Abstract
Protein kinase C (PKC) plays a crucial role(s) in regulation of growth and differentiation of cells. In the present study, we examined possible roles of the α, δ, η, and ζ isoforms of PKC in squamous differentiation by overexpressing these genes in normal human keratinocytes. Because of the difficulty of introducing foreign genes into keratinocytes, we used an adenovirus vector system, Ax, which allows expression of these genes at a high level in almost all the cells infected for at least 72 h. Increased kinase activity was demonstrated in the cells overexpressing the α, δ, and η isoforms. Overexpression of the η isoform inhibited the growth of keratinocytes of humans and mice in a dose (multiplicity of infection [MOI])-dependent manner, leading to G1 arrest. The η-overexpressing cells became enlarged and flattened, showing squamous cell phenotypes. Expression and activity of transglutaminase 1, a key enzyme of squamous cell differentiation, were induced in the η-overexpressing cells in dose (MOI)- and time-dependent manners. The inhibition of growth and the induction of transglutaminase 1 activity were found only in the cells that express the η isoform endogenously, i.e., in human and mouse keratinocytes but not in human and mouse fibroblasts or COS1 cells. A dominant-negative η isoform counteracted the induction of transglutaminase 1 by differentiation inducers such as a phorbol ester, 1α,25-dihydroxyvitamin D3, and a high concentration of Ca2+. Among the isoforms examined, the δ isoform also inhibited the growth of keratinocytes and induced transglutaminase 1, but the α and ζ isoforms did not. These findings indicate that the η and δ isoforms of PKC are involved crucially in squamous cell differentiation.
The epidermis, a major component that exists between the organism and the environment, functions as a barrier to external surroundings due to its architectural structure and immunological competence. The structure of the epidermis is maintained by a finely tuned balance between keratinocyte proliferation and differentiation, which results in a multilayer structure consisting of basal, spinous, granular, and cornified layers. Keratinocyte differentiation progresses through the multiple steps under a tightly regulated program. Once committed to differentiation, the basal cells lose their proliferative potential and move toward the terminally differentiated cornified layer. As this process progresses, differentiation-associated proteins are sequentially induced. A pair of K5/K14 keratin molecules in the basal layer shifts to K1/K10 in the spinous layer (13). Substrate proteins of keratinocyte-specific transglutaminase 1 (TGase 1) are expressed in the spinous and granular layers; these proteins include involucrin, loricrin, filaggrin, and SPR (13, 21, 28, 44). Finally, TGase 1 covalently cross-links its substrate proteins through γ-glutamyl-ɛ-lysine isopeptide bonds, forming a cornified envelope, a chemically resistant membrane structure present beneath the plasma membrane of terminally differentiated keratinocytes (46). However, the mechanisms by which the differentiation process is regulated remain unclear.
Several reports illustrate the emerging importance of protein kinase C (PKC) in signaling pathways of keratinocyte differentiation. 12-O-Tetradecanoylphorbol-13-acetate (TPA), a potent activator of PKC, induces TGase 1 activity and stimulates formation of the cornified envelope (50). The elevated level of Ca2+ ion, a trigger of terminal differentiation of the cultured keratinocytes, accelerates the intracellular phosphatidylinositol turnover (24), yielding diacylglycerol (DAG), an endogenous activator of PKC (34). Dlugosz et al. reported that TPA induces filaggrin and loricrin while it inhibits the expression of K1/K10, which suggests that PKC is involved in the transition process from the spinous to the granular layer (10).
PKC exists as a family composed of at least 10 isoforms. The PKC isoforms are classified into three major groups based on their structures and activation mechanisms (35), i.e., phosphatidylserine (PS)-, DAG- and Ca2+-dependent conventional PKC (α, βI, βII, and γ) isoforms; Ca2+-independent novel PKC (δ, ɛ, η, and θ) isoforms; and DAG- and Ca2+-independent atypical PKC (ζ and λ/ι) isoforms. Some of these isoforms, e.g., α, δ, and ζ, are distributed ubiquitously in almost all tissues, whereas the others are localized in a tissue- or cell-type-specific manner. Furthermore, each isoform possesses discrete properties and functions in the activation and downregulation (2, 9, 17, 32, 33), subcellular localization (3, 12, 15), and induction of differentiation (25–27, 42).
Of these PKC isoforms, we isolated the η and θ isoforms from a cDNA library of mouse skin (39, 40). The η isoform has a unique tissue distribution: it is predominantly expressed in epithelial tissues, including skin, tongue, esophagus, stomach, intestine, trachea, and bronchus (39, 41). In situ hybridization and immunohistochemical staining demonstrated that the η isoform is highly expressed in differentiating and differentiated epithelial cells (41). Furthermore, cholesterol sulfate (CS), an abundant lipid in the granular layer, activates the η and other isoforms (9, 17). It has been shown that CS induces differentiation of mouse keratinocytes in vitro and in vivo (4, 9) and activates transcription of TGase 1 in normal human keratinocytes (23). It also inhibits tumor promotion in mouse skin carcinogenesis (4).
Studies with keratinocytes have been hampered by the difficulty of introducing foreign genes; the transfection efficiency by conventional methods is very low (18), and the use of Ca2+ often induces terminal differentiation (51). Furthermore, the shorter life span of normal human keratinocytes does not allow isolation of stable transformants. In the present study, we used an adenovirus expression vector, Ax, by which PKC genes were expressed at higher levels in almost all of the infected cells. All the data obtained here indicate that the η and δ isoforms play a crucial role in a signal transduction pathway that mediates differentiation in normal human keratinocytes.
