Abstract
Background
Low respiratory function in young adulthood is one of the important factors in the trajectory leading to the future development of COPD, but its morphological characteristics are not well characterised.
Methods
We retrospectively enrolled 172 subjects aged 40–49 years with ≥10 pack-years smoking history who underwent lung cancer screening by computed tomography (CT) and spirometry at two Japanese hospitals. Emphysema was visually assessed according to the Fleischner Society guidelines and classified into two types: centrilobular emphysema (CLE) and paraseptal emphysema (PSE). Airway dysanapsis was assessed with the airway/lung ratio (ALR), which was calculated by the geometric mean of the lumen diameters of the 14 branching segments divided by the cube root of total lung volume on a CT scan.
Results
Among the subjects, CLE and PSE were observed in 20.9% and 30.8%, respectively. The mean ALR was 0.04 and did not differ between those with and without each type of emphysema. Multivariable regression analysis models adjusted for age, sex, body mass index and smoking status indicated that CLE and a low ALR were independently associated with lower forced expiratory volume in 1 s (FEV1)/forced vital capacity (estimate −1.64 (95% CI −2.68– −0.60) and 6.73 (95% CI 4.24–9.24), respectively) and FEV1 % pred (estimate −2.81 (95% CI −5.10– −0.52) and 10.9 (95% CI 5.36–16.4), respectively).
Conclusions
CLE and airway dysanapsis on CT were independently associated with low respiratory function in younger smokers.
Shareable abstract
In smokers aged 40–49 years, centrilobular emphysema and the airway/lung size ratio on CT were independently associated with low respiratory function, one of the key factors leading to the future development of COPD https://bit.ly/4b6Blm5
Introduction
COPD is a worldwide health problem with high prevalence and mortality. COPD is characterised by airflow limitation induced by a combination of emphysema and airway disease, while the clinical presentations and outcomes are very heterogeneous. Heterogeneity is caused by many factors, such as variable host responses to exposure to tobacco and other particles, abnormal lung growth, and congenital components [1–3]. The prevalence of COPD increases at age ≥65 years [4], but some younger subjects aged <50 years develop emphysema and airway disease before COPD diagnosis [5, 6] and are diagnosed with COPD. Further understanding of early changes before the onset of airflow limitation and COPD at a young age is warranted to identify high-risk subjects and establish efficient interventions to prevent COPD development.
There are two major patterns of lung functional trajectory leading to the onset of COPD: 1) normal peak respiratory function followed by rapid functional decline and 2) low attainment of respiratory function in young adulthood followed by normal functional decline [7]. The two patterns are not exclusive. Low functional attainment in young adulthood followed by rapid functional decline leads to severe airflow limitation at a younger age [8]. These findings suggest that the relative contributions of the two patterns to lung function impairment may vary among younger subjects even before the COPD diagnosis. However, their underlying mechanisms are not fully understood.
The concept of disproportional growth of the airway and lung parenchyma, i.e. dysanapsis, was first introduced to describe a mismatch between native tree airway calibre and lung size, which underlies a large variation in lung function in healthy subjects [9]. More recently, a computed tomography (CT) study including middle-aged adults showed that a smaller central airway tree for a given lung size on a CT scan is associated with low baseline lung function followed by normal lung function decline, leading to COPD development [10]. Furthermore, a CT study including smokers of similar age showed that centrilobular emphysema (CLE), a major smoke-related emphysema subtype [11], is associated with a rapid lung function decline, leading to airflow limitation (COPD) [12]. However, no report has examined these two CT factors simultaneously in younger subjects.
It was hypothesised that subclinical spirometric impairment could be induced by a combination of dysanaptic airway/lung growth before adulthood and the emergence of CLE after adulthood in smokers without airflow limitation or COPD. Therefore, this study examined lung cancer screening CT and spirometry in smokers aged <50 years to test whether a smaller airway/lung ratio (ALR) and the presence of CLE were independently associated with forced expiratory volume in 1 s (FEV1) and the FEV1/forced vital capacity (FVC) ratio on spirometry.
Material and methods
Ethics
This retrospective cross-sectional observational cohort study complied the guidelines of the Declaration of Helsinki and was approved by the Ethics Committees of Tsukuba Medical Center Hospital (2022-0029), Takeda Hospital (2019) and Kyoto University (R1660-3). Informed consent was obtained via an opt-out method.
Study design and population
The study enrolled consecutive subjects between 40 and 49 years of age who had a smoking history of ≥10 pack-years and underwent spirometry and chest CT scans in a medical check-up programme at Tsukuba Medical Center Hospital (Tsukuba, Japan) between 2019 and 2021 and at Takeda Hospital (Kyoto, Japan) between 2016 and 2020. In Japan, any participants undergoing a medical check-up can voluntarily select an additional option of lung cancer screening chest CT upon request. The exclusion criteria were: 1) CT abnormal shadow not associated with COPD (such as consolidation, fibrotic change and tumour), 2) lack of demographic data or 3) interval between CT and spirometry >90 days. No subjects had a history of lung resection surgery.
Data measurements
Smoking status and medical history (asthma, diabetes, dyslipidaemia, hypertension, arrhythmia and coronary artery disease) were assessed using self-report questionnaires. Pack-years of smoking were directly calculated at Tsukuba Medical Center Hospital. While the data on smoking duration (<10, 10–19 or ≥20 years) and daily tobacco consumption (<0.5, 0.5–1.0 and ≥1.0 packs per day) were collected at Takeda Hospital, subjects with ≥10 pack-years of smoking were identified by either 1) a combination of smoking duration ≥10 years and tobacco consumption ≥1.0 packs per day or 2) a combination of smoking duration ≥20 years and tobacco consumption ≥0.5 packs per day.
