Abstract
Objective:
SARS-CoV-2 infection increases the risk of diabetes and diabetic ketoacidosis (DKA) in both adults and children. We investigated the clinical course of new-onset type 2 diabetes in obese youth presenting with DKA during the SARS-CoV-2 pandemic.
Methods:
This single-center retrospective cohort study included 148 obese subjects, aged 10–21 years admitted with DKA from January 2018 to January 2022. Groups were defined by presence of DKA precipitant: Any infection (n=38, 26%), which included the COVID (n=10, 7%) and other infection (n=28, 19%) groups, and no infection (n=110, 74%). Primary outcome was insulin discontinuation within 12-month follow-up.
Results:
Mean age was 14.9 years (IQR 13.8, 16.5), age-adjusted BMI% was 99.1 (IQR 98.0, 99.5) with 85.8% identifying as Black or Hispanic. There were no differences in DKA severity among groups. Incidence of DKA was higher during the pandemic (March 2020 to January 2022, n=117) compared to pre-pandemic (January 2018 to February 2020, n=31). Within the first year after the acute DKA episode, 46 discontinued all insulin within 9 months (IQR 4,14). Sixteen subjects restarted insulin 10.0 months (IQR 6.5, 11.0) after insulin discontinuation. Infection with SARS-CoV-2 at diagnosis was not associated with likelihood (p=0.57) or timing (p=0.27) of discontinuing all insulin within 1 year, nor was having any infection.
Conclusions:
The incidence of DKA at onset of type 2 diabetes was higher during the SARS-CoV-2 pandemic than pre-pandemic. SARS-CoV-2 infection was not associated with DKA severity or insulin discontinuation within the first year of diagnosis in youth with new onset type 2 diabetes and DKA.
Keywords: Type 2 Diabetes, Pediatric Diabetes, Remission, SARS-CoV-2, Obesity
Diabetic ketoacidosis (DKA), which is caused by acute deficiency of insulin, has increased in frequency in youth with diabetes mellitus in the United States by 40% from 2006 to 2016(1). Prevalence of DKA at diagnosis had risen to 11% in 2012–2014 in youth with type 2 diabetes compared to 5.5% in years 2006–2012(2, 3). The incidence of type 2 diabetes in children has been climbing before and during the COVID-19 pandemic(4).
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has exacerbated the incidence of type 2 diabetes in youth(5, 6). The incidence of diabetes after infection with SARS-CoV-2 has also increased in both the pediatric and adult populations, with one meta-analysis reporting that SARS-CoV-2 infection is associated with a 66% higher risk of incident diabetes(7, 8, 9). Additionally, presentation with DKA has increased in the COVID-19 pandemic in people with new onset and established diabetes(5, 10, 11). Characteristics and outcomes in children and adults infected with SARS-CoV-2 and DKA vary with some patients requiring short-term insulin use and others with long-term insulin dependence(12, 13). Several reports propose that SARS-CoV-2 may directly affect pancreatic beta (β)-cells(14, 15).
Youth with type 2 diabetes have a worse clinical course and faster decline in β-cell function when compared to adults with type 2 diabetes, about 20–35% decline per year(16, 17, 18). Low et al. reported that half of obese children presenting in DKA are able to achieve insulin remission after several months of follow-up but most relapsed back into hyperglycemia and insulin dependence by 15 months(19). However, there is limited knowledge on the disease course of type 2 diabetes in youth presenting with DKA at diagnosis and whether accelerated deterioration of β-cell function is worsened by SARS-CoV-2 infection. We hypothesized that SARS-CoV-2 infection is associated with higher severity of DKA, increased insulin needs during follow-up and decreased likelihood of insulin discontinuation. The primary objective of this study was to examine the effects of SARS-CoV-2 infection on insulin discontinuation in the pediatric population with type 2 diabetes who present with DKA at new-onset of diabetes.
Research Design and Methods:
Subjects:
This retrospective cohort-study was conducted at an academic pediatric hospital in the Southeast USA. All activities were reviewed and approved by the institutional review board and informed consent was exempted. Inclusion criteria included: ages 10–21 years, overweight (BMI ≥ 85% for age) or obese (BMI ≥ 95% for age) from 10–18 years or BMI ≥ 25 kg/m2 and 30 kg/m2 for age ≥ 18 years, respectively, presentation with DKA at new-onset of diabetes (identified by ICD-10 codes E10.10 and E11.10), and admission between January 2018 to January 2022. BMI percentile for age and sex were calculated by the electronic medical record using growth chart data from the CDC(20). The International Society for Pediatric and Adolescent Diabetes (ISPAD) 2022 Consensus Guidelines define DKA as BG ≥ 200 mg/dl, pH ≤ 7.3 or HCO3 ≤ 18 mEq/L(21).
Exclusion criteria were as follows: presence of antibodies (GAD-65 autoantibody, islet antigen-2 autoantibody or insulin autoantibody), other diabetes type (steroid induced diabetes, diabetes following pancreatic damage), and hemoglobinopathies. GAD-65 autoantibody was detected by enzyme-linked immunosorbent assay, and insulin autoantibody was detected using radioimmunoassay in our laboratories. Patients with combined DKA and hyperosmolar hyperglycemic syndrome were not excluded. Patients with respiratory infection symptoms were screened with a respiratory viral panel using polymerase chain reaction. Those with a positive SARS-CoV-2 test at diagnosis were labeled as COVID-DM. Other infection were patients with non-SARS-CoV-2 viral infections, skin infections and/or candidiasis. The no infection group was asymptomatic or tested negative for infection. Insulin discontinuation was defined as clinical staff stopping all all basal and prandial insulin for a participant at a clinic visit.