MATERIALS AND METHODS
Cell culture.
Normal human keratinocytes were isolated from skin sections discarded during plastic surgery by using a modified method described elsewhere (47). Isolated keratinocytes were cultured in serum-free keratinocyte growth medium (KGM) containing a low level of Ca2+ (0.03 mM). BALB/MK2, a keratinocyte cell line of mice, was cultured in low-Ca2+ (0.05 mM) Eagle’s minimal essential medium (MEM) containing 8% dialyzed fetal calf serum (FCS) and epidermal growth factor (4 ng/ml). Normal human fibroblasts were isolated from skin sections. Normal human fibroblasts, COS1 cells, and 293 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FCS, while BALB 3T3 cells were cultured in MEM containing 10% FCS. All of the cells were grown at 37°C in a 5% CO2-containing atmosphere.
Cell growth assay.
The growth of cells was measured by the MTT assay (Promega, Madison, Wis.). DNA synthesis was monitored by incorporation of 5-bromo-2′-deoxyuridine (BrdU) and visualized with an anti-BrdU antibody (Amersham, Buckinghamshire, United Kingdom). Three hundred or more nuclei were counted for determination of BrdU-positive cells.
Cell cycle analysis.
The cells were stained with propidium iodide (416 μg/ml) for 20 min, and cell cycle distribution was analyzed by flow cytometry with a Becton Dickinson (San Jose, Calif.) FACScan.
Construction of adenovirus vector.
The cosmid cassette pAxCAwt has a nearly full length Ad5 genome but lacks regions E1 and E3 (19). It contains a composite CAG promoter, consisting of cytomegalovirus immediate-early enhancer, chicken β-actin promoter, and rabbit β-globin polyadenylation signal, which strongly induces expression of inserted DNAs (36).
The cDNAs coding for the entire rabbit α isoform (37), the mouse δ isoform (31), the mouse η isoform (39), a dominant-negative mutant of the η isoform, described below, the mouse ζ isoform of PKC (14), and β-galactosidase were inserted into the SwaI site of pAxCAwt (30, 36). By using these cosmids, recombinant adenoviruses containing each PKC gene were generated by the COS-TPC method (30). The cosmids were mixed with the EcoT22I-digested DNA-terminal protein complex of Ad5-dIX (43) and transfected into 293 cells, in which a recombinant adenovirus vector was generated through homologous recombination. Highly concentrated virus was purified, and the titer was determined as described by Kanegae et al. (20). DMEM used for cultivation of 293 cells was replaced with KGM before infection of cells.
Construction of a dominant-negative η isoform of PKC.
The dominant-negative mutant of the η isoform was generated by PCR by the substitution of alanine for the lysine 384 residue at the ATP binding site of the catalytic domain (38). The 192-bp SalI-MaeII fragment corresponding to nucleotide positions 1034 to 1225 of the wild-type mouse η isoform cDNA was replaced with a SalI-MaeII PCR fragment carrying the mutation. The PCR fragment was ligated into the PUC18 vector, and its sequence and orientation were confirmed by the restriction enzyme digestion pattern and sequencing with a set of oligonucleotide primers outside of the PCR fragment. The oligonucleotide primers used for mutagenesis were 5′-CATTTCCAGGTCGACACTAAGACGGCA-3′ and 5′- TGCAGAATCACGTCCTTCTTCAGCACCGCCACGGCGT-3′ (the under- lined sequence was replaced in the mutation). The dominant-negative nature of the η isoform was confirmed functionally by the inability to autophosphorylate, as described below.
PKC activity assay.
PKC activity was assayed by using a partially purified fraction, since immunoprecipitation itself was found to activate the kinase activity. Normal human keratinocytes were disrupted by sonication in homogenizing buffer consisting of 20 mM Tris-HCl (pH 7.5), 0.25 M sucrose, 2 mM EDTA, 0.5 mM EGTA, 50 mM β-mercaptoethanol, 100 μg of leupeptin per ml, and 2 mM phenylmethylsulfonyl fluoride (PMSF). The suspension was incubated in the presence of 0.5% Triton X-100 for 1 h and centrifuged at 5,000 × g for 20 min. PKC was partially purified by DE52 column chromatography as previously described (17). Protein kinase activity was measured by incorporation of 32P from [γ-32P]ATP into myelin basic protein (MBP), the most preferred substrate of the η isoform (17). An aliquot of cell lysate (1.2 μg) was incubated in buffer containing 20 mM Tris-HCl (pH 7.5), 5 mM MgSO4, 1 mM EGTA, 100 mM ATP, 1 mCi of [γ-32P]ATP (30 Ci/mmol), 10 μg of MBP, 50 μg of phosphatidylserine (PS) per ml, and 50 ng of TPA per ml or 25 μM CS. After incubation for 10 min at 30°C, the reaction was stopped by the addition of 75 mM phosphoric acid and the mixture was applied to P81 paper (Whatman, Maidstone, United Kingdom), followed by washing with 75 mM phosphoric acid and Cherenkov counting. One unit of kinase activity corresponds to the incorporation of 1 nmol of radioactive phosphate/min from ATP into MBP.
