Abstract
Although arsenite is an established carcinogen, the mechanisms underlying its tumor-promoting properties are poorly understood. Previously, we reported that arsenite treatment leads to the activation of the extracellular signal-regulated kinase (ERK) in rat PC12 cells through a Ras-dependent pathway. To identify potential mediators of the upstream signaling cascade, we examined the tyrosine phosphorylation profile in cells exposed to arsenite. Arsenite treatment rapidly stimulated tyrosine phosphorylation of several proteins in a Ras-independent manner, with a pattern similar to that seen in response to epidermal growth factor (EGF) treatment. Among these phosphorylated proteins were three isoforms of the proto-oncoprotein Shc as well as the EGF receptor (EGFR). Tyrosine phosphorylation of Shc allowed for enhanced interactions between Shc and Grb2 as identified by coimmunoprecipitation experiments. The arsenite-induced tyrosine phosphorylation of Shc, enhancement of Shc and Grb2 interactions, and activation of ERK were all drastically reduced by treatment of cells with either the general growth factor receptor poison suramin or the EGFR-selective inhibitor tyrphostin AG1478. Down-regulation of EGFR expression through pretreatment of cells with EGF also attenuated ERK activation and Shc tyrosine phosphorylation in response to arsenite treatment. These results demonstrate that the EGFR and Shc are critical mediators in the activation of the Ras/ERK signaling cascade by arsenite and suggest that arsenite acts as a tumor promoter largely by usurping this growth factor signaling pathway.
Arsenite, the trivalent arsenic compound, is a potent carcinogen to which there is significant worldwide exposure through natural contamination of food and drinking water (3). In humans, chronic exposure is associated with an increased incidence of skin and bladder cancers (3, 16). Although it appears to be nonmutagenic alone, in cultured cells arsenite potentiates the mutagenic effects of short-wavelength UV radiation (UV-C) (23, 34, 63). Hence, arsenite has been suggested to act as a tumor promoter in the carcinogenic process (6, 59). While the basis for such tumor-promoting activity is unclear, the ability of arsenite to interact with protein thiol groups and thereby alter the activities of key regulatory proteins is likely to contribute to its carcinogenic properties (6, 37).
The mitogen-activated protein (MAP) kinase cascade leading to the activation of ERK is crucial for regulating cell growth and differentiation. Initiation of this signaling pathway via growth factor receptors has been studied extensively (2, 11, 56). Ligand-mediated dimerization of growth factor receptors triggers the activation of receptor-type tyrosine kinases, resulting in autophosphorylation of tyrosine residues (2, 24, 53, 56). These residues then serve as docking sites for the recruitment of downstream signaling mediators necessary for the activation of membrane-localized Ras (15, 53). For example, the adapter protein Grb2 binds to the phosphotyrosine residues through its Src homology 2 (SH2) domain to bring the Ras guanine nucleotide exchange factor, Son of Sevenless (Sos), from the cytoplasm to the vicinity of Ras through its two Src homology 3 domains. In addition, the Grb2-Sos complexes can be recruited to the phosphotyrosine sites through another adaptor protein, Shc (4). Shc binds to certain receptor phosphotyrosine sites through its phosphotyrosine-binding domain; Shc then becomes tyrosine phosphorylated itself and thereby provides additional docking sites for Grb2 (3, 4, 15, 53). The recruitment of Grb2-Sos complexes to the phosphotyrosine sites leads to Ras activation, which then initiates the phosphorylation cascade starting with the activation of the proto-oncoprotein Raf, a serine kinase that phosphorylates and activates ERK kinase (MEK), which in turn activates ERK (2, 56). ERK is responsible for the phosphorylation of a variety of cellular proteins including downstream kinases, transcription factors, and components of the protein synthesis machinery (11).
In cultured cells, dysregulation of the ERK pathway through alterations in any one of several mediators involved in the cascade (e.g., mutation of growth factor receptors, Shc, Ras, Raf, and MEK) can lead to cellular transformation (7, 14, 15, 33, 40). Indeed, many tumors exhibit elevated ERK activity (32, 54, 57). It is not surprising, therefore, that a common feature of known tumor promoters is their ability to perturb the ERK signaling pathway. For example, although acting through different mechanisms, the tumor promoters phorbol ester, okadaic acid, and butylated hydroxytoluene hydroperoxide all lead to the activation of ERK. Phorbol esters act through activation of protein kinase C (leading to activation of Raf) (29), while okadaic acid acts through the inhibition of serine/threonine phosphatases (18). The mechanism responsible for ERK activation by butylated hydroxytoluene hydroperoxide is not fully understood but appears to involve steps upstream of Ras, as the activation of ERK by this agent is Ras dependent (20). In keeping with arsenite’s putative role as a tumor promoter, we have recently observed that arsenite treatment leads to a significant elevation in ERK activity in Rat1 and PC12 cells (37). This activation is dependent on Ras and is sensitive to the presence of the growth factor receptor poison suramin (60). These findings suggested that arsenite may act early in the pathway, either at a step involving the growth factor receptors directly or during the events serving to activate the growth factor receptors (37). Better understanding of the signal transduction pathway leading to ERK activation by arsenite could provide important insight into its carcinogenic mechanisms. In the present report, we focus on the early events in the signaling pathway leading to ERK activation. We provide evidence that arsenite treatment results in tyrosine phosphorylation of the Shc adapter protein, leading to its enhanced interaction with Grb2. We further demonstrate that epidermal growth factor (EGF) receptor (EGFR) tyrosine kinase is required for these interactions and the subsequent activation of ERK. The ability of arsenite to usurp this growth regulatory pathway is likely to contribute to its tumor-promoting properties.
MATERIALS AND METHODS
Cell culture and treatments.
Wild-type and Ras N17 PC12 cells, kindly provided by G. Cooper (61), were grown in a 37°C humidified atmosphere containing 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Inc., Gaithersburg, Md.) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah) and 5% horse serum (Life Technologies, Inc.). Cells were serum starved by placement in serum-free medium for at least 16 h prior to treatment with arsenite or EGF. Sodium arsenite (Sigma Chemical Co., St. Louis, Mo.) was added directly into the medium to a final concentration of 400 μM. EGF stimulation was carried out by directly adding EGF (Life Technologies) into the medium to a final concentration of 100 ng/ml. Suramin (300 μM) and tyrphostin AG1478 (30 or 40 nM) (Calbiochem, San Diego, Calif.) were added to the medium immediately before addition of sodium arsenite. All reagents were left in the medium until cells were harvested.
Down-modulation of EGFR.
Down-modulation of EGFR was carried out by incubating PC12 cells with EGF essentially as previously described by Carpenter and Cohen (5). PC12 cells cultured in serum-free medium overnight were stimulated with EGF (1 μg/ml) for 1 h. The EGF-containing medium was then removed, and the cells were washed twice with phosphate-buffered saline (PBS). The cells were then kept in serum-free medium for another 2 h at 37°C to allow the internalization of EGF-EGFR complexes before a second treatment with EGF, nerve growth factor (NGF), or arsenite. Replenishment of EGFR was accomplished by incubation of PC12 cells with down-regulated EGFR in DMEM containing 10% fetal bovine serum for 10 h. After replenishment of EGFR, cells were serum starved for another 14 h and subsequently treated with arsenite, EGF, or NGF.
In vitro binding assays.
The glutathione S-transferase (GST)–Grb2 SH2 domain-expressing plasmid was generously provided by H. Gram (43). The GST-Grb2 SH2 domain fusion protein was produced in Escherichia coli and purified by glutathione-Sepharose affinity chromatography followed by fast protein liquid chromatography (FPLC) purification procedures. Twenty micrograms of the glutathione-free GST-Grb2 SH2 domain fusion protein was incubated with 400 μg of soluble cell lysates at 4°C for 2 h. Glutathione-Sepharose beads (Pharmacia Biotech Inc., Piscataway, N.J.) were then added to the samples, and the samples were incubated for an additional hour with gentle rotation. The Sepharose beads were recovered through gentle centrifugation and washed extensively with the lysis buffer. Proteins recovered with the GST-Grb2 SH2 domain fusion protein were separated through a 10% NuPAGE Bis-Tris gel (Novex, San Diego, Calif.) and subjected to Western blot analysis.
Antibodies, immunoprecipitation, and immunoblotting.