In cases with new-onset DKA, the diagnosis of type 2 diabetes is made clinically after discharge from the hospital in most cases and can change over time. Diagnosis codes during hospitalization can be inconsistent, and patients initially classified as type 1 diabetes, type 2 diabetes or other during the initial hospital admission need to be re-classified at later visits (22). Therefore, we combined our initial data set with a manually reviewed list of all new onset type 1 and type 2 diabetes diagnosed in our clinic between January 2019 to December 2021 and extracted participant data, admission data for DKA and antibody status.
Data Collection:
The following data were extracted from the inpatient medical record: demographic characteristics including age, sex, self-reported race/ethnicity, BMI, BMI percentile for age and insurance (public versus private) at diagnosis; chemical and metabolic profiles to assess severity of DKA including initial serum glucose, blood pH, serum bicarbonate concentration, glycosylated hemoglobin (HbA1c); complications of DKA including elevated creatinine, clinical or radiologic evidence of cerebral edema; type of hospitalization including admission to the intensive care unit or floor, length of hospital stay, and basal insulin dosing on discharge. Severity of DKA was based on ISPAD consensus statement(21). Mild, moderate, and severe DKA were defined as pH and bicarbonate levels of PH < 7.3 or serum bicarbonate < 18 mmol/L, pH < 7.2 or serum bicarbonate < 10 mmol/L and pH < 7.1 or serum bicarbonate < 5 mmol/L, respectively. After resolution of DKA, all patients were treated with subcutaneous insulin and hospitalized for two additional days for intensive diabetes education.
Outpatient medical records of these patients who were seen at our pediatric endocrinology clinic for follow-up care were also reviewed to identify the following: change in BMI, ability to discontinue one insulin, ability to stop both basal and prandial insulins, time to stopping one or both insulins, rate of restarting insulin after insulin discontinuation and time from insulin discontinuation to restarting insulin. Patients were followed from diagnosis to May 2023.
Statistical analysis:
The primary outcome was to examine whether SARS-CoV-2 infection affected the rate of insulin discontinuation within one year follow-up and insulin resumption. We compared differences in time to stopping insulin, predictors for stopping insulin (including infection, diagnosis time, BMI), and rate of restarting insulin. Secondary outcomes were differences in clinical course between any infection precipitating DKA compared to no precipitation of DKA, as well rates of DKA prior to and during the pandemic. Pre-pandemic and pandemic groups were divided based on diagnosis of DKA and type 2 diabetes between January 2018 to February 2020 or March 2020 to January 2022, respectively. An additional secondary aim was to combine those in the COVID-DM and other infection group to determine if any infection during diagnosis contributed to the clinical course.
Patient demographic and clinical characteristics were compared using frequency and percentages for categorical variables and median and interquartile ranges (IQR) for continuous variables. Numerical comparisons between groups were conducted through Kruskal-Wallis rank sum tests while categorical comparisons were conducted through Chi-square or Fisher’s exact tests. Analysis was conducted on data from diagnosis until May 2023. The following time frames for follow-up were examined in this analysis: 4–8 weeks, 8–26 weeks, 26–39 weeks, and 39–56 weeks. Thirteen participants were excluded from analysis related to follow-up as they did not receive any follow-up appointment in the specified time frames: 2 in the COVID-DM group, 10 in the no infection group and 1 in the other infection group. We assessed whether change in BMI in the first 6 months was associated with rate or timing of insulin discontinuation using logistic regression analysis and log-rank tests.
Group-based trajectory modeling of likelihood of being off insulin in the year following admission were identified using latent variable mixture modeling implemented by the PROC TRAJ add-on for SAS 9.4(23). This type of statistical modeling allows identification of groups of individuals with similar progressions or outcome over time, and allows for calculating the probability of each individual belonging to distinct subpopulations for a given variable(23). Binary logistic regression was used to assess whether baseline BMI, type of infection, or diagnosis period affected group membership. Additionally, logistic regression was used to assess the association between change in BMI from baseline to 6 months and coming off one or both insulins during follow-up. Data management, cleaning and statistical analysis was performed using SAS 9.4 (Cary, NC) and R v4.1.3 (R Core Team, 2022). A p-value below 0.05 was considered significant and all tests were two-sided.
Results:
One hundred and ninety-two subjects were identified. Of them, 44 did not meet criteria for DKA, had positive diabetes antibodies, or had history of type 2 diabetes or other diabetes prior to admission, leaving 148 patients included for analysis. There were 10 in the COVID-DM group (7%), 28 in the other infection group (19%), and 110 in the no infection group (74%). The other infection group comprised of 2 who had acute respiratory infection, 8 with candidiasis, 13 with bacterial infections, and 5 with multiple infections.
Characteristics and initial hospitalization of the study population summarized is shown in Table 1. Mean age was 15 years (IQR 13.8, 16.5) and BMI was 34 (IQR 31, 40) with BMI adjusted for age in 99% (IQR 98.0, 99.5) with no differences between groups. Most subjects (86%) self-identified as Hispanic or Non-Hispanic Black and 73.1% had public health insurance or were uninsured. Males represented the majority of the COVID-DM group (90%) and no infection group (68%) (p=0.03). There were no differences in admission labs, severity of DKA or insulin dosing at discharge among the infection groups. There was a trend towards increased admission to the intensive care unit in the COVID-DM group (p=0.06). Reasons for ICU admission among the COVID-DM group included severity of DKA, requirement of respiratory support, concern for cerebral edema, and severe hyperglycemia. Creatinine levels, rate neurological deficit and hypertonic saline use did not differ significantly between the groups. The COVID-DM and other infection group each had 1 participant who was intubated (p=0.03). The median length of hospital stay was higher in the COVID-DM group (4.73 days, IQR 3.13, 6.82) compared to the other groups (2.93 and 3.01 days for the no infection and other infection groups, respectively; p=0.02).
Table 1.