Autophosphorylation.
COS1 cells were infected by Ax carrying the η isoform (Ax-PKCη) or its dominant-negative mutant. Cell lysates were prepared as described above and immunoprecipitated with anti-η isoform antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) as described elsewhere (39). An aliquot of the immunoprecipitate was added to the assay mixture of 20 mM Tris-HCl (pH 7.5), 5 mM magnesium acetate, 0.01% leupeptin, 2 mM PMSF, 1 μM ATP, 1 μCi of [γ-32P]ATP (6,000 Ci/mmol), 25 μg of PS per ml, and 50 ng of TPA per ml at 0°C, and a reaction was allowed to take place for 20 min. The resulting pellets were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
Northern blot analysis.
Total RNA was extracted by the guanidinium-hot phenol method. An aliquot (20 μg) of total RNA was used for Northern blot hybridization with 32P-labeled human full-length TGase 1 (49) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (11) as probes. After hybridization at 42°C overnight, the membranes were washed three times at 42°C in 0.2× SSC (1× SSC is 150 mM sodium chloride plus 15 mM sodium citrate) containing 0.1% SDS and autoradiographed.
Immunoblot analysis.
Cells were collected with the standard SDS sample buffer and boiled at 95°C for 5 min. After centrifugation, supernatants were electrophoresed on an SDS–10% PAGE gel and blotted onto a nitrocellulose filter. After blocking with 3% gelatin, the membranes were incubated with anti-α and anti-ζ isoform antibody (Santa Cruz Biotechnology), and anti-δ isoform antibody (Seikagaku Kogyo, Tokyo, Japan), and anti-η isoform antibody raised against synthetic peptides of the D2 and D3 region as described previously (39). The corresponding proteins were visualized with peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibody and an enhanced chemiluminescence (ECL) system (Renaissance; Dupont NEN, Boston, Mass.). Filters were exposed for shorter periods to adjust to the overexpressed gene products.
Immunofluorescence staining.
Cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 20 min and permeabilized with acetone for 30 s for staining of TGase 1 or with cold methanol-acetone (1:1) for 10 min for staining of the η isoform. After blocking with 3% goat serum, the cells were incubated with anti-TGase 1 monoclonal antibody (B.C1; Biomedical Technology Inc., Stoughton, Mass.) or anti-η isoform antibody against the D2 and D3 region in 1% goat serum in PBS for 1 h. After washing, the cells were incubated with goat anti-mouse IgG or anti-rabbit IgG coupled to fluorescein isothiocyanate for 1 h. After five washes of 5 min each in PBS and two washes in water, the stained cells were mounted on glass slides with a PBS-glycerol solution (1:9, vol/vol) and observed under a fluorescence microscope.
Assay of TGase 1 activity.
Cells were sonicated in a buffer consisting of 2 mM HEPES (pH 7.2) containing 2 mM EDTA. After centrifugation at 40,000 × g for 1 h, the resulting pellets were suspended in 2 mM HEPES (pH 7.2) containing 2 mM EDTA, 2 mM dithiothreitol, and 0.3% (vol/vol) Triton X-100 at 0°C for 1 h, followed by centrifugation at 40,000 × g for 1 h. An aliquot of the supernatant containing 20 μg of protein was reacted in 250 μl of reaction buffer (0.25 mM Tris-HCl [pH 8.3], 5 mM CaCl2, 5 mM dithiothreitol, 0.25% Triton X-100, 3.3 mg of α-casein per ml, 0.1 μCi of [3H]putrescine) at 35°C for 30 min. The reaction was stopped by adding 250 μl of 10% (wt/vol) trichloroacetic acid. The pellets were filtered onto glass fiber filters (Whatman GF/A) and washed four times with 5% trichloroacetic acid containing 1% (wt/vol) putrescine. One unit of activity corresponds to 1 nanomole of putrescine incorporated into casein per h per mg of protein.
Transfection to COS1 cells.
The SRD expression plasmids (45) were constructed by introducing cDNAs of the η isoform (39) and TGase 1 (49) and then transfected into COS1 cells as described elsewhere (39). TGase 1 activity was measured 48 h after the transfection with or without treatment with 100 nM TPA for 15 min.
RESULTS
Overexpression of PKC isoforms by adenovirus vector in normal human keratinocytes.
We constructed the adenovirus vectors carrying the cDNAs of the α, δ, η, and the dominant-negative η and ζ isoforms of PKC (Ax-PKCα, Ax-PKCδ, Ax-PKCη, Ax-PKC D/Nη, and Ax-PKCζ, respectively). These four isoforms are expressed in epithelial tissues (39, 41). Throughout the study, the adenovirus vector carrying the β-galactosidase gene, Ax-lacZ, was used as a negative control to exclude possible deleterious effects of the vector itself.
