Summary
Screening of cancers is an important intervention in reducing the incidence and mortality related to cancers. Bhutan is one small country that is witnessing an increasing incidence and mortality related to cancers. The government implemented a time-bound population-level screening for gastric, cervical and breast cancers from 2020 to 2023 with an overall coverage of 91.2% of the eligible population. Among 370,225 individuals screened for H pylori, 32.4% tested positive; among 53,182 who underwent upper gastrointestinal endoscopy and biopsy, 255 (0.07%) had gastric cancer. Among 10,749 tested for HPV DNA, 9.3% were positive; among 9887 evaluated with colposcopy and biopsy, 154 (0.13%) had cervical cancer. For breast cancer screening, 72,283 underwent clinical breast examination, 845 mammography and cancer was detected in 73 (0.10%) individuals. In this article, we review how Bhutan implemented a population-level cancer screening programme with on boarding of multiple stakeholders and the participation of people across all districts.
Keywords: Breast self-examination, Early detection of cancer, Endoscopy, Malignancy, Mammography, Mass screening, Palliative care, Pap smear
Introduction
Cancer is a leading cause of death globally with 19.3 million new cancer cases and 10.0 million cancer deaths in 2020.1 The overall burden of cancer incidence is increasing globally reflecting a shift in the epidemiological risk factors and increasing availability and accessibility to screening and diagnostic tests. Globally, the highest proportions of incident cancer cases were female breast, lung, colorectal, prostate and stomach cancers while the highest proportions of mortality were due to lung, colorectal, liver, stomach and female breast cancers.1 Cancer epidemiology varies by income-level, region and socio-demographic factors.
Bhutan is a small lower-middle income country in the eastern Himalayas with a population of about 0.7 million. The country is reporting an increasing incidence of cancer—2744 new cancer cases were detected between 2014 and 2018 and an age-adjusted incidence rate of 76.4 per 100,000 population among males and 101.7 among females.2 Among the males, the highest age-adjusted incidence rates were cancers of the stomach (16.6 per 100,000 population), oesophagus (6.6), liver (5.1) and lungs (5.1). Among females, the highest age-adjusted incidence rates were cancers of the cervix uteri (20.5 per 100,000 population), stomach (11.7), breast (6.8), thyroid (6.7) and ovary (6.2).2
Addressing the growing burden of cancers is related to Sustainable Development Goal 3.4 in reducing premature mortality from non-communicable diseases. Cancers are the third-leading cause of mortality in Bhutan after alcohol liver disease and cardiovascular diseases.3 Cancer prevention, diagnosis and management face major context-specific challenges. All levels of healthcare services in Bhutan are provided free of cost. Bhutan has a three-tiered healthcare system with the primary healthcare centres at the grassroots level, district and general hospitals at the secondary level and the referral hospitals at the tertiary level, all administered by the National Medical Services since 2023. In 2022, there were 179 Primary Health Centres, 53 sub-posts and 555 outreach clinics, 49 district and general hospitals and three referral hospitals.3 There are vertical programmes implemented by the Ministry of Health that conduct screening programmes including that for cancers.
Patients with cancers may present at any level of hospital for preliminary evaluation and screening. The definitive clinical diagnosis with histopathology can be established in the three referral hospitals. As of 2023, the country had two oncosurgeons (surgical oncologists), two gynaeoncologists (gynecological oncologists) and one head and neck oncosurgeon. The Oncology Ward at the Jigme Dorji Wangchuck National Referral Hospital was established in November 2011 to provide chemotherapy and follow up care to cancer patients.4 The range of oncology-related services currently available in the country is limited, lacking clinical oncology, haemato-oncology and radiation therapy services.5 The Royal Government of Bhutan refers all cancer patients needing radiation therapy and selective cancer patients for chemotherapy, immunotherapy and targeted therapy to medical centres in India with all costs borne by the government. In response to the growing financial and social implications of the rising cancer incidence in the country, the Ministry of Health established the National Cancer Control Program in 2020 to streamline cancer screening and cancer care. Additionally, a time-bound national cancer screening flagship project was executed by the Ministry of Health from 2020 to 2023. In this article, we summarize Bhutan’s cancer control strategy and its population-level screening programmes for gastric, cervical and breast cancers.
Search strategy.
PubMed, Scopus and Web of Science were searched for articles published in English up to 30 November 2023. The terms “cancer, cancer screening, gastric cancer, cervical cancer, breast cancer, human papilloma virus, Helicobacter pylori, cancer control, palliative care” were used in combination with “Bhutan”. We also searched Google Scholar and websites of the Ministry of Health, National Statistics Bureau, Khesar Gyalpo University of Medical Sciences of Bhutan and the national newspapers for information on cancer and the flagship programme.
Bhutan cancer control strategy
The Ministry of Health adopted the Bhutan Cancer Control Strategy 2019–2025 to enhance collaboration and coordination among the stakeholders and reduce cancer incidence and mortality, improve outcome and quality of life among patients.6 The strategic document identifies key opportunities and challenges in primary prevention, early detection, appropriate treatment, palliative care and end-of-life care related services. The six areas of key interventions include enhancement of leadership, governance and resources for prevention and control; implementation of cost-effective prevention and control activities; implementation of evidence-based screening and early detection programmes; strengthening of people-centred healthcare delivery; establishment of integrated palliative care services and strengthening epidemiological surveillance and research.
The National Cancer Control Programme was set up at the Ministry of Health in 2020 with the commissioning of the technical advisory committee. The program serves as the central agency coordinating the government, civil society, cancer survivors and national and international donors to cater to a cascade of activities contributing to reduction of cancer incidence and mortality.
Health Flagship Programme
As a part of the cancer control strategy, the Ministry of Health implemented the Health Flagship Programme that was a part of the nine flagship programmes of the Royal Government for the 12th Five Year Plan (2018–2023). This programme was a nationwide cancer screening programme implemented over three years from 2020 to 2023 with a budget outlay of Nu 1109.572 million (United States dollar 13.095 million).7 Health financing in Bhutan is usually tied to the annual budget appropriation from the planned Five Year Plan. For the implementation of the Health Flagship Programme, a special budget was allocated from the government flagship budget outlay of Nu 15 billion (US dollar 181 million).8 This programme targeted the screening of three cancers—gastric, cervical and breast—with an overall aim of reducing their incidence and mortality. This was an ambitious population-level screening programme followed by linking the screen positive individuals with appropriate care.