Pulmonary function test
Spirometry was performed under pre-bronchodilator conditions with an automated electronic spirometer SYSTEM 7 (Minato Medical Science, Osaka, Japan) at Tsukuba Medical Center Hospital and with Spiro Sift SP-790 COPD (Fukuda Denshi, Tokyo, Japan) at Takeda Hospital following the American Thoracic Society/European Respiratory Society guidelines [13]. The reference values for FEV1 % pred and FVC % pred were based on the LMS (skewness (lambda), mean (mu) and coefficient of variation (sigma)) methods published by the Japanese Respiratory Society [14].
Chest CT scan protocols
A CT scan was conducted at deep inspiration. Aquilion One (Canon Medical Systems, Otawara, Japan) and LightSpeed VCT (GE Healthcare, Waukesha, WI, USA) were used at Tsukuba Medical Center Hospital. Images were acquired at 0.5 mm (Aquilion) or 1.25 mm (VCT) slice thickness with a scan time of 400 ms and tube voltage of 120 kVp. The CT images were reconstructed with sharp kernels (FC53, LUNG) and smoothing kernels (FC02, STANDARD) for visual inspection of emphysema subtypes and calculation of airway dimension, and for quantitative emphysema measurements, respectively. Aquilion Prime (Canon Medical Systems) was used at Takeda Hospital. The CT images were obtained at a slice thickness of 0.5 mm with a scan time of 400 ms and tube voltage of 120 kVp, and reconstructed with sharp kernels (FC51) for visual inspection of emphysema subtypes and calculation of airway dimension. For quantitative emphysema measurements, these images were converted to smoothing kernel-based images using the established deep learning-based kernel conversion method [15]. Reference values for the total lung capacity (TLC) % pred on a CT scan (TLCCT) were determined using the predicted values reported previously [16].
CT measurements
Visual emphysema was assessed based on the Fleischner Society guidelines [17], and assessments were performed by at least two pulmonologists and a chest radiologist [18]. Subjects with trace, mild, moderate, confluent or advanced CLE were regarded as having CLE. Subjects with mild or substantial PSE were regarded as having PSE. Subjects with CLE or PSE were considered to have visual emphysema. Panlobular emphysema was not assessed in this study because its classification is associated with α1-antitrypsin deficiency [17]. The percentage of low attenuation area < −950 HU to the total lung (LAA%) was also calculated to quantify the extent of overall emphysema [19].
As the index of dysanapsis, the ALR was calculated as the geometric mean of the lumen diameters for 14 branch segments (trachea, main bronchus, bronchus intermedius (right median trunk), and third and fourth bronchus of five paths) divided by the cube root of TLCCT, as reported previously [20]. The wall thickness and lumen area of the airway were determined by the mean of the third (segmental) or fourth (subsegmental) bronchus.
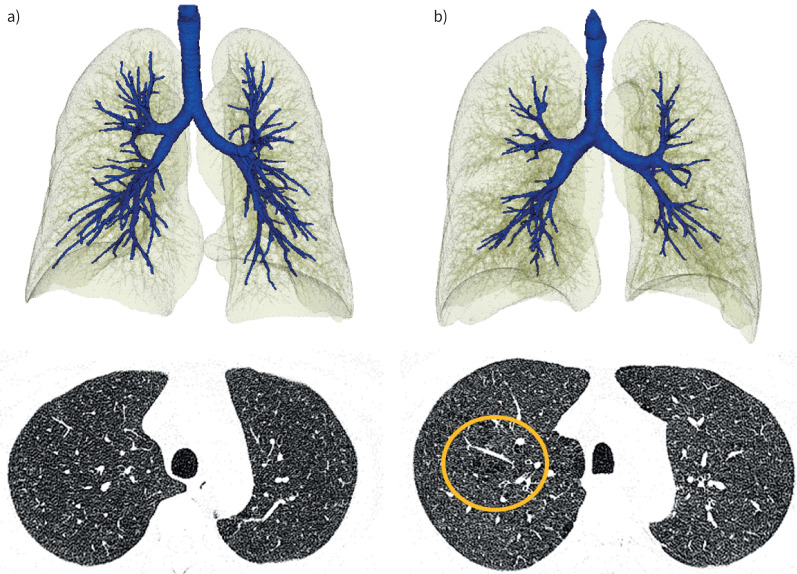
Representative images of visual emphysema and the ALR are shown in figure 1.
FIGURE 1.
Representative images of subjects with or without a low airway/lung ratio (ALR) and centrilobular emphysema (CLE). a) A 47-year-old male who did not have a low ALR (0.042) and no CLE. Forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) was 0.91 and FEV1 % pred was 102%. b) A 49-year-old male presenting with a low ALR (0.033) and mild CLE (circled). FEV1/FVC was 0.74 and FEV1 % pred was 69%, which was lower than that in the subject in a).
Statistical analyses
Interobserver agreements for grades of PSE and CLE between two analysts were evaluated with a weighted κ coefficient. To compare the clinical characteristics, spirometric indices and CT measurements between subjects with and without visual emphysema or its subtype, the t-test and the Chi-squared test were performed. Pearson correlation was used to determine the relationship between spirometric indices and CT measurements. Multivariable regression analysis was performed to investigate factors associated with FEV1/FVC and FEV1 % pred. These multivariable models were evaluated in a sensitivity analysis of subgroups without a history of asthma. JMP version 16.0.0 (SAS Institute, Cary, NC, USA) was used for the statistical analyses. p-values <0.05 were considered to indicate statistical significance.
