Abstract
Background
Previous studies have shown that educational attainment (EA), intelligence and income are key factors associated with mental disorders. However, the direct effects of each factor on major mental disorders are unclear.
Aims
We aimed to evaluate the overall and independent causal effects of the three psychosocial factors on common mental disorders.
Methods
Using genome-wide association study summary datasets, we performed Mendelian randomisation (MR) and multivariable MR (MVMR) analyses to assess potential associations between the 3 factors (EA, N=766 345; household income, N=392 422; intelligence, N=146 808) and 13 common mental disorders, with sample sizes ranging from 9907 to 807 553. Inverse-variance weighting was employed as the main method in the MR analysis.
Results
Our MR analysis showed that (1) higher EA was a protective factor for eight mental disorders but contributed to anorexia nervosa, obsessive-compulsive disorder (OCD), bipolar disorder (BD) and autism spectrum disorder (ASD); (2) higher intelligence was a protective factor for five mental disorders but a risk factor for OCD and ASD; (3) higher household income protected against 10 mental disorders but confers risk for anorexia nervosa. Our MVMR analysis showed that (1) higher EA was a direct protective factor for attention-deficit/hyperactivity disorder (ADHD) and insomnia but a direct risk factor for schizophrenia, BD and ASD; (2) higher intelligence was a direct protective factor for schizophrenia but a direct risk factor for major depressive disorder (MDD) and ASD; (3) higher income was a direct protective factor for seven mental disorders, including schizophrenia, BD, MDD, ASD, post-traumatic stress disorder, ADHD and anxiety disorder.
Conclusions
Our study reveals that education, intelligence and income intertwine with each other. For each factor, its independent effects on mental disorders present a more complex picture than its overall effects.
Keywords: Genetics, Behavioral; Epidemiologic Factors
WHAT IS ALREADY KNOWN ON THIS TOPIC
Educational attainment (EA), intelligence and income are vital psychosocial factors associated with mental disorders. However, the direct effects of each factor on major mental disorders need to be further elucidated.
WHAT THIS STUDY ADDS
Untangled, independent effects of these psychosocial factors on mental disorders are more complex than their overall effects. Our study suggests that higher EA or higher intelligence may exert independent detrimental effects on some mental disorders; while high income is a direct protective factor for most common mental disorders.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In the clinical practice for mental disorders, taking into account each of the psychosocial factors may provide additional granularity necessary for proper disease-specific prevention and management.
Introduction
Intelligence, education and income are three fundamental determinants of human health and well-being. Intelligence is a basic indicator of cognitive ability and a major correlate of human health outcomes.1 Higher intelligence has positive direct effects on educational attainment (EA), higher-status occupations and income.2 The effects of higher EA on occupations and income are also positive and direct. As two vital dimensions of socioeconomic status, EA and income also correlate with a variety of social and health outcomes.3 Social epidemiology demonstrates that both lower-level EA and lower socioeconomic position are strongly associated with poorer health, a higher risk of illness and higher mortality.4
Although the overall effects of each of the three psychosocial factors are deemed beneficial, determining causality in conventional observational studies of mental diseases is a challenge. Recently, the Mendelian randomisation (MR) technique has been widely employed to evaluate causality between risk factors and diseases.5–7 In MR, causality is examined by using germline genetic variants as instruments for quantifying the effect of an exposure on a specific outcome. It was reported that higher EA was associated with an increased risk for bipolar disorder (BD) but a decreased risk for attention-deficit/hyperactivity disorder (ADHD),8 9 while higher EA or intelligence confers a causal effect on autism spectrum disorder (ASD) but protects against ADHD (OR: 0.78).10 11
Here, we hypothesise that the overall effects of each of the three factors on mental disorders may be partially mediated or confounded by each other. To disentangle these relationships, we conducted univariable MR analysis and multivariable MR (MVMR) analyses and compared the overall effects of each of these factors on mental diseases to their direct effects on the same set of conditions.
Methods
Genome-wide association study (GWAS) summary datasets
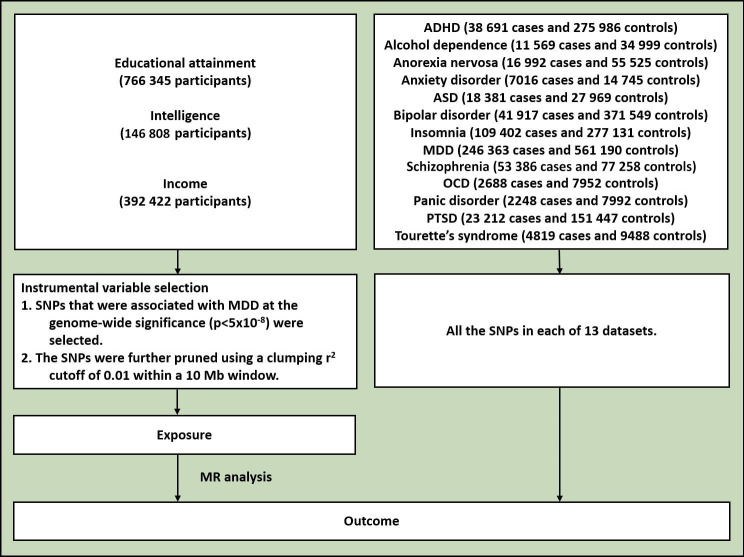
The design of the study is shown in figure 1. The study used publicly available GWAS summary results. Table 1 lists the GWAS datasets used in this study. The datasets on EA contained 766 345 participants.12 The GWAS datasets of household income and intelligence (fluid intelligence score) were obtained from Yang Lab (https://yanglab.westlake.edu.cn/),13 containing 392 422 and 146 808 participants, respectively. The participants of the income and intelligence datasets came from the UK Biobank, consisting of ~500 000 volunteers aged 40–69 recruited between 2006 and 2010 across the United Kingdom.14 Detailed information on the UK Biobank cohort can be found on its website (https://www.ukbiobank.ac.uk/). The insomnia dataset was obtained from the Center for Neurogenomics and Cognitive Research.15 The largest GWAS summary datasets for the other 12 mental disorders, namely, ADHD, alcohol dependence, anorexia nervosa, anxiety disorder, ASD, BD, major depressive disorder (MDD), obsessive-compulsive disorder (OCD), panic disorder, post-traumatic stress disorder (PTSD), schizophrenia and Tourette’s syndrome (TS), were obtained from the Psychiatric Genomics Consortium. Sample sizes ranged from 9907 to 807 553 for the mental disorders. All the participants in the datasets were of European origin.