A Project Management Unit was formed at the Ministry of Health to implement the programme under the guidance of the Project Steering Committee chaired by the Minister of Health. The Project Management Unit was supported by the Technical Working Group. The Project Management Unit reported periodically to the Prime Minister’s Office through the flagship secretariat under erstwhile Gross National Happiness Commission. The primary implementation units were the district health sectors that conducted facility-based and community-outreach screening camps. The screening programme was based on the national cancer screening guideline developed by the Technical Working Group.9
Gastric cancer screening programme
Gastric cancer is the most common cancer reported among males with an age-adjusted incidence of 16.6 per 100,000 population.2 In females, it ranks as the second leading site of cancer with an age-adjusted incidence of 11.7 per 100,000 population. Data from 2008 to 2018 show that Paro, Wangdue Phodrang and Punakha districts report the highest numbers of gastric cancers.2,10 The age-adjusted mortality rates of gastric cancer is 8.3 per 100,000 population among males and 5.2 among females.2 It is more prevalent among males older than 60 years among those evaluated at the National Referral Hospital.11 The most common site of cancer was in the antrum (76.5%) and the most common histopathological phenotype was tubular adenocarcinoma (81.6%) followed by signet-ring cell type (12.8%), with a third of the patients detected with an advanced stage at the time of diagnosis (pT4).11
Gastric cancers in Bhutan are closely linked to H. pylori infection12 and screening and eradication of H pylori is recommended in populations at high risk for gastric carcinoma.13 The serological prevalence of H pylori infection among adults with dyspepsia tested using ELISA was 86% with significantly higher prevalence in the central (97%) and eastern (91%) regions.14 The prevalence was 76.6% by rapid urease test among patients who underwent upper gastrointestinal endoscopy (UGIE) at the National Referral Hospital during routine evaluation.15 Among patients with gastric cancer, the overall prevalence was 87% with the highest proportions in the central (96%) and eastern (91%) regions.10 The seroprevalence was 66% among asymptomatic children aged 4–19 years.16 About 80–90% of H pylori strains isolated in Bhutan were the highly virulent East Asian-type cagA and all strains had the most virulent type of vacA (s1 type).12,17
Prior to 2020, the screening of gastric cancer with UGIE was limited mostly to facility-based services at the three tertiary care hospitals and ad hoc mobile endoscopy camps. Testing for H pylori with rapid urease test was available only to those who underwent UGIE. Likewise, Urea Breath Test made available through external support was limited only to the three tertiary care hospitals and was rarely used. To bridge this gap, the gastric cancer flagship programme was implemented with the objective to reduce gastric cancer incidence and mortality through early screening and prevention. The three key strategic actions were: (i) Mass eradication of H pylori infection (target population of age 18–75 years old) (ii) Early gastric cancer screening and treatment (target population of age 40–75 years old) and (iii) Enhanced advocacy and awareness program.
Screening for H pylori infection was done using stool antigen testing with H pylori Quik ChekTM ELISA in Primary Health Centres and hospitals receiving <12 samples/day and with H pylori ChekTM ELISA in screening camps and in hospitals receiving >12 samples/day (Appendix 1).18,19 Those positive on stool antigen testing were treated with triple therapy for two weeks with Clarithromycin, Amoxicillin and Pantoprazole and re-evaluated after three months.9 If tested positive again, quadruple therapy with Tetracycline, Tinidazole, Pantoprazole and Bismuth was provided for two weeks and re-evaluated after another three months. If tested positive, the patient was subjected to UGIE for biopsy and culture for H pylori for culture-guided therapy.
UGIE was provided to high-risk group with history of atrophic gastritis, past H pylori infection, family history of gastric cancer, those with history of dyspepsia and alarm features and those with significant history of smoking, tobacco and alcohol consumption (Appendix 2). A systematic screening of stomach using UGIE was performed and tissue biopsy taken from all suspicious lesions.20 Those with high grade dysplasia with clinical suspicion of malignancy were referred to oncosurgery review at the National Referral Hospital. Repeat UGIE was performed after 6 months in case of high grade dysplasia and after 12 months in those with low grade dysplasia. In case of severe atrophic gastritis or intestinal metaplasia, repeat UGIE were performed in 12 months in those with family history of gastric cancer and were recommended repeat UGIE after 3 years.9
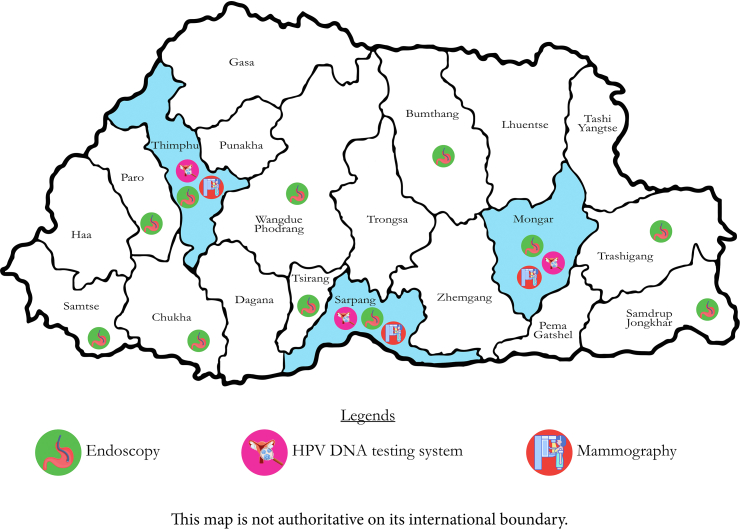
As a part of this flagship programme, H pylori stool antigen testing and triple therapy have been made available in all health facilities. UGIE services were made available in 12 hospitals across 11 districts with availability of equipment and health professionals to handle them (Fig. 1). The programme also helped strengthen histopathology laboratories in the three referral hospitals. Human resources including health assistants, endoscopy nurses, laboratory technicians, endoscopists and pathologists have been provided appropriate training. Endoscopists were also trained in the technique of systematic screening of stomach.20 This has greatly improved equitable access to services to people living away from the capital city.9 The Khesar Gyalpo University of Medical Sciences of Bhutan in collaboration with experts from Japan are piloting a project to produce a rapid diagnosis kit at the Royal Centre for Disease Control and provide an in-house manufacturing capacity. This project also aims to improve capability for genomic analysis and perform rapid antimicrobial susceptibility testing against antimicrobial resistant H pylori. All these efforts are aimed at sustainable delivery of these services through the routine healthcare delivery system after the flagship programme has ended.21
Fig. 1.