Results
Characteristics of subjects
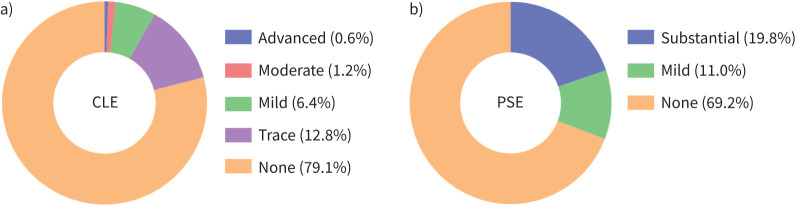
As shown in figure 2, of 661 subjects with ≥10 pack-years who underwent lung cancer screening CT and spirometry, 191 were aged between 40 and 49 years. Following exclusion of 19 subjects due to abnormal CT shadows, lack of demographic data, and long intervals (>90 days) between CT and spirometry, 172 subjects were included in this study. As shown in table 1, the rates of males and current smokers were 89% and 84%, respectively, and the mean FEV1/FVC and FEV1 % pred were 0.81 and 94.6%, respectively. The mean±sd ALR was 0.04±0.00. Any findings of emphysema, CLE and PSE were observed in 39.0%, 20.9% and 30.8% of subjects, respectively. Figure 3 shows the distribution of CLE and PSE grades in all subjects. Trace CLE accounted for the majority of those with CLE (12.8%), followed by mild CLE (6.4%), moderate CLE (1.2%) and advanced CLE (0.6%). The proportion of subjects with mild PSE was 11.0%, which was less than that of those with substantial PSE (19.8%). The range of weighted κ coefficients for grades of PSE was from 0.87 to 0.90 and that for CLE was from 0.84 to 1.00 (supplementary table S1).
FIGURE 2.
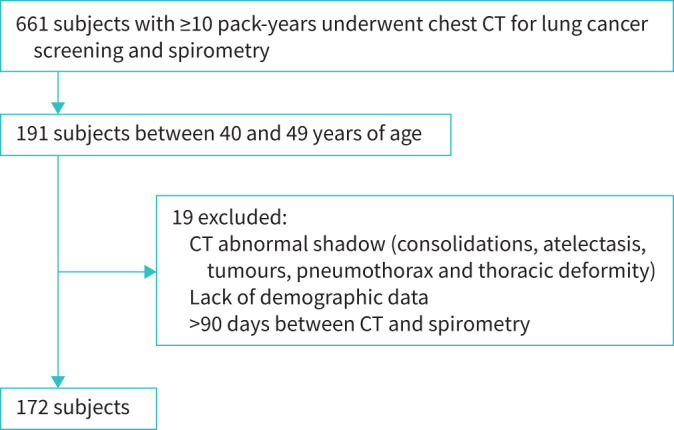
Flowchart of subject selection. CT: computed tomography.
TABLE 1.
Characteristics of the subjects (n=172)
| Age, years | 45.3±2.7 |
| Male | 89.0 |
| Height, cm | 171.1±6.5 |
| BMI, kg·m−2 | 24.1±3.7 |
| Current smoker | 84.3 |
| FEV1/FVC | 0.81±0.06 |
| FEV1, % pred | 94.6±11.9 |
| FVC, % pred | 94.8±10.5 |
| Medical history | |
| Asthma | 3.5 |
| Diabetes | 2.9 |
| Dyslipidaemia | 10.4 |
| Hypertension | 10.5 |
| Arrhythmia | 5.8 |
| Coronary artery disease | 1.2 |
| CT measurements | |
| Visual emphysema | 39.0 |
| CLE | 20.9 |
| PSE | 30.8 |
| WT segmental, mm | 1.34±0.12 |
| WT subsegmental, mm | 1.19±0.08 |
| LA segmental, mm2 | 23.0±5.8 |
| LA subsegmental, mm2 | 13.8±3.5 |
| ALR | 0.04±0.00 |
| TLCCT, mL | 5362.4±944.5 |
| TLCCT, % pred | 82.8±11.7 |
Data are presented as mean±sd or %. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; CT: computed tomography; CLE: centrilobular emphysema; PSE: paraseptal emphysema; WT: wall thickness; LA: lumen area; ALR: airway/lung ratio; TLCCT: total lung capacity on CT.
FIGURE 3.
Prevalence of emphysema subtypes in all subjects: a) centrilobular emphysema (CLE) and b) paraseptal emphysema (PSE).
Supplementary table S2 shows the FEV1/FVC and FEV1 % pred by each clinical feature. In all subjects, males had a lower FEV1/FVC than females. There were no differences in respiratory function between subjects with or without a history of asthma. No other medical history was associated with respiratory function.