Figure 1.
Flowchart of the study. ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; MDD, major depressive disorder; MR, Mendelian randomisation; OCD, obsessive-compulsive disorder; PTSD, post-traumatic stress disorder; SNPs. single-nucleotide polymorphisms.
Table 1.
Summary information of the datasets
| Trait | Year | First author | PMID | Ncase | Ncontrol | N |
| EA | 2018 | Lee JJ | 30038396 | NA | NA | 766 345 |
| Intelligence | 2019 | Jiang L | 31768069 | NA | NA | 146 808 |
| Income | 2019 | Jiang L | 31768069 | NA | NA | 392 422 |
| ADHD | 2023 | Demontis D | 36702997 | 38 691 | 275 986 | 314 677 |
| Alcohol dependence | 2018 | Walters RK | 30482948 | 11 569 | 34 999 | 46 568 |
| Anorexia nervosa | 2019 | Watson HJ | 31308545 | 16 992 | 55 525 | 72 517 |
| Anxiety disorder | 2016 | Otowa T | 26857599 | 7016 | 14 745 | 21 761 |
| ASD | 2019 | Grove J | 30804558 | 18 381 | 27 969 | 46 350 |
| BD | 2021 | Mullins N | 34002096 | 41 917 | 371 549 | 413 466 |
| Insomnia | 2019 | Jansen PR | 30804565 | 109 402 | 277 131 | 386 533 |
| MDD | 2019 | Howard DM | 30718901 | 246 363 | 561 190 | 807 553 |
| OCD | 2017 | Arnold PD | 28761083 | 2688 | 7952 | 10 640 |
| Panic disorder | 2021 | Forstner AJ | 31712720 | 2248 | 7992 | 9907 |
| PTSD | 2019 | Nievergelt CM | 31594949 | 23 212 | 151 447 | 174 659 |
| Schizophrenia | 2022 | Trubetskoy V | 35396580 | 53 386 | 77 258 | 130 644 |
| TS | 2019 | Yu D | 30818990 | 4819 | 9488 | 14 307 |
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; BD, bipolar disorder; EA, educational attainment; MDD, major depressive disorder; OCD, obsessive-compulsive disorder; PTSD, post-traumatic stress disorder; TS, Tourette’s syndrome.
Genetic correlation analysis
The genetic correlations between the 3 factors and the 13 mental disorders were appraised by linkage disequilibrium (LD) score regression.16 The LD structure of the genetic variants was derived from the 1000 Genome Project Phase 3 in European populations. Multiple testing was accounted for by the false discovery rate (FDR) procedure (FDR<0.05).
MR analysis
The MR analyses were performed using three techniques implemented in TwoSampleMR,17 including inverse-variance weighted (IVW), weighted median and MR-Egger. The IVW model was used as the main method. Directional pleiotropy was evaluated by the intercept from the MR-Egger regression. The heterogeneity of the MR analysis was evaluated by Cochran’s Q test (p<0.05) and I2 statistics (I2>0.25). The significant associations were determined by an IVW-based FDR <0.05. For each MR analysis, genome-wide significant single-nucleotide polymorphisms (p<5×10−8) in the exposure dataset were used to select independent variables (IVs) by a clumping r2 value of 0.01 in a 10 Mb window.
MVMR analysis
An MVMR analysis was used to estimate the direct effect of each exposure on each of the mental disorders. The MVMR analyses were performed using the TwoSampleMR package.17
Results
Genetic correlations
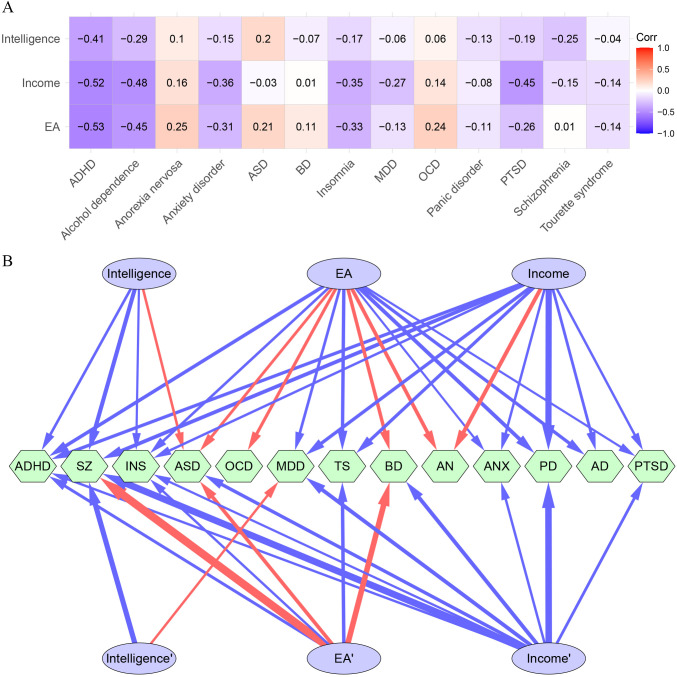
For the three psychosocial factors, high and positive intercorrelations were detected (rg: 0.63–0.81) (online supplemental table 1). The three psychosocial factors had overall negative genetic correlations with the 13 mental disorders (figure 2A and online supplemental table 2). Notably, ADHD demonstrated the strongest negative correlation with all the three psychosocial factors (rg: −0.41 to −0.53). ASD had positive correlations with EA (rg: 0.21) and intelligence (rg: 0.20). OCD was positively correlated with EA (rg: 0.24) and income (rg: 0.14). Anorexia nervosa was also positively correlated with EA (rg: 0.25) and income (rg: 0.16).