Upper gastrointestinal endoscopy is available at 11 districts and HPV DNA testing and mammography available at the three tertiary care hospitals in Bhutan, 202321
During this flagship programme (Table 1), 370,255 individuals were tested (90.2% coverage) for H pylori stool antigen test of which 119,854 tested positive (32.4%) with the highest proportions in Zhemgang (49.8%), Haa (49.7%), Bumthang (46.9%) and Dagana (44.5%) districts. There were 53,182 upper gastrointestinal endoscopies performed (94.2% of the eligible population), of which there were 111 (0.2%) persons with low grade dysplasia, 64 (0.1%) with pre-cancerous lesions and 255 (0.07%) with gastric cancers. Among those with gastric cancers, 116 were males and the majority were detected among those older than 60 years (Table 2).21
Table 1.
Screening for Helicobacter pylori stool antigen and upper gastrointestinal endoscopy for gastric cancer screening during the population-level flagship screening programme in Bhutan, 2020–2023.21
| Districts | Target population (18–75 years) | H pylori test |
H pylori positive |
Line list for UGIE | UGIE donea |
Programme coverage (%) | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Bumthang | 9770 | 9729 | 4566 | 46.9 | 1859 | 838 | 45.1 | 99.6 |
| Chhukha | 39,328 | 35,777 | 11,654 | 32.6 | 3269 | 2159 | 66.0 | 91.0 |
| Dagana | 13,098 | 11,926 | 5312 | 44.5 | 3262 | 2132 | 65.4 | 91.1 |
| Gasa | 2327 | 2287 | 550 | 24.0 | 453 | 405 | 89.4 | 98.3 |
| Haa | 5431 | 5100 | 2536 | 49.7 | 1119 | 992 | 88.7 | 93.9 |
| Lhuentse | 7705 | 7085 | 2589 | 36.5 | 1525 | 1459 | 95.7 | 92.0 |
| Monggar | 20,055 | 19,795 | 5359 | 27.1 | 2200 | 3701 | 168.2 | 98.7 |
| Paro | 22,847 | 19,495 | 4714 | 24.2 | 2670 | 3978 | 149.0 | 85.3 |
| Pema Gatshel | 10,345 | 10,151 | 3284 | 32.4 | 2534 | 1797 | 70.9 | 98.1 |
| Punakha | 10,787 | 9871 | 2890 | 29.3 | 5802 | 5109 | 88.1 | 91.5 |
| Samdrup Jongkhar | 20,233 | 15,436 | 5803 | 37.6 | 3292 | 3219 | 97.8 | 76.3 |
| Samtse | 36,364 | 27,440 | 6006 | 21.9 | 3061 | 2877 | 94.0 | 75.5 |
| Sarpang | 29,740 | 28,056 | 10,053 | 35.8 | 6349 | 6433 | 101.3 | 94.3 |
| Thimphu | 100,103 | 90,625 | 26,470 | 29.2 | 3333 | 2582 | 77.5 | 90.5 |
| Trashigang | 22,516 | 22,478 | 7809 | 34.7 | 5062 | 4255 | 84.1 | 99.8 |
| Trashi Yangtse | 9305 | 9062 | 3063 | 33.8 | 1751 | 1603 | 91.5 | 97.4 |
| Trongsa | 8366 | 7998 | 2483 | 31.0 | 1034 | 1417 | 137.0 | 95.6 |
| Tsirang | 13,587 | 12,468 | 3531 | 28.3 | 3194 | 2382 | 74.6 | 91.8 |
| Wangdue Phodrang | 19,841 | 16,814 | 6883 | 40.9 | 2919 | 3523 | 120.7 | 84.7 |
| Zhemgang | 8798 | 8632 | 4299 | 49.8 | 1789 | 2321 | 129.7 | 98.1 |
| Total | 410,546 | 370,225 | 119,854 | 32.4 | 56,477 | 53,182 | 94.2 | 90.2 |
UGIE = upper gastrointestinal endoscopy.
More number of individuals were screened than line listed.
Table 2.
Cancer cases detected during the population-level flagship screening programme in Bhutan, 2020–2023.21
| Age group (years) | Gastric cancer |
Cervical cancer |
Breast cancer |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 20–29 | 3 | 1.2 | 4 | 2.6 | 3 | 4.1 |
| 30–39 | 8 | 3.1 | 32 | 20.8 | 12 | 16.4 |
| 40–49 | 20 | 7.8 | 45 | 29.2 | 21 | 28.8 |
| 50–59 | 40 | 15.7 | 36 | 23.4 | 20 | 27.4 |
| ≥60 | 184 | 72.2 | 37 | 24.0 | 17 | 23.3 |
Efforts of mass screening and eradication of H pylori is shown to reduce its prevalence.13 In a cohort followed up in Matsu Islands that covered 85.5% of its population with six rounds of screening and eradication, the prevalence of H pylori had decreased from 64.2% in 2004 to 15.0% in 2018 with 53% reduction of gastric cancer incidence.22 The rate of H pylori reinfection was <1% per person-year and had no significant changes in antibiotic resistance rate of H pylori.22 Population-wide gastric cancer screening have been available in Japan since 1983, South Korea since 1999 and in selected high-risk areas in China since 2008.23 In South Korea, this intervention has reduced the mortality of gastric cancer by up to 21% among the screened population.24 UGIE remains the procedure of choice in clinical characterization, tumour localisation and tissue diagnosis of gastric carcinoma.25,26 It is highly sensitive and specific diagnostic test, especially when it is combined with endoscopic biopsy for tissue diagnosis.27,28
Cervical cancer screening programme
Cervical cancer is the most frequent cancer diagnosed among women with an age adjusted incidence rate of 20.5 per 100,000 among and an age-adjusted mortality rate of 5.3 per 100,000.2 The highest numbers of cases are reported from Trashigang, Monggar, Wangdue Phodrang and Chhukha districts.
For the screening of cervical cancer, Pap smear services were available in bigger hospitals since the 1990s with the first set of cervical cancer screening protocol adopted in 1999. Visual inspection under acetic acid and cytology were piloted in three districts in 2002–2004 and conventional Pap smear was adopted as a part of the protocol in 2006 with gradual expansion of the services across all health centres. Conventional Pap smear was performed every 3 years from age 25 years till 65 years of age if negative cytology.
After piloting catch up human papillomavirus (HPV) vaccination in 2009 in Paro district, a nationwide vaccination (quadrivalent) for girls was adopted in 2010 and gender-neutral vaccination since September 2020.29,30 In 2019, the crude HPV vaccination coverage among girls was 90.5%.31 The implementation of HPV vaccine has led to a decrease in the prevalence of HPV vaccine types from 8.3% in 2011/2012 to 1.4% in 2018 among sexually active women aged 17–29 years with an adjusted vaccine effectiveness of 88%.32 Among women younger than 27 years who were targeted by the vaccination programme, the overall vaccine effectiveness was 93%.32
However, in a review of Pap smear coverage in 2019, it was found that the uptake of Pap smear was poor and also had poor sensitivity in detection of (precancerous cervical lesion) carcinoma in situ (CIN) III.33 The overall percentage of unsatisfactory smear was high indicating a need for quality improvement. With the adoption of the national strategy to eliminate cervical cancer in the country by 2030, HPV DNA testing along with options for self-sampling and HPV genotyping was introduced in 2021 through the Health Flagship Programme. At the same time for the triaging purpose, the conventional Pap smear method was replaced with the liquid-based cytology (LBC).
