a mechanism referred to as photoactivation, thereby preserving their liquid crystal phase.
Figure 8.
Applications of 1,3-diene-based AIEgens in OLED and photoresponsive materials
(A) Photophysical properties of (E,E)-1,4-DPB and (Z,E)-1,4-DPB. Reproduced with permission from ref.121 Copyright 2003 American Chemical Society.
(B) Effects of double bond geometry on PLQY. Reproduced with permission from ref.60 Copyright 2020 Elsevier.
(C) Effects of double bond geometry on piezochromism phenomenon. Reproduced with permission from ref.122 Copyright 2016 Elsevier.
(D) The structure of diene skeleton. Reproduced with permission from ref.123 Copyright 2012 Royal Society of Chemistry.
(E) Images of individual crystals captured using fluorescence microscopy (upper row) and powders illuminated with 365 nm UV light (lower row), depicting the respective small molecular derivatives. Reproduced with permission from ref.123 Copyright 2012 Royal Society of Chemistry.
(F) Images depicting the polymer films (uppermost row), and the emission color observed in DCM along with the film’s condition (lower two rows) when exposed to 365 nm UV light. Reproduced with permission from ref.123 Copyright 2012 Royal Society of Chemistry.
(G) 1,3-diene used in photoresponsive materials. Reproduced with permission from ref.124 Copyright 2020 Elsevier.
(H) The structure of TABD-COOH. Reproduced with permission from ref.125 Copyright 2013 Royal Society of Chemistry.
(I) AIE properties of TABD-COOH. Reproduced with permission from ref.125 Copyright 2013 Royal Society of Chemistry.
(J) Piezochromic effect of TABD-COOH. Reproduced with permission from ref.125 Copyright 2013 Royal Society of Chemistry.
Feng and Lin et al. chose the 1,1,4-TriPB skeleton to explore the influence of varying double bond geometry on the solid-state quantum efficiency (Figure 8B).60 When compared to 3Z-TriPB (ΦF = 3.1%) and 1Z-TriPB-F (ΦF = 12.0%), both 3E-TriPB (ΦF = 23.0%) and 1E-TriPB-F (ΦF = 41.5%) demonstrated significantly higher ΦF values, suggesting significant performance enhancements. However, the authors did not provide a crystal structure to explain this intriguing phenomenon.
Shi and Dong et al. conducted a study on the influence of double bond geometry on the photophysical properties of 1,1,2,3,4,4-HPBs (Figure 8C).122 The synthesis of (E,E)-HPB-CHO, (E,Z)-HPB-CHO, and (Z,Z)-HPB-CHO was carried out, followed by their structural characterization using diverse analytical methods. An examination of the fluorescence spectra revealed that the (E,E)-isomer displayed the shortest emission wavelength and weakest intensity. In contrast, the (Z,Z)-isomer resulted in a more potent emission at 540 nm due to its extended conjugated structure. Furthermore, (E,E)-HPB-CHO exhibited AIE activity in water/THF system, while both (E,Z) and (Z,Z)-isomers demonstrated typical Aggregation-enhanced emission (AEE) behavior with weak luminescence in pure THF. Dynamic light scattering (DLS) measurements indicated that the aggregates formed by (E,Z) and (Z,Z)-isomers were larger than those formed by the (E,E)-isomer due to their looser molecular packing caused by twisted conformations. RIR was identified as the primary factor influencing the AIE/AEE characteristics of these compounds. Notably, compared to the other two isomers, (Z,Z)-HPB-CHO displayed significant mechanochromic performance due to its twisted conformation and relatively loose packing, whereas the (E,E)-isomer showed minimal mechanochromic performance.
OLEDs
The challenge of ACQ poses a significant obstacle in the advancement of optoelectronic devices, especially those relying on organic materials as solid thin films. The formation of these films inherently triggers aggregation, which frequently results in diminished fluorescence quantum yields. To reduce nonradiative decay in the solid state, three approaches are employed: 1) increasing molecular conjugation, 2) achieving a finely-tuned electron donor (D) and acceptor (A) unit system, and 3) reducing intermolecular interactions. Malik et al. employed the third approach, incorporating 1,3-diene into polymers as a building block. The partially twisted structure of 1,3-diene significantly mitigated intermolecular interactions, resulting in AIE phenomenon. Color tuning was achieved by introducing different functional groups to the central core, causing the emission color of the organic fluorophores to vary from blue to red based on the substituents attached.126
Organic fluorophores, particularly MPBs, play a vital role in OLEDs or polymeric light-emitting diodes (PLEDs) by effectively addressing the issue of ACQ phenomenon. OLED device fabrication has garnered substantial attention since the successful disclosure of the first electroluminescence (EL) device by vapor deposition.126 To achieve a full-color display, achieving pure blue, green, and red colors is essential. In the early 2000s, Shin et al. successfully used a structure combining 1,3-diene to achieve pure blue luminescence.127 Subsequently, Sudip Malik et al. reported a series of conjugated copolymers featuring a (1Z,3Z)-1,1,4,4-TPB unit as a central core, demonstrating tunability across a broad color spectrum (Figures 8D–8F).123 These organic solid fluorophores are not only highly emissive in the condensed phase but also allow color tuning from blue to red within the visible region. The emission color of fluorophores can be modulated by the substituent attached to the central core, ranging from blue (444 nm) to red (613 nm). The electrochemical measurements demonstrate the potential of polymer-based derivatives of the abovementioned central unit as promising candidates for applications in organic photovoltaics (OPVs).
Stimuli-responsive materials
Photoresponsive materials
The significance of photoresponsive materials has grown substantially across multiple domains, including national security, environmental preservation, chemical and biological sensing, and medical quarantine diagnostics.128,129,130 Two isomers of HPB derivatives incorporating dicyanoacetate have been observed to display a fluorescent response when exposed to UV radiation. Dong et al. previously described the properties of heat-induced recrystallization and photo-induced intramolecular cyclization of these isomers.124 Utilizing ZZ-HPB–NC–based strips is seen as a promising approach to advance deep-UV detectors. Researchers employed single crystals to reveal a distorted conformation, suggesting that the phenyl group in the molecule can freely rotate in the solvent. However, it exhibits pronounced emission in the solid state due to constraints on RIM and the formation of orderly and compact intermolecular arrangements, which effectively reduce non-radiative energy transfer. When exposed to an irradiance of 18 μW/cm2 at a wavelength of 254 nm, the fluorescent spectrum reached its peak intensity after 60 min. Conversely, exposure to an irradiance of 50 μW/cm2 at the same wavelength resulted in a significantly reduced response time of only 20 min. Additional experiments confirmed that a change in wavelength of 10 nm served as the threshold for UV detection, with an LOD of approximately 8.2 μW/cm2 (Figure 8G).
Piezochromic materials
Piezochromic materials, characterized by their luminescence color changes in response to mechanical forces, possess substantial practical implications for diverse applications.131,132 These include the development of mechano-sensors, optical data storage systems, secure document technologies, and optoelectronic devices. Han et al. reported their design and synthesis of TABD-COOH, which demonstrated reversible mechano-responsive abilities upon grinding (Figures 9H–9J). Intermolecular hydrogen bonding was assumed to play a pivotal role in color alteration. The application of pressure can disrupt the H-bond interactions and modify molecular packing. However, exposure to vapors of polar solvents can help reestablish the H-bonds and restore the crystal arrangement, returning the sample to its original color.125
Figure 9.
Applications of 1,3-diene-based AIEgens in piezochromic materials and sensors
(A) The solid surface temperature detection. Reproduced with permission from ref.133 Copyright 2014 Royal Society of Chemistry.
(B) The temperature sensing arrays, fabricated using MCBD in the three solutions. Reproduced with permission from ref.133 Copyright 2014 Royal Society of Chemistry.
(C) The structure of MCBD. Reproduced with permission from ref.133 Copyright 2014 Royal Society of Chemistry.
(D) The structure of TPB-COOH. Reproduced with permission from ref.134 Copyright 2018 Elsevier.
(E) Fluorescence response of TPB-COOH upon the addition of different metal ions. Reproduced with permission from ref.134 Copyright 2018 Elsevier.
(F) Fluorescence response of TPB-COOH toward Ce4+ with the different alcohols. Reproduced with permission from ref.134 Copyright 2018 Elsevier.
(G) Depiction of 1D zigzag chains of MOF. Reproduced with permission from ref.135 Copyright 2018 American Chemical Society.
(H) Alterations in the fluorescence intensity of MOF are observed when specific metal ions are introduced, as well as when competing metal ions are added to water in the presence of MOF+Al3+. Reproduced with permission from ref.135 Copyright 2018 American Chemical Society.
(I) Illustration depicting the strategy of substituting metal coordination through competitive competition. Reproduced with permission from ref.135 Copyright 2018 American Chemical Society.
(J) The structure of 1,2,3,4-TPB. Reproduced with permission from ref.136 Copyright 2004 American Chemical Society.
(K) The diagram illustrates the detection effect of volatile gas for 1,2,3,4-TPB. Reproduced with permission from ref.136 Copyright 2004 American Chemical Society. The testing strips of Z,Z–HPB–CN undergo a color (L) and wavelength (M) alteration when exposed to amine vapor (10 ppm) for 20 s under UV-light. The sequence from left to right represents the strip’s appearance before and after exposure to various substances, namely NH3·H2O, hydrazine hydrate, ethylenediamine, di-n-propylamine, diethylamine, and trimethylamine. Reproduced with permission from ref.137 Copyright 2017 Royal Society of Chemistry.