Figure 2.
Genetic correlations and causal associations between the three psychosocial factors and the mental disorders. (A) Genetic correlations between the three psychosocial factors and the mental disorders. (B) Significant overall and independent causal effects of the three factors on the mental disorders. The upper lines are causal effects in the Mendelian randomisation analysis, and the bottom lines are causal effects in the multivariable Mendelian randomisation analysis. The red colour represents positive causal effects, and the blue colour represents negative causal effects. The width of the edges is relative to the absolute value of the effect sizes. AD, alcohol dependence; ADHD, attention-deficit/hyperactivity disorder; AN, anorexia nervosa; ANX, anxiety disorder; ASD, autism spectrum disorder; BD, bipolar disorder; EA, educational attainment; INS, insomnia; MDD, major depressive disorder; MR, Mendelian randomisation; OCD, obsessive-compulsive disorder; PD, panic disorder; PTSD, post-traumatic stress disorder; SZ, schizophrenia; TS, Tourette’s syndrome.
gpsych-2023-101080supp001.pdf (81KB, pdf)
MR analysis
Considering the genetic correlations between the three factors and the intertwined influences of these factors on each mental disorder, we called the associations revealed by the MR analysis ‘overall causal effects’.
For the three psychosocial factors, their causal associations with each other were strong and bidirectional (OR: 1.44–2.17) (online supplemental table 3). Specifically, intelligence confers causal effects on EA (OR: 1.50) and income (OR: 1.44), EA confers causal effects on intelligence (OR: 2.17) and income (OR: 1.99), and income confers causal effects on EA (OR: 1.88) and intelligence (OR: 1.99).
The causal effects of the three studied factors on the mental disorders are shown in table 2, figure 2 and online supplemental table 4. Higher EA served as a protective factor for eight mental disorders, including panic disorder (OR: 0.68), ADHD (OR: 0.72), TS (OR: 0.73), alcohol dependence (OR: 0.75), MDD (OR: 0.84), insomnia (OR: 0.86), PTSD (OR: 0.94) and anxiety disorder (OR: 0.95), but as a risk factor for anorexia nervosa (OR: 1.44), OCD (OR: 1.29), BD (OR: 1.38) and ASD (OR: 1.21).
Table 2.
Causal effects of the three factors on the mental disorders in the Mendelian randomisation analysis
| Exposure | Outcome | B (SE) | OR (95% CI) | N_IV | P value | FDR |
| EA | ADHD | −0.333 (0.018) | 0.72 (0.69 to 0.74) | 457 | <0.001 | 6.63E−78 |
| EA | Alcohol dependence | −0.286 (0.042) | 0.75 (0.69 to 0.82) | 460 | <0.001 | 1.57E−10 |
| EA | Anorexia nervosa | 0.364 (0.077) | 1.44 (1.24 to 1.67) | 458 | <0.001 | 7.64E−06 |
| EA | Anxiety disorder | −0.056 (0.020) | 0.95 (0.91 to 0.98) | 454 | <0.001 | 8.83E−03 |
| EA | ASD | 0.192 (0.036) | 1.21 (1.13 to 1.30) | 460 | <0.001 | 5.15E–07 |
| EA | BD | 0.323 (0.066) | 1.38 (1.21 to 1.57) | 463 | <0.001 | 3.90E−06 |
| EA | Insomnia | −0.154 (0.013) | 0.86 (0.84 to 0.88) | 458 | <0.001 | 1.13E−32 |
| EA | MDD | −0.175 (0.028) | 0.84 (0.79 to 0.89) | 461 | <0.001 | 2.63E−09 |
| EA | OCD | 0.253 (0.064) | 1.29 (1.14 to 1.46) | 461 | <0.001 | 2.06E−04 |
| EA | Panic disorder | −0.390 (0.160) | 0.68 (0.49 to 0.93) | 462 | 0.015 | 0.023 |
| EA | PTSD | −0.064 (0.015) | 0.94 (0.91 to 0.97) | 462 | <0.001 | 5.99E−05 |
| EA | Schizophrenia | 0.050 (0.082) | 1.05 (0.90 to 1.23) | 461 | 0.536 | 0.674 |
| EA | TS | −0.313 (0.056) | 0.73 (0.66 to 0.82) | 460 | <0.001 | 1.36E−07 |
| Income | ADHD | −0.280 (0.035) | 0.76 (0.70 to 0.81) | 86 | <0.001 | 2.69E−14 |
| Income | Alcohol dependence | −0.191 (0.085) | 0.83 (0.70 to 0.98) | 88 | 0.024 | 0.036 |
| Income | Anorexia nervosa | 0.421 (0.119) | 1.52 (1.21 to 1.92) | 87 | <0.001 | 8.43E−04 |