Prior to the flagship programme, a national review noted that there was a poor coordination between screening centres at the districts and treatment centres at the obstetric-gynaecologic centres leading to attrition of patients from the care cascade. The objective for cervical cancer screening programme was to reduce cervical cancer incidence and mortality through early screening and prevention with two key strategic actions: i) early screening and treatment of cervical pre-cancers/cancers (target population of age 30–65 years old); ii) enhanced advocacy and awareness program.
As a part of the flagship programme, HPV DNA testing and LBC were introduced at the three tertiary hospitals and HPV genotyping at the National Referral Hospital as a part of routine services. Gynaecology, colposcopy and treatment services were strengthened and expanded to ten hospitals. Health assistants were trained in HPV DNA/LBC sampling and cytotechnicians were trained on HPV DNA genotyping and LBC assessment (Appendix 3).
In the flagship programme (Table 3), 116,493 women were screened for cervical cancer (90.8% of the eligible population) of which 10,749 (9.2%) were HPV DNA positive. The highest proportions of HPV positive rate were detected in Punakha (14.4%), Wangdue Phodrang (13.7%), Zhemgang (13.6%), Lhuentse (12.5%) and Trashi Yangtse (12.0%). To prevent attrition from care, see and treat policy was adopted where treatment services (loop electrosurgical excision procedure and thermocoagulation) were provided to 9887 individuals. There were 154 (0.13%) cases of cervical cancer detected with the highest proportion among those aged 40–49 years (Table 2).21
Table 3.
Screening for human papillomavirus DNA followed by colposcopic evaluation and treatment (see and treat principle) for cervical cancer screening during the population-level flagship screening programme in Bhutan, 2020–2023.21
| District | Target population (30–65 years) | HPV DNA test | HPV DNA positive |
Colposcopy | LEEP | Thermo-coagulation | Treatment coverage (%)a | Program coverage (%)a | |
|---|---|---|---|---|---|---|---|---|---|
| n | % | ||||||||
| Bumthang | 3064 | 2865 | 274 | 9.6 | 272 | 24 | 6 | 99.3 | 93.5 |
| Chhukha | 11,694 | 10,360 | 733 | 7.1 | 387 | 53 | 193 | 52.8 | 88.6 |
| Dagana | 4334 | 4311 | 408 | 9.5 | 401 | 39 | 0 | 98.3 | 99.5 |
| Gasa | 756 | 742 | 74 | 10.0 | 74 | 11 | 2 | 100.0 | 98.1 |
| Haa | 2070 | 1825 | 157 | 8.6 | 150 | 22 | 23 | 95.5 | 88.2 |
| Lhuentse | 2502 | 2179 | 273 | 12.5 | 234 | 44 | 3 | 85.7 | 87.1 |
| Monggar | 6872 | 6461 | 333 | 5.2 | 490 | 28 | 55 | 147.1 | 94.0 |
| Paro | 4583 | 4512 | 351 | 7.8 | 376 | 67 | 85 | 107.1 | 98.5 |
| Pema Gatshel | 4008 | 4112 | 231 | 5.6 | 277 | 8 | 101 | 119.9 | 102.6 |
| Punakha | 3513 | 3406 | 489 | 14.4 | 445 | 105 | 41 | 91.0 | 97.0 |
| Samdrup Jongkhar | 6467 | 5840 | 60 | 1.0 | 220 | 1 | 48 | 366.7 | 90.3 |
| Samtse | 12,696 | 11,850 | 768 | 6.5 | 654 | 3 | 81 | 85.2 | 93.3 |
| Sarpang | 9361 | 7989 | 907 | 11.4 | 775 | 23 | 259 | 85.4 | 85.3 |
| Thimphu | 27,879 | 22,267 | 2480 | 11.1 | 1991 | 374 | 1 | 80.3 | 79.9 |
| Trashigang | 7750 | 7760 | 889 | 11.5 | 844 | 18 | 1 | 94.9 | 100.1 |
| Trashi Yangtse | 2906 | 2928 | 351 | 12.0 | 339 | 19 | 28 | 96.6 | 100.8 |
| Trongsa | 2756 | 2508 | 207 | 8.3 | 229 | 43 | 7 | 110.6 | 91.0 |
| Tsirang | 4438 | 4004 | 322 | 8.0 | 304 | 25 | 72 | 94.4 | 90.2 |
| Wangdue Phodrang | 7195 | 7325 | 1001 | 13.7 | 1015 | 203 | 105 | 101.4 | 101.8 |
| Zhemgang | 3441 | 3249 | 441 | 13.6 | 410 | 82 | 83 | 93.0 | 94.4 |
| Total | 128,285 | 116,493 | 10,749 | 9.2 | 9887 | 1192 | 1194 | 92.0 | 90.8 |
HPV DNA = human papilloma virus deoxyribonucleic acid; LEEP = loop electrosurgical excision procedure.
More number of individuals availed the services than line listed.
In a cluster-randomized trial in India, cervical cancer screening with HPV testing was shown to have better performance (incidence of stage II or higher cervical cancer 14.5 per 100,000 person-year and hazard ratio of 0.47) compared to cytologic testing (incidence of 23.2 and hazard ratio 0.75) and visual inspection of cervix with acetic acid (incidence of 32.2 and hazard ratio 1.04).34 The World Health Organization suggests using an HPV DNA testing as primary screening test with or without triaging.35 HPV DNA-based screening methods are feasible to be rolled out at population level36, 37, 38, 39 with 17 countries recommending self-sampling as of 2021.40 Towards meeting the cervical cancer elimination targets by 2030, the World Health Organization recommends at least 70% women are screened with a high-performance test at least by 35 years of age.41 However, for many countries including Bhutan, health financing remains a major problem in continuing the momentum on cervical cancer elimination.
Breast cancer screening programme
Breast cancer is the fourth leading cancer among women with an age-adjusted incidence of 6.9 per 100,000 population. There were 73 cases of breast cancer detected between 2014 and 2018. Mammography services for screening for breast cancer was available at the National Referral Hospital since July 2018 funded by the Japanese government. However, there was no dedicated breast cancer screening programme.