Polyclonal anti-Shc, monoclonal anti-Grb2, and anti-pan ERK antibodies were purchased from Transduction Laboratories (Lexington, Ky.). Polyclonal antibodies against EGFR and ERK2 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Polyclonal anti-phospho-p42/p44 ERK antibody was purchased from Promega Co. (Madison, Wis.). Antiphosphotyrosine monoclonal antibody 4G10 was purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Amersham Co. (Arlington Heights, Ill.).
Immunoblotting was performed as described previously (35–37). Briefly, PC12 cells were washed twice with ice-cold PBS and lysed in 1 ml of lysis buffer (20 mM HEPES [pH 7.4], 50 mM β-glycerophosphate, 1% Triton X-100, 10% glycerol, 2 mM EGTA, 1 mM dithiothreitol, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 2 μM leupeptin, 2 μM aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.5 μM okadaic acid). The cell lysates were clarified by centrifugation at 14,000 rpm for 10 min. Samples normalized for total protein content were resolved by electrophoresis through either straight or gradient NuPAGE Bis-Tris gels (10% or 4 to 12%) (Novex) and transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). Enhanced chemiluminescence reagent (Amersham Co.) was used for the detection of the immunoreactive bands. For reprobing, blots were stripped with a buffer containing 50 mM Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), and 0.1 M β-mercaptoethanol.
For immunoprecipitation, soluble cell lysates normalized for total protein content were precleaned by incubation with 20 μl of protein A-Sepharose beads (Pharmacia Biotech) at 4°C for 30 min. After the incubation, the supernatant was collected by brief centrifugation and incubated with the indicated antibody and 20 μl of protein A-Sepharose beads for 16 h at 4°C while gently rotating. The immunoprecipitates were washed four times with 1 ml of lysis buffer and separated through the NuPAGE gel system (Novex). Immunoblotting analysis of the immunoprecipitates was carried out as described above.
Immune complex kinase assay.
The kinase activity of ERK was assessed by an immune complex kinase assay as previously described, using myelin basic protein (MBP) as a substrate (35–37). Briefly, soluble cell lysates containing 400 μg of protein were incubated with 1 μg of rabbit polyclonal anti-ERK2 antibody and 20 μl of protein A-Sepharose beads at 4°C for 16 h with gentle rotation. The immunoprecipitates were then washed three times with lysis buffer, three times with wash buffer (500 mM LiCl, 100 mM Tris-HCl [pH 7.6], 0.1% Triton X-100, 1 mM dithiothreitol), and three times with kinase assay buffer (20 mM morpholinepropanesulfonic acid [pH 7.2], 2 mM EGTA, 10 mM MgCl2, 1 mM dithiothreitol, 0.1% Triton X-100). The kinase reactions were carried out at 30°C for 20 min in 55 μl of kinase assay buffer containing 10 μM ATP, 10 μCi of [γ-32P]ATP, 20 mM MgCl2, and 6 μg of MBP (Sigma).
Northern blot analysis.
Northern blot analysis was carried out as previously described (36). Briefly, serum-starved PC12 cells were pretreated with or without tyrphostin AG1478 (40 nM) for 30 min prior to arsenite treatment. Arsenite was directly added to the medium to a final concentration of 400 μM. Cells were harvested 30 min after the addition of arsenite, and total RNA was extracted by using Stat 60 (Teltest “B,” Friendswood, Tex.). Northern blot analysis was performed with rat c-fos and c-jun cDNAs and 18S oligonucleotides as probes.
Cell cycle distribution analysis.
PC12 cells were plated into six-well plates and cultured until reaching 50 to 60% confluence. Cells were serum starved by being placed in serum-free medium for 48 h and were then treated with 400 μM arsenite for another 24 h. Cells were harvested before and after the treatment. Cell cycle distribution was analyzed by flow cytometry as previously described (17). Briefly, 106 to 2 × 106 cells were collected and fixed in 70% ethanol for 30 min. Fixed cells were washed with PBS once, incubated with 1 μg of RNase A per ml for 30 min at 37°C, and then stained with propidium iodide (Boehringer Mannheim, Indianapolis, Ind.). The stained cells were analyzed on a FACscan flow cytometer for relative DNA content based on red fluorescence. The percentages of the cells in the various cell cycle compartments were determined by using the MULTICYCLE software program (Phoenix Flow Systems, San Diego, Calif.).
RESULTS
Arsenite activates ERK through a signaling pathway mediated by Ras and MEK.
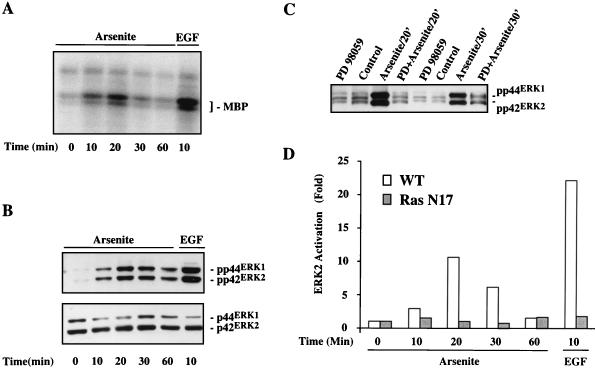
We have previously reported that arsenite can activate ERK MAP kinase through a Ras-dependent and suramin-sensitive signaling pathway (37). Results presented in Fig. 1 confirmed these earlier observations and provided additional information regarding the kinetics of ERK activation by arsenite and its dependency on MEK in PC12 cells. The kinase activity of ERK was assessed by an immune complex kinase assay using MBP as a substrate. An increase in ERK activity was apparent within 10 min of exposure to 400 μM arsenite (Fig. 1A). Maximum activity was observed between 20 and 30 min of treatment, with a return to near baseline levels by 60 min. This time course is similar to that seen following treatment with EGF, but the magnitude of activation by arsenite is somewhat lower than that seen with the growth factor. Since ERK activation requires dual phosphorylation of both threonine and tyrosine residues in the kinase subdomain VIII (2, 11, 56), active ERK can be detected by the antibody specific for dually phosphorylated ERK. Consistent with the time course for ERK activation detected by immunocomplex kinase assay, Western blot analysis of cell lysates using the anti-active ERK (phospho-ERK) antibody also showed a transient increase in ERK phosphorylation (Fig. 1B, upper panel). In contrast to the time-dependent increase in the level of active ERK, Western blot analysis with a pan-ERK antibody which recognizes both the phosphorylated and nonphosphorylated forms of ERK1 and ERK2 demonstrated that total ERK protein levels did not change in response to arsenite treatment (Fig. 1B, lower panel).
FIG. 1.
Arsenite-stimulated ERK activation depends on upstream signals. Serum-starved PC12 cells were treated with either 400 μM arsenite or 100 ng of EGF per ml for the indicated times (in all figures, ′ denotes minutes). (A) Time course of ERK activation following arsenite treatment of PC12 cells. Kinase activity of ERK was measured by an immune complex kinase assay using MBP as a substrate. (B) Western blot analysis of phosphorylated and total ERK protein in arsenite-treated cells. Upper panel, phospho-ERK detected with an anti-phospho-ERK antibody; lower panel, total ERK levels detected with an anti-pan-ERK antibody. (C) Effect of MEK inhibitor PD98059 on phosphorylation of ERK in response to arsenite. Cells were preincubated with 50 μM PD98059 for 30 min before addition of arsenite to the medium. Phospho-ERK was detected by Western blot analysis using the phospho-ERK-specific antibody. (D) Comparison of ERK kinase activities in wild-type (WT) and Ras N17 mutant PC12 cells. ERK activity was assessed by an immune complex kinase assay using MBP as a substrate and quantitated by using ImageQuant software from Molecular Dynamics (Sunnyvale, Calif.). Results are representative of three separate experiments.