| Income | Anxiety disorder | −0.121 (0.033) | 0.89 (0.83 to 0.95) | 87 | <0.001 | 5.53E−04 |
| Income | ASD | −0.010 (0.068) | 0.99 (0.87 to 1.13) | 88 | 0.884 | 0.907 |
| Income | BD | −0.069 (0.130) | 0.93 (0.72 to 1.20) | 88 | 0.595 | 0.682 |
| Income | Insomnia | −0.121 (0.022) | 0.89 (0.85 to 0.93) | 88 | <0.001 | 1.91E−07 |
| Income | MDD | −0.302 (0.047) | 0.74 (0.67 to 0.81) | 86 | <0.001 | 1.26E−09 |
| Income | OCD | 0.151 (0.102) | 1.16 (0.95 to 1.42) | 87 | 0.139 | 0.192 |
| Income | Panic disorder | −0.885 (0.255) | 0.41 (0.25 to 0.68) | 87 | <0.001 | 1.06E−03 |
| Income | PTSD | −0.116 (0.025) | 0.89 (0.85 to 0.93) | 89 | <0.001 | 1.01E−05 |
| Income | Schizophrenia | −0.382 (0.156) | 0.68 (0.50 to 0.93) | 88 | 0.014 | 0.023 |
| Income | TS | −0.282 (0.111) | 0.75 (0.61 to 0.94) | 87 | 0.011 | 0.019 |
| Intelligence | ADHD | −0.158 (0.028) | 0.85 (0.81 to 0.90) | 78 | <0.001 | 6.24E−08 |
| Intelligence | Alcohol dependence | −0.115 (0.055) | 0.89 (0.80 to 0.99) | 78 | 0.035 | 0.051 |
| Intelligence | Anorexia nervosa | 0.160 (0.109) | 1.17 (0.95 to 1.45) | 77 | 0.143 | 0.192 |
| Intelligence | Anxiety disorder | −0.005 (0.026) | 0.99 (0.95 to 1.05) | 76 | 0.833 | 0.879 |
| Intelligence | ASD | 0.144 (0.048) | 1.15 (1.05 to 1.27) | 78 | <0.001 | 4.66E−03 |
| Intelligence | BD | −0.032 (0.096) | 0.97 (0.80 to 1.17) | 78 | 0.741 | 0.826 |
| Intelligence | Insomnia | −0.068 (0.018) | 0.93 (0.90 to 0.97) | 78 | <0.001 | 2.75E−04 |
| Intelligence | MDD | −0.005 (0.044) | 0.99 (0.91 to 1.09) | 78 | 0.909 | 0.909 |
| Intelligence | OCD | −0.047 (0.084) | 0.95 (0.81 to 1.12) | 78 | 0.575 | 0.682 |
| Intelligence | Panic disorder | −0.046 (0.217) | 0.96 (0.62 to 1.46) | 78 | 0.834 | 0.879 |
| Intelligence | PTSD | −0.011 (0.021) | 0.99 (0.95 to 1.03) | 78 | 0.579 | 0.682 |
| Intelligence | Schizophrenia | −0.454 (0.135) | 0.63 (0.49 to 0.83) | 78 | <0.001 | 1.49E−03 |
| Intelligence | TS | −0.052 (0.081) | 0.95 (0.81 to 1.11) | 77 | 0.519 | 0.674 |
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; BD, bipolar disorder; CI, confidence interval; EA, educational attainment; FDR, false discovery rate; IVW, inverse-variance weighted; MDD, major depressive disorder; N_IV, number of instrumental variable; OCD, obsessive-compulsive disorder; OR, odds ratio; PTSD, post-traumatic stress disorder; SE, standard error; TS, Tourette’s syndrome.
Higher intelligence was a protective factor for five mental disorders, including schizophrenia (OR: 0.63), ADHD (OR: 0.85) and insomnia (OR: 0.93), but a risk factor for ASD (OR: 1.15).
Higher income conferred a protective effect on 9 out of the 13 mental disorders, including panic disorder (OR: 0.41), schizophrenia (OR: 0.68), MDD (OR: 0.74), ADHD (OR: 0.76), TS (OR: 0.75), alcohol dependence (OR: 0.83), PTSD (OR: 0.89), anxiety disorder (OR: 0.89) and insomnia (OR: 0.89). However, higher income was a risk factor for anorexia nervosa (OR: 1.52).
The average overall causal effects (OR) were 0.99 (0.26), 0.96 (0.13) and 0.88 (0.26) for EA, intelligence and income, respectively.
MR sensitivity analysis showed that the directions from different models were largely the same (online supplemental table 4). The MR-Egger regression did not support directional pleiotropy in the MR analysis (MR-Egger intercept <0.01). The evidence of directional pleiotropy for the IVs from the MR-Egger regression was minimal, indicating the robustness of the main results. There was evidence supporting the potential heterogeneity among the individual IV effect estimates.
MVMR analysis
Further exploration of the direct causal effects of each factor on the mental disorders was conducted with the aid of MVMR, which is capable of accounting for the influence of synergistic factors. Here, we called the associations revealed by MVMR ‘direct’ or ‘independent’ causal effects.