Associations of emphysema and the ALR with lung function
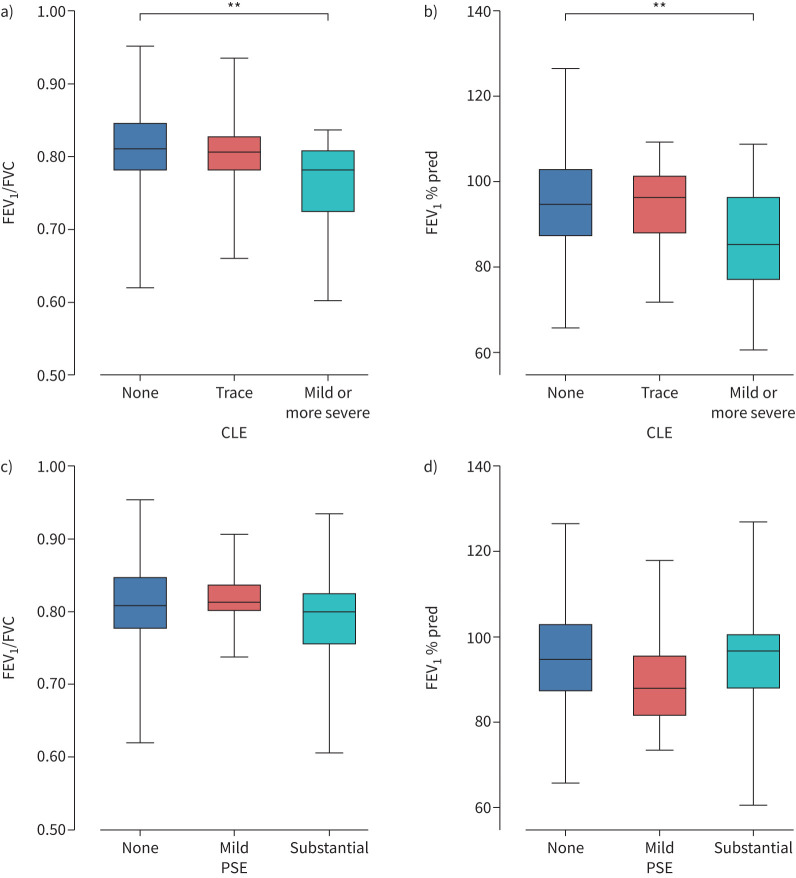
To explore whether emphysema is associated with lung function, subjects were divided into two groups based on visual emphysema, CLE or PSE, as shown in table 2. Subjects with CLE had a lower FEV1/FVC and FEV1 % pred than subjects without CLE but not PSE. The percentages of FEV1/FVC <0.70 or FEV1/FVC <0.75 were higher in subjects with CLE than those without. Moreover, figure 4 shows that FEV1/FVC and FEV1 % pred were significantly lower in subjects with mild or more severe CLE than those without CLE. In contrast, FEV1/FVC and FEV1 % pred did not differ between those without PSE, those with trace PSE and those with substantial PSE. A total of 61.1% of subjects with CLE and 41.5% of subjects with PSE had other visual emphysema. The ALR did not differ between those with and without CLE.
TABLE 2.
Characteristics of subjects with and without each type of emphysema
| Visual emphysema | CLE | PSE | ||||
| + | − | + | − | + | − | |
| Subjects | 67 (39.0) | 105 (61.0) | 36 (20.9) | 136 (79.1) | 53 (30.8) | 119 (69.2) |
| Age, years | 45.3±2.6 | 45.2±2.7 | 45.8±2.5 | 45.2±2.7 | 45.1±2.7 | 45.4±2.7 |
| Male | 92.5 | 86.7 | 88.9 | 89.0 | 94.3 | 86.6 |
| Height, cm | 172.0±6.1 | 170.5±6.8 | 172.7±5.6 | 170.7±6.7 | 171.7±6.3 | 170.9±6.6 |
| BMI, kg·m−2 | 23.2±3.1* | 24.7±3.8 | 22.9±3.3* | 24.5±3.7 | 23.1±3.1* | 24.6±3.8 |
| Current smoker | 88.1 | 81.9 | 86.1 | 83.8 | 90.6 | 81.5 |
| FEV1/FVC | 0.80±0.06 | 0.81±0.05 | 0.78±0.07* | 0.81±0.05 | 0.80±0.06 | 0.81±0.06 |
| FEV1/FVC <0.70 | 4.5 | 1.9 | 8.3* | 1.5 | 3.8 | 2.5 |
| FEV1/FVC <0.75 | 19.4* | 7.6 | 22.2* | 9.6 | 17.0 | 10.1 |
| FEV1, % pred | 92.5±12.2 | 95.9±11.6 | 90.4±12.2* | 95.7±11.6 | 92.7±12.2 | 95.5±11.7 |
| CLE | 53.7 | 0.0 | 41.5* | 11.8 | ||
| PSE | 79.1 | 0.0 | 61.1* | 22.8 | ||
| LAA% ≥5 | 23.9* | 12.4 | 27.8* | 14.0 | 22.6 | 14.3 |
| WT segmental, mm | 1.35±0.12 | 1.33±0.11 | 1.36±0.13 | 1.33±0.11 | 1.35±0.13 | 1.33±0.11 |
| WT subsegmental, mm | 1.19±0.08 | 1.19±0.08 | 1.19±0.08 | 1.19±0.08 | 1.18±0.09 | 1.19±0.08 |
| LA segmental, mm2 | 23.3±5.6 | 22.8±5.9 | 23.6±5.6 | 22.8±5.8 | 22.9±5.0 | 23.0±6.1 |
| LA subsegmental, mm2 | 14.2±3.7 | 13.5±3.3 | 14.2±4.0 | 13.6±3.3 | 13.8±3.4 | 13.7±3.5 |
| ALR | 0.04±0.00 | 0.04±0.00 | 0.04±0.00 | 0.04±0.00 | 0.04±0.00 | 0.04±0.00 |
| TLCCT, L | 5.49±1.01 | 5.28±0.90 | 5.53±0.96 | 5.32±0.94 | 5.52±1.02 | 5.29±0.90 |
| TLCCT, % pred | 83.4±13.3 | 82.3±10.6 | 84.1±14.0 | 82.4±11.1 | 83.8±13.1 | 82.3±11.0 |
Data are presented as n (%), mean±sd or %. CLE: centrilobular emphysema; PSE: paraseptal emphysema; BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; LAA: percentage of low attenuation area < −950 HU to the total lung; WT: wall thickness; LA: lumen area; ALR: airway/lung ratio; TLCCT: total lung capacity on computed tomography. *: p<0.05 compared with subjects without the same type of emphysema.