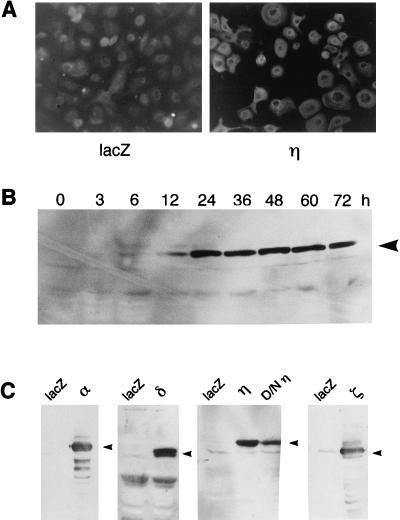
We first examined the expression levels of the PKC isoform proteins in normal human keratinocytes after infection with the above-mentioned Ax adenovirus vectors. By immunofluorescence staining, the η isoform was found to be expressed strongly in all cells of the infected cultures, while in the control cells without infection, weak signals of the endogenous η isoform were seen (Fig. 1A). Figure 1C demonstrates the overexpression of the α, δ, η, and ζ isoforms and the dominant-negative mutant of the η isoform. The expression lasted for at least 72 h; as shown in Fig. 1B, immunoblots of the η isoform appeared within 12 h after infection, reaching a maximal level at 24 h and maintaining a high level up to 72 h after infection. Signals of the endogenous isoforms were barely visible due to shorter exposure.
FIG. 1.
Expression of PKC isoforms in adenovirus-infected normal human keratinocytes. (A) Immunofluorescence staining of the η isoform in the Ax-PKCη-infected cells. Normal human keratinocytes were infected with Ax-lacZ (lacZ) or Ax-PKCη (η) for 48 h (MOI = 6). (B) Time course of the immunoblots of the η isoform protein in Ax-PKCη-infected cells (MOI = 6). (C) Immunoblotting of PKC isoforms in adenovirus-infected cells. Cells were infected with Ax-lacZ (lacZ), Ax-PKCη (η), Ax-D/NPKCη (D/Nη), Ax-PKCα (α), Ax-PKCδ (δ), and Ax-PKCζ (ζ) for 48 h (MOI = 6).
Such a high efficiency of gene transfer by the adenovirus vectors makes it possible to study the possible functions of PKC isoforms in growth and differentiation of keratinocytes.
Kinase activity.
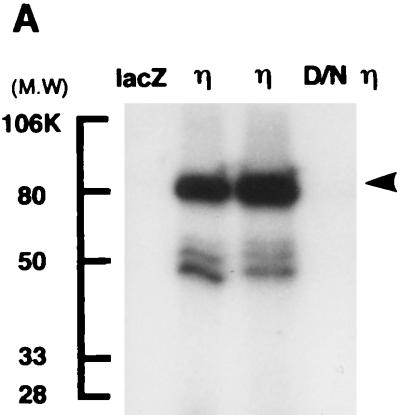
The kinase activity of the η isoform introduced by Ax-PKCη was demonstrated by autophosphorylation of the immunoprecipitated material from COS1 cells and also with a partially purified fraction of normal human keratinocytes. As shown in Fig. 2A, a phosphorylated band with a molecular mass of 82 kDa was immunoprecipitated with the anti-η antibody from Ax-PKCη-infected COS1 cells but not from the dominant-negative η isoform- or Ax-lacZ-infected control cells.
FIG. 2.
Kinase activity in adenovirus-infected cells. (A) Autophosphorylation of the η isoform expressed in COS1 cells. COS1 cells were infected with Ax-lacZ (lacZ; MOI = 6), Ax-PKCη (η; lane 2, MOI = 3; lane 3, MOI = 6), or the dominant-negative mutant of PKCη (D/Nη; MOI = 6) for 48 h. The extracts were immunoprecipitated with antibody against the η isoform and reacted with [γ-32P]ATP; this was followed by SDS-PAGE and autoradiography. The arrowhead indicates the position of the η isoform. Molecular weight (MW) standards in thousands (K) are shown on the left. (B) Kinase activities of the cells overexpressing the α, δ, η, and ζ isoforms. Partially purified cell extracts were incubated for kinase assay in the presence of PS (50 μg/ml) plus TPA (50 ng/ml) or CS (25 μM). (C) MOI-dependent kinase activity of the Ax-PKCη-infected normal human keratinocytes. Partially purified cell extracts were incubated for assay of kinase activity in the presence (hatched bars) or absence (stippled bars) of PS (50 μg/ml) plus TPA (50 ng/ml).
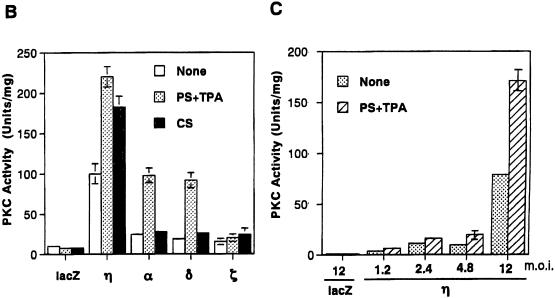
In the experiment shown in Fig. 2B and C, DEAE-purified cell lysates were prepared from normal human keratinocytes infected with the PKC adenovirus vectors and assayed for kinase activity in the presence or absence of the activators. As shown in Fig. 2B, infection with the adenovirus vectors of the α, δ, and η isoforms markedly induced the kinase activity whereas a marginal increase was observed with infection by Ax-PKCζ. The activity of the η isoform was highly stimulated in the presence of PS plus TPA and CS, being 20- to 30-fold higher than the activity of nontreated or lacZ-infected cells. In the α- and δ-overexpressing cells, the activity was stimulated only by PS plus TPA. Figure 2C shows that the kinase activity increased with increasing multiplicity of infection (MOI). These results indicate that the overexpressed η isoform, as well as the α and δ isoforms, is enzymatically active and responds to the activators.