(N) Tetrasubstituted-1,3-diene used in N2H4 detection. Reproduced with permission from ref.138 Copyright 2020 Elsevier.
(O) Fluorescence enhancement efficiencies achieved using TABD-MOF with various analytes. Reproduced with permission from ref.139 Copyright 2014 American Chemical Society.
(P) The PL spectra of E,E-HPB-ID in mixtures of THF and water were measured. Reproduced with permission from ref.140 Copyright 2018 Royal Society of Chemistry.
(Q) The correlation between the relative change in PL intensity [(I - I0)/I0] was analyzed. Reproduced with permission from ref.140 Copyright 2018 Royal Society of Chemistry.
(R) The variation in wavelength of E,E-HPB-ID was studied with different fractions of water in the THF/water mixtures. [E,E-HPB-ID] concentration was 1 × 10−5 mol/L, and the excitation wavelength used was 410 nm. Reproduced with permission from ref.140 Copyright 2018 Royal Society of Chemistry.
Sensors
Temperature sensors
In recent times, there has been a significant pursuit of fluorescent temperature sensors owing to their numerous advantages, such as their affordability, heightened sensitivity, non-contact mode of operation, ability to facilitate large-scale imaging, and resistance to robust electromagnetic fields. The application of a developed sensor array, consisting of dimalononitrile-dicarbazole-substituted 1,4-butadiene (MCBD) molecules with push-pull electronic structures is described for non-invasive temperature mapping on material surfaces (Figures 9A–9C). The sensor array operates within three solvation envelopes, ensuring accurate temperature measurements without any risk of contamination. These arrays possess the ability to generate a unique fingerprint that corresponds to cryogenic temperatures. The precise measurement of temperature was accomplished by observing significant fluctuations in both fluorescence intensity and wavelength in these solvents in response to temperature variations. This advancement enabled the creation of a comprehensive array of temperature sensors, capable of producing a distinct visual color pattern of signals that can be readily perceived without the need for supplementary instruments.133
Chemical sensors
The cost-effective, efficient, and straightforward advantages of AIEgens have sparked significant interest in their chemical sensing capabilities.141,142 These AIEgens can effectively detect specific chemicals without requiring complex equipment, intricate components, or complicated procedures. In contrast, conventional organic materials often suffer from ACQ, which limits the use of traditional luminophores in solid states. Moreover, using a solid matrix for detection, rather than a solution, offers a more practical approach, avoiding the need for cumbersome optical instruments.
Detection of ions
The TPB derivative, which incorporates two carboxyl groups as substituents, exhibits notable AIE characteristics when dissolved in a mixture of THF and water.134 The objective of this design is to create a fluorescent probe that possesses exceptional selectivity, rapid response time, and an extensive linear response range for Ce3+ exhibiting a remarkably low detection limit of 2.27 mol/L and Ce4+ ions by adding 1,2-propanediol. During the process of titrating TPB-COOH with Ce3+ in a water/THF mixture, the fluorescence exhibited a significant increase of 5-fold. While after conducting an examination of 13 metal cations with varying valences, the findings unequivocally illustrate the commendable selectivity of compound TPB-COOH toward Ce3+ (Figures 9D–9F).
Metal-organic frameworks (MOFs) are precisely structured crystalline solids formed by the bonding of metal ions and organic linkers through covalent interactions. The emissive properties of MOFs can be adjusted from highly emissive to totally nonemissive by strategically modifying the metal ion through ligand-to-metal charge transfer. Zhao, Lin, and Zhao et al. synthesized MOF material exhibits the capability to function as a fluorescence ‘off-on’ probe, enabling the detection of Al3+ in aqueous environments with high sensitivity (Figures 9G–9I).135 The proposed interpretation for the fluorescence off state involves a fluorescence quenching mechanism that relies on photoinduced electron transfer from the excited state of the linker (active fluorophore) to the heterocyclic auxiliary ligand 4,4′-bipyridine (Bpy), which was further supported by TD-DFT calculations.
Detection of volatile gases
AIEgens are capable of emitting light in an aggregated state, yet it does not emit light when in a solution state. This characteristic makes it suitable for the detection of volatile organic gases. Chen et al. described the on-off fluorescence switching behavior of TPB with four phenyl substituents on the butadiene core: due to the restriction of the intramolecular phenyl rotations, the twisted structure showed an intense emission in the solid state but virtually invisible in the common organic solvents or solvent vapor (Figures 9J and 9K).136 For this, this structure of AIE materials can be utilized to sense the organic vapor (such as dichloromethane).
Dong et al. conducted a study in which they employed an AIE probe, namely ZZ-HPB–CN, for the detection of volatile amines (Figures 9L and 9M).137 The probe achieved a limit of detection (LOD) of 1–10 ppm, 1–10 ppm, and 1 ppb using chromogenic naked-eye, ultraviolet, and fluorescence techniques, respectively. The probe demonstrated the ability to engage in a Schiff base reaction with primary amines and distinguish secondary and tertiary amines through charge transfer complexing (CTC) interactions. The stability of the ZZ-HPB-CN framework was achieved through Knoevenagel condensation reactions, while the properties associated with AIE were preserved by RIR mechanism.
Hydrazine (N2H4) has been utilized as a propellant in onboard space vehicles, known for its high reducibility and alkalinity. However, Liu, Xu, and Zhao et al. conducted a study in which they developed a probe capable of detecting hydrazine with a LOD of 90 nM (2.88 ppb). The researchers noted that the use of hydrazine led to a substantial increase, approximately 40-fold, in the intensity ratio (I515/I650) of the probe. Furthermore, the probe demonstrated the capability to detect hydrazine and trigger a fluorescence shift from red to cyan. Due to its swift response and excellent temporal accuracy, this probe shows promise as a robust analytical tool and sensor for hydrazine(Figure 9N).138
Detection of explosives
The organic linkers within the TPB-MOFs undergo competitive coordination substitution, leading to their release and subsequent reassembly into emissive aggregates as a result of aggregation-induced emission (AIE), which means the utilization of AIE fluorophore in combination with Mg2+ results in the synthesis of highly luminescent MOF (ΦF = 38.5%). While, substitution of Mg2+ with Ni2+ and Co2+, both possessing incomplete d subshells, leads to the formation of weakly fluorescent and nonfluorescent MOFs. The investigation enabling rapid and precise identification of explosives, specifically five-membered-ring energetic heterocyclic compounds, within a short time frame and with minimal detection thresholds. This achievement is attributed to the modification of emission and/or activation processes. Notably, the cobalt-based MOF demonstrates selective detection capability for the potent explosive 5-nitro-2,4-dihydro-3H-1,2,4-triazole-3-one at a discernible level visible to the naked eye, as well as spectroscopic sensitivity at parts per billion scale indicated by significant fluorescence emission (Figure 9O).139
A study conducted by Zhang, Shi, and Dong et al. examined the characteristics of E,E-HPB-ID, a derivative of HPB, concerning its application in the solid-state detection of polyazoles (Figures 9P–9R). The study’s findings demonstrated that E,E-HPB-ID exhibited remarkable sensitivity and specificity, with an LOD as low as 7 nM. The molecular structure of E,E-HPB-ID was found to possess torsion, primarily due to substantial steric hindrance caused by the inclusion of six phenyl rings. This structural attribute of E,E-HPB-ID played a pivotal role in advancing AIE fluorophores, enabling the creation of highly luminescent AIE nanoparticles. It is noteworthy that despite the inherent susceptibility of polyazoles, commonly encountered explosive substances like imidazoles showed minimal interference effects. This discovery suggests that E,E-HPB-ID holds significant promise as a sensor for chemical components in water, facilitating the monitoring of water quality.140
Biosensors
Detection of proteins
Tong and Dong et al. identified a group of HPB derivatives containing methylpyridinium that can effectively detect hemagglutinin proteins (H5) in avian influenza viruses (Figures 10A–10C). This novel probe exhibits several advantages over traditional detection methods, including real-time monitoring, high selectivity, and sensitivity toward H5. Additionally, it possesses the ability to detect H5 concentrations through a colorimetric approach over a wide range. The observed “turn-on” effect occurs within a remarkably short time frame of 10 s and rapidly reaches its maximum intensity. The findings of this study demonstrate a direct relationship between the intensities of fluorescence and the concentration of H5 within the range of 0–5.00 μg/mL/mL. The estimated LOD is determined to be 179.5 ng/mL (6.08 nM). This application introduces an innovative approach for the development of therapeutic interventions targeting virus detection and inhibition of viral infections.143
Figure 10.
Applications of 1,3-diene-based AIEgens in and biosensors, bioimaging, and PDT
(A) The structure of HPB-X. Reproduced with permission from ref.143 Copyright 2021 Elsevier.
(B) Fluorescence response of HPB-I, HPB-P, HPB-C and HPB-B (1.0 × 10−6 mol/L in DMSO/H2O mixture: 1/9999 v/v) upon the addition of different proteins (2.50 μg/mL). Reproduced with permission from ref.143 Copyright 2021 Elsevier.
(C) the mechanism of the turn-on detection of H5 by HPB-I. Reproduced with permission from ref.143 Copyright 2021 Elsevier.
(D) The synthesis route of TABD-Py-PF6. Reproduced with permission from ref.144 Copyright 2017 Elsevier.