Baseline Characteristics of Participants, compared by infection status.
| Characteristic | Overall N = 148 | COVID-19 N = 10 | Other Infection N = 28 | No Infection N = 110 | p-value |
|---|---|---|---|---|---|
| Age, years | 14.92 (13.75, 16.52) | 15.42 (13.81, 16.49) | 14.29 (13.16, 16.10) | 14.95 (13.94, 16.59) | 0.33 |
| Sex | 0.03 | ||||
| Female | 51 (34%) | 1 (10%) | 15 (54%) | 35 (32%) | |
| Male | 97 (66%) | 9 (90%) | 13 (46%) | 75 (68%) | |
| BMI (Missing) | 34 (31, 40) 6 | 33 (32, 43) 0 | 36 (32, 40) 1 | 34 (30, 40) 5 | 0.38 |
| BMI (% for age) (Missing) | 99.06 (98.02, 99.53) 8 | 99.03 (98.82, 99.53) 0 | 99.22 (98.37, 99.57) 1 | 99.00 (97.66, 99.52) 7 | 0.36 |
| Race | 0.62 | ||||
| Hispanic | 10 (6.8%) | 2 (20%) | 3 (11%) | 5 (4.5%) | |
| Non-Hispanic black | 117 (79%) | 8 (80%) | 22 (79%) | 87 (79%) | |
| Non-Hispanic white | 2 (1.4%) | 0 (0%) | 0 (0%) | 2 (1.8%) | |
| Other | 10 (6.8%) | 0 (0%) | 2 (7.1%) | 8 (7.3%) | |
| White | 9 (6.1%) | 0 (0%) | 1 (3.6%) | 8 (7.3%) | |
| Insurance | 0.62 | ||||
| Private | 40 (27%) | 3 (30%) | 9 (32%) | 28 (25%) | |
| Public | 102 (69%) | 6 (60%) | 18 (64%) | 78 (71%) | |
| Uninsured/Unknown | 6 (4.1%) | 1 (10%) | 1 (3.6%) | 4 (3.6%) | |
| Admission | |||||
| pH | 7.19 (7.13, 7.25) | 7.20 (7.06, 7.24) | 7.19 (7.13, 7.26) | 7.19 (7.13, 7.24) | 0.84 |
| HCO3(mEq/L) (Missing) | 10.0 (7.0, 13.0) 6 | 8.0 (5.2, 10.0) 0 | 11.0 (7.0, 12.5) 1 | 10.0 (7.0, 13.0) 5 | 0.37 |
| Creatinine (mg/dL) | 0.88 (0.67, 1.32) | 1.04 (0.78, 1.35) | 0.78 (0.59, 1.19) | 0.88 (0.67, 1.37) | 0.36 |
| Glucose (mg/dL) | 540 (393, 786) | 540 (442, 869) | 525 (385, 602) | 550 (393, 832) | 0.68 |
| Anion gap (Missing) | 22 (19, 26) 6 | 24 (22, 26) 0 | 22 (18, 26) 1 | 22 (19, 26) 5 | 0.74 |
| BOHB (mmol/L) (Missing) | 7.50 (5.50, 8.30) 46 | 6.65 (4.22, 8.10) 2 | 7.69 (5.65, 8.38) 8 | 7.50 (5.80, 8.30) 36 | 0.62 |
| DKA Severity | 0.44 | ||||
| High | 28 (19%) | 4 (40%) | 6 (21%) | 18 (16%) | |
| Mild | 54 (36%) | 2 (20%) | 11 (39%) | 41 (37%) | |
| Moderate | 66 (45%) | 4 (40%) | 11 (39%) | 51 (46%) | |
| Initial admission | 0.06 | ||||
| Floor | 47 (32%) | 0 (0%) | 10 (36%) | 37 (34%) | |
| ICU | 101 (68%) | 10 (100%) | 18 (64%) | 73 (66%) | |
| Hospital length of stay, days | 3.00 (2.45, 3.85) | 4.73 (3.13, 6.82) | 3.01 (2.41, 3.90) | 2.93 (2.38, 3.62) | 0.020 |
| Diabetes | |||||
| HbA1c (Missing) | 12.40 (11.10, 13.90) 9 | 11.60 (10.80, 12.90) 1 | 12.70 (11.10, 14.20) 3 | 12.20 (11.10, 13.90) 5 | 0.21 |
| Discharge basal insulin (Units/kg) | 0.56 (0.50, 0.65) | 0.63 (0.55, 0.74) | 0.54 (0.45, 0.66) | 0.55 (0.50, 0.65) | 0.30 |
Legend: Median (IQR), n (%), BMI: body mass index, HCO3: bicarbonate, BOHB: beta hydroxybutyrate, HbA1c: glycosylated hemoglobin, ICU: intensive care unit, LOS: length of stay, DKA: diabetic ketoacidosis
Subjects were followed for up to 53 months after diagnosis. Median follow-up was 19 months (IQR 11, 27). One hundred and twenty subjects were seen on follow-up within the 1–2 month after discharge, 98 subjects at 2–6 months, 78 subjects at 6–9 months, and 65 at 9–13 months. Clinical practice varied and insulin therapy was individualized according to the treating physician’s discretion rather than a target HbA1c. Table 2 shows the primary outcome, patients who were able to discontinue one or both types of insulin within one year of diabetes diagnosis: 53% discontinued 1 insulin and 31% achieved insulin discontinuation. There were a total of 60% of patients who were able to stop at least one insulin with median time to discontinuation of 5.0 months (IQR 3.0, 8.0) and 43% who were able to discontinue both insulins within a median time of 9 months (IQR 4, 14). Of the subjects able to discontinue both insulins, 25% (n=16) had relapse 10.0 months (IQR 6.5–11.0) after discontinuing insulin. Participants were separated into all infection and no infection groups. There was significant difference in time to stopping one insulin between the groups but no differences in rate of stopping one or both insulins, time to stopping both insulins or restarting insulin. Likewise, those admitted during the pandemic did not significantly differ from those admitted prior to the pandemic in terms of coming off one (OR=0.55, 95% CI: 0.23–1.29, p-value=0.17) or both insulins (OR=0.47, 95% CI: 0.21–1.05, p-value=0.06). Demographics and initial admission characteristics were not associated with insulin discontinuation (Supplemental Table 1). Lastly, change in BMI from baseline to 6 months was not associated with coming off one (Odds ratio [OR]= 1.00, 95% CI: 0.90–1.11, p-value=0.99) or both insulins (OR=0.91, 95% CI: 0.82–1.00, p-value=0.06).