Recent studies have suggested that arsenite activates JNK through inhibition of a JNK phosphatase rather than through activation of upstream kinases (6). In an effort to determine whether upstream mediators were involved in ERK activation by arsenite, we examined the effect of the MEK-specific inhibitor PD98059 (13, 44) on arsenite-induced ERK phosphorylation and activation. Pretreatment of PC12 cells with PD98059 resulted in complete inhibition of arsenite-induced ERK phosphorylation at the 20-min time point following arsenite exposure and greatly reduced ERK activity at 30 min (Fig. 1C). The sensitivity of arsenite-triggered ERK activation to the MEK inhibitor indicated that it was mediated through this upstream kinase. Supporting this observation, Fig. 1D shows a direct comparison of ERK activity in arsenite-treated wild-type PC12 cells with that in PC12 cells expressing a dominant negative Ras mutant (Ras N17) (15, 61), which confirmed our earlier observation that Ras was required for arsenite-induced ERK activation (37). Thus, these results showed the ERK activation was dependent on both MEK and Ras and further established that an upstream signal(s) was involved in the activation of the ERK pathway by arsenite.
Arsenite induces tyrosine phosphorylation in PC12 cells.
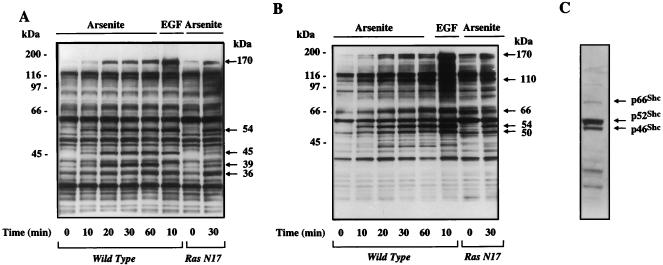
Tyrosine phosphorylation plays an essential role in ERK activation by growth factors and by G-protein-mediated events (10, 15, 39, 53, 64). To investigate the role of tyrosine-phosphorylated proteins in the response to arsenite, a protein tyrosine phosphorylation profile was generated. PC12 cells were treated with 400 μM arsenite and then harvested at different times. The lysates were examined by Western blot analysis using the antiphosphotyrosine antibody 4G10. As shown in Fig. 2A, several proteins (marked by arrows) were found to undergo rapid tyrosine phosphorylation upon arsenite stimulation, similar to that seen following EGF treatment. The apparent molecular masses of the affected proteins on the 10% NuPAGE blot were 170, 54, 45, 39, and 36 kDa, respectively. EGF stimulation did not increase the tyrosine phosphorylation of the 39-kDa protein but did result in tyrosine phosphorylation of a 41-kDa protein that was not affected by arsenite treatment. Interestingly, tyrosine phosphorylation of the 45-kDa (hereafter referred to as p45) and 39-kDa (hereafter referred to as p39) proteins by arsenite was drastically reduced in the Ras N17 cells, while tyrosine phosphorylation of the 170-, 54-, and 36-kDa proteins was unchanged relative to parental cells. The identity of the p39 protein is unclear, but its tyrosine phosphorylation pattern suggests that it may represent a component of a stress-specific signaling pathway that is partially mediated through Ras.
FIG. 2.
Protein tyrosine phosphorylation profile of arsenite-treated PC12 cells. (A) Wild-type and Ras N17 PC12 cells were treated with 400 μM arsenite or 100 ng of EGF per ml for the indicated times. The cell extracts were separated on 10% NuPAGE Bis-Tris gel. Tyrosine-phosphorylated proteins were detected by immunoblotting with the antiphosphotyrosine antibody 4G10. The major tyrosine-phosphorylated bands induced by arsenite or EGF are indicated by arrows. (B) Protein tyrosine phosphorylation profile in arsenite-treated PC12 cells was generated on a 4 to 12% gradient NuPAGE Bis-Tris gel. Tyrosine-phosphorylated proteins were detected by immunoblotting with the antiphosphotyrosine antibody 4G10. Arrows indicate the major tyrosine-phosphorylated bands induced by arsenite or EGF. (C) Shc isoforms comigrate with tyrosine-phosphorylated proteins induced by arsenite or EGF. The blot shown in panel B was stripped and reprobed with an Shc-specific rabbit polyclonal antibody. Shown is a single lane from this blot (wild-type, arsenite-treated cells), as all lanes showed identical patterns.
To improve the resolution of proteins in the high-molecular-weight range, samples were separated through a 4 to 12% gradient NuPAGE Bis-Tris gel and then analyzed for protein tyrosine phosphorylation (Fig. 2B). In addition to the 170- and 54-kDa tyrosine-phosphorylated proteins detected with the 10% gel, three additional bands with apparent molecular masses of 110, 66, and 50 kDa were evident on the gradient gel. All five of these proteins (170, 110, 66, 54, and 50 kDa) were further heavily tyrosine phosphorylated after EGF stimulation. The striking similarity between the protein tyrosine phosphorylation profiles seen with arsenite-treated and EGF-stimulated cells strongly supports the hypothesis that stress and mitogens share many components of their signaling pathways.
EGF is known to stimulate tyrosine phosphorylation of Shc (4). Three Shc isoforms, with molecular masses of 46, 52, and 66 kDa, have been described (4). Since three of the proteins that underwent tyrosine phosphorylation following arsenite treatment had similar molecular weights, we addressed whether these three proteins belonged to the Shc family. Western blot analysis using an anti-Shc polyclonal antibody indicated that the three tyrosine-phosphorylated proteins (apparent molecular weights of 66,000, 54,000, and 50,000) comigrated with the three isoforms of Shc (Fig. 2C). Given that Shc plays an important role in linking growth factor receptor tyrosine kinases to the Ras/ERK pathway (4), and that Shc was potently tyrosine phosphorylated in arsenite-treated cells in a Ras-independent manner, it is likely that Shc serves a similar role in transducing the arsenite signal to the Ras/ERK MAP kinase cascade.
Arsenite induces tyrosine phosphorylation of Shc and enhances its association with Grb2.
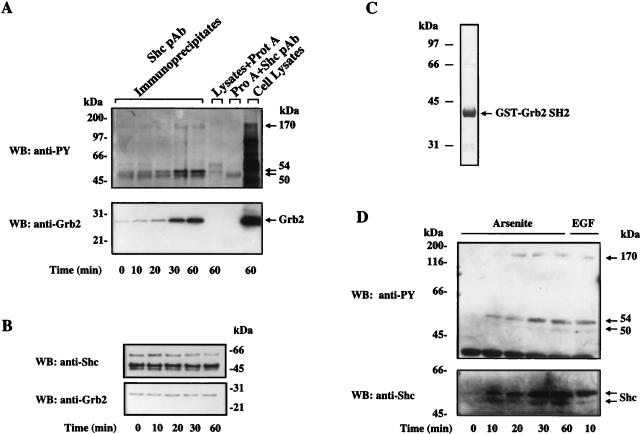
Shc serves as an adapter protein in growth factor signaling through its interaction with Grb2 (4). To determine if it provides a similar function during stress signaling, we examined whether Shc indeed associated with Grb2 following arsenite treatment. Shc was immunoprecipitated from the cell lysates by using the rabbit anti-Shc antibody that recognizes all three Shc isoforms. The immunoprecipitates were then separated by NuPAGE Bis-Tris gel electrophoresis, and tyrosine-phosphorylated proteins in the immune complexes were detected by Western blot analysis using the mouse monoclonal antiphosphotyrosine antibody 4G10. As shown in the upper panel of Fig. 3A, arsenite treatment led to an enrichment in tyrosine-phosphorylated Shc proteins (p52Shc and p46Shc) which were absent in control cells. Interestingly, a 170-kDa tyrosine-phosphorylated protein (p170) was also detected in the Shc immune complexes isolated from the arsenite-treated cells but not from the control cells (Fig. 3A, upper panel). This result suggested that arsenite treatment led to an interaction between the tyrosine-phosphorylated protein p170 and Shc. Western blot analysis of the anti-Shc immunoprecipitates with an anti-Grb2 antibody indicated that Grb2 did coimmunoprecipitate with Shc in the arsenite-treated samples (Fig. 3A, lower panel). Although a small amount of Grb2 in the Shc immune complexes could be detected in the Shc immune complexes as early as 10 min following arsenite exposure, the amount of Grb2 in the Shc immunocomplexes increased markedly after 20 min (Fig. 3A, lower panel; compare the first three lanes). Again, little Grb2 protein was detectable in the negative controls (time zero). Importantly, the enrichment of Grb2 protein in the Shc immune complexes correlated precisely with the kinetics of Shc tyrosine phosphorylation (compare the upper and lower panels in Fig. 3A), while the amounts of Shc and Grb2 detected by Western blot analysis in the total cell lysates remained unchanged throughout the entire treatment period (Fig. 3B).