FIGURE 4.
Lung function among each grade of visual emphysema: a, b) centrilobular emphysema (CLE) and c, d) paraseptal emphysema (PSE). Comparisons of a) forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) and b) FEV1 % pred in subjects without CLE, those with trace CLE and those with mild or more severe CLE (mild, moderate, confluent and advanced). Comparisons of c) FEV1/FVC and d) FEV1 % pred in subjects without PSE, those with mild PSE and those with substantial PSE. **: p<0.01.
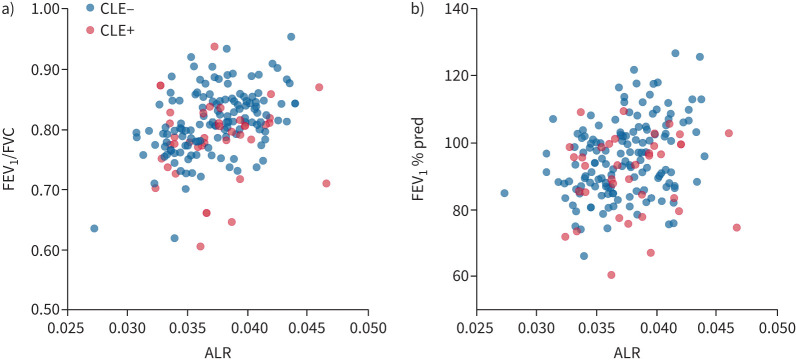
Figure 5 shows that the ALR was significantly associated with FEV1/FVC (r=0.35, p<0.0001) and FEV1 % pred (r=0.26, p=0.0007) (supplementary table S3).
FIGURE 5.
Association of the airway/lung ratio (ALR) on computed tomography with lung function: a) forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) and b) FEV1 % pred. Pearson correlation coefficients and p-values for a) and b) were r=0.35, p<0.0001 and r=0.26, p=0.0007, respectively. CLE: centrilobular emphysema.
Subjects with LAA% ≥5% had lower FEV1/FVC, but no difference in FEV1 % pred (supplementary table S4).
Relative associations of emphysema and the ALR with lung function in multivariable models
Table 3 shows the multivariable regression analysis exploring factors associated with FEV1/FVC. The presence of visual emphysema was associated with a low FEV1/FVC (estimate −0.88 (95% CI −1.72– −0.04)). When examined by subtype of emphysema, CLE was associated with a low FEV1/FVC, whereas PSE was not (estimate −1.64 (95% CI −2.68– −0.60) and 0.07 (95% CI −0.86–1.00), respectively). A low ALR was associated with a low FEV1/FVC in all models. The associations of FEV1 % pred with visual emphysema, its subtypes and the ALR showed similar trends (table 4). The associations of FEV1/FVC and FEV1 % pred with CLE and the ALR were confirmed in the model using segmental wall thickness (supplementary tables S5 and S6). There was no interaction between CLE and the ALR (supplementary tables S7 and S8).
TABLE 3.
Multivariable regression analysis for forced expiratory volume in 1 s/forced vital capacity
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Visual emphysema+ | −0.88 (−1.72– −0.04)* | |||
| CLE+ | −1.61 (−2.59– −0.63)** | −1.64 (−2.68– −0.60)** | ||
| PSE+ | −0.40 (−1.30–0.50) | 0.07 (−0.86–1.00) | ||
| WT subsegmental per 1 mm increase | 9.65 (−1.98–21.3) | 10.3 (−1.11–21.7) | 8.96 (−2.77–20.7) | 10.3 (−1.17–21.7) |
| ALR per 0.01 increase | 6.63 (4.10–9.17)**** | 6.72 (4.23–9.21)**** | 6.46 (3.90–9.03)**** | 6.73 (4.23–9.23)**** |
| Age per 1 year increase | −0.14 (−0.43–0.16) | −0.09 (−0.39–0.20) | −0.15 (−0.45–0.15) | −0.09 (−0.39–0.20) |
| Male | −2.08 (−3.39– −0.78)** | −2.23 (−3.50– −0.95)*** | −2.12 (−3.44– −0.80)** | −2.24 (−3.53– −0.95)*** |
| BMI per 1 kg·m−2 increase | −0.06 (−0.32–0.19) | −0.08 (−0.33–0.17) | −0.02 (−0.28–0.23) | −0.08 (−0.33–0.17) |
| Smoking status, current | 0.23 (−0.87–1.34) | 0.20 (−0.88–1.28) | 0.18 (−0.94–1.29) | 0.19 (−0.90–1.28) |
Data are presented as estimate (95% CI). CLE: centrilobular emphysema; PSE: paraseptal emphysema; WT: wall thickness; ALR: airway/lung ratio; BMI: body mass index. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001.
TABLE 4.