Table 2:
Participants who were able to stop one or both types of insulins according to infection status
| Characteristic | Overall, N = 148 | Infection, N = 38 | No Infection, N = 110 | p-value |
|---|---|---|---|---|
| Stopped One Insulin | 89 (60%) | 22 (58%) | 67 (61%) | 0.74 |
| Within one year | 78 (53%) | 18 (47%) | 60 (55%) | 0.44 |
| Time to stopping one insulin, months | 5.0 (3.0, 8.0) | 7.0 (4.0, 10.0) | 4.0 (2.0, 8.0) | 0.03 |
| Insulin discontinuation | 64 (43%) | 15 (39%) | 49 (45%) | 0.59 |
| Within one year | 46 (31%) | 9 (24%) | 37 (34%) | 0.25 |
| Time to insulin discontinuation, months | 9 (4, 14) | 11 (8, 17) | 8 (4, 12) | 0.11 |
| Restarted insulin | 16 (25%) | 2 (13%) | 14 (29%) | 0.32 |
| Time to restarting insulin, months | 10.0 (6.5, 11.0) | 11.0 (11.0, 11.0) | 9.5 (5.5, 11.0) | 0.38 |
To confirm that asymptomatic SARS-CoV-2 participants who were not tested or had infection prior to diagnosis did not skew results, the analysis was repeated with patients split into pre-pandemic (n=31) and pandemic groups (n=117), Table 3. Subjects in both groups had similar demographic characteristics, severity of DKA, hospitalization, HbA1c, and length of stay.
Table 3.
Baseline characteristics of participants, comparing those who were admitted pre-pandemic (1/2018–2/2020) and those admitted after the start of the pandemic (3/2020–1/2022). Median (IQR); n (%)
| Characteristic | Overall, N = 148 | Pre-Pandemic, N = 31 | Pandemic, N = 117 | p-value |
|---|---|---|---|---|
| Age, years | 14.92 (13.75, 16.52) | 14.92 (13.94, 16.93) | 14.92 (13.58, 16.33) | 0.46 |
| Sex | 0.47 | |||
| Female | 51 (34%) | 9 (29%) | 42 (36%) | |
| Male | 97 (66%) | 22 (71%) | 75 (64%) | |
| BMI (Missing) | 34 (31, 40) 6 | 35 (31, 40) 0 | 34 (30, 40) 6 | 0.97 |
| BMI (% for age) (Missing) | 99.06 (98.02, 99.53) 8 | 99.22 (98.06, 99.58) 1 | 99.04 (98.01, 99.53) 7 | 0.74 |
| Race | 0.86 | |||
| Hispanic | 10 (6.8%) | 2 (6.5%) | 8 (6.8%) | |
| Non-Hispanic black | 117 (79%) | 24 (77%) | 93 (79%) | |
| Non-Hispanic white | 2 (1.4%) | 0 (0%) | 2 (1.7%) | |
| Other | 10 (6.8%) | 2 (6.5%) | 8 (6.8%) | |
| White | 9 (6.1%) | 3 (9.7%) | 6 (5.1%) | |
| Insurance | 0.11 | |||
| Private | 40 (27%) | 10 (32%) | 30 (26%) | |
| Public | 102 (69%) | 18 (58%) | 84 (72%) | |
| Uninsured/Unknown | 6 (4.1%) | 3 (9.7%) | 3 (2.6%) | |
| Admission Labs | ||||
| pH | 7.19 (7.13, 7.25) | 7.20 (7.15, 7.22) | 7.19 (7.12, 7.25) | 0.94 |