(E) Exploring the impact of diverse amino acids, DNA molecules, and proteins on the intensity of PL. Reproduced with permission from ref.144 Copyright 2017 Elsevier.
(F) Investigating the impact of varying γ-globulin concentrations on the PL intensity at 502 nm for TABD-Py-PF6, both in the presence and absence of NH4Cl. Reproduced with permission from ref.144 Copyright 2017 Elsevier.
(G) The Structure of TABD-Py. Reproduced with permission from ref.145 Copyright 2019 John Wiley and Sons.
(H) AIE property of TABD-Py. Reproduced with permission from ref.145 Copyright 2019 John Wiley and Sons.
(I) CLSM images of a) NIH 3T3 and b) HeLa cells at room temperature. CLSM images of c) NIH 3T3 and d) HeLa cells in the frozen state. Both NIH 3T3 and HeLa cells were incubated with TABD-Py. Reproduced with permission from ref.145 Copyright 2019 John Wiley and Sons.
Li and Dong et al. introduced a novel fluorescent probe, TABD-Py-PF6, designed for the in situ quantitative detection of gamma-globulin in serum (Figures 10D–10F). This probe is characterized by its AIE properties. The mechanism of protein detection capabilities hinges on specific interactions with the binding sites of gamma-globulins, as opposed to non-specific interactions with amino acid side chains. The findings indicate that the highly specific binding between TABD-Py-PF6 and gamma-globulin confines molecular rotation, resulting in fluorescence switching behavior. Furthermore, electrostatic interactions between methylpyridine group in TABD-Py-PF6 and gamma-globulins were noted. In PBS, these electrostatic interactions predominantly govern the binding interaction between TABD-Py-PF6 and gamma-globulin. The benefits of this detection method encompass: 1) High sensitivity: Data indicates a linear correlation between the fluorescence emission intensity of the TABD-Py-PF6 probe and gamma-globulin concentration within a range of 7.89–300 μg/mL, boasting a detection limit of 7.89 μg/mL. 2) Real-time detection capability: TABD-Py-PF6 responds to gamma-globulin with a fluorescence response time under 5 s, facilitating real-time monitoring. 3) No separation required: The in-situ detection obviates the need for separating gamma-globulin from serum, significantly streamlining experimental procedures. 4) High specificity: Other serum components such as albumin, fibrinogen, glucose, urea, and cholesterol exhibit minimal interference with the molecular interaction between TABD-Py-PF6 and gamma-globulin. This heightened specificity facilitates the quantitative in situ detection of gamma-globulin in serum using this probe. 5) Promising clinical applications: TABD-Py-PF6 presents significant potential for diverse clinical applications.144
Bioimaging
Fluorescence imaging predominantly utilizes organic dyes and proteins, such as green fluorescent protein, as reagents. However, these materials exhibit limitations in terms of light absorption capacity and resistance to photobleaching. The application of inorganic quantum dots (QDs) is also challenging due to their toxicity when exposed to oxidative environments. The advancement of organic dyes has been hindered by the ACQ effect observed in conventional fluorophore systems. In contrast, AIEgens display remarkable brightness in aggregated state and exhibit a distinct characteristic: they become intense emitters at higher concentrations. This makes them particularly promising for fluorescence imaging applications.146
ZZ-HPB-NC exhibited a straightforward, remarkably discerning, precise, and immediate reaction in the intraoperative frozen-section slides of hepatocellular carcinoma (HCC) specimens, whereas the adjacent tissue and non-tumor tissues did not elicit any alteration in its fluorescence. The fluorescence imaging of ZZ-HPB-NC serves as a non-invasive diagnostic technique that poses no harm to the patient. The utilization of this probe holds great potential as an intraoperative diagnostic tool for HCC. However, the underlying mechanism by which ZZ-HPB-NC selectively activates HCC tissues remains unclear and necessitates further investigation. Considering the heterogeneity of tumor tissue and the intricate nature of the tumor microenvironment, it is plausible that this phenomenon could be attributed to electrostatic interactions involving specific proteins within the tissue.147
Cryosurgery is a widely utilized technique for tumor treatment; however, its therapeutic efficacy is often compromised due to the difficulty in accurately determining the volume of frozen tissue in real-time. To address this issue, Cai and Wang et al. have developed TABD-Py, a molecule that exhibits luminescence when exposed to freezing conditions, and employed it for live imaging during cryosurgery (Figures 10G–10I).145 TABD-Py is a novel fluorescence agent with AIE properties, which can form aggregates at the interface between ice crystals and water. This unique interaction allows TABD-Py to emit light when subjected to freezing temperatures. By specifically interacting with ice crystals and forming aggregates at the ice/water interface, TABD-Py limits its internal rotation as the ice crystals grow, leading to energy loss through radiative transitions. Due to its excellent biocompatibility and preferential accumulation within cancer cells, TABD-Py enables easy identification of cancer cells during freezing procedures. This innovative imaging approach has significant practical value for real-time visualization during surgical interventions.
The coumarin luciferin and its derivatives, in addition to MPBs, represent an exceptional class of bioimaging materials. The employment of luciferase enzymes to facilitate the oxidation of luciferin molecules, leading to the emission of light that is perceptible to the human eye, can contribute to the bioluminescence imaging (BLI). Luciferases commonly display wide and overlapping emission spectra, which present difficulties in achieving resolution through conventional bioluminescence imagers with surface read-outs. Consequently, the discrimination of distinct colors is impeded. Additionally, as the depth of luminescent probes increases, a more pronounced “red” appearance becomes evident at the surface. A novel series of coumarin luciferin analogues (CouLuc-3s) with near-infrared (NIR) emission properties were synthesized. The analogues and their complementary mutant luciferases were identified to meet the need for supplementary NIR emitting probes. The distinctive chemical characteristics of CouLuc-3 probes facilitated the utilization of multicomponent imaging, enabling the examination of up to five luciferase species within cells. This presented an advantageous avenue for exploring the influence of heterogeneous mixtures on the proliferation of cancer cells, leveraging the orthogonality of these probes.148
PDT
The compound 1,1,4,4-TPB, previously recognized as a potential AIE material, has recently emerged as a promising candidate for PDT in cancer treatment. PDT is well-known for its non-invasive and low-toxicity nature, necessitating highly efficient photosensitizers (PSs) to generate reactive oxygen species (ROS). However, conventional PSs often experience fluorescence quenching, commonly referred to as ACQ, due to the formation of π-π stacking aggregates in aqueous solutions. In contrast, 1,1,4,4-tetraphenylbuta-1,3-diene exhibits remarkable AIE characteristics and has been successfully synthesized into self-assembled nanoparticles, displaying inhibitory effects on HeLa cells. Subsequent in vivo investigations evaluated its efficacy and organ toxicity, revealing minimal toxicities and significant cancer cell inhibition.149
Conclusions
The extensive applications of 1,3-diene compounds in the field of AIE underscore the importance of exploring novel synthesis strategies and investigating their diverse applications across various fields. This review provides a systematic overview of the stereoselective synthesis strategies for 1,3-diene-based AIEgens. Currently, two primary approaches are employed: firstly, C2 synthons react with other C2 synthons through processes such as alkene-alkene reactions or alkyne-alkene reactions; secondly, the derivatization of C4 synthons is carried out, which includes enyne/diyne additions, diene derivatization, and the isomerization or rearrangement of other π systems. Transition metal catalysts play a pivotal role in these synthetic strategies by broadening substrate scope and controlling stereo-selectivity of the two double bonds. Despite the maturity achieved in stereoselective synthesis of dienes, there remains significant potential for progress. Firstly, the development of greener and more convenient synthesis methods is crucial not only for sustainable development but also for materials scientists to spontaneously create more diene scaffolds. Secondly, further advancements in stereo-convergent synthesis of dienes are required, as some cases necessitate the use of stereo-defined substrates to construct diene compounds, as previously discussed. Such single configuration substrates are often difficult to obtain, which exacerbates the complexity associated with diene synthesis. Lastly, achieving stereo-divergent synthesis of 1,3-dienes would be highly valuable given their two double bonds and four possible stereoisomers. The development of stereo-divergent synthetic strategies to obtain pure isomers would greatly contribute to studying the influence of double bond geometry on properties.
1,3-Diene-based AIEgens have attracted considerable interest from materials scientists due to their unique AIE/AEE effect and adjustable emission wavelength. Our review also provides a comprehensive overview of the key applications of this framework in AIE field. In second part, we summarize various MPBs skeletons with an AIE effect, including DPBs, TriPBs, TPBs, among others, and discuss the photophysical properties of these compounds, as well as the reasons for their AIE effect. We also offer a detailed analysis of how double bond geometry affects photophysical properties. Following this, we examine the diverse applications of these compounds in OLEDs, stimuli-responsive materials (such as photochromic and piezochromic materials), sensors (ion detection, volatile gas detection, explosive detection, protein detection), bioimaging, and PDT. Despite significant progress in this field to date, research on MPBs as traditional AIE star molecule TPE is still in its early stages. The quest for designing high quantum yield and longer wavelength-emitting AIEgens remains a critical objective that requires focused efforts at this juncture. It is essential to further understand the mechanism of AIE effect and establish a relationship between structure and properties within this class of skeleton to successfully develop novel fluorescent materials. Additionally, studying the impact of double bonds on the properties and applications of diene compounds with a single stereo-isomer is also crucial. It should be noted that some diene compounds exhibit relatively poor stability and are susceptible to photoisomerization thermal isomerization air oxidation reactions; therefore, it is advisable to conduct stability assessments under inert gas protection while maintaining them at low temperatures.