FIG. 3.
Arsenite stimulates Shc tyrosine phosphorylation and its interaction with Grb2. (A) Upper panel, arsenite-induced tyrosine phosphorylation of Shc. Cellular extracts from arsenite-treated PC12 cells were immunoprecipitated with anti-Shc polyclonal antibody (pAb) and protein (Prot or Pro) A-Sepharose beads. Shc immunoprecipitates were resolved by NuPAGE Bis-Tris gel and Western blotted (WB) with the antiphosphotyrosine (anti-PY) antibody 4G10. Cell lysates (60-min arsenite treatment) and immunoprecipitates obtained in the absence of either Shc antibody or lysates were run as controls. Arrows indicate the major tyrosine-phosphorylated bands detected. Lower panel, arsenite-enhanced interaction between Grb2 and Shc. The immunoblot used in the upper panel was stripped and then blotted with a monoclonal anti-Grb2 antibody. The position of Grb2 is indicated. (B) Western blot analysis of total Shc and Grb2 levels in control and arsenite-treated cells. (C) Coomassie blue staining of the GST-Grb2 SH2 domain fusion protein. Recombinant GST-Grb2 SH2 domain fusion protein was produced in E. coli and purified by glutathione-Sepharose affinity column followed by FPLC. The fusion protein was then separated by SDS-polyacrylamide gel electrophoresis and stained. (D) Arsenite stimulates interaction between Grb2 SH2 domain and Shc in vitro. Cell lysates prepared from arsenite- or EGF-treated cells were incubated with the glutathione-free GST-Grb2 SH2 fusion protein for 2 h at 4°C. GST-Grb2 SH2 domain fusion protein and proteins associated with it were recovered by glutathione-Sepharose affinity chromatography and subjected to immunoblotting with the antiphosphotyrosine 4G10 or the anti-Shc antibodies.
In response to growth factor stimulation, Grb2 interacts with phosphotyrosine residues of both growth factor receptor tyrosine kinases and Shc through its SH2 domain, bridging the tyrosine phosphorylation signal to the Ras/ERK pathway (4, 53). To determine whether Shc plays a prominent role in recruiting Grb2 to the phosphotyrosine sites in arsenite-treated cells, an experiment was carried out to detect all of the tyrosine-phosphorylated proteins capable of interacting with the SH2 domain of Grb2 in arsenite-treated cells. A recombinant GST-Grb2 SH2 domain fusion protein was produced in E. coli as previously described (43) and purified by glutathione-Sepharose chromatography followed by passage through an FPLC ion-exchange column to remove glutathione from the fusion protein (Fig. 3C). Twenty micrograms of the GST-Grb2 SH2 domain fusion protein was then incubated with 400 μg of soluble cell lysate at 4°C for 2 h. The GST-Grb2 SH2 domain fusion protein and proteins interacting with it were recovered by incubation with glutathione-Sepharose beads, separated through a 10% NuPAGE Bis-Tris gel, and subjected to immunoblotting analysis using the antiphosphotyrosine antibody 4G10. Three major tyrosine-phosphorylated proteins with molecular weights of about 170,000, 54,000, and 50,000 were detected in preparations from arsenite-treated cells (Fig. 3D, upper panel). Essentially no tyrosine-phosphorylated proteins were brought down by the GST-Grb2 SH2 domain fusion protein from untreated control cells (Fig. 3D, upper panel, first lane). Reprobing the same blot with the polyclonal anti-Shc antibody indicated that the two smaller tyrosine-phosphorylated proteins corresponded to the p52 and the p46 isoforms of Shc (Fig. 3D, lower panel). This in vitro binding experiment further established that Shc constituted the major tyrosine-phosphorylated protein interacting with the SH2 domain of Grb2 in arsenite-treated PC12 cells.
Activation of EGFR is required for arsenite-induced Shc tyrosine phosphorylation and ERK activation.
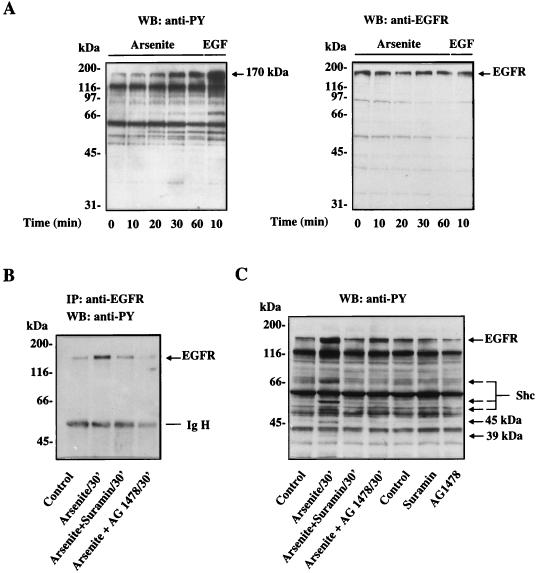
Tyrosine phosphorylation of Shc can be regulated by both receptor-type and nonreceptor-type protein tyrosine kinases (4). It has been shown that the EGFR tyrosine kinase can directly phosphorylate Shc (55). The observations that the tyrosine-phosphorylated protein p170 was found to coimmunoprecipitate with tyrosine-phosphorylated Shc in arsenite-treated cells (Fig. 3A) and also to copurify with tyrosine-phosphorylated Shc by using the Grb2 SH2 domain fusion protein (Fig. 3D) raised the possibility that the 170-kDa tyrosine-phosphorylated protein p170 might be the tyrosine kinase responsible for Shc phosphorylation. Because EGF treatment also enhanced the tyrosine phosphorylation of this protein and its size was similar to that of the EGFR, we investigated whether EGFR was involved in the arsenite-triggered signaling cascade. Western blot analysis using a polyclonal anti-EGFR antibody indicated that EGFR did indeed comigrate with the 170-kDa tyrosine-phosphorylated protein seen in both arsenite- and EGF-treated cells (Fig. 4A, right panel).
FIG. 4.
Role of EGFR in mediating arsenite-induced protein tyrosine phosphorylation. (A) Control and arsenite-treated PC12 cells were subjected to Western blot (WB) analysis using the antiphosphotyrosine (anti-PY) monoclonal antibody 4G10 and an anti-EGFR polyclonal antibody. The same blot was sequentially probed with the two antibodies. The band recognized by the EGFR antibody is indicated by an arrow. EGFR detected in the right panel comigrates with the 170-kDa tyrosine-phosphorylated protein seen in the left panel. (B) Effects of suramin and tyrphostin AG1478 on the tyrosine phosphorylation of EGFR. Suramin (300 μM) or tyrphostin AG1478 (40 nM) was added at the same time as arsenite (400 μM). Cell lysates were immunoprecipitated (IP) with the anti-EGFR polyclonal antibody, and the immunoprecipitates were subjected to Western blot analysis using the antiphosphotyrosine antibody 4G10. (C) Effect of suramin and tyrphostin AG1478 on overall arsenite-induced protein tyrosine phosphorylation. PC12 cells were treated with arsenite in the presence or absence of suramin or tyrphostin AG1478 under the conditions described above. Cell lysates were analyzed by Western blotting with the antiphosphotyrosine antibody 4G10. Arrows denote proteins whose tyrosine phosphorylation was affected by suramin or tyrphostin AG1478.
To more directly examine the role of the EGFR in the response to arsenite, we sought to inhibit the function of the EGFR. Two inhibitory agents were used for this purpose: suramin, a general growth factor receptor inhibitor (60), and tyrphostin AG1478, a selective inhibitor of EGFR (33). The ability of suramin and tyrphostin AG1478 to inhibit the tyrosine phosphorylation of EGFR was demonstrated by immunoprecipitation of EGFR followed by Western blot analysis using the antiphosphotyrosine antibody 4G10 (Fig. 4B). Arsenite treatment of PC12 cells stimulated the phosphorylation of EGFR on tyrosine residues, but this was significantly diminished in the presence of 300 μM suramin and completely abolished in the presence of 40 nM tyrphostin AG1478. Figure 4C shows the overall protein tyrosine phosphorylation profile obtained after arsenite treatment in the presence or absence of these inhibitors. As noted earlier, in the absence of these inhibitors, arsenite treatment resulted in tyrosine phosphorylation of the p170/EGFR protein as well as p66Shc, p52Shc, p46Shc, p45, and p39. Treatment of cells with suramin or tyrphostin AG1478 not only greatly diminished the tyrosine phosphorylation of p170/EGFR induced by arsenite but also prevented arsenite-triggered tyrosine phosphorylation of the other five proteins. This finding provides strong evidence that EGFR is the major tyrosine kinase involved in transducing the arsenite signal.