| HCO3 (mEq/L) (Missing) | 10.0 (7.0, 13.0) 6 | 10.0 (7.2, 12.0) 1 | 10.0 (7.0, 13.0) 5 | 0.71 |
| Creatinine (mg/dL) | 0.88 (0.67, 1.32) | 0.85 (0.70, 1.30) | 0.89 (0.64, 1.31) | 0.90 |
| Glucose (mg/dL) | 540 (393, 786) | 548 (427, 742) | 539 (373, 790) | 0.72 |
| Anion gap (Missing) | 22 (19, 26) 6 | 22 (20, 25) 1 | 22 (18, 27) 5 | 0.95 |
| BOHB (mmol/L) (Missing) | 7.50 (5.50, 8.30) 46 | 7.56 (6.60, 8.03) 11 | 7.48 (5.31, 8.40) 35 | 0.93 |
| DKA Severity | ||||
| High | 28 (19%) | 3 (9.7%) | 25 (21%) | 0.26 |
| Mild | 54 (36%) | 11 (35%) | 43 (37%) | |
| Moderate | 66 (45%) | 17 (55%) | 49 (42%) | |
| Initial admission | 0.17 | |||
| Floor | 47 (32%) | 13 (42%) | 34 (29%) | |
| ICU | 101 (68%) | 18 (58%) | 83 (71%) | |
| Hospital LOS, days | 3.00 (2.45, 3.85) | 2.88 (2.44, 3.92) | 3.02 (2.46, 3.83) | 0.65 |
| Diabetes | ||||
| HbA1c (Missing) | 12.40 (11.10, 13.90) 9 | 11.60 (11.05, 13.15) 0 | 12.55 (11.10, 13.90) 9 | 0.37 |
| Discharge basal insulin (units/kg) | 0.56 (0.50, 0.65) | 0.54 (0.46, 0.61) | 0.57 (0.50, 0.66) | 0.20 |
Legend: Median (IQR), n (%), BMI: body mass index, HCO3: bicarbonate, BOHB: beta hydroxybutyrate, HbA1c: glycosylated hemoglobin, ICU: intensive care unit, LOS: length of stay, DKA: diabetic ketoacidosis
We identified two trajectory groups of likelihood of insulin discontinuation in the year following admission as shown in Figure 1: early discontinuation with high likelihood of being off by 2 months (n=21 or 14%) and a late discontinuation group with late or low likelihood of stopping insulin therapy (n=127 or 86%). Eventually 68 (54%) participants in the late discontinuation group discontinued one insulin, and 43 (34%) discontinued both. BMI (OR=1.02, 95% CI: 0.96–1.10, p=0.45), infection group (OR=1.76 for COVID-DM, 95% CI: 0.21–14.78; OR=2.54 for other infection, 95% CI: 0.55–11.68), and diagnosis period (OR=1.63, 95% CI: 0.58–4.63) were not associated with early or late discontinuation groups
Figure 1:
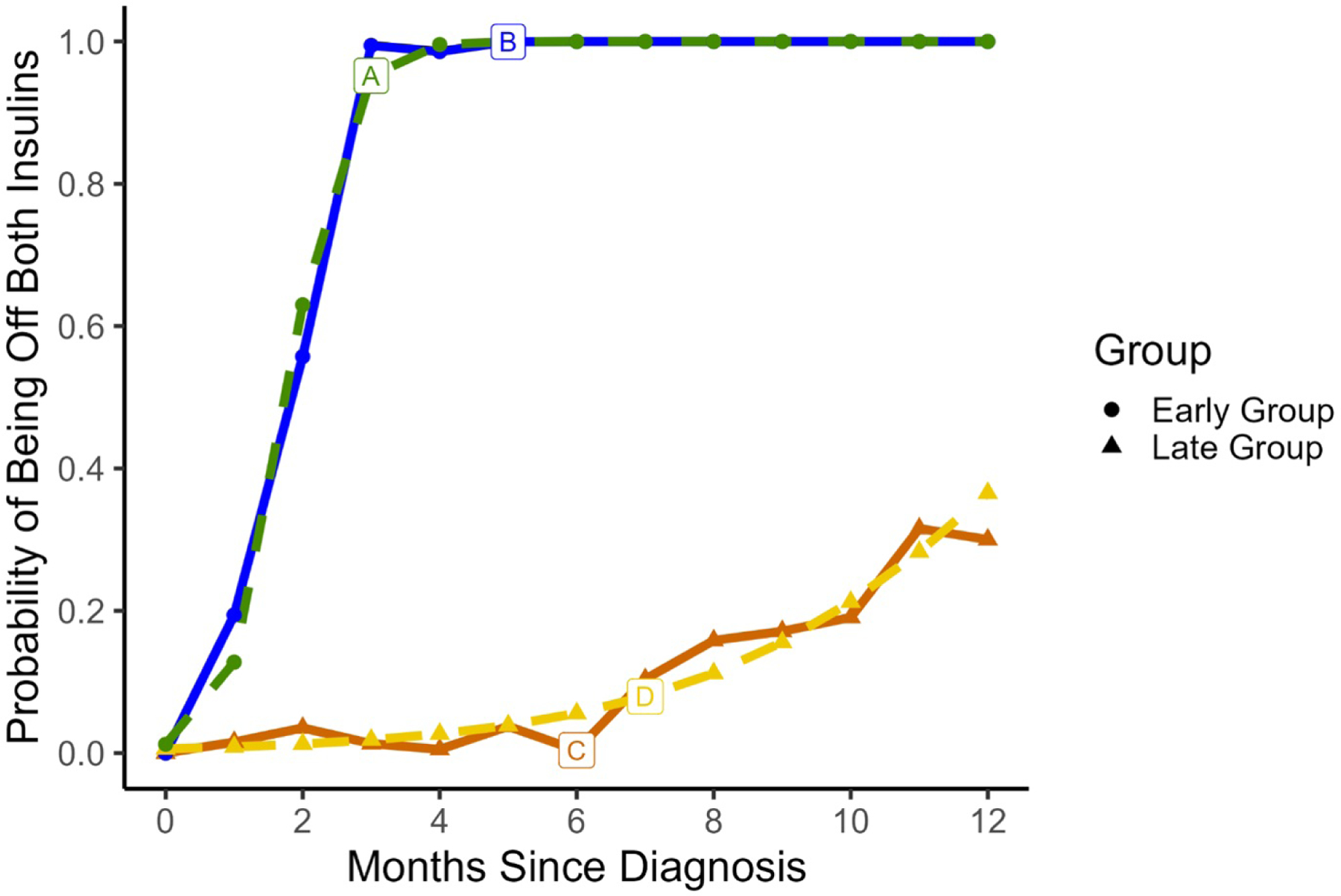
We identified two trajectory groups of likelihood of being off both insulin types in the year following admission. The solid lines (B and C) represent average observed data while the dotted lines (A and D) represent predicted trajectory for the group. Group 1 had early transition off with high likelihood of being off by 2 months shown by line A (n=21 or 14%) and Group 2 was a late transition group with later and lower likelihood of ever coming off both insulins shown by line C (n=127 or 86%).