Evidently, the exploration of diene compound synthesis and its subsequent applications is pivotal for societal advancement. Despite significant strides made by chemists and materials scientists in their respective domains, a discernible gap persists between the realms of synthesis and application. For instance, chemists have yet to fully comprehend the impact of substituents on applications and struggle to develop necessary methodologies around useful skeletons. Similarly, materials scientists face challenges in selecting appropriate methods to synthesize required skeletons. In the realm of synthesis research, there exists a notable scarcity of exploration into the practical application value of the resultant product. In applied research endeavors, there is a prevailing preference for established classical multi-step reactions over the nascent transition metal catalytic synthesis methodologies. Enhancing collaboration and communication between synthetic and materials scientists could pave the way for novel opportunities in this field.
To summarize, this review holds the potential to aid synthetic chemists in effectively navigating the systematic synthesis and corresponding applications of 1,3-dienes. This, in turn, can facilitate the development of improved methods and modifications of AIEgens with more suitable structures tailored for specific applications. Furthermore, it provides material and biochemical scientists with the opportunity to thoughtfully select the most appropriate synthesis approach and desired framework.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (91940305). The authors would like to thank Shiyanjia Lab (www.shiyanjia.com) for the language editing service.
Author contributions
M.-Y.L. and Y.L. conceived the project and overall supervised the project. X.-M.N. prepared the ‘stereo-synthesis of 1,3-diene based AIEgens’ part. A.G. prepared the ‘application’ part. M.-Y.L. prepared the introduction and conclusion parts. Z.-Y.Y. made significant contributions during the revision process. X.-M.N., A.G., S.Z., J.L., Z.-Y.Y., and M.-Y.L. collected the references.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Zhu-Ying Yue, Email: yuezhuying@renji.com.
Meng-Yao Li, Email: limy@sioc.ac.cn.
Yingbin Liu, Email: laoniulyb@shsmu.edu.cn.
References
- 1.MacMillan J.B., Molinski T.F. Majusculoic Acid, a Brominated Cyclopropyl Fatty Acid from a Marine Cyanobacterial Mat Assemblage. J. Nat. Prod. 2005;68:604–606. doi: 10.1021/np049596k. [DOI] [PubMed] [Google Scholar]
- 2.Marville K.I., McLean S., Reynolds W.F., Tinto W.F. New Cembrane Diterpenes of the Marine Octocoral Eunicea tourniforti from the Eastern Caribbean. J. Nat. Prod. 2003;66:1284–1287. doi: 10.1021/np030091o. [DOI] [PubMed] [Google Scholar]
- 3.Zuck K.M., Shipley S., Newman D.J. Induced Production of N-Formyl Alkaloids from Aspergillus fumigatus by Co-culture with Streptomyces peucetius. J. Nat. Prod. 2011;74:1653–1657. doi: 10.1021/np200255f. [DOI] [PubMed] [Google Scholar]
- 4.Li J., Gu A., Nong X.-M., Zhai S., Yue Z.-Y., Li M.-Y., Liu Y. Cover Picture: Six-Membered Aromatic Nitrogen Heterocyclic Anti-Tumor Agents: Synthesis and Applications (Chem. Rec. 12/2023) Chem. Rec. 2023;23 doi: 10.1002/tcr.202300293. [DOI] [PubMed] [Google Scholar]
- 5.Soengas R.G., Rodríguez-Solla H. Modern Synthetic Methods for the Stereoselective Construction of 1,3-Dienes. Molecules. 2021;26:249. doi: 10.3390/molecules26020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubert P., Seibel E., Beemelmanns C., Campagne J.M., de Figueiredo R.M. Stereoselective Construction of (E,Z)-1,3-Dienes and Its Application in Natural Product Synthesis. Adv. Synth. Catal. 2020;362:5532–5575. [Google Scholar]
- 7.Maikhuri V.K., Maity J., Srivastava S., Prasad A.K. Transition metal-catalyzed double Cvinyl–H bond activation: synthesis of conjugated dienes. Org. Biomol. Chem. 2022;20:9522–9588. doi: 10.1039/d2ob01646j. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Mao H., Xu W., Shi J., Cai Z., Tong B., Dong Y. Aggregation-Induced Emission of Multiphenyl-Substituted 1,3-Butadiene Derivatives: Synthesis, Properties and Application. Chem. Eur J. 2018;24:15965–15977. doi: 10.1002/chem.201802114. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y., Tan Y., Yan D., Gui Y., Luo W., Zhu D., Wang D., Tang B.Z. Recent advances of AIE-active materials for orthotopic tumor phototheranostics. WIREs Nanomed. Nanobiotechnol. 2023;15 doi: 10.1002/wnan.1906. [DOI] [PubMed] [Google Scholar]
- 10.Wang H., Li Q., Alam P., Bai H., Bhalla V., Bryce M.R., Cao M., Chen C., Chen S., Chen X., et al. Aggregation-Induced Emission (AIE), Life and Health. ACS Nano. 2023;17:14347–14405. doi: 10.1021/acsnano.3c03925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C., Shen H., Liu T.-M., Kwok R.T., Lam J.W., Tang B.Z. Restriction of molecular motion to a higher level: Towards bright AIE dots for biomedical applications. iScience. 2023;26 doi: 10.1016/j.isci.2023.106568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J., Xie Z., Lam J.W.Y., Cheng L., Tang B.Z., Chen H., Qiu C., Kwok H.S., Zhan X., Liu Y., Zhu D. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001:1740–1741. doi: 10.1039/b105159h. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X.-S., Han Y.-P., Zhang Y., Liang Y.-M. Transition-Metal-Catalyzed Transformations Involving the Heck Reaction. Adv. Synth. Catal. 2023;365:2436–2466. [Google Scholar]
- 14.Karimi B., Behzadnia H., Elhamifar D., Akhavan P., Esfahani F., Zamani A. Transition-Metal-Catalyzed Oxidative Heck Reactions. Synthesis. 2010;2010:1399–1427. [Google Scholar]
- 15.Yang X., Ma S., Du Y., Tao Y. Progress in Reductive Heck Reaction. Chin. J. Org. Chem. 2013;33:2325–2333. [Google Scholar]
- 16.Dounay A.B., Overman L.E. Chem. Rev. 2003;103:2945–2963. doi: 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]
- 17.Beletskaya I.P., Cheprakov A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 18.Kurandina D., Chuentragool P., Gevorgyan V. Transition-Metal-Catalyzed Alkyl Heck-Type Reactions. Synthesis. 2019;51:985–1005. doi: 10.1055/s-0037-1611659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doucet H., Santelli M., Lemhadri M., Battace A., Berthiol F., Zair T. Palladium-Tetraphosphine Complex Catalysed Heck Reaction of Vinyl Bromides with Alkenes: A Powerful Access to Conjugated Dienes. Synthesis. 2008;2008:1142–1152. [Google Scholar]
- 20.Shang X., Liu Z. Recent Developments in Pd-Catalyzed Oxidative Heck Cross Coupling Reaction of Arenes/Alkenes with Allylic Esters. Chin. J. Org. Chem. 2015;35:522–527. [Google Scholar]
- 21.Zheng C., Wang D., Stahl S.S. Catalyst-Controlled Regioselectivity in the Synthesis of Branched Conjugated Dienes via Aerobic Oxidative Heck Reactions. J. Am. Chem. Soc. 2012;134:16496–16499. doi: 10.1021/ja307371w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang J., Wang B., Huang C., Ye X., Wen Y. Synthesis of Symmetrical (E,E)-1,4-Diaryl-1,3-butadienes by One-Pot Method. Chin. J. Org. Chem. 2021;41:2116–2120. [Google Scholar]
- 23.Manabe K., Wang J.R. Transition-Metal-CatalyzedSite-Selective Cross-Coupling of Di- and PolyhalogenatedCompounds. Synthesis. 2009;2009:1405–1427. [Google Scholar]
- 24.Molander G.A., Felix L.A. Stereoselective Suzuki−Miyaura Cross-Coupling Reactions of Potassium Alkenyltrifluoroborates with Alkenyl Bromides. J. Org. Chem. 2005;70:3950–3956. doi: 10.1021/jo050286w. [DOI] [PubMed] [Google Scholar]