The objective of the breast cancer screening programme was to reduce breast cancer incidence and mortality through early screening and prevention with two key strategic actions: i) early screening and treatment of breast cancer; ii) enhanced advocacy and awareness program. This included all women in the target age group and those with high risk factors such as family history of cancer, genetic predisposition, nulliparity, early menarche or late menopause, advanced age at first childbirth, prolonged used of hormonal contraceptives and lifestyle related risk-factors.
Through the flagship programme, mammography services were established at the Regional Referral Hospitals towards the end of 2021 with screening activities taking place from 2022. Health workers were trained on clinical breast examination and x-ray technicians trained on mammography machines. The only interventional radiologist in the country was trained on stereotactic biopsy of breast lesions (Appendix 4).
In this flagship programme (Table 4), 72,283 women (92.7% of the eligible population) were screened through clinical breast examination and comprehensive risk factor assessment. There were 932 women identified with high risk of developing breast cancer among which 845 individuals underwent mammography. There were 73 cases (0.10%) of breast cancer, the highest proportion detected among those aged 40–59 years (Table 2).21
Table 4.
Screening for breast cancer with clinical breast examination and comprehensive risk assessment and mammography for the population-level flagship screening programme in Bhutan, 2020–2023.21
| Districts | Target population (40–65 years) | Women screened | Line list for mammography | Mammography | Treatment coverage (%) | Program coverage (%)a |
|---|---|---|---|---|---|---|
| Bumthang | 1701 | 1602 | 17 | 14 | 82.4 | 94.2 |
| Chhukha | 6449 | 5790 | 62 | 58 | 93.5 | 89.8 |
| Dagana | 2760 | 2723 | 63 | 60 | 95.2 | 98.7 |
| Gasa | 400 | 415 | 40 | 33 | 82.5 | 103.8 |
| Haa | 1043 | 946 | 22 | 19 | 86.4 | 90.7 |
| Lhuentse | 1552 | 1529 | 10 | 9 | 90.0 | 98.5 |
| Monggar | 5647 | 5201 | 70 | 70 | 100.0 | 92.1 |
| Paro | 2725 | 2628 | 43 | 43 | 100.0 | 96.4 |
| Pema Gatshel | 2692 | 2679 | 28 | 25 | 89.3 | 99.5 |
| Punakha | 2464 | 2386 | 65 | 57 | 87.7 | 96.8 |
| Samdrup Jongkhar | 3926 | 3600 | 10 | 8 | 80.0 | 91.7 |
| Samtse | 8018 | 7415 | 81 | 75 | 92.6 | 92.5 |
| Sarpang | 7058 | 6753 | 82 | 73 | 89.0 | 95.7 |
| Thimphu | 13,906 | 11,501 | 89 | 76 | 85.4 | 82.7 |
| Trashigang | 4460 | 4428 | 36 | 33 | 91.7 | 99.3 |
| Trashi Yangtse | 1801 | 1732 | 14 | 12 | 85.7 | 96.2 |
| Trongsa | 1807 | 1980 | 73 | 59 | 80.8 | 109.6 |
| Tsirang | 3040 | 2802 | 23 | 21 | 91.3 | 92.2 |
| Wangdue Phodrang | 4571 | 4286 | 52 | 48 | 92.3 | 93.8 |
| Zhemgang | 1974 | 1887 | 52 | 52 | 100.0 | 95.6 |
| Total | 77,994 | 72,283 | 932 | 845 | 90.7 | 92.7 |
CBE = clinical breast examination.
More number of individuals were screened than line listed.
Screening for breast cancers with mammography is shown to reduce the risk of mortality by 20%42 but have sometimes been questioned about over diagnosis of breast cancer.43 However, for a population-level screening, mammography remains the most preferred recommendation with screening recommended at a younger age in those with high risk.44
Cancer treatment services
The Health Flagship Programme ended in 2023 with an overall programmatic coverage of 91.2% of the eligible population across all districts. This programme helped establish services in newer centres allowing for easier accessibility. All the screening services are integrated with the routine healthcare service delivery. To enhance the treatment capabilities in the country, the Oncology Ward at the National Referral Hospital is being strengthened with training of appropriate health care professionals to be able to provide medical, surgical and radiation therapies. Given that there are no oncologists in the country, gastric and breast cancer treatment including risk stratification, staging and chemotherapeutic treatments are led by surgeons. For cervical cancers, all forms of treatment are decided by gynaeoncologic surgeons. The follow up of patients on chemotherapy regimens are coordinated by the Oncology Ward. In patients requiring oncology review and radiation therapy, patients are referred to oncology centres outside Bhutan with all costs borne by the Royal Government of Bhutan.
To be able to provide better quality cancer care, there is a need to enhance diagnostic capabilities, additional services for histopathology and nuclear imaging. In the coming years, a multi-disciplinary hospital with all capabilities may be established. With this, it is hoped that the referral of patients outside the country for cancer care may be reduced. The cancer control strategy envisions the expansion of oncology services to Regional Referral Hospitals, establish district oncology hubs and involve the district and the primary care hospitals to provide screening and palliative care services and community education and awareness.
Improvement in cancer treatment services are key to improving cancer survival and enhancement of quality of life. In the current settings, there is very limited information on cancer survival rates. Among patients with cervical cancers, the one-year survival rate was 82.1% and the five-year survival rate was 55.4%.45
Palliative care services
In Bhutan, palliative care services have incorporated therapies provided under the Bhutanese Traditional Medicine. The service package includes relaxation massage, trigger release, cupping, moxabustion, hot and cold compression, acupuncture and meditation practices. These are aimed at treating chronic pain and provide psychological support. The oncology unit at the National Referral Hospital offers home-based palliative care to patients located in Thimphu.46,47 As many of the cancers are diagnosed only at terminal stages, there is a need to expand the programme beyond the capital city to meet the needs of both the patients and their care givers.47
Cancer surveillance and research
With improved capability for radioimaging and oncopathology services, it has become easier to establish the diagnosis of cancers. Cancer reporting is incorporated into the District Health Information System (DHIS) 2. This is an online reporting platform capturing data from screening stage till treatment with the database controlled by the Ministry of Health. The Digital Drukyul project has rolled out the Electronic Patient Information System, an electronic health record with ICD-11 coding, in 2023. With improved data capture system, it is hoped that the next version of the population-based cancer registry will have robust data that includes the measure of incidence and mortality among the screened population. It is recommended to follow up the cancer positive patients for the treatment outcomes and survival analysis.