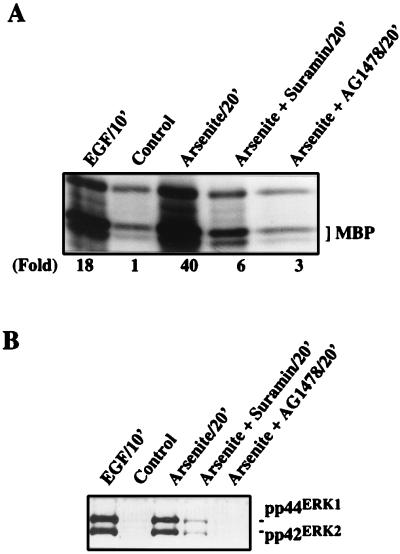
The importance of EGFR in arsenite-induced ERK activation was further indicated by the ERK immunocomplex kinase assays and Western blot analysis using the phospho-ERK-specific antibody. Treatment of PC12 cells with suramin reduced arsenite-induced ERK activation by about 85% (Fig. 5A). Tyrphostin AG1478 treatment resulted in even greater inhibition of arsenite-triggered ERK activation (93% inhibition) (Fig. 5A). The inhibition on ERK kinase activity by suramin and tyrphostin AG1478 was consistent with their inhibitory effects on arsenite-induced ERK phosphorylation (Fig. 5B). Neither suramin nor tyrphostin AG1478 alone had any effect on ERK activity (data not shown). Taken together, our data strongly suggest that the activation of EGFR by arsenite is critical for downstream ERK activation.
FIG. 5.
Central role of EGFR in mediating arsenite-induced ERK activation. (A) Effects of suramin and tyrphostin AG1478 on arsenite-induced ERK activation. PC12 cells were treated with arsenite (400 μM) in the presence or absence of 300 μM suramin or 40 nM tyrphostin AG1478 for 30 min. ERK activity was analyzed by an immune complex kinase assay using MBP as a substrate and quantitated by using ImageQuant software (Molecular Dynamics). (B) Effects of suramin and tyrphostin AG1478 on arsenite-induced ERK phosphorylation. Cell lysates from cells treated with arsenite in the absence or presence of suramin or tyrphostin AG1478 were analyzed by Western blotting using the phospho-ERK-specific antibody.
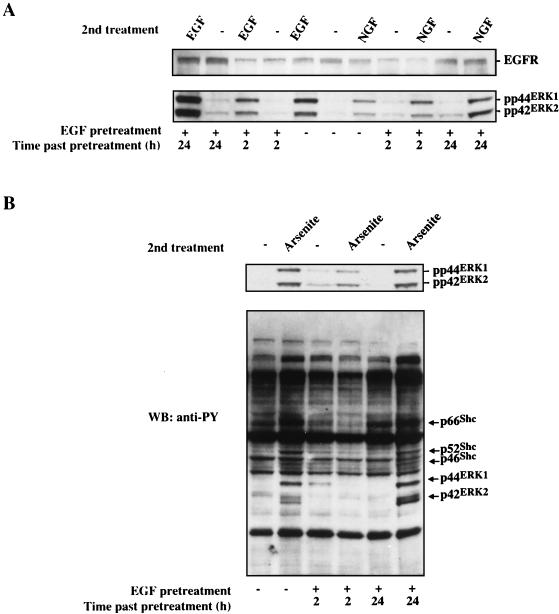
The continued presence of a growth factor such as EGF in the culture medium often results in decreased receptor levels on the cell surface due to internalization and proteolysis. Such receptor down-modulation is associated with transient nonresponsiveness to subsequent stimulation (5, 41). Removal of the growth factor results in the replenishment of surface receptors either through the recycling of internalized receptors or through expression and synthesis of new receptor molecules. To further examine the role of EGFR in mediating the ERK signaling cascade following arsenite treatment, we examined the influence of down-modulating the EGFR on this response. Serum-starved PC12 cells were pretreated with 1 μg of EGF per ml for 1 h. The EGF-containing medium was then removed, and cells were kept in serum-free medium for an additional 2 or 24 h prior to a second stimulation with either EGF, NGF, or arsenite. As shown in Fig. 6A (upper panel), 2 h after the EGF pretreatment, EGFR levels were reduced to about 30% of those present in control cells (no pretreatment, no second treatment). This was accompanied by a significant inhibition of ERK activation by both EGF (Fig. 6A, lower panel) and arsenite (Fig. 6B, upper panel) treatment. Replacing the EGF-containing medium with serum-free medium for 24 h led to replenishment of EGFR levels and restoration of the responses to both EGF stimulation and arsenite treatment. Importantly, alterations of the EGFR levels by EGF pretreatment did not significantly affect the responsiveness to subsequent stimulation with NGF (Fig. 6A, lower panel), consistent with the fact that EGF and NGF utilize different receptors to activate the ERK cascade. Down-regulation of the arsenite-induced ERK activation by EGF pretreatment might also occur through the induction of an inhibitory mechanism which acts at a later stage in the signaling pathways. For example, the dual-specificity phosphatase MKP-1, which is capable of inactivating ERK, has been shown to be induced by a variety of growth factors and is thereby implicated in feedback control of ERK activity (35). Furthermore, interaction between Grb2 and Sos can also be inhibited by an ERK-mediated mechanism (46, 62). However, the observation that arsenite-induced Shc tyrosine phosphorylation was also significantly inhibited by EGF pretreatment (Fig. 6B, lower panel) strongly argues that this down-modulation of the EGFR accounts for the reduced ERK activation by arsenite. This finding, taken together with the influence of suramin and tyrphostin AG1478 on the response, supports the conclusion that the EGFR plays a central role in initiating arsenite-induced ERK activation.
FIG. 6.
Pretreatment of cells with EGF inhibits a subsequent arsenite response. (A) PC12 cells were pretreated with EGF (1 μg/ml) for 1 h (+) or not pretreated (−), as indicated at the bottom. The cells were then washed and incubated in EGF-free medium for the time intervals shown at the bottom. The cells were further treated with EGF (100 ng/ml) for 10 min or NGF (100 ng/ml) for 5 min (second treatment, shown at the top). Cell lysates following these different treatments were subjected to Western blot analysis using anti-EGFR polyclonal antibody (upper panel). The same blot was sequentially probed with anti-phospho-ERK-specific antibody (lower panel). (B) PC12 cells were pretreated with EGF (1 μg/ml) for 1 h (+) or not pretreated (−), as indicated at the bottom. The cells were then washed and incubated in EGF-free medium for the time intervals shown at the bottom. The cells were further treated (second treatment) with 400 μM arsenite for an additional 30 min. Total cellular proteins were subjected to Western blot (WB) analysis using anti-phospho-ERK-specific antibody (upper panel) or the antiphosphotyrosine (anti-PY) monoclonal antibody 4G10 (lower panel). EGFR-mediated protein tyrosine phosphorylation stimulated by arsenite is indicated by arrows.