The results of the MVMR analysis are shown in table 3 and figure 2B. We found that higher EA is independently associated with an increased risk for schizophrenia (OR: 3.43), BD (OR: 2.11) and ASD (OR: 1.43) and a decreased risk for ADHD (OR: 0.80) and insomnia (OR: 0.90). In the MVMR analysis, the average effect sizes (OR values) of EA on the mental disorders increased substantially and became largely detrimental (OR_MR: 0.99 vs OR_MVMR: 1.30). In particular, the effects of EA on schizophrenia and BD remarkably increased, and the protective effects of EA on alcohol dependence, panic disorder, PTSD and MDD disappeared.
Table 3.
Causal effects of the three factors on the mental disorders in the multivariable Mendelian randomisation analysis
| Exposure | Outcome | B (SE) | OR (95% CI) | N_IV | P value | FDR |
| EA | ADHD | −0.22 (0.04) | 0.80 (0.74 to 0.87) | 435 | <0.001 | 1.88E−07 |
| EA | Alcohol dependence | −0.15 (0.10) | 0.86 (0.71 to 1.04) | 441 | 0.125 | 0.212 |
| EA | Anorexia nervosa | 0.16 (0.17) | 1.17 (0.84 to 1.63) | 436 | 0.358 | 0.499 |
| EA | Anxiety disorder | 0.01 (0.04) | 1.01 (0.93 to 1.10) | 430 | 0.788 | 0.884 |
| EA | ASD | 0.36 (0.08) | 1.43 (1.22 to 1.67) | 441 | <0.001 | 2.85E−05 |
| EA | BD | 0.75 (0.15) | 2.11 (1.57 to 2.83) | 444 | <0.001 | 3.83E−06 |
| EA | Insomnia | −0.11 (0.03) | 0.90 (0.85 to 0.95) | 439 | <0.001 | 7.50E−04 |
| EA | MDD | 0.02 (0.06) | 1.02 (0.90 to 1.15) | 443 | 0.795 | 0.884 |
| EA | OCD | 0.17 (0.14) | 1.19 (0.90 to 1.57) | 443 | 0.219 | 0.356 |
| EA | Panic disorder | 0.20 (0.36) | 1.22 (0.61 to 2.47) | 443 | 0.573 | 0.721 |
| EA | PTSD | 0.06 (0.03) | 1.07 (1.00 to 1.14) | 443 | 0.046 | 0.092 |
| EA | Schizophrenia | 1.23 (0.17) | 3.43 (2.44 to 4.83) | 443 | <0.001 | 6.32E−11 |
| EA | TS | −0.33 (0.13) | 0.72 (0.56 to 0.92) | 442 | <0.001 | 0.034 |
| Income | ADHD | −0.11 (0.04) | 0.90 (0.82 to 0.98) | 63 | 0.012 | 0.037 |
| Income | Alcohol dependence | −0.22 (0.11) | 0.80 (0.65 to 1.00) | 63 | 0.047 | 0.092 |
| Income | Anorexia nervosa | 0.33 (0.19) | 1.39 (0.95 to 2.02) | 61 | 0.087 | 0.162 |
| Income | Anxiety disorder | −0.12 (0.05) | 0.88 (0.80 to 0.97) | 62 | 0.013 | 0.037 |
| Income | ASD | −0.31 (0.09) | 0.73 (0.61 to 0.87) | 63 | <0.001 | 1.41E−03 |
| Income | BD | −0.41 (0.17) | 0.67 (0.48 to 0.92) | 62 | 0.015 | 0.037 |
| Income | Insomnia | −0.08 (0.03) | 0.93 (0.87 to 0.98) | 63 | 0.015 | 0.037 |
| Income | MDD | −0.40 (0.07) | 0.67 (0.59 to 0.77) | 61 | <0.001 | 1.45E−07 |
| Income | OCD | 0.15 (0.16) | 1.16 (0.86 to 1.58) | 63 | 0.333 | 0.481 |
| Income | Panic disorder | −0.95 (0.40) | 0.39 (0.18 to 0.85) | 62 | 0.017 | 0.039 |
| Income | PTSD | −0.19 (0.04) | 0.83 (0.77 to 0.89) | 62 | <0.001 | 2.29E−06 |
| Income | Schizophrenia | −0.99 (0.20) | 0.37 (0.25 to 0.55) | 62 | <0.001 | 2.68E−06 |
| Income | TS | −0.11 (0.14) | 0.90 (0.68 to 1.18) | 63 | 0.445 | 0.598 |
| Intelligence | ADHD | −0.06 (0.03) | 0.95 (0.89 to 1.00) | 52 | 0.047 | 0.092 |
| Intelligence | Alcohol dependence | 0.01 (0.07) | 1.01 (0.88 to 1.16) | 52 | 0.848 | 0.894 |
| Intelligence | Anorexia nervosa | −0.02 (0.12) | 0.98 (0.77 to 1.24) | 52 | 0.873 | 0.896 |
| Intelligence | Anxiety disorder | 0.01 (0.03) | 1.01 (0.95 to 1.07) | 52 | 0.816 | 0.884 |
| Intelligence | ASD | 0.06 (0.06) | 1.07 (0.95 to 1.19) | 52 | 0.266 | 0.407 |
| Intelligence | BD | −0.18 (0.11) | 0.84 (0.68 to 1.03) | 52 | 0.100 | 0.177 |
| Intelligence | Insomnia | 0.01 (0.02) | 1.01 (0.97 to 1.05) | 52 | 0.806 | 0.884 |
| Intelligence | MDD | 0.11 (0.04) | 1.12 (1.02 to 1.22) | 52 | 0.014 | 0.037 |
| Intelligence | OCD | −0.06 (0.10) | 0.94 (0.77 to 1.15) | 52 | 0.561 | 0.721 |
| Intelligence | Panic disorder | 0.06 (0.26) | 1.06 (0.64 to 1.76) | 52 | 0.815 | 0.884 |
| Intelligence | PTSD | 0.00 (0.02) | 1.00 (0.96 to 1.05) | 52 | 0.992 | 0.992 |
| Intelligence | Schizophrenia | −0.67 (0.13) | 0.51 (0.40 to 0.66) | 52 | <0.001 | 1.38E−06 |
| Intelligence | TS | 0.10 (0.09) | 1.11 (0.92 to 1.32) | 52 | 0.271 | 0.407 |
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; BD, bipolar disorder; CI, confidence interval; EA, educational attainment; FDR, false discovery rate; MDD, major depressive disorder; N_IV, number of instrumental variable; OCD, obsessive-compulsive disorder; OR, odds ratio; PTSD, post-traumatic stress disorder; SE, standard error; TS, Tourette’s syndrome.