Multivariable regression analysis for forced expiratory volume in 1 s percentage predicted
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Visual emphysema+ | −2.00 (−3.84– −0.17)* | |||
| CLE+ | −3.01 (−5.18– −0.85)** | −2.81 (−5.10– −0.52)* | ||
| PSE+ | −1.37 (−3.33–0.59) | −0.57 (−2.61–1.47) | ||
| WT subsegmental per 1 mm increase | −4.81 (−30.2–20.6) | −3.97 (−29.1–21.2) | −6.07 (−31.6–19.5) | −3.79 (−29.0–21.4) |
| ALR per 0.01 increase | 10.8 (5.31–16.4)*** | 11.0 (5.47–16.4)*** | 10.4 (4.84–16.0)*** | 10.9 (5.36–16.4)*** |
| Age per 1 year increase | −0.26 (−0.92–0.39) | −0.19 (−0.84–0.46) | −0.30 (−0.96–0.35) | −0.20 (−0.86–0.45) |
| Male | −1.14 (−3.99–1.70) | −1.45 (−4.26–1.35) | −1.15 (−4.03–1.73) | −1.35 (−4.19–1.49) |
| BMI per 1 kg·m−2 increase | −0.31 (−0.86–0.25) | −0.32 (−0.87–0.23) | −0.25 (−0.81–0.31) | −0.34 (−0.90–0.21) |
| Smoking status, current | −0.44 (−2.85–1.97) | −0.55 (−2.92–1.83) | −0.49 (−2.93–1.94) | −0.47 (−2.86–1.93) |
Data are presented as estimate (95% CI). CLE: centrilobular emphysema; PSE: paraseptal emphysema; WT: wall thickness; ALR: airway/lung ratio; BMI: body mass index. *: p<0.05; **: p<0.01; ***: p<0.001.
Discussion
This study used lung cancer screening CT data in younger smokers and showed that CLE, but not PSE, and the ALR were significantly associated with FEV1/FVC and FEV1 % pred. Moreover, multivariable analyses showed that CLE and a smaller ALR on a CT scan were independently associated with lower respiratory function in young smokers. These findings suggest that a combination of a native airway structure characterised by a smaller airway tree calibre relative to lung size and smoking-related ongoing lung destruction characterised by CLE emergence could impair lung function in younger smokers.
Early detection of COPD and establishment of an early interventional strategy, especially at a younger age, is of great interest to change the natural course of the disease [21, 22]. Moreover, since many pathophysiological changes, such as emphysema, are observed before COPD diagnosis, the concepts of pre-COPD and early COPD have been recently proposed to identify subjects with a high risk of COPD [23, 24]. It is critical to understand the structural changes underlying subclinical lung function impairment before COPD diagnosis. To the best of our knowledge, this is the first study to show the relative associations of the ALR and CLE with FEV1/FVC and FEV1 % pred in smokers before age 50 years. Our data substantially extend the understanding of the structure‒function relationship in smokers before COPD onset by showing that CT dysanapsis and CLE independently affected expiratory airflow on spirometry.
In the population for the present study, ∼90% of subjects with CLE were found to have a degree of CLE <5% (trace/mild) and even the mild form of CLE affected respiratory function. A cut-off value of 5% has been proposed to decrease clinically relevant emphysema, particularly in quantitative analyses [25], and this study also showed subjects with LAA% ≥5% had lower FEV1/FVC. A previous report revealed that trace or greater visual emphysema on a CT scan predicted lower pulmonary function and progressive respiratory disease [12]. On the visual emphysema subtyping, CLE, but not PSE, was associated with a lower FEV1/FVC and FEV1 % pred. This is consistent with previous CT studies showing that physiological impacts are greater in CLE than in PSE [11, 17, 26, 27]. Small airways <2 mm in internal diameter are the major site of airflow obstruction in COPD [28]. Small airway disease is more prominent in CLE than in PSE [29]. Subjects with CLE have fewer terminal bronchioles on micro-CT [30]. Collectively, we think that while the current criteria for pre-COPD and early COPD include the extent of overall emphysema [22, 24, 31], the presence of trace or more CLE should be added to the criteria for better identification of high-risk subjects. This should be further investigated, particularly in longitudinal studies.
In a recent report, FEV1/FVC <0.75 was cited as one of the predictors of future chronic airflow limitation in middle-aged ever-smokers [32]. Our data extend this by showing that the prevalence of CLE was higher in subjects with FEV1/FVC <0.75 than those without.
A smaller ALR was also associated with a lower FEV1/FVC and FEV1 % pred in this study. One would argue that the difference in the ALR might be affected by total lung volume because emphysema may cause lung hyperexpansion due to loss of elastic properties. However, we think this factor should have a minimal effect. The data showed no differences in TLCCT between subjects with or without emphysema. Therefore, the association between a lower ALR and lower lung function could suggest that an intersubject variation in airway tree size rather than lung size may be a determinant of lung function irrespective of the presence of emphysema.
A history of asthma is associated with impaired lung growth and small airway trees, and increases the risk for developing COPD [33, 34]. The association with dysanapsis was also shown in atopic asthma [35]. In this study, six subjects (3.5%) had a history of asthma and their respiratory function tended to be lower (without statistical significance), while the ALR did not differ (supplementary table S1). The results of multivariate regression analysis for the FEV1/FVC and FEV1 % pred values were invariant in sensitivity analyses in subjects without a history of asthma (supplementary tables S9 and S10).
The associations of wall thickness of the segmental and subsegmental airways with FEV1 % pred and FEV1/FVC were absent or minimal in this study. This is inconsistent with a previous report on an association between airway wall thickness and respiratory function in patients with COPD [36]. This discrepancy might be because the population in this study was younger than that in the previous study. Additionally, a report showed that airway wall thickness on CT scan was associated with a decline in FEV1 in smokers [37]. This should be further investigated in younger populations in future studies.