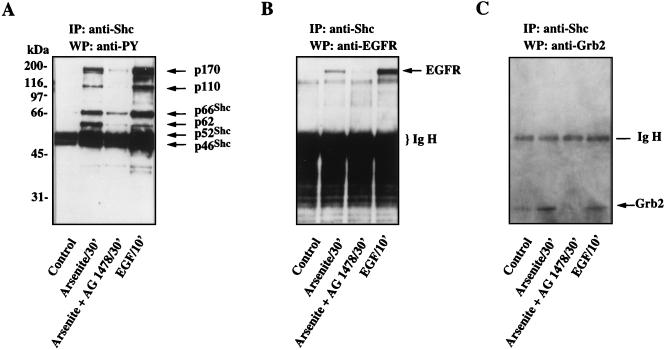
We next examined whether the tyrosine kinase activity of EGFR was required for EGFR-Shc interaction, Shc tyrosine phosphorylation, and/or Grb2 translocation to the membrane in response to arsenite treatment. PC12 cells were treated with arsenite for 30 min in the presence or absence of tyrphostin AG1478, after which Shc was immunoprecipitated from the cell extracts with the polyclonal Shc antibody. As a comparison, PC12 cells treated with EGF for 10 min were also examined. The coimmunoprecipitating tyrosine-phosphorylated proteins in the Shc immune complexes were detected with the antiphosphotyrosine antibody 4G10 (Fig. 7A). Six tyrosine-phosphorylated proteins, p46Shc, p52Shc, p66Shc, and three proteins with molecular weights of 170,000, 110,000, and 62,000, were detected in the Shc immunoprecipitates from arsenite-treated cells. This pattern was very similar to that observed in EGF-treated samples. In contrast, only residual tyrosine phosphorylation of p46Shc and p52Shc was observed in Shc immunoprecipitates from untreated control cells. In the presence of tyrphostin AG1478 (30 nM), the arsenite-induced tyrosine phosphorylation of the proteins was reduced to near basal levels (Fig. 7A). Immunoblotting of the same membrane with anti-EGFR antibody detected the p170EGFR in both the arsenite-treated and EGF-stimulated samples, although more p170EGFR was complexed with Shc in EGF-stimulated cells than in arsenite-treated cells (Fig. 7B). No p170EGFR was detected in Shc immunoprecipitates from either the control cells or cells treated with arsenite in the presence of tyrphostin AG1478. Finally, in addition to its effects on Shc tyrosine phosphorylation and EGFR association, tyrphostin AG1478 prevented the enrichment of Grb2 in the Shc immunocomplexes (Fig. 7C). These data indicate that arsenite activates the Ras/ERK cascade primarily through the EGFR tyrosine kinase and the adapter protein Shc.
FIG. 7.
Treatment of PC12 cells with the EGFR-selective inhibitor tyrphostin AG1478 prevents arsenite-induced Shc tyrosine phosphorylation and its interaction with EGFR and Grb2. PC12 cells were treated with EGF or arsenite in the presence or absence of 30 nM tyrphostin AG1478. Cell lysates containing 4.5 mg of protein were subjected to immunoprecipitation (IP) by using 1 μg of anti-Shc polyclonal antibody. Immunoprecipitates were subjected to Western blot (WB) analysis using the antiphosphotyrosine (anti-PY) antibody 4G10 (A), anti-EGFR antibody (B), and anti-Grb2 antibody (C). The same blot was sequentially probed with the three antibodies.
Arsenite induces expression of c-fos and c-jun and stimulates DNA synthesis.
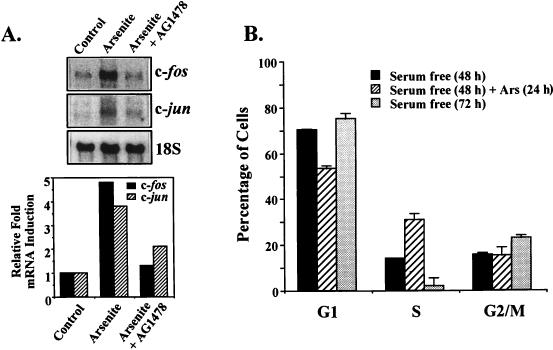
Growth factors and other mitogens have been shown to stimulate the expression of a number of immediate-early genes that play a critical role in mediating proliferation (1). Among these are the c-fos and c-jun genes, whose inductions are largely dependent on MAP kinase pathways (1, 11, 27). Since arsenite treatment is also known to induce the expression of c-fos and c-jun (6), we investigated the potential role of EGFR in mediating this response. Arsenite treatment of PC12 cells resulted in the rapid induction of both c-fos and c-jun mRNA levels (4.8- and 3.8-fold, respectively [Fig. 8A]). Pretreatment of cells with tyrphostin AG1478 almost completely abolished c-fos induction and partially inhibited c-jun expression, supporting a role for the EGFR in mediating these effects (Fig. 8A). To gain further evidence for the ability of arsenite to influence cell proliferation, cells were examined by fluorescence-activated cell sorting analysis for changes in cell cycle distribution following treatment with the agent. Cells were first subjected to 48 h of serum starvation to enrich for cells in the G1 phase. At this point only a small number of cells were present in S phase (14%). Cells were then either left untreated or treated with arsenite and examined 24 h later. As shown in Fig. 8B, in the absence of arsenite treatment there was a further reduction in the number of cells in S phase. In contrast, arsenite treatment led to a significant increase in the number of cells in S phase (31%), indicative of increased DNA synthesis. Thus, arsenite does appear to enhance cell proliferation, consistent with its role as a tumor promoter.
FIG. 8.
Arsenite induces c-fos and c-jun expression and enhances DNA synthesis. (A) Northern blot analysis of RNA extracted from arsenite-stimulated PC12 cells in the presence or absence of tyrphostin AG1478. PC12 cells were treated (Arsenite) or untreated (Control) with 400 μM arsenite for 30 min in the presence (Arsenite + AG1478) or absence of tyrphostin AG1478. The cells were harvested and analyzed for expression of c-fos mRNA, c-jun mRNA, and 18S rRNA by Northern blot analysis. The signals were quantitated with a PhosphorImager, and following normalization to 18S rRNA, relative fold mRNA induction for c-fos and c-jun mRNA expression was determined (bottom panel). (B) Arsenite increases cell population in S phase. PC12 cells were starved in serum-free medium for 48 h. Starved cells were either treated with 400 μM arsenite for 24 h or continual kept in serum-free medium for another 24 h. For cell cycle distribution analysis (as described in Materials and Methods), the cells were collected before arsenite treatment [Serum free (48 h)], after arsenite treatment [Serum free (48 h) + Ars (24 h)], or without treatment [serum free (72 h)]. The percentages of the cells in various cell cycle phases are indicated as means ± standard deviations. Results were from three different experiments.
DISCUSSION
We have previously demonstrated that arsenite treatment results in the activation of ERK MAP kinase through a Ras-dependent, suramin-sensitive pathway (37). Based on this observation, we hypothesized that the arsenite signal was transmitted through a pathway mediated by growth factor receptor-type tyrosine kinase(s). In this report, we have provided direct evidence to support this view. In particular, we have shown that EGFR undergoes tyrosine phosphorylation following arsenite treatment and that this event is associated with enhanced tyrosine phosphorylation of the Shc adapter protein, allowing for its increased interaction with Grb2.
The EGFR has been implicated in ERK activation by other extracellular stress signals including UV-C, H2O2, and asbestos (22, 25, 28, 47, 50, 52, 65). However, in none of the previous studies was the link between EGFR phosphorylation and activation of the ERK pathway established. Phosphorylation of Shc has been demonstrated to be a crucial step in the activation of the Ras/MEK/ERK pathway in response to growth factor stimulation (4). Our findings indicate that Shc is also an important mediator in arsenite-induced ERK activation, serving as an adapter for the recruitment of Grb2 and Sos to the membrane. Shc can be phosphorylated by both receptor-type and nonreceptor-type tyrosine kinases (4). Since a variety of stresses are known to activate nonreceptor-type tyrosine kinases (e.g., Src), it remains possible that these, rather than the intrinsic EGFR kinase activity, are responsible for Shc phosphorylation following arsenite treatment (12, 52). It is also clear that EGFR can be phosphorylated by nonreceptor-type tyrosine kinases, and in certain instances, EGFR does not rely on its intrinsic kinase activity for its signaling functions (39, 64). For example, Src family tyrosine kinases have been shown to be responsible for tyrosine phosphorylation of EGFR regulated by G-protein-coupled receptors (39). Similarly, JAK2, another nonreceptor tyrosine kinase, was found to phosphorylate EGFR in response to growth hormone treatment (64). In both instances, the phosphorylated EGFR was shown to be important for mediating downstream events, including Shc tyrosine phosphorylation and activation of the ERK pathway. In our case, abrogation of EGFR autophosphorylation by treatment with the highly specific EGFR inhibitor tyrphostin AG1478 also prevented the arsenite-induced Shc tyrosine phosphorylation and ERK activation. These findings argue strongly that EGFR phosphorylation is crucial for activation of the ERK signaling cascade in response to arsenite and also that intrinsic EGFR tyrosine kinase activity is required.