Discussion:
Diabetes course after presentation with new onset DKA and SARS-CoV-2 infection in obese youth has not been reported previously. This cohort study showed increased incidence of DKA during the pandemic period, but rate and severity of DKA were not associated with an infection group. COVID-19 was not associated with insulin discontinuation during follow-up. However, because of the small sample size of participants in the COVID-DM group, we were unable to power the study to adequately to make inferences about SARS-CoV-2 infection on a larger scale. We therefore combined the COVID-DM and other infection groups. Having any infection also did not change the likelihood or timing of insulin discontinuation. A total of 64 participants in our cohort was able to discontinue insulin during follow-up, 46 within the first year. In those with insulin discontinuation, there was a high relapse rate to insulin use (25%). We identified 2 trajectory groups: those with early insulin discontinuation and those with late or no insulin discontinuation.
There was an increased incidence of new onset type 2 diabetes and DKA during the pandemic period, which is consistent with data from our clinic in which the number of patients with new onset type 2 diabetes increased from 174 in 2019 to 369 in 2021, and the literature(5, 6). There was a similar reported increase in incidence of type 1 diabetes during the pandemic era and after infection with SARS-CoV-2 (6, 24). Prevalence of DKA was already increasing prior to the pandemic from 19.5 to 30.2 per 1,000 persons from 2009–2014, most notably in those aged under 45 years old(25). Presentation with DKA at new-onset of diabetes is more likely to occur in the Black population(26). Furthermore, Misra et al. reported that rate of DKA increased during the COVID-19 pandemic period in adults, with highest rates in non-White ethnicities and those with type 2 diabetes and new onset diabetes(27). Similarly, DKA has increased in pediatric patients with type 2 diabetes, with the highest in the Black population (2, 6). Data from our cohort study confirmed increased DKA incidence during the pandemic period, with most cases occurring in Black and Hispanic patients. SARS-CoV-2 infection did not affect severity of DKA, consistent with previous reporting by Pasquel et al. which reported that metabolic parameters were similar on admission for patients with and without SARS-CoV-2 infection but those with SARS-CoV-2 had a higher mortality rate (28). One would also expect delayed access to care would cause worsening acidosis, which we did not detect. The COVID-DM group required longer hospitalization in our study. It has been reported that SARS-CoV-2 prolongs duration of acidosis but this was not analyzed in our study(29).
Several mechanisms have been proposed for the increased incidence of DKA and new diagnosis of type 2 diabetes. Whether SARS-CoV-2 directly or indirectly affects insulin-producing pancreatic β-cells is still under debate. The etiology is likely complex (30). SARS-CoV-2 can bind the ACE-II receptors which are expressed in the pancreas, lungs, small intestine, and other organs(14). There may also be alternative receptors and mediators facilitating SARS-CoV-2 entry(15). Infection can cause β-cell identity loss, cellular transdifferentiation, impairment of insulin and protein secretion and reduced blood flow to pancreatic islet cells(14, 31, 32). Long-term follow-up and comparisons to uninfected individuals is unknown.
The increase in DKA during COVID-19 pandemic is multidimensional. One possibility for the increase in DKA during the pandemic is undiagnosed SARS-CoV-2 due to the scarcity of viral testing at the beginning of the pandemic. In our cohort, patients presented at a similar age and HbA1c. If SARS-CoV-2 inflicted acute damage on β-cells triggering DKA, HbA1c at presentation may have been lower in the COVID-DM group. Instead, the high and similar HbA1c across groups suggest hyperglycemia for at least 3 months prior to infection. Misra et al. found increased rate of DKA in type 2 diabetes during the pandemic compared to pre-pandemic, even in post-infection waves when new cases were lower suggesting that there are factors other than acute SARS-CoV-2 infection rates driving prevalence of DKA(27). In one study with 128 patients with COVID-19, inflammatory biomarkers were measured in diabetic and non-diabetic patients at 3 and 6 months after recovery. Although there was an increase in fasting blood glucose in 35% and HbA1c in 50% of non-diabetic patients, C-peptide tests showed similar β-cell function in both diabetic and non-diabetic patients(33). Our study found that SARS-CoV-2 infection did not change the clinical course suggesting that β-cell stress caused by SARS-CoV-2 infection is likely transient and mild(34).
Our results in youth presenting with DKA at new-onset of diabetes are similar to previous reports in adults who present with DKA at new-onset of diabetes; there is a Black predominance, obesity, and males are more likely to present in unprovoked DKA(26, 35). Adult patients subsequently recover and have long-term clinical course similar to that of patients with type 2 diabetes who do not initially present with DKA, with up to 70% of patients achieving insulin independence in one group and 40% retaining insulin independence at 10 years(26). However, youth with type 2 diabetes have a three to fourfold faster decline in β-cell function and have higher therapeutic failure rates(36). In regards to percentage of patients able to achieve insulin discontinuation, our results are worse than previous reports of youth with type 2 diabetes. The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study reported that 90% of insulin dependent children with type 2 diabetes were able to discontinue insulin after an 8-week run-in period(37). Although not a direct comparison, only 43% of our cohort had insulin discontinuation. Perhaps this is due to presenting HbA1c being much higher (12.50, IQR 11.10, 13.9) than in the TODAY cohort (6.9, IQR 6, 8.9), signifying greater β-cell damage prior to diagnosis(37). Ethnic differences may have also played a role. The TODAY study reported a higher rate of glycemic failure in Non-Hispanic Black and Hispanic participants(38). The time to insulin discontinuation was also longer in our cohort, with a median of 9 months. In the paper by Low and colleagues, half of obese patients who presented in DKA were able to discontinue insulin after 2.2 ± 2.3 months and majority of patients required reinstitution of insulin treatment within 15 months whereas our patients restarted insulin in a median of 10 months after discontinuation (19). Early intensive insulin therapy can be critical in improving β-cell function and prolonging insulin remission(39). Possibly our patients with early insulin discontinuation had better initial insulin compliance, quickly reversing glucose β-cell toxicity.