The average causal effect of intelligence on the mental disorders did not change after MVMR (OR_MR: 0.96 vs OR_MVMR: 0.97). The protective effects of intelligence vanished in ADHD and insomnia. Notably, in MVMR, higher intelligence was a risk factor for MDD (OR: 1.12). Higher intelligence remained a protective factor for schizophrenia (OR: 0.51).
The overall effects of household income on the mental disorders had little change in the MVMR analysis (OR_MR: 0.88 vs OR_MVMR: 0.82). After adjustments for EA and intelligence in the MVMR analysis, higher income ceased to serve as a protective or risk factor for anorexia nervosa, alcohol dependence and TS but turned into a protective factor for BD (OR: 0.67) and ASD (OR: 0.73). After the MVMR adjustment, higher income remained a protective factor for schizophrenia (OR: 0.37), insomnia (OR: 0.93), MDD (OR: 0.67), anxiety disorder (OR: 0.88), PTSD (OR: 0.83), panic disorder (OR: 0.39) and ADHD (OR: 0.90).
Discussion
Main findings
In this study, we comprehensively explored the genetic relationships between 3 psychosocial factors and 13 common mental disorders. We observed overall negative genetic correlations between three psychosocial factors and the mental disorders. However, several mental disorders have positive correlations with EA or income, including ASD, anorexia nervosa, BD and OCD. These genetic correlations were largely consistent with those uncovered by MR analysis.
Of note, the strongest negative genetic correlations with all three psychosocial factors were observed for ADHD. This finding should be interpreted as the protection offered by the genetic components associated with high EA (OR: 0.72), intelligence (OR: 0.85) and income (OR: 0.76). This observation is consistent with results from previous studies.10 18 19 However, in the MVMR, the observed protective effect of intelligence on ADHD was diminished (OR: 0.95). Our results indicate the strong confounding effects of the three psychosocial factors on ADHD. It seems that the protective effects of intelligence on ADHD are chiefly mediated by EA and income.
In some cases, the results of MR and MVMR analyses differed substantially. For example, our MR analysis highlighted the protective effects of EA on eight mental disorders, which were somewhat counterbalanced by the risks for anorexia nervosa (OR: 1.44), OCD (OR: 1.29), BD (OR: 1.38) and ASD (OR: 1.21). However, the MVMR analysis pointed towards the direct detrimental effects of EA on several mental disorders. In other words, the unfavourable effects of EA on mental disorders became apparent after subtracting the confounding influences of the other two factors. Although the MR results did support the protective effects of EA on MDD (OR: 0.84), this protection disappeared after MVMR-guided dissection (OR: 1.02). Our results suggest that the survey-observed negative associations between EA and MDD should be attributed to the confounding protective effect of income.
Our MR analysis showed that higher intelligence was a protective factor for three mental disorders (schizophrenia, ADHD and insomnia) but a risk factor for ASD (OR: 1.15). In MVMR, higher intelligence turned from a neutral to a risk factor for MDD (OR: 1.12). Notably, higher intelligence remained an independent protective factor for schizophrenia (OR: 0.51). After MVMR-guided adjustment for confounders, higher intelligence was no longer marked as a protective factor for ADHD and insomnia.
After MVMR-guided adjustment for confounders, higher income behaved as a protective factor for 9 out of the 13 mental disorders, including schizophrenia (OR: 0.37), BD (OR: 0.67), ASD (OR: 0.73), MDD (OR: 0.67), anxiety disorder (OR: 0.88), insomnia (OR: 0.93), panic disorder (OR: 0.39), PTSD (OR: 0.83) and ADHD (OR: 0.90). In summary, both the overall and the independent effects of income on the mental disorders were beneficial (OR_MR: 0.88 vs OR_MVMR: 0.82).
The observed causal influences of the three factors on schizophrenia are worth specific attention. In the MR analysis, the effects of EA on schizophrenia were non-significant, while the other two factors conferred robust protective effects on this psychiatric condition. In the MVMR analysis, EA conferred the highest causal effect on schizophrenia (OR: 3.43), while the other two factors strongly protected against schizophrenia, with the protective effect of higher income being the highest (OR: 0.37).