Challenges in cancer screening and care
Cancer care remains a major challenge in the country. As of 2023, there are no oncologists, radiation specialists, medical physicist or oncology trained nurses in the country. Radiation therapy was briefly available in the country but got discontinued in August 2021.48,49 Currently, for the majority of cancers, the patients are referred to India for treatment and follow up. This reflects a need to establish a functional oncology centre with the establishment of infrastructures and training of appropriate specialist human resources through collaboration with donor partners. For those that require palliative care, there is a need to strengthen and expand the services to other districts and incorporate culturally appropriate interventions.
A multidisciplinary team with tumour board forms an essential component of cancer treatment. We do not have adequate number of trained human resources to start a tumour board. All patients requiring tumour board decision are referred to India, with increased cost to the government.
The cancer strategy has received high-level political support for cancer advocacy and mobilization of social support for cancer patients and their families. A civil society organization, Bhutan Cancer Society, has been reaching out to underprivileged cancer patients and care givers and help them cope with the diagnosis and treatment processes. While the district health sectors implement the main community-reach programmes on raising awareness about cancer prevention and early diagnosis, Bhutan Cancer Society also supplements these efforts. There needs to be continued efforts towards improving health literacy among the population and connect the eligible population to the nearest health centre.
As a part of routine health services, cancer screening may not receive adequate and rigorous monitoring of its coverage and performance as it were done in during the flagship programme. As the National Medical Services leads the clinical health service delivery, it is expected that the oncology services will be strengthened in the country so that early cancer detection, cancer and palliative care become more accessible. The National Cancer Control Programme must identify innovative measures in retaining the patient in the screening cascade so that adequate follow up services are provided. For cervical cancer elimination, Bhutan has achieved 90-70-90 WHO targets as of 202341 but sustaining the achievement requires streamlined efforts.50
Conclusion
Bhutan implemented a nationwide population-level cancer screening programme for gastric, cervical and breast cancers. The programme had a very high coverage and brought cancer patients into medical care. The programme also helped establish infrastructure such as upper gastrointestinal endoscopy, colposcopy and mammography in selected centres and trained a pool of human resources. This has led to improved access to cancer screening. However, the country still faces major challenges lacking dedicated oncology services at the tertiary level hospitals while palliative care services remain available only at the capital city. With the adoption of the cancer strategy and high-level advocacy on cancer screening, it is expected that oncology services will improve in the coming years.
Contributors
Conceptualization: Thinley Dorji, SW, P, ND, YD, BP, KJ.
Methodology, Resources, Data curation: Thinley Dorji, P, KJ.
Software, Validation, Formal analysis: Thinley Dorji.
Investigation, Writing original draft, Visualization and Supervision: Thinley Dorji.
Writing—Review and editing: Thinley Dorji, SW, SD, ND, YD, BP, DP, CD, JC, Tandin Dorji, MLM, P, KJ.
Project administration: Not applicable.
Funding acquisition: Not applicable.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
We thank the Ministry of Health, Royal Government of Bhutan for their support in writing this article.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2024.100370.
Contributor Information
Thinley Dorji, Email: dorji.thinleydr@gmail.com.
Sangay Wangmo, Email: wangmo.sangaydr@gmail.com.
Sonam Dargay, Email: sdargay@jdwnrh.gov.bt.
Namkha Dorji, Email: namji2002@gmail.com.
Yeshey Dorjey, Email: yesheydorjey@gmail.com.
Birendra Pradhan, Email: birenpradhan@gmail.com.
Dechen Pema, Email: dpema1278@gmail.com.
Choney Dema, Email: choneyd@nms.gov.bt.
Jamyang Choden, Email: jchoden@health.gov.bt.
Tandin Dorji, Email: tandorji@gmail.com.
Mimi Lhamu Mynak, Email: mlmynak@nms.gov.bt.
Pempa, Email: pemba@health.gov.bt.
Kinga Jamphel, Email: kjamphel@gmail.com.
Appendix A. Supplementary data
Appendix 1.
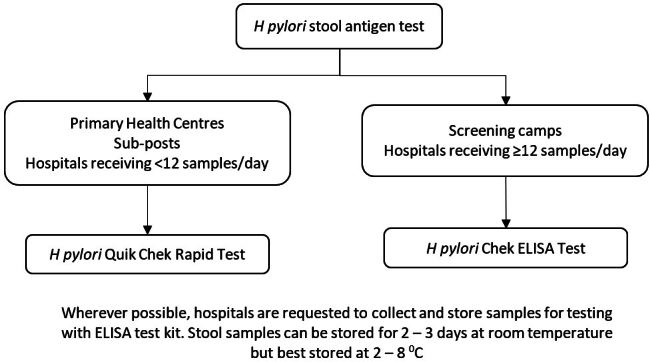
Testing algorithm for H pylori using stool antigen for the gastric cancer screening programme in Bhutan, 2020.18
Appendix 2.
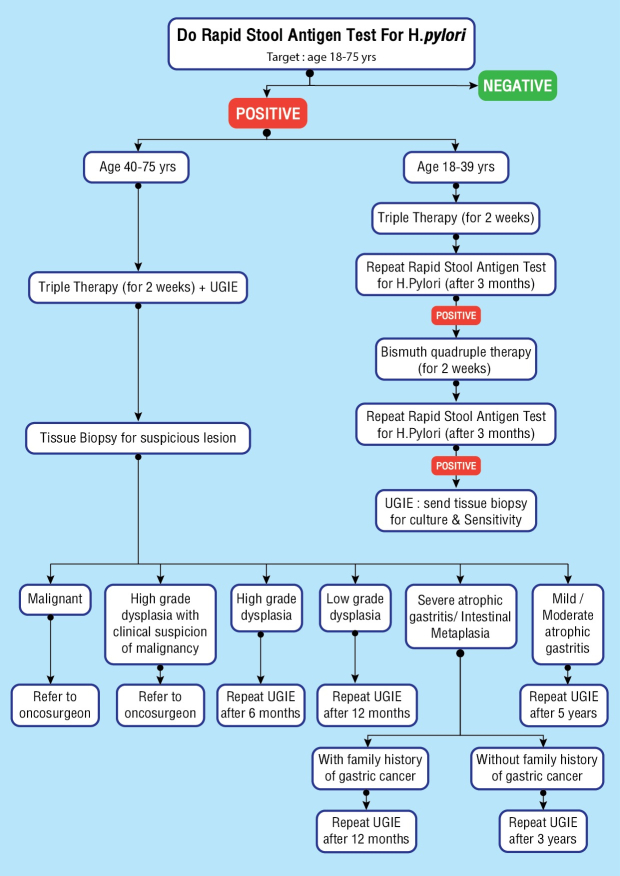
Gastric cancer screening protocol for Bhutan, 2020.9 Those individuals positive for stool antigen for H pylori are treated and offered upper gastrointestinal endoscopy (UGIE). Follow up after endoscopy depends on the findings and histopathology report.