Induction of morphological differentiation.
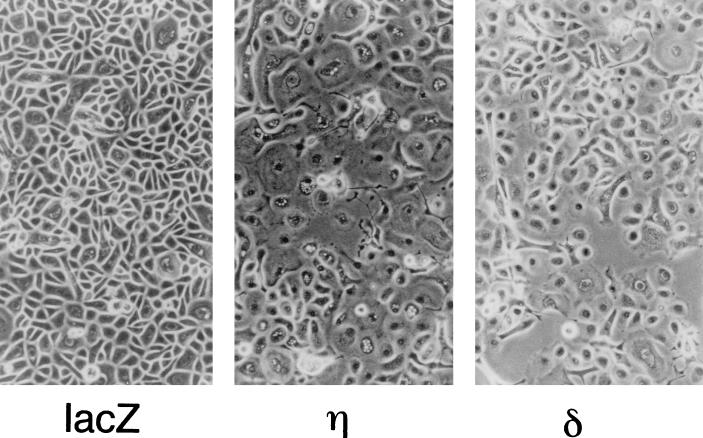
Normal human keratinocytes grown in low-Ca2+ medium (0.03 mM) have characteristics of basal cells with regard to cell morphology, growth potential, and expression of the basal cell-specific genes (47). We examined the effects of individual PKC isoforms on cell morphology when the cells were infected with the adenovirus vectors (Fig. 3). At 24 to 36 h after infection with Ax-PKCη, an apparent morphological change was observed, and at 48 h, most cells became enlarged and flattened, suggesting squamous cell differentiation (Fig. 3). These phenotypic changes became more remarkable with an increase in MOI. Overexpression of the δ isoform also induced morphological changes, with cells showing a spindle shape rather than being enlarged and flattened. On the other hand, no morphological changes were induced by the overexpression of the α or ζ isoform (data not shown).
FIG. 3.
Morphology of normal human keratinocytes overexpressing the η and δ isoforms. Cells were infected with Ax-lacZ (lacZ), Ax-PKCη (η), or Ax-PKCδ (δ) at an MOI of 12 and cultured for 48 h.
Keratinocyte-specific growth inhibition.
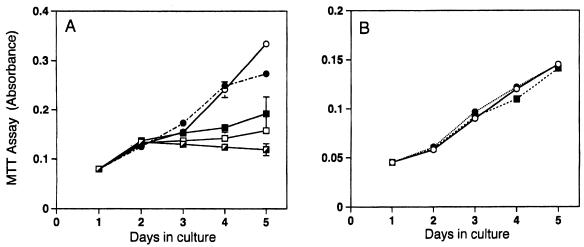
In the process toward terminal differentiation, keratinocytes undergo irreversible growth inhibition. We investigated whether PKC isoforms inhibit the growth of normal human keratinocytes. When infected with the η isoform, growth was suppressed with increasing MOI; no or little growth was observed with an MOI higher than 6 (Fig. 4A). In contrast, normal human fibroblasts were not inhibited by overexpression of the η isoform (Fig. 4B). Similarly, infection with Ax-PKCη suppressed the growth of BALB/MK2 mouse keratinocytes to an extent similar to that of human keratinocytes but not that of BALB 3T3 fibroblasts (data not shown). The above data indicate that the inhibitory effect of the η isoform is cell type specific, being observed in cells that express it endogenously.
FIG. 4.
Growth inhibition by the η isoform of normal human keratinocytes but not of normal human fibroblasts. Cells were infected on day 1 and cultured for 5 days. Cell growth was monitored by the MTT assay. Values represent means ± standard deviations of three cultures. The experiments were repeated three times with reproducible results. (A) Normal human keratinocytes. Symbols: ○, no infection; •, Ax-lacZ (MOI = 12); ■, Ax-PKCη (MOI = 3); □, Ax-PKCη (MOI = 6); ┌, Ax-PKCη (MOI = 12). (B) Normal human fibroblasts. Symbols: ○, no infection; •, Ax-lacZ (MOI = 12); ■, Ax-PKCη (MOI = 12).
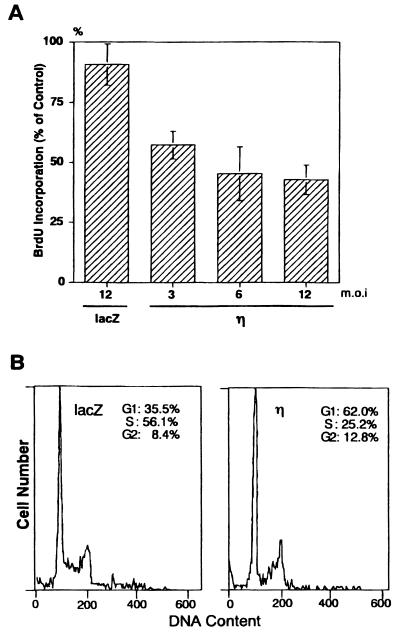
Inhibition of cell growth was further confirmed by the incorporation of BrdU. Overexpression of the η isoform reduced incorporation of BrdU to approximately 50% of that of the Ax-lacZ-infected control cells at 48 h after infection (Fig. 5A).
FIG. 5.