- 25.Drouet K.E., Theodorakis E.A. Stereoselective Total Synthesis of Reveromycin B and C19-epi-Reveromycin B. Chem. Eur J. 2000;6:1987–2001. doi: 10.1002/1521-3765(20000602)6:11<1987::aid-chem1987>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 26.Su M., Kang Y., Yu W., Hua Z., Jin Z. Negishi Coupling between α-Alkyl(aryl)thio Vinyl Zinc Chloride and α-Bromo Vinyl Ether: A Convergent Synthesis of 2-Alkoxy-3-alkyl(aryl)thiobuta-1,3-dienes1. Org. Lett. 2002;4:691–694. doi: 10.1021/ol010252k. [DOI] [PubMed] [Google Scholar]
- 27.Aufdermarsh C.A. Preparation and Some Reactions of 2-(1,3-Butadienyl)magnesium Chloride. J. Org. Chem. 1964;29:1994–1996. [Google Scholar]
- 28.Nunomoto S., Kawakami Y., Yamashita Y. Synthesis of 2-Substituted 1,3-Butadienes by Cross-coupling Reaction of 2-(1,3-Butadienyl)magnesium Chloride with Alkyl or Aryl Iodides. Bull. Chem. Soc. Jpn. 1981;54:2831–2832. [Google Scholar]
- 29.Fiorito D., Folliet S., Liu Y., Mazet C. A General Nickel-Catalyzed Kumada Vinylation for the Preparation of 2-Substituted 1,3-Dienes. ACS Catal. 2018;8:1392–1398. [Google Scholar]
- 30.Duncton M.A.J., Pattenden G. The intramolecular Stille reaction. J. Chem. Soc. Perkin. 1999;1:1235–1246. [Google Scholar]
- 31.Maleczka R.E., Lavis J.M., Clark D.H., Gallagher W.P. Microwave-Assisted One-Pot Hydrostannylation/Stille Couplings. Org. Lett. 2000;2:3655–3658. doi: 10.1021/ol006559l. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee T., Dey R., Ranu B.C. An easy access to styrenes: trans aryl 1,3-1,4- and 1,5-dienes, and 1,3,5-trienes by Hiyama cross-coupling catalyzed by palladium nanoparticles. New J. Chem. 2011;35:1103–1110. [Google Scholar]
- 33.McAdam C.A., McLaughlin M.G., Cook M.J. An alkyne hydrosilylation–Hiyama coupling approach to highly functionalised 1,3-dienes. Org. Chem. Front. 2015;2:510–514. [Google Scholar]
- 34.Hornillos V., Giannerini M., Vila C., Fañanás-Mastral M., Feringa B.L. Direct catalytic cross-coupling of alkenyllithium compounds. Chem. Sci. 2015;6:1394–1398. doi: 10.1039/c4sc03117b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q., Wang Z.Y., Peng X.S., Wong H.N.C. Ligand-Free Iron-Catalyzed Carbon(sp2)–Carbon(sp2) Cross-Coupling of Alkenyllithium with Vinyl Halides. J. Org. Chem. 2018;83:6325–6333. doi: 10.1021/acs.joc.8b00510. [DOI] [PubMed] [Google Scholar]
- 36.Qian Q., Zang Z., Chen Y., Tong W., Gong H. Nickel and Cobalt-Catalyzed Coupling of Alkyl Halides with Alkenes via Heck Reactions and Radical Conjugate Addition. Mini-Rev. Med. Chem. 2013;13:802–813. doi: 10.2174/1389557511313060003. [DOI] [PubMed] [Google Scholar]
- 37.Wang S.-S., Yang G.-Y. Recent developments in low-cost TM-catalyzed Heck-type reactions (TM = transition metal, Ni, Co, Cu, and Fe) Catal. Sci. Technol. 2016;6:2862–2876. [Google Scholar]
- 38.Olivares A.M., Weix D.J. Multimetallic Ni- and Pd-Catalyzed Cross-Electrophile Coupling To Form Highly Substituted 1,3-Dienes. J. Am. Chem. Soc. 2018;140:2446–2449. doi: 10.1021/jacs.7b13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sha Y., Liu J., Wang L., Liang D., Wu D., Gong H. Nickel-catalyzed reductive 1,3-diene formation from the cross-coupling of vinyl bromides. Org. Biomol. Chem. 2021;19:4887–4890. doi: 10.1039/d1ob00791b. [DOI] [PubMed] [Google Scholar]
- 40.Shang X., Liu Z.-Q. Transition metal-catalyzed Cvinyl–Cvinyl bond formation via double Cvinyl–H bond activation. Chem. Soc. Rev. 2013;42:3253–3260. doi: 10.1039/c2cs35445d. [DOI] [PubMed] [Google Scholar]
- 41.Tang S., Liu K., Liu C., Lei A. Olefinic C-H functionalization through radical alkenylation. Chem. Soc. Rev. 2015;44:1070–1082. doi: 10.1039/c4cs00347k. [DOI] [PubMed] [Google Scholar]
- 42.Wang S., Yan F., Wang L., Zhu L. Recent Advances in Directing Group-Induced C-H Activation Reactions. Chin. J. Org. Chem. 2018;38:291–303. [Google Scholar]
- 43.Rasheed O.K., Sun B. Advances in Development of C–H Activation/Functionalization Using a Catalytic Directing Group. ChemistrySelect. 2018;3:5689–5708. [Google Scholar]
- 44.Wu Y., Shi B. Transition Metal-Catalyzed C-H Activation via Imine-Based Transient Directing Group Strategy. Chin. J. Org. Chem. 2020;40:3517–3535. [Google Scholar]
- 45.Zarkadoulas A., Zgouleta I., Tzouras N.V., Vougioukalakis G.C. Traceless Directing Groups in Sustainable Metal-Catalyzed C–H Activation. Catalysts. 2021;11:554. [Google Scholar]
- 46.Gandeepan P., Ackermann L. Transient Directing Groups for Transformative C–H Activation by Synergistic Metal Catalysis. Chem. 2018;4:199–222. [Google Scholar]
- 47.Shan C., Zhu L., Qu L.-B., Bai R., Lan Y. Mechanistic view of Ru-catalyzed C-H bond activation and functionalization: computational advances. Chem. Soc. Rev. 2018;47:7552–7576. doi: 10.1039/c8cs00036k. [DOI] [PubMed] [Google Scholar]
- 48.Ma W., Gandeepan P., Li J., Ackermann L. Recent advances in positional-selective alkenylations: removable guidance for twofold C–H activation. Org. Chem. Front. 2017;4:1435–1467. [Google Scholar]
- 49.Jin L., Zhang P., Li Y., Yu X., Shi B.-F. Atroposelective Synthesis of Conjugated Diene-Based Axially Chiral Styrenes via Pd(II)-Catalyzed Thioether-Directed Alkenyl C–H Olefination. J. Am. Chem. Soc. 2021;143:12335–12344. doi: 10.1021/jacs.1c06236. [DOI] [PubMed] [Google Scholar]
- 50.Dai D.T., Yang M.W., Chen Z.Y., Wang Z.L., Xu Y.H. Chelation-Controlled Stereospecific Cross-Coupling Reaction between Alkenes for Atroposelective Synthesis of Axially Chiral Conjugated Dienes. Org. Lett. 2022;24:1979–1984. doi: 10.1021/acs.orglett.2c00386. [DOI] [PubMed] [Google Scholar]
- 51.Shen C., Zhu Y., Shen W., Jin S., Zhong G., Luo S., Xu L., Zhong L., Zhang J. Access to axially chiral aryl 1,3-dienes by transient group directed asymmetric C–H alkenylations. Org. Chem. Front. 2022;9:2109–2115. [Google Scholar]
- 52.Liu M., Yang P., Karunananda M.K., Wang Y., Liu P., Engle K.M. C(alkenyl)–H Activation via Six-Membered Palladacycles: Catalytic 1,3-Diene Synthesis. J. Am. Chem. Soc. 2018;140:5805–5813. doi: 10.1021/jacs.8b02124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang F., Spring D.R. Arene C–H functionalisation using a removable/modifiable or a traceless directing group strategy. Chem. Soc. Rev. 2014;43:6906–6919. doi: 10.1039/c4cs00137k. [DOI] [PubMed] [Google Scholar]
- 54.Rani G., Luxami V., Paul K. Traceless directing groups: a novel strategy in regiodivergent C–H functionalization. Chem. Commun. 2020;56:12479–12521. doi: 10.1039/d0cc04863a. [DOI] [PubMed] [Google Scholar]
- 55.Font M., Quibell J.M., Perry G.J.P., Larrosa I. The use of carboxylic acids as traceless directing groups for regioselective C–H bond functionalisation. Chem. Commun. 2017;53:5584–5597. doi: 10.1039/c7cc01755c. [DOI] [PubMed] [Google Scholar]
- 56.Li M.-Y., Wei D., Feng C.-G., Lin G.-Q. Tandem Reactions involving 1,4-Palladium Migrations. Chem. Asian J. 2022;17 doi: 10.1002/asia.202200456. [DOI] [PubMed] [Google Scholar]
- 57.Hu T.J., Li M.Y., Zhao Q., Feng C.G., Lin G.Q. Highly Stereoselective Synthesis of 1,3-Dienes through an Aryl to Vinyl 1,4-Palladium Migration/Heck Sequence. Angew. Chem. Int. Ed. 2018;57:5871–5875. doi: 10.1002/anie.201801963. [DOI] [PubMed] [Google Scholar]