In addition to EGFR, other growth factor receptors including the T-cell receptor complex, interleukin-1α receptor, and the NGF receptor TrkA have been implicated in the transduction of stress signals by other toxic agents (21, 28, 30, 48, 50, 51). In particular, we have found that overexpression of TrkA in PC12 cells leads to enhanced activation of ERK in response to hydrogen peroxide treatment (21). Hence, it was somewhat surprising in this study to find that the EGFR tyrosine kinase-selective inhibitor tyrphostin AG1478 completely abolished the ERK activation in response to arsenite. This observation suggests that at least for PC12 cells, the response to arsenite is highly dependent on EGFR. Further support for this notion was obtained in experiments in which down-regulation of EGFR led to an attenuation of Shc tyrosine phosphorylation and ERK activation.
The arsenite-induced activation of ERK is not restricted to PC12 cells, as we have observed that arsenite produces similar effects in other cell types, including Rat1 fibroblasts (37), human epidermoid carcinoma A431 cells, and transformed human embryonal kidney 293 cells (data not shown). However, our findings do contrast with several reports by others. For example, Rouse et al. (49) failed to detect ERK activation in arsenite-treated PC12 cells. Similarly, Cavigelli et al. (6) did not observe activation of ERK in arsenite-treated HeLa cells, although JNK was highly activated. While the reasons for the differences between our studies and these remain unclear, they may reflect differences in the EGFR content of the different cell lines or strains used. In support of this notion, we have observed that compared to our PC12 cells, HeLa cells contain very low levels of EGFR (data not shown). Like Cavigelli et al. (6), we too have reported that JNK is highly activated in response to arsenite treatment in HeLa cells, as is p38 (37). However, the activation of both JNK and p38 occurs through mechanisms which do not involve EGFR, as the activation cannot be blocked by treatment with either suramin (37) or AG1478 (unpublished observations), nor is it dependent on Ras (37). This is consistent with the suggestion by Cavigelli et al. that arsenite-induced JNK activation occurs primarily through inhibition of a specific JNK phosphatase (6).
What is the mechanism through which arsenite activates EGFR? At least two distinct possibilities exist: (i) inhibition of tyrosine phosphatases that are involved in the inactivation of the EGFR tyrosine kinase, and (ii) up-regulation of intrinsic EGFR tyrosine kinase activity through direct interaction with the receptor. The first scenario assumes that cells exhibit significant spontaneous tyrosine kinase activity that is normally kept in check by protein tyrosine phosphatases which serve to inactivate the kinase. Studies of Knebel et al. (28) have provided strong evidence that certain treatments such as radiation, oxidants, and alkylating agents act through such a mechanism. Essential thiol groups on the tyrosine phosphatases are the presumed targets of the adverse agents, but the specific phosphatases involved have not yet been identified. Given the high reactivity of arsenite for vicinal dithiols, it is possible that arsenite also produces such effects, leading to elevated EGFR tyrosine kinase activity. The second possibility is that arsenite can actually mimic the action of ligands to activate the receptor tyrosine kinase. EGFR contains extracellular cysteine-rich domains that are important for ligand-triggered dimerization (24, 53). By reacting with vicinal dithiols in these domains, arsenite could alter the conformation of EGFR, resulting in an increase of its intrinsic tyrosine kinase activity. In support of this model, we observed that cotreatment of cells with arsenite and orthovanadate, a general tyrosine phosphatase inhibitor, synergistically enhanced the protein tyrosine phosphorylation (data not shown).
Arsenite is a potent carcinogen (3) that is believed to play an important role in the development of certain cancers such as skin and bladder tumors (3, 8, 9, 26). For example, arsenite ingested through contaminated water supplies was found to be associated with a high rate of bladder and skin cancer in regions of developing countries. In addition, a high rate of these cancers has also been reported in patients receiving Fowler’s solution (potassium arsenite) for the treatment of psoriasis in the 1940s to 1970s (8, 31). The potential role of EFGR in mediating arsenite’s carcinogenic effects is intriguing. Consistent with its tumor-promoting properties, arsenite treatment led to enhanced DNA synthesis and induction of the proliferation-associated genes c-fos and c-jun (Fig. 8). That the induction of c-fos was almost completely abolished in the presence of tyrphostin AG1478 highlights the essential role of the EGFR in mediating this response. Abnormal activation of EGFR and related tyrosine kinases such as Neu, ErbB-3, and ErbB-4 has been reported for a variety of human cancers (19, 33, 38, 42, 58). In many cases, elevated Shc tyrosine phosphorylation has also been observed (45). Finally, suramin as well as antibodies targeting members of the EGFR tyrosine kinase family have proven effective in the treatment of certain tumors (33, 60). Our findings indicating that arsenite usurps the EGF/EGFR signaling pathway to activate ERK provides a mechanism for its tumor-promoting properties and offers a potential target for therapeutic strategies aimed at preventing or inhibiting arsenite-induced tumor growth.
ACKNOWLEDGMENTS
We are very grateful to H. Gram and G. Cooper for providing valuable reagents. We are grateful to R. Wange, J. Staros, and M. Bernier for stimulating discussion and valuable suggestions. We thank F. Chrest for technical support.
REFERENCES
- 1.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 2.Avruch J, Zhang X F, Kyriakis J M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 3.Bagla P, Kaiser J. India’s spreading health crisis draws global arsenic experts. Science. 1996;274:174–175. doi: 10.1126/science.274.5285.174. [DOI] [PubMed] [Google Scholar]
- 4.Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci P G. Not all Shc’s roads lead to Ras. Trends Biochem Sci. 1996;21:257–261. [PubMed] [Google Scholar]
- 5.Carpenter G, Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976;71:159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavigelli M, Li W W, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- 7.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Evans S, Gillman M, Price E D. Medicinal arsenic and internal malignancies. Br J Cancer. 1982;45:904–911. doi: 10.1038/bjc.1982.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuzick J, Sasieni P, Evans S. Ingested arsenic, keratoses, and bladder cancer. Am J Epidemiol. 1992;136:417–421. doi: 10.1093/oxfordjournals.aje.a116514. [DOI] [PubMed] [Google Scholar]
- 10.Daub H, Weiss F U, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 11.Davis R J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 12.Devary Y, Gottlieb R A, Smeal T, Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992;71:1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 13.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan S E, Weinberg R A. The pathway to signal achievement. Nature. 1993;365:781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- 15.Feig L A. The many roads that lead to Ras. Science. 1993;260:767–768. doi: 10.1126/science.8484117. [DOI] [PubMed] [Google Scholar]
- 16.Germolec D R, Yoshida T, Gaido K, Wilmer J L, Simeonova P P, Kayama F, Burleson F, Dong W, Lange R W, Luster M I. Arsenic induces overexpression of growth factors in human keratinocytes. Toxicol Appl Pharmacol. 1996;141:308–318. doi: 10.1006/taap.1996.0288. [DOI] [PubMed] [Google Scholar]
- 17.Gorospe M, Liu Y, Xu Q, Chrest F J, Holbrook N J. Inhibition of G1 cyclin-dependent kinase activity during growth arrest of human breast carcinoma cells by prostaglandin A2. Mol Cell Biol. 1996;16:762–770. doi: 10.1128/mcb.16.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh Y, Nishida E, Sakai H. Okadaic acid activates microtubule-associated protein kinase in quiescent fibroblastic cells. Eur J Biochem. 1990;193:671–674. doi: 10.1111/j.1432-1033.1990.tb19385.x. [DOI] [PubMed] [Google Scholar]