Inhibition of DNA synthesis (A) and cell cycle progression (B) by overexpression of the η isoform in normal human keratinocytes. (A) Cells were infected with Ax-lacZ (lacZ) or Ax-PKCη (η) for 44 h and then incubated for 4 h with BrdU. Values are means ± standard deviations of triplicate determinations and are shown as a percentage relative to the value for noninfected cells. (B) Cells were infected with Ax-lacZ (lacZ) or Ax-PKCη (η) at an MOI of 12 for 24 h. Cell cycle distribution was determined by flow cytometry.
Cell cycle distribution of the η isoform-overexpressing cells was analyzed by flow cytometry (Fig. 5B). Ax-PKCη-infected keratinocytes accumulated in G1 phase; 62% of cells were in G1 phase in the Ax-PKCη-infected cells compared to 35.5% in the Ax-lacZ-infected cells. In contrast, there were 25.2% cells in S phase in the Ax-PKCη-infected cells and 56.1% in the Ax-lacZ-infected cells. When stained with Hoechst 33258, fragmentation of nuclei was not observed (data not shown), indicating that the η isoform of PKC causes G1 arrest rather than apoptotic cell death.
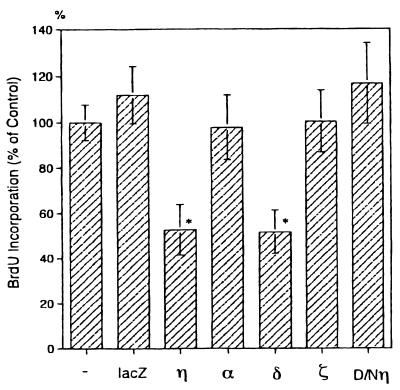
Among the four isoforms examined, the δ isoform was found to suppress the DNA synthesis of keratinocytes to the same extent as the η isoform did (Fig. 6). In contrast, overexpression of α and ζ isoforms had no effect on DNA synthesis.
FIG. 6.
Effect of various PKC isoforms on DNA synthesis in normal human keratinocytes. Cells were infected with Ax-lacZ (lacZ), Ax-PKCη (η), Ax-PKCα (α), Ax-PKCδ (δ), Ax-PKCζ (ζ), or Ax-D/Nη (D/Nη) for 44 h and then incubated for 4 h with BrdU. Values represent means ± standard deviations of triplicate determinations and are shown as a percentage relative to the value for noninfected cells (−). Asterisks indicate a significant difference from value of control cells (−), P < 0.01.
Induction and activation of TGase 1.
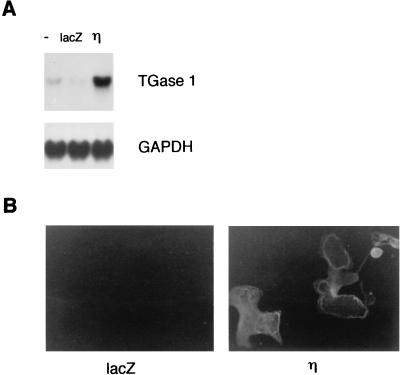
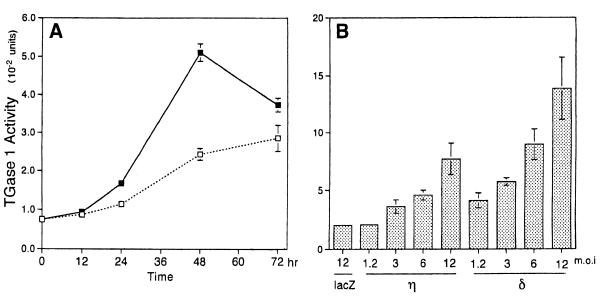
We found that infection of normal human keratinocytes with Ax-PKCη induced the expression of TGase 1 at the mRNA and protein levels and stimulated its activity. As shown in Fig. 7A, Northern blot analysis shows that mRNA of TGase 1 was strongly induced at 36 h after the infection. The expression of TGase 1 protein was confirmed by immunofluorescence staining, in which larger cells were stained preferentially (Fig. 7B). As shown in Fig. 8, the activity of TGase 1 increased in time-dependent and MOI-dependent manners, being maximal at 48 h after infection and when infected at an MOI of 12.
FIG. 7.
Expression of TGase 1 in normal human keratinocytes overexpressing the η isoform. (A) Northern blot analysis of TGase 1. Normal human keratinocytes were either noninfected (−) or infected with Ax-lacZ (lacZ) or Ax-PKCη (η) for 36 h (MOI = 12). (B) Immunofluorescence staining of TGase 1 protein. Cells were infected with Ax-lacZ (lacZ) or Ax-PKCη (η) for 60 h (MOI = 12) and stained by indirect immunofluorescence.
FIG. 8.
Activation of TGase 1 in normal human keratinocytes overexpressing the η isoform. (A) Time course of the induction of TGase 1 activity. Normal human keratinocytes were infected with Ax-lacZ (□) or Ax-PKCη (■) at an MOI of 12, and cell lysate was extracted at the indicated times. Values are means ± standard deviations of triplicate determinations. (B) MOI-dependent activation of TGase 1 by η and δ isoforms. Cells were infected with Ax-lacZ (lacZ), Ax-PKCη (η), or Ax-PKCδ (δ) at the indicated MOIs for 48 h.