Strengths of this study included describing a population of minorities and those on public insurance. Both are populations who have been most disparately affected by the COVID-19 pandemic (40). This study is also able to fill a gap in the literature in describing youths with DKA at new onset type 2 diabetes. While no association was found between SARS-CoV-2 infection and insulin independence, we did identify a group of subjects with rapid β-cell recovery who were able to quickly discontinue insulin, a group with poor β-cell recovery who were unable to discontinue insulin, and an intermediate group who were able to discontinue only one insulin or took months to discontinue both. Future studies need to focus on studying these endotypes to predict disease course and increase insulin discontinuation. The TODAY study identified youth with type 2 diabetes with early and late loss of glycemic control off insulin. Higher HbA1c at baseline, measures of insulin secretion, proinsulin and change in HbA1c predicted those who experienced metabolic decompensation(41). Predictors of recurrent DKA remain to be identified to determine who can safely discontinue insulin.
Limitations of our study are the retrospective nature of the study with small sample size in the COVID-DM group. It is difficult to distinguish type 1 and type 2 diabetes at hospitalization for new onset DKA, and all patients are discharged on insulin. True diabetes type may not be determined until months later so patients may have been mislabeled as having type 1 diabetes and therefore not included in the study. Autoantibody testing of patients with poorly defined diabetes type and risk of type 2 diabetes reduces the risk of misclassification. Treatment was not protocolized and assessment of adherence to prescribed insulin regimens was not measured. Overall follow-up was poor with only 65 of the 148 original subjects having follow up in the 9–13 month window. While only 16 (25%) of those who discontinued insulin needed to restart insulin, this may be due to inadequate follow up time. It is possible that the subjects with poor follow-up may have remained off insulin and did not seek care. Therefore, the amount of subjects with insulin independence was possibly underestimated. In our experience, those who stop insulin are also less likely to follow up, or are discharged from the endocrinology to continue follow up with their primary care doctor.
Conclusion:
In summary, prevalence of DKA at diagnosis increased during the pandemic period in new-onset pediatric subjects with type 2 diabetes. Our results indicate no evidence to support that SARS-CoV-2 infection is associated with severity of DKA, or frequency of DKA related comorbidities including elevated creatinine, cerebral edema or altered mental status and intubation. We observed two trajectories predicting insulin discontinuation emerged but were not associated with any infection status. SARS-CoV-2 does not appear to have long lasting impact on pancreatic β-cell function. Larger multicenter prospective studies examining diabetes endotypes are needed to determine whether SARS-CoV-2 affects clinical trajectory of youth diagnosed with type 2 diabetes.
Supplementary Material
Article Highlights:
There is limited data on new onset diabetes course after SARS-CoV-2 infection
Infection was not associated with diabetic ketoacidosis severity
Infection was not associated with insulin discontinuation
SARS-CoV-2 does not appear to have long lasting impact on beta-cell function
Clinical Relevance.
During the COVID-19 pandemic, the rate of new onset diabetes and diabetic ketoacidosis increased significantly. Our study suggests that infection with SARS-CoV-2 did not increase severity of DKA or affect long-term insulin use.
Personal thanks:
The authors thank Andrew B. Muir, Emory University School of Medicine, Atlanta, GA for reviewing and editing the manuscript, and Tanicia Daley, Emory University School of Medicine, Atlanta, GA, for helpful discussions during the development of the study. Drs. Muir and Daley received no financial support for their participation.
Funding and Assistance:
This study was supported by the Emory Department of Pediatrics and Children’s Healthcare of Atlanta. PV was supported in part by a grant from the National Institutes of Health (K23 DK11324–01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentation:
Part of this study was presented at the Southern Regional Meeting on 2 February 2023 in New Orleans, Louisiana.
References:
- 1.Everett EM, Copeland TP, Moin T, Wisk LE. National Trends in Pediatric Admissions for Diabetic Ketoacidosis, 2006–2016. J Clin Endocrinol Metab 2021;106(8):2343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klingensmith GJ, Connor CG, Ruedy KJ, Beck RW, Kollman C, Haro H, et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes 2016;17(4):266–73. [DOI] [PubMed] [Google Scholar]
- 3.Praveen PA, Hockett CW, Ong TC, Amutha A, Isom SP, Jensen ET, et al. Diabetic ketoacidosis at diagnosis among youth with type 1 and type 2 diabetes: Results from SEARCH (United States) and YDR (India) registries. Pediatr Diabetes 2021;22(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magge SN, Wolf RM, Pyle L, Brown EA, Benavides VC, Bianco ME, et al. The COVID-19 pandemic is associated with a substantial rise in frequency and severity of presentation of youth-onset type 2 diabetes. J Pediatr 2022. [DOI] [PMC free article] [PubMed]
- 6.Marks BE, Khilnani A, Meyers A, Flokas ME, Gai J, Monaghan M, et al. Increase in the Diagnosis and Severity of Presentation of Pediatric Type 1 and Type 2 Diabetes during the COVID-19 Pandemic. Horm Res Paediatr 2021;94(7–8):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol 2022;10(5):311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLacey S, Arzu J, Levin L, Ranganna A, Swamy A, Bianco ME. Impact of SARS-CoV2 on youth onset type 2 diabetes new diagnoses and severity. J Diabetes 2022;14(8):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ssentongo P, Zhang Y, Witmer L, Chinchilli VM, Ba DM. Association of COVID-19 with diabetes: a systematic review and meta-analysis. Sci Rep 2022;12(1):20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab 2020;22(10):1935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elgenidy A, Awad AK, Saad K, Atef M, El-Leithy HH, Obiedallah AA, et al. Incidence of diabetic ketoacidosis during COVID-19 pandemic: a meta-analysis of 124,597 children with diabetes. Pediatr Res 2022. [DOI] [PMC free article] [PubMed]
