Risankizumab is a humanized monoclonal antibody that binds to the IL-23 p19 subunit, inhibiting IL-23–mediated signaling and the related inflammatory cytokine cascade.1 The INSPIRE study, a phase 3, double-blind, placebo-controlled trial, was designed to evaluate the efficacy and safety of risankizumab as induction therapy in patients with moderately to severely active UC. In a presentation by Loftus and colleagues, the primary efficacy and safety results at week 12 from the INSPIRE study were reported.2
Patients enrolled in INSPIRE had an intolerance or inadequate response to conventional and/or advanced therapies for UC. No prior exposure to ustekinumab or IL-23 inhibitors was permitted. A total of 975 patients comprised the intention-to-treat population; patients were randomized in a 2:1 fashion to either placebo or 1200 mg risankizumab IV administered at weeks 0, 4, 8, and 12. Following induction therapy, responding patients were eligible for enrollment in the COMMAND maintenance study, whereas nonresponding patients were eligible for an additional 12 weeks of induction treatment.
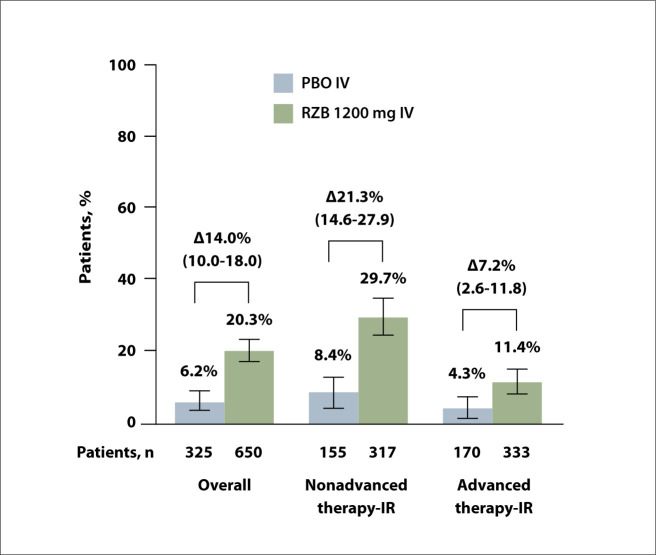
The primary endpoint of clinical remission at week 12 was significantly higher with risankizumab vs placebo (20.3% vs 6.2%; 95% CI, 10.0-18.0; P<.00001) (Figure 5). A benefit with risankizumab compared with placebo was observed in patients with an inadequate response to prior nonadvanced therapy (29.7% vs 8.4%) as well as in patients with an inadequate response to prior advanced therapy (11.4% vs 4.3%). The secondary endpoints of clinical response were also significantly improved with risankizumab vs placebo both at week 4 (52.2% vs 30.5%; 95% CI, 15.6-28.1; P<.00001) and week 12 (64.3% vs 35.7%; 95% CI, 22.3-34.8; P<.00001).
Figure 5.
Clinical remissiona at week 12 of RZB induction therapy in patients with moderately to severely active ulcerative colitis from the phase 3 INSPIRE study.
aClinical remission per Adapted Mayo score: SFS ≤1 and not greater than baseline, RBS of 0, and endoscopic subscores ≤1 without friability. Results reported as adjusted treatment difference RZB vs PBO, % (95 CI) and are based on nonresponder imputation incorporating multiple imputation (NRI-MI) to handle missing data owing to COVID-19 or owing to geopolitical conflict in Ukraine or surrounding areas.
IR, inadequate response; IV, intravenous; PBO, placebo; RBS, rectal bleeding subscore; RZB, risankizumab; SFS, stool frequency subscore.
Adapted from Loftus et al. Abstract 35. Presented at: ACG 2023; October 20-25, 2023; Vancouver, Canada.2
Several measures of endoscopic and histologic improvements at week 12 were also significantly improved with risankizumab compared with placebo. These included endoscopic improvement (36.5% vs 12.1%; 95% CI, 19.3-29.4; P<.00001), endoscopic remission (10.6% vs 3.4%; 95% CI, 4.2-10.2; P<.00001), HEMI (24.5% vs 7.7%; 95% CI, 12.3-21.0; P<.00001), and histologic-endoscopic mucosal remission (HEMR; 6.3% vs 0.6%; 95% CI, 3.5-7.7; P<.00001). Risankizumab was associated with improvements in a number of patient-reported outcomes, such as no bowel urgency (44.1% vs 27.7%; 95% CI, 10.3-22.4; P<.00001), no nocturnal bowel movements (67.3% vs 43.1%; 95% CI, 17.9-30.5; P<.00001), and no abdominal pain (35.8% vs 26.5%; 95% CI, 3.4-15.3; P<.00001).
Reported safety results were consistent with the already known toxicity profile of risankizumab across other indications. The 3 most common adverse events (reported by ≥5% in either treatment arm) were COVID-19 infection (4.8% with risankizumab vs 5.9% with placebo), anemia (3.4% vs 6.5%), and worsening of UC (1.7% vs 10.2%). There were no adjudicated cases of major cardiovascular event, active tuberculosis, opportunistic infections (excluding tuberculosis and herpes zoster), nonmelanoma skin cancer, serious hypersensitivity reactions, or anaphylactic reactions reported in either treatment group. Overall, 9.4% of patients in the risankizumab arm and 8.0% of patients in the placebo arm experienced an adverse event that was potentially drug related. More patients in the placebo arm experienced a severe or serious adverse event (10.2% for both) vs the risankizumab arm (2.5% and 2.3%, respectively). Discontinuations owing to an adverse event occurred in 3.7% of the placebo arm and 0.6% of the risankizumab arm.
References
- Verstockt B, Salas A, Sands BE et al. Alimentiv Translational Research Consortium (ATRC). IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2023;20(7):433–446. doi: 10.1038/s41575-023-00768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus E, Panaccione R, Parkes G Risankizumab induction therapy in patients with moderately to severely active ulcerative colitis: efficacy and safety in the randomized phase 3 INSPIRE study. Abstract 35. 2023. Presented at: ACG 2023 Annual Scientific Meeting; October 20-25, Vancouver, Canada. [PMC free article] [PubMed]