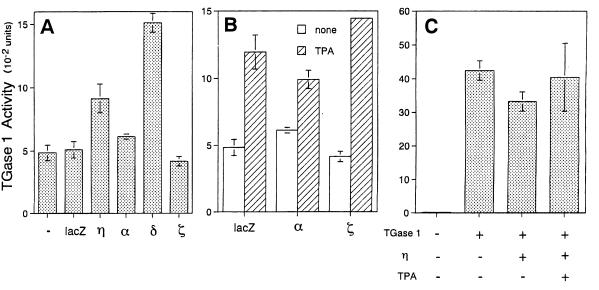
Of the four PKC isoforms examined, overexpression of the δ isoform was also found to stimulate TGase 1 activity in an MOI-dependent manner, with the extent of stimulation being higher than that resulting from η isoform overexpression (Fig. 8B and 9A). In contrast, overexpression of the α and ζ isoforms did not activate TGase 1 activity even in the presence of TPA (Fig. 9).
FIG. 9.
Effect of various PKC isoforms on TGase 1 activity. (A) Normal human keratinocytes were noninfected (−) or infected with Ax-lacZ (lacZ), Ax-PKCη (η), Ax-PKCα (α), Ax-PKCδ (δ), or Ax-PKCζ (ζ) at an MOI of 12 for 48 h. The results show the means ± standard deviations of three cultures. (B) Effect of TPA on TGase 1 activity in the α or ζ isoform-overexpressed cells. Normal human keratinocytes were infected with Ax-lacZ (lacZ), Ax-PKCα (α), or Ax-PKCζ (ζ) at an MOI of 12 for 48 h. TPA (2 ng/ml) was added at 24 h after infection. The results shows the means ± standard deviations of three cultures. (C) Effect of ectopic expression of the η isoform on TGase 1 activity in COS1 cells. COS1 cells were transfected with the TGase 1 gene with or without the PKCη gene using SRD isoform expression vector. TGase 1 activity was measured 48 h after transfection with or without the treatment with 100 nM TPA.
In the experiment shown in Fig. 9C, COS1 cells were cotransfected with cDNAs of the η isoform and TGase 1 by the conventional calcium phosphate method, followed by measurement of TGase 1 activity. Although no or little TGase 1 activity was found in COS1 cells, introduction of the TGase 1 gene greatly increased its activity. Cotransfection of the η isoform gene did not result in a further increase in TGase 1 activity even when the cells were treated with TPA. This result suggests that ectopic expression of the η isoform does not stimulate TGase 1 activity.
Expression of differentiation markers.
During the process of terminal differentiation, various marker proteins are expressed; these include K1/K10 keratins, loricrin, involucrin, SPR, and TGase 1. Of these markers, the expression of K1 keratin and loricrin was examined. Unlike TGase 1 expression, the expression levels of these markers were not changed in the overexpressing keratinocytes of all four isoforms examined (data not shown).
A dominant-negative mutant of the η isoform.
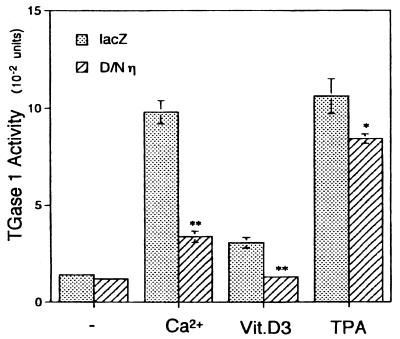
Further supporting evidence for the participation of the η isoform in keratinocyte differentiation was provided by the use of a dominant-negative mutant of the η isoform in which the lysine residue at the ATP binding site is replaced by alanine, abrogating the autophosphorylation activity (Fig. 2A). When normal human keratinocytes were treated with inducers of differentiation, e.g., TPA (50), 1α,25-dihydroxyvitamin D3 (16), and high concentrations of Ca2+ (51), TGase 1 activity was induced. However, expression of the dominant-negative η isoform diminished the activation of TGase 1 by these differentiation inducers (Fig. 10). The extent of inhibition was most remarkable in the differentiation induced by Ca2+.
FIG. 10.
Suppression of differentiation by the dominant-negative η isoform. Twelve hours after infection with Ax-lacZ (stippled bars) or Ax-D/Nη (hatched bars) at an MOI of 12, normal human keratinocytes grown in KGM were exposed to CaCl2 (Ca2+; 0.2 mM), 1α,25-dihydroxyvitamin D3 (Vit.D3; 12 nM), or TPA (1 ng/ml) for 24 h; measurement of TGase 1 activity followed. Values shown are the means ± standard deviations of three cultures. Asterisks indicate values that are significantly different from the values of Ax-lacZ-infected cells (∗, P < 0.05; ∗∗, P < 0.01).
DISCUSSION
In this report, we examined the role of the η and other isoforms in squamous cell differentiation by overexpressing them in normal human keratinocytes. We found that the η and δ isoforms induced a set of differentiation phenotypes, including morphological changes, growth inhibition, and the increased expression and activation of TGase 1 that are a late-stage marker of differentiation. Our observation implys that these isoforms are crucially involved in squamous cell differentiation. This argument is strengthened by the use of the dominant-negative η isoform that inhibits induction of TGase 1 by the known inducers of squamous cell differentiation.