- 58.Xue Z.J., Li M.Y., Zhu B.B., He Z.T., Feng C.G., Lin G.Q. A 1,4-Palladium Migration/Heck Sequence with Unactivated Alkenes: Stereoselective Synthesis of Trisubstituted 1,3-Dienes. Adv. Synth. Catal. 2021;363:2089–2092. [Google Scholar]
- 59.Xue Z.J., Li M.Y., Zhu B.B., He Z.T., Feng C.G., Lin G.Q. Stereoselective synthesis of conjugated trienes via 1,4-palladium migration/Heck sequence. Chem. Commun. 2020;56:14420–14422. doi: 10.1039/d0cc06452a. [DOI] [PubMed] [Google Scholar]
- 60.Li M.-Y., Han P., Hu T.-J., Wei D., Zhang G., Qin A., Feng C.-G., Tang B.Z., Lin G.-Q. Suzuki-Miyaura Coupling Enabled by Aryl to Vinyl 1,4-Palladium Migration. iScience. 2020;23 doi: 10.1016/j.isci.2020.100966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin J., Huang Z., Ma J., Xu B.-H., Zhou Y.-G., Yu Z. Tunable Construction of Multisubstituted 1,3-Dienes and Allenes via a 1,4-Palladium Migration/Carbene Insertion Cascade. J. Org. Chem. 2022;87:12019–12035. doi: 10.1021/acs.joc.2c01019. [DOI] [PubMed] [Google Scholar]
- 62.Abderrezak M., Kabouche Z., Bruneau C., Fischmeister C. Ene-yne Cross-Metathesis for the Preparation of 2,3-Diaryl-1,3-dienes. Catalysts. 2017;7:365. [Google Scholar]
- 63.Rohde L.N., Wild T.H., Diver S.T. Ene–Yne Metathesis of Allylphosphonates and Allylphosphates: Synthesis of Phosphorus-Containing 1,3-Dienes. J. Org. Chem. 2021;86:1371–1384. doi: 10.1021/acs.joc.0c02886. [DOI] [PubMed] [Google Scholar]
- 64.Shibata Y., Hirano M., Tanaka K. Rhodium-Catalyzed Regio- and Stereoselective Codimerization of Alkenes and Electron-Deficient Internal Alkynes Leading to 1,3-Dienes. Org. Lett. 2008;10:2829–2831. doi: 10.1021/ol800995r. [DOI] [PubMed] [Google Scholar]
- 65.Hilt G., Treutwein J. Cobalt-Catalyzed Alder–Ene Reaction. Angew. Chem. Int. Ed. 2007;46:8500–8502. doi: 10.1002/anie.200703180. [DOI] [PubMed] [Google Scholar]
- 66.Mannathan S., Cheng C.H. Cobalt-catalyzed regio- and stereoselective intermolecular enyne coupling: an efficient route to 1,3-diene derivatives. Chem. Commun. 2010;46:1923–1925. doi: 10.1039/b920071a. [DOI] [PubMed] [Google Scholar]
- 67.Horie H., Koyama I., Kurahashi T., Matsubara S. Nickel-catalyzed intermolecular codimerization of acrylates and alkynes. Chem. Commun. 2011;47:2658–2660. doi: 10.1039/c0cc04061d. [DOI] [PubMed] [Google Scholar]
- 68.Hansen E.C., Lee D. Synthesis of β,β-Disubstituted Vinyl Boronates via the Ruthenium-Catalyzed Alder Ene Reaction of Borylated Alkynes and Alkenes. J. Am. Chem. Soc. 2005;127:3252–3253. doi: 10.1021/ja0424629. [DOI] [PubMed] [Google Scholar]
- 69.Saito N., Saito K., Shiro M., Sato Y. Regio- and Stereoselective Synthesis of 2-Amino-1,3-diene Derivatives by Ruthenium-Catalyzed Coupling of Ynamides and Ethylene. Org. Lett. 2011;13:2718–2721. doi: 10.1021/ol200812y. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J., Ugrinov A., Zhang Y., Zhao P. Exploring Bis(cyclometalated) Ruthenium(II) Complexes as Active Catalyst Precursors: Room-Temperature Alkene–Alkyne Coupling for 1,3-Diene Synthesis. Angew. Chem. Int. Ed. 2014;53:8437–8440. doi: 10.1002/anie.201402098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindhardt neé Hansen A., Mantel M., Skrydstrup T. Palladium-Catalyzed Intermolecular Ene–Yne Coupling: Development of an Atom-Efficient Mizoroki–Heck-Type Reaction. Angew. Chem. Int. Ed. 2008;47:2668–2672. doi: 10.1002/anie.200705558. [DOI] [PubMed] [Google Scholar]
- 72.Fang S., Ling H., Zeng C., Li M., Jiang H., Wu W. Palladium-Catalyzed Sequential Three-Component Cross-Coupling to 1,3-Dienes: Employing Alkenes as Hydride and Alkenyl Donors. J. Org. Chem. 2022;87:12816–12830. doi: 10.1021/acs.joc.2c01406. [DOI] [PubMed] [Google Scholar]
- 73.Shibata Y., Otake Y., Hirano M., Tanaka K. Amide-Directed Alkenylation of sp2 C−H Bonds Catalyzed by a Cationic Rh(I)/BIPHEP Complex Under Mild Conditions: Dramatic Rate Acceleration by a 1-Pyrrolidinecarbonyl Group. Org. Lett. 2009;11:689–692. doi: 10.1021/ol802767s. [DOI] [PubMed] [Google Scholar]
- 74.Meng K., Zhang J., Li F., Lin Z., Zhang K., Zhong G. Amide Directed Cross-Coupling between Alkenes and Alkynes: A Regio- and Stereoselective Approach to Substituted (2Z,4Z)-Dienamides. Org. Lett. 2017;19:2498–2501. doi: 10.1021/acs.orglett.7b00695. [DOI] [PubMed] [Google Scholar]
- 75.Neisius N., Plietker B. The Ruthenium-Catalyzed Hydrovinylation of Internal Alkynes by Acrylates: An Atom Economic Approach to Highly Substituted 1,3-Dienes. Angew. Chem. Int. Ed. 2009;48:5752–5755. doi: 10.1002/anie.200901928. [DOI] [PubMed] [Google Scholar]
- 76.Li S.S., Xia Y.Q., Liu C.F., Zhang G.T., Su F., Zhang X.M., Dong L. Diverse Reactivity in a Rhodium(III)-Catalyzed Vinylic sp2 C–H Bond Functionalization: Synthesis of Fused Polycyclic Heteroarenes or Conjugated Dienes. Adv. Synth. Catal. 2016;358:3724–3729. [Google Scholar]
- 77.Thiel N.O., Kemper S., Teichert J.F. Copper(I)-catalyzed stereoselective hydrogenation of 1,3-diynes and enynes. Tetrahedron. 2017;73:5023–5028. [Google Scholar]
- 78.Gorgas N., Brünig J., Stöger B., Vanicek S., Tilset M., Veiros L.F., Kirchner K. Efficient Z-Selective Semihydrogenation of Internal Alkynes Catalyzed by Cationic Iron(II) Hydride Complexes. J. Am. Chem. Soc. 2019;141:17452–17458. doi: 10.1021/jacs.9b09907. [DOI] [PubMed] [Google Scholar]
- 79.Liu J., Yang J., Baumann W., Jackstell R., Beller M. Stereoselective Synthesis of Highly Substituted Conjugated Dienes via Pd-Catalyzed Carbonylation of 1,3-Diynes. Angew. Chem. Int. Ed. 2019;58:10683–10687. doi: 10.1002/anie.201903533. [DOI] [PubMed] [Google Scholar]
- 80.Gao S., Liu H., Yang C., Fu Z., Yao H., Lin A. Accessing 1,3-Dienes via Palladium-Catalyzed Allylic Alkylation of Pronucleophiles with Skipped Enynes. Org. Lett. 2017;19:4710–4713. doi: 10.1021/acs.orglett.7b01960. [DOI] [PubMed] [Google Scholar]
- 81.Trost B.M. Chem. Eur J. 1998;4:2405–2412. [Google Scholar]
- 82.Guo Z., Wen H., Liu G., Huang Z. Iron-Catalyzed Regio- and Stereoselective Hydrosilylation of 1,3-Enynes To Access 1,3-Dienylsilanes. Org. Lett. 2021;23:2375–2379. doi: 10.1021/acs.orglett.1c00670. [DOI] [PubMed] [Google Scholar]
- 83.Huang F., Huang Z., Liu G., Huang Z. Iridium-Catalyzed Selective trans-Semihydrogenation of 1,3-Enynes with Ethanol: Access to (E,E)-1,4-Diarylbutadienes. Org. Lett. 2022;24:5486–5490. doi: 10.1021/acs.orglett.2c02327. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z., Zhang C., Wu J., Li B., Chrostowska A., Karamanis P., Liu S.-Y. trans-Hydroalkynylation of Internal 1,3-Enynes Enabled by Cooperative Catalysis. J. Am. Chem. Soc. 2023;145:5624–5630. doi: 10.1021/jacs.3c00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Negishi E.-I., Huang Z., Wang G., Mohan S., Wang C., Hattori H. Recent Advances in Efficient and Selective Synthesis of Di-Tri-and Tetrasubstituted Alkenes via Pd-Catalyzed Alkenylation−Carbonyl Olefination Synergy. Acc. Chem. Res. 2008;41:1474–1485. doi: 10.1021/ar800038e. [DOI] [PubMed] [Google Scholar]
- 86.Furuichi N., Hara H., Osaki T., Nakano M., Mori H., Katsumura S. Stereocontrolled Total Synthesis of a Polyfunctional Carotenoid, Peridinin. J. Org. Chem. 2004;69:7949–7959. doi: 10.1021/jo048852v. [DOI] [PubMed] [Google Scholar]
- 87.Luh T.Y., Wong K.T. Silyl-Substituted Conjugated Dienes: Versatile Building Blocks in Organic Synthesis. Synthesis. 1993;1993:349–370. [Google Scholar]