The present findings have clinical relevance. First, CT findings of CLE and a low ALR can be used to identify young smokers with subclinical lung function abnormalities and to provide them with intense smoking cessation programmes. Moreover, although there are no currently approved medications to prevent COPD development in younger subjects, the present findings can be used to help high-risk subjects by testing whether new medications can be effective for preventing COPD development in young subjects. Second, considering that dysanapsis reflects a disproportionate growth pattern in the shape of the airway tree and lung [9], and that dysanapsis on a CT scan is stable in adulthood [10], the observed association between a low ALR and low lung function elucidates the importance of early intervention to facilitate normal airway/lung growth before adulthood to prevent lung function impairment in individuals in their 40s. The causes of abnormal lung growth range from the prenatal period (in utero smoking exposure) to childhood (pre-term birth, bronchopulmonary dysplasia and childhood asthma) [38, 39]. Smoking may also cause lung growth failure and an accelerated decline in FEV1 in subjects with early exposures, such as infant lower respiratory tract infection, manual social class, household overcrowding and pollution exposure [8]. Thus, smoking cessation is important in these subjects.
There are some limitations of this study. First, the target population included subjects who had undergone health check-ups and was therefore more limited than studies on the entire population. Second, spirometry was not performed under the use of bronchodilators because post-bronchodilator spirometry is not routinely conducted at medical check-up programmes. Third, multiple CT scanners with different conditions were used to assess visual emphysema and the ALR. Fourth, since expiratory CT was not performed and data of forced expiratory flow at 25–75% of FVC (FEF25–75%) were not available, the extent of small airway disease could not be estimated in this study. Fifth, while information on early-life disadvantage as a cause of lung growth abnormalities was not obtained, the association with the ALR could not be examined.
In conclusion, this study used chest CT and spirometry in young smokers to show that the presence of CLE and a low ALR are associated with a lower FEV1 % pred and FEV1/FVC. CT findings of CLE and the ALR might be useful to separately evaluate factors associated with smoking-related lung destruction and abnormal airway/lung growth due to congenital factors and childhood medical events, and to define younger subjects who have a higher risk of COPD development.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00695-2023.SUPPLEMENT (226.7KB, pdf)
Acknowledgements
The authors thank Masanori Yasuda and Keiko Mitsuhata at Tsukuba Medical Center Hospital (Tsukuba, Japan) for their assistance in data collection. We also thank the members of the Japan-Respiratory Imaging and Function Research Network (J-RIF-neT) group for their help in data interpretation: Kazuya Tanimura (Department of Respiratory Medicine, Nara Medical University); Akira Oguma (Department of Respiratory Medicine, Faculty of Medicine, Hokkaido University); and Satoru Terada, Yi Zhang and Hiroshi Shima (Department of Respiratory Medicine, Graduate School of Medicine, Kyoto University).
Provenance: Submitted article, peer reviewed.
Ethics statement: This retrospective cross-sectional observational cohort study complied with the guidelines of the Declaration of Helsinki, and was approved by the Ethics Committees of Tsukuba Medical Center Hospital (2022-0029), Takeda Hospital (2019) and Kyoto University (R1660-3). Informed consent was obtained via an opt-out method.
M. Suzuki passed away on 12 January 2024. Dr Suzuki contributed substantially to the manuscript.
Conflict of interest: T. Oguma reports lecture fees from Kyowa Kirin, AstraZeneca, Kyorin Pharmaceutical, GlaxoSmithKline, Sanofi and Boehringer Ingelheim. S. Sato reports grants or contracts from Nippon Boehringer Ingelheim, FujiFilm, Philips Japan, Fukuda Denshi, Fukuda Lifetec Keiji and ResMed. The other authors report no relevant conflicts of interest.
Support statement: This study was supported by the Japan Society for the Promotion of Science (Grants-in-Aid for scientific research 22K08233). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.de Marco R, Accordini S, Marcon A, et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med 2011; 183: 891–897. doi: 10.1164/rccm.201007-1125OC [DOI] [PubMed] [Google Scholar]
- 2.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009; 374: 733–743. doi: 10.1016/S0140-6736(09)61303-9 [DOI] [PubMed] [Google Scholar]
- 3.Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010; 65: 14–20. doi: 10.1136/thx.2008.112136 [DOI] [PubMed] [Google Scholar]
- 4.Soriano JB, Maier WC, Egger P, et al. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax 2000; 55: 789–794. doi: 10.1136/thorax.55.9.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JA, Dunnill MS, Ryder RC. Dependence of the incidence of emphysema on smoking history, age, and sex. Thorax 1972; 27: 547–551. doi: 10.1136/thx.27.5.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auerbach O, Stout AP, Hammond EC, et al. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med 1961; 265: 253–267. doi: 10.1056/NEJM196108102650601 [DOI] [PubMed] [Google Scholar]
- 7.Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med 2015; 373: 111–122. doi: 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 8.Allinson JP, Hardy R, Donaldson GC, et al. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med 2017; 196: 1021–1030. doi: 10.1164/rccm.201703-0506OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green M, Mead J, Turner JM. Variability of maximum expiratory flow-volume curves. J Appl Physiol 1974; 37: 67–74. doi: 10.1152/jappl.1974.37.1.67 [DOI] [PubMed] [Google Scholar]