The observed protective roles of higher income and higher intelligence in schizophrenia were consistent with the large body of accumulated evidence.20–23 In our study, EA behaved as a risk factor for schizophrenia, which is consistent with the genetic findings that additional years of schooling are genetically associated with an increased risk for this psychiatric condition.21 24 However, previous cohort survey studies revealed lower risks of schizophrenia in cohorts with additional years of education.25 The paradoxical relationships between genetic prediction and cohort observations may be due to a reverse causation bias, namely, obvious impairments of school performance after the onset of psychotic symptoms in individuals with schizophrenia. Moreover, patients with schizophrenia are less likely to enter higher education due to premorbid cognitive impairments.26
BD genetically overlaps with schizophrenia. While being associated with some cognitive deficits, BD predisposes individuals to certain creativity and cognitive adeptness, thus being distinct from schizophrenia in its neurodevelopmental aspects. The distribution of educational levels of patients with BD is similar to that in the general population.27 A detrimental effect of EA on BD was detectable in our analyses, although at a smaller scale than that seen for schizophrenia. Our MR analysis showed that EA was an overall risk factor for BD (OR: 1.38). In MVMR, its causal effects were much stronger than those of BD (OR: 2.11). Therefore, higher EA is a remarkable risk factor for BD.
Another interesting case is anorexia nervosa, where the detrimental effects of both EA and income were relatively strong in MR but diminished to non-significance in the results of MVMR. Performance at school or educational level is positively associated with the risk of eating disorders, and striving for perfection is proposed as an explanation for this association.28 29 In a previous MR study, a higher EA adjusted for intelligence was reported to be associated with a higher risk of anorexia nervosa.30 Our MR analysis indicates that, in addition to higher EA, higher household income is also an overall risk factor for anorexia nervosa. However, neither EA nor income could independently confer risk for anorexia nervosa in the MVMR analysis.
In summary, when the independent effects of the three factors were accounted for, higher EA represented a risk factor for mental health, higher income served as a definite protective factor, and the impacts of intelligence on the mental disorders appeared to be mixed. However, when the much higher prevalence and population disease burden of MDD are taken into consideration along with the detrimental effects of intelligence on MDD, an overall trend describing the influence of intelligence on these mental disorders becomes unfavourable.
To some extent, intelligence represents the inherent mental capacity of a human being, EA may reflect a process of socialisation from birth to adulthood, and income denotes the outcome of this socialisation. Our results suggest that the protective effects of EA and intelligence on mental disorders, as observed in the MR analysis, mainly stem from the mediating effects of income. It seems that higher intelligence or high EA will be of little benefit to mental health when they cannot be translated into higher socioeconomic status. Education is a long and stress-increasing process. While a majority of mental problems occur at the life stage of studying, the higher socioeconomic outcomes of EA compensate for the toll the study period took on mental health, with an outcome depending on all of the above and reflected in a cumulative incidence of mental disorders within the lifetime.
Limitations
Although the MR framework provides an advantage over traditionally designed epidemiological studies, several limitations of our study should be noted. In particular, both MR and MVMR analyses concentrate exclusively on the genetic liability to studied traits, with no regard to their environmental components. Moreover, as the heritability of intelligence may be in a non-linear relationship with socioeconomic status, a more granular view on the correlation puzzle of multiple factors connecting mental disorders with psychosocial factors is warranted. We should also emphasise that observed relationships may not be translatable into actionable items through socioeconomic interventions. Pleiotropy is a well-known source of bias in MR analyses, especially in non-homogenous datasets. While the MR-Egger procedure did not support directional pleiotropy in our study, some evidence supporting the potential heterogeneity among the individual IV effect estimates was reported.
Implications
Here, we dissect the complexity of causal connections between EA, intelligence, income and 13 common mental disorders. The results of this study may aid in clarifying inconsistencies noted in previously published research and add some insights to the current understanding of the relationship between psychosocial factors and mental disorders.
Acknowledgments
The authors thank all investigators and participants from the Psychiatric Genomics Consortium and other groups for sharing these data.
Biography
Ancha Baranova graduated with a PhD from Moscow State University in 1998 and with a DSci from the Russian Academy of Science. Currently, she is a professor in the School of Systems Biology at George Mason University in Virginia. She is an expert in human pathophysiology and human genetics, with an emphasis on chronic liver diseases, cancer and anti-ageing research. Her lab has discovered many novel biomarkers, the biosynthesis of melanin in human adipose, some properties of cell-free DNA and a variety of novel functions for known biomolecules. Her main research interests include major pathophysiological components of human ageing: systemic inflammation, insulin resistance and organ fibrosis. Her works in personalised medicine emphasise longitudinal monitoring and management of health in pre-symptomatic individuals and augmenting the body’s homeostasis by non-pharmacological means.

Footnotes
AB and HC contributed equally.
Contributors: FZ conceived the project and supervised the study. AB, HC and FZ wrote the manuscript. FZ analysed the data and created the figures and tables. All authors read and approved the final manuscript. FZ is
responsible for the overall content as the guarantor.