- 88.Li H., Fiorito D., Mazet C. Exploring Site Selectivity of Iridium Hydride Insertion into Allylic Alcohols: Serendipitous Discovery and Comparative Study of Organic and Organometallic Catalysts for the Vinylogous Peterson Elimination. ACS Catal. 2017;7:1554–1562. [Google Scholar]
- 89.Fischmeister C., Bruneau C. Ene–yne cross-metathesis with ruthenium carbene catalysts. Beilstein J. Org. Chem. 2011;7:156–166. doi: 10.3762/bjoc.7.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang W., He S., Zhong Y., Chen J., Cai C., Luo Y., Xia Y. Cobalt-Catalyzed Z to E Geometrical Isomerization of 1,3-Dienes. J. Org. Chem. 2022;87:4712–4723. doi: 10.1021/acs.joc.1c03164. [DOI] [PubMed] [Google Scholar]
- 91.Diver S.T., Glickert E., Rohde L.N., Wild T. Stereoconvergent Synthesis of Z-1,3-Disubstituted-1,3-Dienes by Uphill Photocatalysis. Chem. Eur J. 2022;29 doi: 10.1002/chem.202202635. [DOI] [PubMed] [Google Scholar]
- 92.Kudo E., Sasaki K., Kawamata S., Yamamoto K., Murahashi T. Selective E to Z isomerization of 1,3-Dienes Enabled by A Dinuclear Mechanism. Nat. Commun. 2021;12:1473. doi: 10.1038/s41467-021-21720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark J.R., Griffiths J.R., Diver S.T. Ruthenium Hydride-Promoted Dienyl Isomerization: Access to Highly Substituted 1,3-Dienes. J. Am. Chem. Soc. 2013;135:3327–3330. doi: 10.1021/ja4011207. [DOI] [PubMed] [Google Scholar]
- 94.Scaringi S., Mazet C. Kinetically Controlled Stereoselective Access to Branched 1,3-Dienes by Ru-Catalyzed Remote Conjugative Isomerization. ACS Catal. 2021;11:7970–7977. [Google Scholar]
- 95.Zhao J., Xu G., Wang X., Liu J., Ren X., Hong X., Lu Z. Cobalt-Catalyzed Migration Isomerization of Dienes. Org. Lett. 2022;24:4592–4597. doi: 10.1021/acs.orglett.2c01701. [DOI] [PubMed] [Google Scholar]
- 96.Hearne Z., Li C.-J. Palladium-catalysed atom-economical synthesis of conjugated dienals from terminal acetylenes and acrolein. Chem. Commun. 2017;53:6136–6139. doi: 10.1039/c7cc02767b. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z., Wang Y., Zhang L. Soft Propargylic Deprotonation: Designed Ligand Enables Au-Catalyzed Isomerization of Alkynes to 1,3-Dienes. J. Am. Chem. Soc. 2014;136:8887–8890. doi: 10.1021/ja503909c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ting C.M., Hsu Y.L., Liu R.S. Gold-catalyzed isomerization of unactivated allenes into 1,3-dienes under ambient conditions. Chem. Commun. 2012;48:6577–6579. doi: 10.1039/c2cc32131a. [DOI] [PubMed] [Google Scholar]
- 99.Al-Jawaheri Y., Kimber M.C. Synthesis of 1,3-Dienes via a Sequential Suzuki–Miyaura Coupling/Palladium-Mediated Allene Isomerization Sequence. Org. Lett. 2016;18:3502–3505. doi: 10.1021/acs.orglett.6b01841. [DOI] [PubMed] [Google Scholar]
- 100.Farinola G., Babudri F., Naso F., Ragni R., Spina G. A Novel Stereoselective Synthesis of Symmetrical (1E,3E)-1,4-Diarylbuta-1,3-dienes. Synthesis. 2007;2007:3088–3092. [Google Scholar]
- 101.Zhang W.S., Ji D.W., Li Y., Zhang X.X., Mei Y.K., Chen B.Z., Chen Q.A. Nickel-catalyzed divergent Mizoroki–Heck reaction of 1,3-dienes. Nat. Commun. 2023;14:651. doi: 10.1038/s41467-023-36237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H., Wu X., Wei Y., Zhu C. Radical-Mediated Heck-Type Alkylation: Stereoconvergent Synthesis of Functionalized Polyenes. Org. Lett. 2019;21:7568–7572. doi: 10.1021/acs.orglett.9b02838. [DOI] [PubMed] [Google Scholar]
- 103.Liao L., Guo R., Zhao X. Organoselenium-Catalyzed Regioselective C−H Pyridination of 1,3-Dienes and Alkenes. Angew. Chem. Int. Ed. 2017;56:3201–3205. doi: 10.1002/anie.201610657. [DOI] [PubMed] [Google Scholar]
- 104.Funk T.W., Efskind J., Grubbs R.H. Chemoselective Construction of Substituted Conjugated Dienes Using an Olefin Cross-Metathesis Protocol. Org. Lett. 2005;7:187–190. doi: 10.1021/ol047929z. [DOI] [PubMed] [Google Scholar]
- 105.Luo S.X.L., Cannon J.S., Taylor B.L.H., Engle K.M., Houk K.N., Grubbs R.H. Z-Selective Cross-Metathesis and Homodimerization of 3E-1,3-Dienes: Reaction Optimization, Computational Analysis, and Synthetic Applications. J. Am. Chem. Soc. 2016;138:14039–14046. doi: 10.1021/jacs.6b08387. [DOI] [PubMed] [Google Scholar]
- 106.McAlpine N.J., Wang L., Carrow B.P. A Diverted Aerobic Heck Reaction Enables Selective 1,3-Diene and 1,3,5-Triene Synthesis through C–C Bond Scission. J. Am. Chem. Soc. 2018;140:13634–13639. doi: 10.1021/jacs.8b10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nguyen V.T., Dang H.T., Pham H.H., Nguyen V.D., Flores-Hansen C., Arman H.D., Larionov O.V. Highly Regio- and Stereoselective Catalytic Synthesis of Conjugated Dienes and Polyenes. J. Am. Chem. Soc. 2018;140:8434–8438. doi: 10.1021/jacs.8b05421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dang H.T., Nguyen V.D., Pham H.H., Arman H.D., Larionov O.V. Highly stereoselective and catalytic desulfitative C O and C I dienylation with sulfolenes: The importance of basic additives. Tetrahedron. 2019;75:3258–3264. doi: 10.1016/j.tet.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dang H.T., Nguyen V.D., Haug G.C., Vuong N.T.H., Arman H.D., Larionov O.V. Z-Selective Dienylation Enables Stereodivergent Construction of Dienes and Unravels a Ligand-Driven Mechanistic Dichotomy. ACS Catal. 2021;11:1042–1052. doi: 10.1021/acscatal.0c05574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li M.-Y., Nong X.-M., Xiao H., Gu A., Zhai S., Li J., Zhang G., Xue Z.-J., Liu Y., Li C., et al. Aggregation-enabled alkene insertion into carbon–halogen bonds. Aggregate. 2023;4:e346. [Google Scholar]
- 111.Li M.-Y., Li J., Gu A., Nong X.-M., Zhai S., Yue Z.-Y., Feng C.-G., Liu Y., Lin G.-Q. Solvent-free and catalyst-free direct alkylation of alkenes. Green Chem. 2023;25:7073–7078. [Google Scholar]
- 112.Zhang H., Zheng X., Kwok R.T.K., Wang J., Leung N.L.C., Shi L., Sun J.Z., Tang Z., Lam J.W.Y., Qin A., Tang B.Z. In situ monitoring of molecular aggregation using circular dichroism. Nat. Commun. 2018;9:4961. doi: 10.1038/s41467-018-07299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu B., Tang B.Z. Aggregation-Induced Emission: More Is Different. Angew. Chem. Int. Ed. 2020;59:9788–9789. doi: 10.1002/anie.202005345. [DOI] [PubMed] [Google Scholar]
- 114.Tu Y., Liu J., Zhang H., Peng Q., Lam J.W.Y., Tang B.Z. Restriction of Access to the Dark State: A New Mechanistic Model for Heteroatom-Containing AIE Systems. Angew. Chem. Int. Ed. 2019;58:14911–14914. doi: 10.1002/anie.201907522. [DOI] [PubMed] [Google Scholar]
- 115.Roy E., Nagar A., Chaudhary S., Pal S. Advanced Properties and Applications of AIEgens-Inspired Smart Materials. Ind. Eng. Chem. Res. 2020;59:10721–10736. [Google Scholar]
- 116.Li M.-Y., Zhai S., Nong X.-M., Gu A., Li J., Lin G.-Q., Liu Y. Trisubstituted alkenes featuring aryl groups: stereoselective synthetic strategies and applications. Sci. China Chem. 2023;66:1261–1287. [Google Scholar]
- 117.Bacchi A., Brillante A., Crocco D., Chierotti M.R., Della Valle R.G., Girlando A., Masino M., Pelagatti P., Venuti E. Exploration of the polymorph landscape for 1,1,4,4-tetraphenyl-1,3-butadiene. CrystEngComm. 2014;16:8205–8213. [Google Scholar]
- 118.Davis R., Saleesh Kumar N.S., Abraham S., Suresh C.H., Rath N.P., Tamaoki N., Das S. Molecular Packing and Solid-State Fluorescence of Alkoxy-Cyano Substituted Diphenylbutadienes: Structure of the Luminescent Aggregates. J. Phys. Chem. C. 2008;112:2137–2146. [Google Scholar]