- 12.Kuchay MS, Reddy PK, Gagneja S, Mathew A, Mishra SK. Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19. Diabetes Metab Syndr 2020;14(6):2039–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seow CJ, Wei Choon Koh A, Lian JX, Dalan R, Boehm BO. Non autoimmune type 1B diabetes after mild COVID-19: Report of three cases. Diabetes Metab Res Rev 2021;37(5):e3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller JA, Gross R, Conzelmann C, Kruger J, Merle U, Steinhart J, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab 2021;3(2):149–65. [DOI] [PubMed] [Google Scholar]
- 15.Rangu R, Wander PL, Barrow BM, Zraika S. Going viral in the islet: mediators of SARS-CoV-2 entry beyond ACE2. J Mol Endocrinol 2022;69(2):R63–R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacha F, Gungor N, Lee S, Arslanian SA. Progressive deterioration of beta-cell function in obese youth with type 2 diabetes. Pediatr Diabetes 2013;14(2):106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett T, Jalaludin MY, Turan S, Hafez M, Shehadeh N, Novo Nordisk Pediatric Type 2 Diabetes Global Expert P. Rapid progression of type 2 diabetes and related complications in children and young people-A literature review. Pediatr Diabetes 2020;21(2):158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Group TS. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care 2013;36(6):1749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low JC, Felner EI, Muir AB, Brown M, Dorcelet M, Peng L, et al. Do obese children with diabetic ketoacidosis have type 1 or type 2 diabetes? Prim Care Diabetes 2012;6(1):61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Center for Disease Control and Prevention NCfHS. CDC growth charts: United States http://www.cdc.gov/growthcharts/2000
- 21.Glaser N, Fritsch M, Priyambada L, Rewers A, Cherubini V, Estrada S, et al. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 2022;23(7):835–56. [DOI] [PubMed] [Google Scholar]
- 22.Tosur M, Huang X, Inglis AS, Aguirre RS, Redondo MJ. Imprecise Diagnosis of Diabetes Type in Youth: Prevalence, Characteristics, and Implications 2023. [DOI] [PMC free article] [PubMed]
- 23.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociological Methods & Research 2007;35(4):542–71. [Google Scholar]
- 24.Weiss A, Donnachie E, Beyerlein A, Ziegler AG, Bonifacio E. Type 1 Diabetes Incidence and Risk in Children With a Diagnosis of COVID-19. JAMA 2023. [DOI] [PMC free article] [PubMed]
- 25.Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in Diabetic Ketoacidosis Hospitalizations and In-Hospital Mortality - United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2018;67(12):362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vellanki P, Umpierrez GE. Diabetic Ketoacidosis: A Common Debut of Diabetes among African Americans with Type 2 Diabetes. Endocr Pract 2017;23(8):971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misra S, Barron E, Vamos E, Thomas S, Dhatariya K, Kar P, et al. Temporal trends in emergency admissions for diabetic ketoacidosis in people with diabetes in England before and during the COVID-19 pandemic: a population-based study. Lancet Diabetes Endocrinol 2021;9(10):671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasquel FJ, Messler J, Booth R, Kubacka B, Mumpower A, Umpierrez G, et al. Characteristics of and Mortality Associated With Diabetic Ketoacidosis Among US Patients Hospitalized With or Without COVID-19. JAMA Netw Open 2021;4(3):e211091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armeni E, Aziz U, Qamar S, Nasir S, Nethaji C, Negus R, et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet Diabetes Endocrinol 2020;8(8):660–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Guerreiro SG, Cruz-Martins N, Batiha GE. COVID-19 in Relation to Hyperglycemia and Diabetes Mellitus. Front Cardiovasc Med 2021;8:644095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang X, Uhl S, Zhang T, Xue D, Li B, Vandana JJ, et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab 2021;33(8):1577–91 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook JR, Ausiello J. Functional ACE2 deficiency leading to angiotensin imbalance in the pathophysiology of COVID-19. Rev Endocr Metab Disord 2022;23(2):151–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberca RW, Ramos YAL, Pereira NZ, Beserra DR, Branco A, Leao Orfali R, et al. Long-term effects of COVID-19 in diabetic and non-diabetic patients. Front Public Health 2022;10:963834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology 2020;159(1):367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter WE, Maclaren NK, Riley WJ, Clarke DW, Kappy MS, Spillar RP. Maturity-onset diabetes of youth in black Americans. N Engl J Med 1987;316(6):285–91. [DOI] [PubMed] [Google Scholar]
- 36.Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci 2015;1353:113–37. [DOI] [PubMed] [Google Scholar]
- 37.Kelsey MM, Geffner ME, Guandalini C, Pyle L, Tamborlane WV, Zeitler PS, et al. Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatr Diabetes 2016;17(3):212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366(24):2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao S, Umpierrez GE, Daley T, Vellanki P. Intervention with Therapeutic Agents, Understanding the Path to Remission in Type 2 Diabetes: Part 1. Endocrinology and Metabolism Clinics 2022. [DOI] [PubMed]
- 40.Antoon JW, Grijalva CG, Thurm C, Richardson T, Spaulding AB, Teufel RJ 2nd, et al. Factors Associated With COVID-19 Disease Severity in US Children and Adolescents. J Hosp Med 2021;16(10):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeitler P, El Ghormli L, Arslanian S, Caprio S, Isganaitis E, Kelsey MK, et al. Deterioration of Glycemic Control in Youth-Onset Type 2 Diabetes: What Are the Early and Late Predictors? J Clin Endocrinol Metab 2022;107(8):e3384–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