- 19.Gullick W J. The c-erbB3/HER3 receptor in human cancer. Cancer Surv. 1996;27:339–349. [PubMed] [Google Scholar]
- 20.Guyton K Z, Gorospe M, Kensler T W, Holbrook N J. Mitogen-activated protein kinase (MAPK) activation by butylated hydroxytoluene hydroperoxide: implications for cellular survival and tumor promotion. Cancer Res. 1996;56:3480–3485. [PubMed] [Google Scholar]
- 21.Guyton, K. Z., M. Gorospe, X. Wang, Y. D. Mock, G. C. Kokkonen, Y. Liu, G. S. Roth, and N. J. Holbrook. Age-related changes in activation of mitogen activated protein kinase cascades by oxidative stress. J. Invest. Dermatol. Symp. Proc., in press. [PubMed]
- 22.Guyton K Z, Liu Y, Gorospe M, Xu Q, Holbrook N J. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 23.Hartwig A, Groblinghoff U D, Beyersmann D, Natarajan A T, Filon R, Mullenders L H. Interaction of arsenic (III) with nucleotide excision repair in UV-irradiated human fibroblasts. Carcinogenesis. 1997;18:399–405. doi: 10.1093/carcin/18.2.399. [DOI] [PubMed] [Google Scholar]
- 24.Heldin C H. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 25.Huang R P, Wu J X, Fan Y, Adamson E D. UV activates growth factor receptors via reactive oxygen intermediates. J Cell Biol. 1996;133:211–220. doi: 10.1083/jcb.133.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson S L, Cohen S M. Epidemiology and etiology of bladder cancer. Semin Surg Oncol. 1997;13:291–298. doi: 10.1002/(sici)1098-2388(199709/10)13:5<291::aid-ssu2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 28.Knebel A, Rahmsdorf H J, Ullrich A, Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- 29.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp U R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 30.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 31.Lander J J, Stanley R J, Sumner H W, Boswell D C, Aach R D. Angiosarcoma of the liver associated with Fowler’s solution (potassium arsenite) Gastroenterology. 1975;68:1582–1586. [PubMed] [Google Scholar]
- 32.Lengyel E, Wang H, Gum R, Simon C, Wang Y, Boyd D. Elevated urokinase-type plasminogen activator receptor expression in a colon cancer cell line is due to a constitutively activated extracellular signal-regulated kinase-1-dependent signaling cascade. Oncogene. 1997;14:2563–2573. doi: 10.1038/sj.onc.1201098. [DOI] [PubMed] [Google Scholar]
- 33.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 34.Li J H, Rossman T G. Mechanism of comutagenesis of sodium arsenite with n-methyl-n-nitrosourea. Biol Trace Elem Res. 1989;21:373–381. doi: 10.1007/BF02917278. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Gorospe M, Yang C, Holbrook N J. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Guyton K Z, Gorospe M, Xu Q, Kokkonen G C, Mock Y D, Roth G S, Holbrook N J. Age-related decline in mitogen-activated protein kinase activity in epidermal growth factor-stimulated rat hepatocytes. J Biol Chem. 1996;271:3604–3607. [PubMed] [Google Scholar]
- 37.Liu Y, Guyton K Z, Gorospe M, Xu Q, Lee J C, Holbrook N J. Differential activation of ERK, JNK/SAPK and P38/CSBP/RK map kinase family members during the cellular response to arsenite. Free Radical Biol Med. 1996;21:771–781. doi: 10.1016/0891-5849(96)00176-1. [DOI] [PubMed] [Google Scholar]
- 38.Lupu R, Cardillo M, Harris L, Hijazi M, Rosenberg K. Interaction between erbB-receptors and heregulin in breast cancer tumor progression and drug resistance. Semin Cancer Biol. 1995;6:135–145. doi: 10.1006/scbi.1995.0016. [DOI] [PubMed] [Google Scholar]
- 39.Luttrell L M, Della R G, van B T, Luttrell D K, Lefkowitz R J. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 40.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande W G, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 41.Masui H, Castro L, Mendelsohn J. Consumption of EGF by A431 cells: evidence for receptor recycling. J Cell Biol. 1993;120:85–93. doi: 10.1083/jcb.120.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitra A B, Murty V V, Pratap M, Sodhani P, Chaganti R S. ERBB2 (HER2/neu) oncogene is frequently amplified in squamous cell carcinoma of the uterine cervix. Cancer Res. 1994;54:637–639. [PubMed] [Google Scholar]
- 43.Muller K, Gombert F O, Manning U, Grossmuller F, Graff P, Zaegel H, Zuber J F, Freuler F, Tschopp C, Baumann G. Rapid identification of phosphopeptide ligands for SH2 domains. Screening of peptide libraries by fluorescence-activated bead sorting. J Biol Chem. 1996;271:16500–16505. [PubMed] [Google Scholar]
- 44.Pang L, Sawada T, Decker S J, Saltiel A R. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 45.Pelicci G, Lanfrancone L, Salcini A E, Romano A, Mele S, Grazia B M, Segatto O, Di F P, Pelicci P G. Constitutive phosphorylation of Shc proteins in human tumors. Oncogene. 1995;11:899–907. [PubMed] [Google Scholar]
- 46.Porfiri E, McCormick F. Regulation of epidermal growth factor receptor signaling by phosphorylation of the ras exchange factor hSOS1. J Biol Chem. 1996;271:5871–5877. doi: 10.1074/jbc.271.10.5871. [DOI] [PubMed] [Google Scholar]
- 47.Rao G N. Hydrogen peroxide induces complex formation of SHC-Grb2-SOS with receptor tyrosine kinase and activates Ras and extracellular signal-regulated protein kinases group of mitogen-activated protein kinases. Oncogene. 1996;13:713–719. [PubMed] [Google Scholar]
- 48.Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 49.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 50.Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf H J. Involvement of growth factor receptors in the mammalian UVC response. Cell. 1994;78:963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 51.Schieven G L, Ledbetter J A. Activation of tyrosine kinase signal pathways by radiation and oxidative stress. Trends Endocrinol Metab. 1994;5:383–387. doi: 10.1016/1043-2760(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 52.Schieven G L, Mittler R S, Nadler S G, Kirihara J M, Bolen J B, Kanner S B, Ledbetter J A. ZAP-70 tyrosine kinase, CD45, and T cell receptor involvement in UV- and H2O2-induced T cell signal transduction. J Biol Chem. 1994;269:20718–20726. [PubMed] [Google Scholar]
- 53.Schlessinger J. How receptor tyrosine kinases activate Ras. Trends Biochem Sci. 1993;18:273–275. doi: 10.1016/0968-0004(93)90031-h. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt C M, McKillop I H, Cahill P A, Sitzmann J V. Increased MAPK expression and activity in primary human hepatocellular carcinoma. Biochem Biophys Res Commun. 1997;236:54–58. doi: 10.1006/bbrc.1997.6840. [DOI] [PubMed] [Google Scholar]
- 55.Segatto O, Pelicci G, Giuli S, Digiesi G, Di F P, McGlade J, Pawson T, Pelicci P G. Shc products are substrates of erbB-2 kinase. Oncogene. 1993;8:2105–2112. [PubMed] [Google Scholar]
- 56.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 57.Sivaraman V S, Wang H, Nuovo G J, Malbon C C. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slamon D J, Godolphin W, Jones L A, Holt J A, Wong S G, Keith D E, Levin W J, Stuart S G, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 59.Snow E T. Metal carcinogenesis: mechanistic implications. Pharmacol Ther. 1992;53:31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- 60.Stein C A. Suramin: a novel antineoplastic agent with multiple potential mechanisms of action. Cancer Res. 1993;53:2239–2248. [PubMed] [Google Scholar]
- 61.Szeberenyi J, Cai H, Cooper G M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990;10:5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waters S B, Holt K H, Ross S E, Syu L J, Guan K L, Saltiel A R, Koretzky G A, Pessin J E. Desensitization of Ras activation by a feedback disassociation of the SOS-Grb2 complex. J Biol Chem. 1995;270:20883–20886. doi: 10.1074/jbc.270.36.20883. [DOI] [PubMed] [Google Scholar]
- 63.Wiencke J K, Yager J W, Varkonyi A, Hultner M, Lutze L H. Study of arsenic mutagenesis using the plasmid shuttle vector pZ189 propagated in DNA repair proficient human cells. Mutat Res. 1997;386:335–344. doi: 10.1016/s1383-5742(97)00016-1. [DOI] [PubMed] [Google Scholar]
- 64.Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, Honjo M, Takahashi M, Takahashi T, Hirai H, Tushima T, Akanuma Y, Fujita T, Komuro I, Yazaki Y, Kadowaki T. Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature. 1997;390:91–96. doi: 10.1038/36369. [DOI] [PubMed] [Google Scholar]
- 65.Zanella C L, Posada J, Tritton T R, Mossman B T. Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996;56:5334–5338. [PubMed] [Google Scholar]