- 10.Smith BM, Kirby M, Hoffman EA, et al. Association of dysanapsis with chronic obstructive pulmonary disease among older adults. JAMA 2020; 323: 2268–2280. doi: 10.1001/jama.2020.6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BM, Austin JH, Newell JD Jr, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med 2014; 127: 94.e7–94.e23. doi: 10.1016/j.amjmed.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh AS, Strand M, Pratte K, et al. Visual emphysema at chest CT in GOLD stage 0 cigarette smokers predicts disease progression: results from the COPDGene study. Radiology 2020; 296: 641–649. doi: 10.1148/radiol.2020192429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 14.Kubota M, Kobayashi H, Quanjer PH, et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig 2014; 52: 242–250. doi: 10.1016/j.resinv.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 15.Tanabe N, Kaji S, Shima H, et al. Kernel conversion for robust quantitative measurements of archived chest computed tomography using deep learning-based image-to-image translation. Front Artif Intell 2021; 4: 769557. doi: 10.3389/frai.2021.769557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Eur Respir J 1993; 6: Suppl. 16, 5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 17.Lynch DA, Austin JH, Hogg JC, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology 2015; 277: 192–205. doi: 10.1148/radiol.2015141579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiraishi Y, Shimada T, Tanabe N, et al. The prevalence and physiological impacts of centrilobular and paraseptal emphysema on computed tomography in smokers with preserved ratio impaired spirometry. ERJ Open Res 2022; 8: 00063-2022. doi: 10.1183/23120541.00063-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1996; 154: 187–192. doi: 10.1164/ajrccm.154.1.8680679 [DOI] [PubMed] [Google Scholar]
- 20.Maetani T, Tanabe N, Terada S, et al. Physiological impacts of computed tomography airway dysanapsis, fractal dimension, and branch count in asymptomatic never smokers. J Appl Physiol 2023; 134: 20–27. doi: 10.1152/japplphysiol.00385.2022 [DOI] [PubMed] [Google Scholar]
- 21.Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet 2022; 400: 921–972. doi: 10.1016/S0140-6736(22)01273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez FJ, Agusti A, Celli BR, et al. Treatment trials in young patients with chronic obstructive pulmonary disease and pre-chronic obstructive pulmonary disease patients: time to move forward. Am J Respir Crit Care Med 2022; 205: 275–287. doi: 10.1164/rccm.202107-1663SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han MK, Agusti A, Celli BR, et al. From GOLD 0 to pre-COPD. Am J Respir Crit Care Med 2021; 203: 414–423. doi: 10.1164/rccm.202008-3328PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez FJ, Han MK, Allinson JP, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1540–1551. doi: 10.1164/rccm.201710-2028PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han MK, Tayob N, Murray S, et al. Association between emphysema and chronic obstructive pulmonary disease outcomes in the COPDGene and SPIROMICS cohorts: a post hoc analysis of two clinical trials. Am J Respir Crit Care Med 2018; 198: 265–267. doi: 10.1164/rccm.201801-0051LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J, Hobbs BD, Crapo JD, et al. Subtyping COPD by using visual and quantitative CT imaging features. Chest 2020; 157: 47–60. doi: 10.1016/j.chest.2019.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiraishi Y, Tanabe N, Shimizu K, et al. Stronger associations of centrilobular than paraseptal emphysema with longitudinal changes in diffusing capacity and mortality in COPD. Chest 2023; 164: 327–338. doi: 10.1016/j.chest.2023.01.034 [DOI] [PubMed] [Google Scholar]
- 28.Hogg JC, Paré PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev 2017; 97: 529–552. doi: 10.1152/physrev.00025.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim WD, Eidelman DH, Izquierdo JL, et al. Centrilobular and panlobular emphysema in smokers. Two distinct morphologic and functional entities. Am Rev Respir Dis 1991; 144: 1385–1390. doi: 10.1164/ajrccm/144.6.1385 [DOI] [PubMed] [Google Scholar]
- 30.Tanabe N, Vasilescu DM, Hague CJ, et al. Pathological comparisons of paraseptal and centrilobular emphysema in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2020; 202: 803–811. doi: 10.1164/rccm.201912-2327OC [DOI] [PubMed] [Google Scholar]
- 31.Celli B, Fabbri L, Criner G, et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med 2022; 206: 1317–1325. doi: 10.1164/rccm.202204-0671PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Divo MJ, Liu C, Polverino F, et al. From pre-COPD to COPD: a Simple, Low cost and easy to IMplement (SLIM) risk calculator. Eur Respir J 2023; 62: 2300806. doi: 10.1183/13993003.00806-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai A, Tran H, Roberts M, et al. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax 2014; 69: 805–810. doi: 10.1136/thoraxjnl-2013-204815 [DOI] [PubMed] [Google Scholar]
- 34.Diaz AA, Hardin ME, Come CE, et al. Childhood-onset asthma in smokers. Association between CT measures of airway size, lung function, and chronic airflow obstruction. Ann Am Thorac Soc 2014; 11: 1371–1378. doi: 10.1513/AnnalsATS.201403-095OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munakata M, Ohe M, Homma Y, et al. Pulmonary dysanapsis, methacholine airway responsiveness and sensitization to airborne antigen. Respirology 1997; 2: 113–118. doi: 10.1111/j.1440-1843.1997.tb00063.x [DOI] [PubMed] [Google Scholar]
- 36.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000; 162: 1102–1108. doi: 10.1164/ajrccm.162.3.9907120 [DOI] [PubMed] [Google Scholar]
- 37.Mohamed Hoesein FAA, de Jong PA, Lammers JW, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J 2015; 45: 644–651. doi: 10.1183/09031936.00020714 [DOI] [PubMed] [Google Scholar]
- 38.Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax 2013; 68: 760–766. doi: 10.1136/thoraxjnl-2012-203079 [DOI] [PubMed] [Google Scholar]
- 39.Liu GY, Kalhan R. Impaired respiratory health and life course transitions from health to chronic lung disease. Chest 2021; 160: 879–889. doi: 10.1016/j.chest.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00695-2023.SUPPLEMENT (226.7KB, pdf)