- 119.Zhang Y., Xu H., Xu W., Zhang C., Shi J., Tong B., Cai Z., Dong Y. Conformational sensitivity of tetraphenyl-1,3-butadiene derivatives with aggregation-induced emission characteristics. Sci. China Chem. 2019;62:1393–1397. [Google Scholar]
- 120.Nirmalananthan-Budau N., Budau J.H., Moldenhauer D., Hermann G., Kraus W., Hoffmann K., Paulus B., Resch-Genger U. Substitution pattern controlled aggregation-induced emission in donor–acceptor–donor dyes with one and two propeller-like triphenylamine donors. Phys. Chem. Chem. Phys. 2020;22:14142–14154. doi: 10.1039/d0cp00413h. [DOI] [PubMed] [Google Scholar]
- 121.Davis R., Mallia V.A., Das S. Reversible Photochemical Phase Transition Behavior of Alkoxy-Cyano-Substituted Diphenylbutadiene Liquid Crystals. Chem. Mater. 2003;15:1057–1063. [Google Scholar]
- 122.Zhang Y., Mao H., Kong L., Tian Y., Tian Z., Zeng X., Zhi J., Shi J., Tong B., Dong Y. Effect of E/Z isomerization on the aggregation-induced emission features and mechanochromic performance of dialdehyde-substituted hexaphenyl-1,3-butadiene. Dyes Pigm. 2016;133:354–362. [Google Scholar]
- 123.Bera M.K., Chakraborty C., Malik S. Solid state emissive organic fluorophores with remarkable broad color tunability based on aryl-substituted buta-1,3-diene as the central core. J. Mater. Chem. C. 2017;5:6872–6879. [Google Scholar]
- 124.Mao H., Li Y., Zhang Y., Kong L., Tian Y., Shi J., Cai Z., Tong B., Dong Y. UV-detecting dual-responsive strips based on dicyanoacetate-containing hexaphenylbutadiene with aggregation-induced emission characteristic. Dyes Pigm. 2020;175 [Google Scholar]
- 125.Han T., Zhang Y., Feng X., Lin Z., Tong B., Shi J., Zhi J., Dong Y. Reversible and hydrogen bonding-assisted piezochromic luminescence for solid-state tetraaryl-buta-1,3-diene. Chem. Commun. 2013;49:7049–7051. doi: 10.1039/c3cc42644k. [DOI] [PubMed] [Google Scholar]
- 126.Zhao Z., Lam J.W.Y., Tang B.Z. Tetraphenylethene: a versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J. Mater. Chem. 2012;22:23726–23740. [Google Scholar]
- 127.Kim J.H., Noh S., Kim K., Lim S.T., Shin D.M. Blue light emitting diode with 1,1,4,4-tetraphenyl-1,3-butadiene (TPB) Synth. Met. 2001;117:227–228. [Google Scholar]
- 128.Wang C., O’Hagan M.P., Li Z., Zhang J., Ma X., Tian H., Willner I. Photoresponsive DNA materials and their applications. Chem. Soc. Rev. 2022;51:720–760. doi: 10.1039/d1cs00688f. [DOI] [PubMed] [Google Scholar]
- 129.Danowski W., van Leeuwen T., Browne W.R., Feringa B.L. Photoresponsive porous materials. Nanoscale Adv. 2021;3:24–40. doi: 10.1039/d0na00647e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhuang Y., Ren X., Che X., Liu S., Huang W., Zhao Q. Organic photoresponsive materials for information storage: a review. Adv. Photonics. 2021;3 [Google Scholar]
- 131.Li C., Lv C., Ouyang M., Zhang Y. Near-infrared Piezochromic Materials at High Pressure. ChemPhysChem. 2023;24 doi: 10.1002/cphc.202200922. [DOI] [PubMed] [Google Scholar]
- 132.Yao X., Chi Z. Piezochromic aggregation-induced emission materials. Sci. Sin. -Chim. 2013;43:1090–1104. [Google Scholar]
- 133.Guo Y., Gu S., Feng X., Wang J., Li H., Han T., Dong Y., Jiang X., James T.D., Wang B. 3D cross-correlative matrix temperature detection and non-invasive thermal mapping based on a molecular probe. Chem. Sci. 2014;5:4388–4393. [Google Scholar]
- 134.Wang Y., Pan X., Peng Z., Zhang Y., Liu P., Cai Z., Tong B., Shi J., Dong Y. A “Turn-On” fluorescent chemosensor with the aggregation-induced emission characteristic for high-sensitive detection of Ce ion. Sensor Actuat. B Chem. 2018;267:351–356. [Google Scholar]
- 135.Li Q., Wu X., Huang X., Deng Y., Chen N., Jiang D., Zhao L., Lin Z., Zhao Y. Tailoring the Fluorescence of AIE-Active Metal–Organic Frameworks for Aqueous Sensing of Metal Ions. ACS Appl. Mater. Interfaces. 2018;10:3801–3809. doi: 10.1021/acsami.7b17762. [DOI] [PubMed] [Google Scholar]
- 136.Chen J., Xu B., Ouyang X., Tang B.Z., Cao Y. Aggregation-Induced Emission of cis,cis-1,2,3,4-Tetraphenylbutadiene from Restricted Intramolecular Rotation. J. Phys. Chem. A. 2004;108:7522–7526. [Google Scholar]
- 137.Kong L., Zhang Y., Mao H., Pan X., Tian Y., Tian Z., Zeng X., Shi J., Tong B., Dong Y. Dimalononitrile-containing probe based on aggregation-enhanced emission features for the multi-mode fluorescence detection of volatile amines. Faraday Discuss. 2017;196:101–111. doi: 10.1039/c6fd00178e. [DOI] [PubMed] [Google Scholar]
- 138.Lai Q., Si S., Qin T., Li B., Wu H., Liu B., Xu H., Zhao C. A novel red-emissive probe for colorimetric and ratiometric detection of hydrazine and its application in plant imaging. Sensor Actuat. B Chem. 2020;307 [Google Scholar]
- 139.Guo Y., Feng X., Han T., Wang S., Lin Z., Dong Y., Wang B. Tuning the Luminescence of Metal–Organic Frameworks for Detection of Energetic Heterocyclic Compounds. J. Am. Chem. Soc. 2014;136:15485–15488. doi: 10.1021/ja508962m. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Y., Xu W., Kong L., Han B., Cai Z., Shi J., Tong B., Dong Y., Tang B. Turn-on fluorescent probe with aggregation-induced emission characteristics for polyazoles. Mater. Chem. Front. 2018;2:1779–1783. [Google Scholar]
- 141.Wan H., Xu Q., Gu P., Li H., Chen D., Li N., He J., Lu J. AIE-based fluorescent sensors for low concentration toxic ion detection in water. J. Hazard Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123656. [DOI] [PubMed] [Google Scholar]
- 142.Zhou H., Chua M.H., Tang B.Z., Xu J. Aggregation-induced emission (AIE)-active polymers for explosive detection. Polym. Chem. 2019;10:3822–3840. [Google Scholar]
- 143.Pan X., Liu P., Wu X., Zhang Y., Cai Z., Shi J., Zhi J., Li Z., Wang D., Tong B., Dong Y. A “Turn-on” fluorescent bioprobe with aggregation-induced emission characteristics for detection of influenza virus-specific hemagglutinin protein. Sensor Actuat. B Chem. 2021;345 [Google Scholar]
- 144.Liu P., Chen D., Wang Y., Tang X., Li H., Shi J., Tong B., Dong Y. A highly sensitive “turn-on” fluorescent probe with an aggregation-induced emission characteristic for quantitative detection of γ-globulin. Biosens. Bioelectron. X. 2017;92:536–541. doi: 10.1016/j.bios.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 145.He Z., Liu P., Zhang S., Yan J., Wang M., Cai Z., Wang J., Dong Y. A Freezing-Induced Turn-On Imaging Modality for Real-Time Monitoring of Cancer Cells in Cryosurgery. Angew. Chem. Int. Ed. 2019;58:3834–3837. doi: 10.1002/anie.201813239. [DOI] [PubMed] [Google Scholar]
- 146.Wang S., Zhou K., Lyu X., Li H., Qiu Z., Zhao Z., Tang B.Z. The Bioimaging Story of AIEgens. Chem. Biomed. Imaging. 2023;1:509–521. [Google Scholar]
- 147.Chen D., Mao H., Hong Y., Tang Y., Zhang Y., Li M., Dong Y. Hexaphenyl-1,3-butadiene derivative: a novel “turn-on” rapid fluorescent probe for intraoperative pathological diagnosis of hepatocellular carcinoma. Mater. Chem. Front. 2020;4:2716–2722. [Google Scholar]
- 148.Love A.C., Caldwell D.R., Kolbaba-Kartchner B., Townsend K.M., Halbers L.P., Yao Z., Brennan C.K., Ivanic J., Hadjian T., Mills J.H., et al. Red-Shifted Coumarin Luciferins for Improved Bioluminescence Imaging. J. Am. Chem. Soc. 2023;145:3335–3345. doi: 10.1021/jacs.2c07220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wen H., Ma J., Chen J., Ke Z., Zou D., Li Q. Heavy atom free 1,1,4,4-tetraphenylbuta-1,3-diene with aggregation induced emission for photodynamic cancer therapy. New J. Chem. 2019;43:9183–9187. [Google Scholar]