Appendix 3.
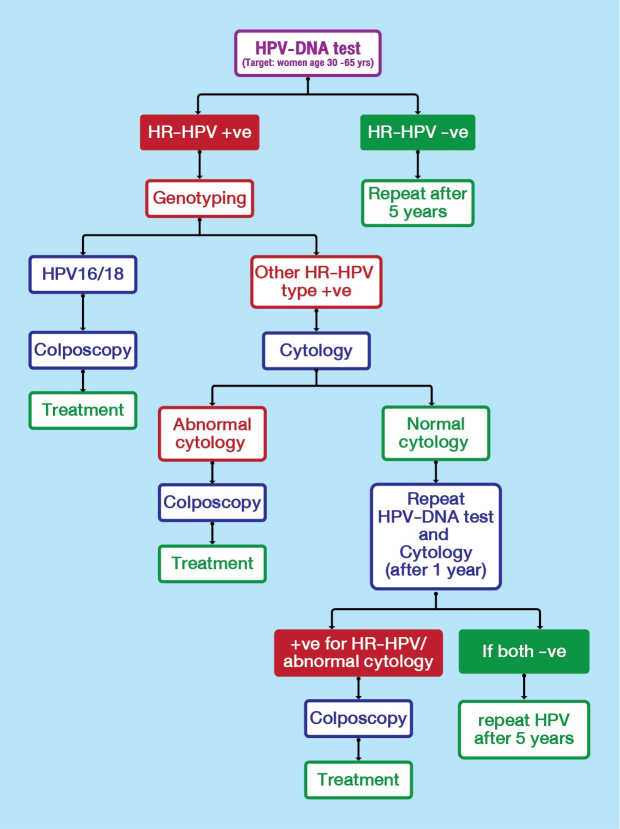
Cervical cancer screening protocol for Bhutan based on HPV DNA testing, 2020.9 Follow up of women are based on detection of high risk human papillomavirus (HPV) types.
Appendix 4.
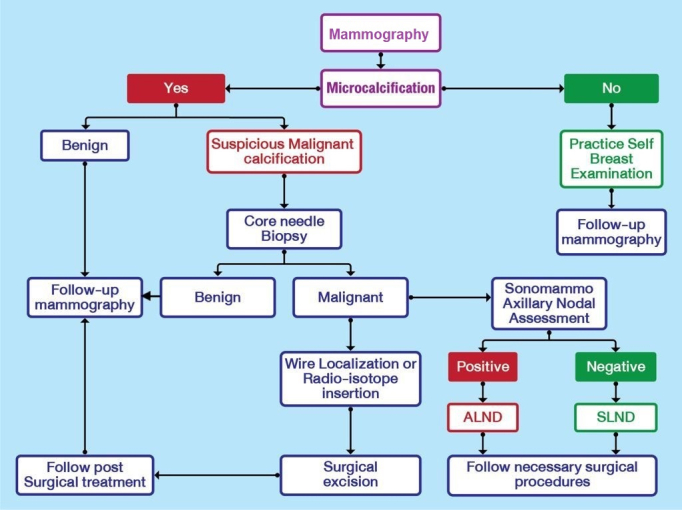
Breast cancer screening protocol for Bhutan, 2020.9 Follow up of women with suspicious or malignant calcification depend on the histopathology findings on core needle biopsy of the lesion. ALND = axillary lymph node dissection; SLND = sentinel node lymph node dissection.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2018. Cancer incidence and mortality in Bhutan: 2014 – 2018. [Google Scholar]
- 3.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2022. Annual Health Bulletin 2022. [Google Scholar]
- 4.Wangdi T.D. Cancer care in Bhutan. Bhutan Heal J. 2018;4:1–2. [Google Scholar]
- 5.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2019. Health Service Standard 2019. [Google Scholar]
- 6.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2019. Bhutan Cancer Control Strategy 2019–2025. [Google Scholar]
- 7.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2020. Health Flagship Blueprint 2020 - 2023. [Google Scholar]
- 8.Royal Government of Bhutan . Royal Government of Bhutan; Thimphu: 2013. Twelfth Five Year Plan (2017 - 2023) [Google Scholar]
- 9.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2020. Guideline for screening gastric cancer, cervical cancer and breast cancer. [Google Scholar]
- 10.Dendup T., Richter J.M., Yamaoka Y., Wangchuk K., Malaty H.M. Geographical distribution of the incidence of gastric cancer in Bhutan. World J Gastroenterol. 2015;21:10883–10889. doi: 10.3748/wjg.v21.i38.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choden S., Wangmo C. The histopathological characteristics of gastric carcinoma in Bhutanese population: a Retrospective study. Bhutan Health J. 2022;8:27–33. [Google Scholar]
- 12.Shiota S., Mahachai V., Vilaichone R.-K., et al. Seroprevalence of Helicobacter pylori infection and gastric mucosal atrophy in Bhutan, a country with a high prevalence of gastric cancer. J Med Microbiol. 2013;62(Pt 10):1571–1578. doi: 10.1099/jmm.0.060905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liou J.-M., Malfertheiner P., Lee Y.-C., et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093–2112. doi: 10.1136/gutjnl-2020-322368. [DOI] [PubMed] [Google Scholar]
- 14.Dorji D., Dendup T., Malaty H.M., Wangchuk K., Yangzom D., Richter J.M. Epidemiology of Helicobacter pylori in Bhutan: the role of environment and Geographic location. Helicobacter. 2014;19:69–73. doi: 10.1111/hel.12088. [DOI] [PubMed] [Google Scholar]
- 15.Adhikari C.L., Dhakal G.P., Suwisith N., Dargay S., Sharma K.P. Characteristics of Helicobacter pylori infection in a national referral hospital in Bhutan. Bhutan Heal J. 2021;6:6–10. [Google Scholar]
- 16.Wangda S., Richter J.M., Kuenzang P., et al. Epidemiology of Helicobacter pylori infection in asymptomatic schoolchildren in Bhutan. Helicobacter. 2017;22 doi: 10.1111/hel.12439. [DOI] [PubMed] [Google Scholar]
- 17.Matsunari O., Miftahussurur M., Shiota S., et al. Rare Helicobacter pylori virulence genotypes in Bhutan. Sci Rep. 2016;6 doi: 10.1038/srep22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2021. Standard Operating Procedure for H pylori antigen testing in stool. [Google Scholar]
- 19.Chophel T., Tshering S., Dorji N., Tshomo U. Stomach cancer screening services of Bhutan. Indian J Surg. 2022;85:489–494. doi: 10.1007/s12262-022-03519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013;26:11–22. [PMC free article] [PubMed] [Google Scholar]
- 21.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2023. The Health Flagship Report 2020 - 2023. [Google Scholar]
- 22.Chiang T.-H., Chang W.-J., Chen S.L.-S., et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut. 2021;70:243–250. doi: 10.1136/gutjnl-2020-322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Januszewicz W., Turkot M.H., Malfertheiner P., Regula J. A global perspective on gastric cancer screening: which concepts are feasible, and when? Cancers. 2023;15:664. doi: 10.3390/cancers15030664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jun J.K., Choi K.S., Lee H.-Y., et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328.e7. doi: 10.1053/j.gastro.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Xia J.Y., Aadam A.A. Advances in screening and detection of gastric cancer. J Surg Oncol. 2022;125:1104–1109. doi: 10.1002/jso.26844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yashima K., Shabana M., Kurumi H., Kawaguchi K., Isomoto H. Gastric cancer screening in Japan: a narrative review. J Clin Med. 2022;11:4337. doi: 10.3390/jcm11154337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamashima C., Okamoto M., Shabana M., Osaki Y., Kishimoto T. Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer. 2013;133:653–659. doi: 10.1002/ijc.28065. [DOI] [PubMed] [Google Scholar]