Funding: This study was funded by Nanjing Medical Science and Technology Development Project (ZKX20027).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Wraw C, Deary IJ, Gale CR, et al. Intelligence in youth and health at age 50. Intelligence 2015;53:23–32. 10.1016/j.intell.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clouston SAP, Richards M, Cadar D, et al. Educational inequalities in health behaviors at midlife: is there a role for early-life cognition? J Health Soc Behav 2015;56:323–40. 10.1177/0022146515594188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conti G, Heckman J, Urzua S. The education-health gradient. Am Econ Rev 2010;100:234–8. 10.1257/aer.100.2.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosworth B. Increasing disparities in mortality by socioeconomic status. Annu Rev Public Health 2018;39:237–51. 10.1146/annurev-publhealth-040617-014615 [DOI] [PubMed] [Google Scholar]
- 5. Baranova A, Zhao Y, Cao H, et al. Causal associations between major depressive disorder and COVID-19. Gen Psychiatr 2023;36:e101006. 10.1136/gpsych-2022-101006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao H, Baranova A, Song Y, et al. Causal associations and genetic overlap between COVID-19 and intelligence. QJM 2023;116:766–73. 10.1093/qjmed/hcad122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baranova A, Chandhoke V, Cao H, et al. Shared genetics and bidirectional causal relationships between type 2 diabetes and attention-deficit/hyperactivity disorder. Gen Psychiatr 2023;36:e100996. 10.1136/gpsych-2022-100996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai J, Wei Z, Chen M, et al. Socioeconomic status, individual behaviors and risk for mental disorders: a Mendelian randomization study. Eur Psychiatry 2022;65:e28. 10.1192/j.eurpsy.2022.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michaëlsson M, Yuan S, Melhus H, et al. The impact and causal directions for the associations between diagnosis of ADHD, socioeconomic status, and intelligence by use of a bi-directional two-sample Mendelian randomization design. BMC Med 2022;20:106. 10.1186/s12916-022-02314-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rao S, Baranova A, Yao Y, et al. Genetic relationships between attention-deficit/hyperactivity disorder, autism spectrum disorder, and intelligence. Neuropsychobiology 2022;81:484–96. 10.1159/000525411 [DOI] [PubMed] [Google Scholar]
- 11. Dardani C, Riglin L, Leppert B, et al. Is genetic liability to ADHD and ASD causally linked to educational attainment? Int J Epidemiol 2022;50:2011–23. 10.1093/ije/dyab107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee JJ, Wedow R, Okbay A, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 2018;50:1112–21. 10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang L, Zheng Z, Qi T, et al. A resource-efficient tool for mixed model association analysis of large-scale data. Nat Genet 2019;51:1749–55. 10.1038/s41588-019-0530-8 [DOI] [PubMed] [Google Scholar]
- 14. Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–9. 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jansen PR, Watanabe K, Stringer S, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet 2019;51:394–403. 10.1038/s41588-018-0333-3 [DOI] [PubMed] [Google Scholar]
- 16. Bulik-Sullivan BK, Loh P-R, Finucane HK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 2015;47:291–5. 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sauver JLSt, Barbaresi WJ, Katusic SK, et al. Early life risk factors for attention-deficit/hyperactivity disorder: a population-based cohort study. Mayo Clinic Proceedings 2004;79:1124–31. 10.1016/S0025-6196(11)62594-9 [DOI] [PubMed] [Google Scholar]
- 19. Keilow M, Wu C, Obel C. Cumulative social disadvantage and risk of attention deficit hyperactivity disorder: results from a nationwide cohort study. SSM Popul Health 2020;10:100548. 10.1016/j.ssmph.2020.100548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hakulinen C, Webb RT, Pedersen CB, et al. Association between parental income during childhood and risk of schizophrenia later in life. JAMA Psychiatry 2020;77:17–24. 10.1001/jamapsychiatry.2019.2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams CD. A multivariable mendelian randomization to appraise the pleiotropy between intelligence, education, and bipolar disorder in relation to schizophrenia. Sci Rep 2020;10:6018. 10.1038/s41598-020-63104-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hill WD, Davies NM, Ritchie SJ, et al. Genome-wide analysis identifies molecular systems and 149 genetic loci associated with income. Nat Commun 2019;10:5741. 10.1038/s41467-019-13585-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dohrenwend BP, Levav I, Shrout PE, et al. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science 1992;255:946–52. 10.1126/science.1546291 [DOI] [PubMed] [Google Scholar]
- 24. Bansal V, Mitjans M, Burik CAP, et al. Genome-wide association study results for educational attainment aid in identifying genetic heterogeneity of schizophrenia. Nat Commun 2018;9:3078. 10.1038/s41467-018-05510-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo Y, Pang L, Zhao Y, et al. Gender difference in the association between education and schizophrenia in Chinese adults. BMC Psychiatry 2020;20:296. 10.1186/s12888-020-02700-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dickson H, Hedges EP, Ma SY, et al. Academic achievement and schizophrenia: a systematic meta-analysis. Psychol Med 2020;50:1949–65. 10.1017/S0033291720002354 [DOI] [PubMed] [Google Scholar]
- 27. Schoeyen HK, Vaaler AE, Auestad BH, et al. Despite clinical differences, bipolar disorder patients from acute wards and outpatient clinics have similar educational and disability levels compared to the general population. J Affect Disord 2011;132:209–15. 10.1016/j.jad.2011.02.025 [DOI] [PubMed] [Google Scholar]
- 28. Sundquist J, Ohlsson H, Winkleby MA, et al. School achievement and risk of eating disorders in a Swedish national cohort. J Am Acad Child Adolesc Psychiatry 2016;55:41–6. 10.1016/j.jaac.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schilder CMT, Sternheim LC, Aarts E, et al. Relationships between educational achievement, intelligence, and perfectionism in adolescents with eating disorders. Int J Eat Disord 2021;54:794–801. 10.1002/eat.23482 [DOI] [PubMed] [Google Scholar]
- 30. Yuan S, Xiong Y, Michaëlsson M, et al. Genetically predicted education attainment in relation to somatic and mental health. Sci Rep 2021;11:4296. 10.1038/s41598-021-83801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2023-101080supp001.pdf (81KB, pdf)
Data Availability Statement
No data are available.