- 28.Hamashima C. Update version of the Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2018;48:673–683. doi: 10.1093/jjco/hyy077. [DOI] [PubMed] [Google Scholar]
- 29.Dorji T., Tshomo U., Gyamtsho S., Tamang S.T., Wangmo S., Pongpirul K. Gender-neutral HPV elimination, cervical cancer screening, and treatment: experience from Bhutan. Int J Gynaecol Obstet. 2022;156:425–429. doi: 10.1002/ijgo.13728. [DOI] [PubMed] [Google Scholar]
- 30.Dorji T., Tshomo U., Phuntsho S., et al. Introduction of a National HPV vaccination program into Bhutan. Vaccine. 2015;33:3726–3730. doi: 10.1016/j.vaccine.2015.05.078. [DOI] [PubMed] [Google Scholar]
- 31.Ministry of Health . Ministry of Health, Royal Government of Bhutan; Thimphu: 2021. Annual health bulletin 2021. [Google Scholar]
- 32.Baussano I., Tshomo U., Tenet V., et al. Prevalence of human papillomavirus and estimation of human papillomavirus vaccine effectiveness in Thimphu, Bhutan, in 2011-2012 and 2018 : a cross-sectional study. Ann Intern Med. 2020;173:888–894. doi: 10.7326/M20-2849. [DOI] [PubMed] [Google Scholar]
- 33.Tshomo U., Franceschi S., Tshokey T., et al. Evaluation of cytology versus human papillomavirus-based cervical cancer screening algorithms in Bhutan. Oncotarget. 2017;8:72438–72446. doi: 10.18632/oncotarget.19783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sankaranarayanan R., Nene B.M., Shastri S.S., et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization . 2nd ed. World Health Organization; 2021. WHO guideline for screening and treatment of cervical pre-cancerous lesions for cervical cancer prevention. [PubMed] [Google Scholar]
- 36.Teixeira J.C., Vale D.B., Campos C.S., Bragança J.F., Discacciati M.G., Zeferino L.C. Organization of cervical cancer screening with DNA–HPV testing impact on early–stage cancer detection: a population–based demonstration study in a Brazilian city. Lancet Reg Health Am. 2022;5:1–9. doi: 10.1016/j.lana.2021.100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perera K.C.M., Mapitigama N., Abeysena H. The feasibility of new HPV/DNA test as a primary cervical cancer screening method among 35- years- old ever-married women in Kalutara district; a cross-sectional study. BMC Publ Health. 2021;21:131. doi: 10.1186/s12889-021-10190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baussano I., Tshering S., Choden T., et al. Cervical cancer screening in rural Bhutan with the careHPV test on self-collected samples: an ongoing cross-sectional, population-based study (REACH-Bhutan) BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maver P.J., Poljak M. Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect. 2020;26:579–583. doi: 10.1016/j.cmi.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Serrano B., Ibáñez R., Robles C., Peremiquel-Trillas P., de Sanjosé S., Bruni L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev Med. 2022;154 doi: 10.1016/j.ypmed.2021.106900. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization . World Health Organization; Geneva: 2020. Global strategy to accelerate the elimination of cervical cancer as a public health problem and its associated goals and targets for the period 2020 – 2030. [Google Scholar]
- 42.Gøtzsche P.C., Jørgensen K.J. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;2013 doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clift A.K. Population-level breast screening: how to salvage the concept? J R Soc Med. 2020;113:306–309. doi: 10.1177/0141076820910317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren W., Chen M., Qiao Y., Zhao F. Global guidelines for breast cancer screening: a systematic review. Breast. 2022;64:85–99. doi: 10.1016/j.breast.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tshewang U., Satiracoo P., Lenbury Y. Survival analysis of cervical cancer patients: a case study of Bhutan. Asian Pac J Cancer Prev. 2021;22:2987–2993. doi: 10.31557/APJCP.2021.22.9.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bay J., Yangden Y., Sherpa N., Luitel A. Palliative care: the emerging field in Bhutan. Clin J Oncol Nurs. 2019;23:108–111. doi: 10.1188/19.CJON.108-111. [DOI] [PubMed] [Google Scholar]
- 47.Laabar T.D., Saunders C., Auret K., Johnson C.E. Palliative care needs among patients with advanced illnesses in Bhutan. BMC Palliat Care. 2021;20:8. doi: 10.1186/s12904-020-00697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tshedup Y. 2021. Tshedup 2021 Radiation therapy fail JD.pdf. Kuen.https://kuenselonline.com/radiation-treatment-at-jdwnrh-not-benefited-patients/ [Google Scholar]
- 49.Dolkar D. Kuensel; 2022. Millions lost in JDWNRH’s failed radiation therapy.https://kuenselonline.com/millions-lost-in-jdwnrhs-failed-radiation-therapy/ [Google Scholar]
- 50.Lee Y.-C., Chiang T.-H., Liou J.-M., Chen H.-H., Wu M.-S., Graham D.Y. Mass eradication of Helicobacter pylorito prevent gastric cancer: theoretical and practical considerations. Gut Liver. 2016;10:12–26. doi: 10.5009/gnl15091. [DOI] [PMC free article] [PubMed] [Google Scholar]