We reported here that overexpression of the η isoform increased the TGase 1 mRNA level and its activity in normal human keratinocytes. It seems likely that the activity of TGase 1 is controlled by the transcription of the TGase 1 gene. Ueda et al. (48) reported that the η isoform induced luciferase activity under control of the 5′ upstream region of the human TGase 1 gene when transfected into the FRSK rat keratinocyte cell line. We further reported that CS activates transcription of TGase 1 in normal human keratinocytes (23). Alternatively, the increase of TGase 1 activity might be due to the phosphorylation of TGase 1 protein by the η isoform. However, this possibility is unlikely because Chakravarty et al. (1) reported that TGase 1 was phosphorylated by treatment with a phorbol ester but the activity remained unchanged.
Biological functions of the η isoform seem to be limited to keratinocytes that express this isoform endogenously: inhibition of cell growth was not observed in fibroblastic cells. Ectopic expression of TGase 1 and the η isoform in COS1 cells did not stimulate the activity of TGase 1. The report by Ueda et al. (48) indicates no increase of transcriptional activity of the TGase 1 gene in HT-1080 fibrosarcoma cells. These observations suggest the requirement of keratinocyte-specific cellular machineries for exhibition of physiological functions of the η isoform.
In keeping with the present observations, we found that a cell cycle machinery(ies) of mouse keratinocytes is regulated negatively by the η and δ isoforms (unpublished data). Previous studies have shown that the growth arrest of keratinocytes by transforming growth factor β or Ca2+ is associated with decreased activity of Cdk2 and increased expression of p21 (WAF1, Cip1, Sdi1) (8, 29). Our preliminary data show that Cdk2 activity decreased in the η isoform-overexpressing cells without induction of p21.
In the present study, we demonstrated that the overexpression of the δ isoform also induced growth inhibition and TGase 1 activity. The δ isoform is a major isoform expressed in the skin along with the η isoform (41). However, these isoforms show distinct tissue distribution, subcellular localization, and downregulation: the η isoform is highly expressed in the differentiated epithelial tissues (41), localizes to rough endoplasmic reticulum (3), and lacks downregulation and membrane translocation upon activation (32). In contrast, the δ isoform is distributed ubiquitously to almost all tissues, is present in cytoplasm (12, 15), and undergoes membrane translocation and downregulation. Furthermore, we noted some differences in the morphological changes demonstrated by the δ and η isoforms: the η-overexpressing cells were enlarged, whereas the δ-overexpressing cells showed a spindle shape. These differences suggest that δ and η isoform share some functions but might play distinct roles in keratinocyte differentiation.
In contrast to the η and δ isoforms, the α and ζ isoforms exerted no effects on differentiation of human keratinocytes under the same conditions. However, Lee and Yuspa reported that antisense oligonucleotides of the α isoform inhibited the induction of late-stage markers, including TGase 1 and loricrin, in mouse keratinocytes (25). The possibility remains that multiple PKC isoforms are involved in the differentiation process. This notion is suggested by the findings that the η isoform induced growth inhibition and TGase 1 expression but was not associated with the expression of K1 keratin and loricrin. These observations may imply that the η isoform contributes to a certain stage in the progression of keratinocyte differentiation.
Functions of each PKC isoform rely on activation and substrate specificity. Our earlier study revealed substrates for PKC in mouse skin and keratinocytes, i.e., MARCKS protein, nuclear envelope protein lamin B2, creatinine phosphokinase B, HSP28, and an unidentified 34-kDa protein (5–7, 22), although it is unknown whether these proteins are involved in keratinocyte differentiation. Further studies on the isoform-specific substrates should provide essential information in detail on epithelial differentiation, skin diseases, and carcinogenesis.
We previously reported that CS, a membrane lipid formed during keratinocyte differentiation, stimulates the activity of the η isoform (17). Our observation was confirmed and extended by Denning et al. (9): CS activates the α, δ, ɛ, and ζ isoforms along with the η isoform and induces the late differentiation markers. Further, we demonstrated that CS acts as a transcriptional activator of TGase 1 in normal human keratinocytes (23). On the basis of all the existing data, we propose the following model of a signal transduction pathway in keratinization: CS is synthesized by cholesterol sulfotransferase in response to differentiation signals, resulting in the activation of the η and δ isoforms of PKC, which in turn activates transcription of TGase 1, leading to squamous cell differentiation.
ACKNOWLEDGMENTS
We are grateful to Yumi Kanegae and Izumu Saito of the Institute of Medical Science, University of Tokyo, for their kind help in adenovirus vector construction and their gift of the pAxCAwt cosmid cassette. We thank N. Shishido for her extensive secretarial assistance.
This work was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Education, Science, Sports and Culture of Japan (no. 06281216).
REFERENCES
- 1.Chakravarty R, Rong X H, Rice R H. Phorbol ester-stimulated phosphorylation of keratinocyte transglutaminase in the membrane anchorage region. Biochem J. 1990;271:25–30. doi: 10.1042/bj2710025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C C, Wang J K, Chen W C. TPA induces translocation but not down-regulation of new PKC isoform η in macrophages, MDCK cells and astrocytes. FEBS Lett. 1997;412:30–34. doi: 10.1016/s0014-5793(97)00697-2. [DOI] [PubMed] [Google Scholar]
- 3.Chida K, Sagara H, Suzuki Y, Murakami A, Osada S, Ohno S, Hirosawa K, Kuroki T. The η isoform of protein kinase C is localized on rough endoplasmic reticulum. Mol Cell Biol. 1994;14:3782–3790. doi: 10.1128/mcb.14.6.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chida K, Murakami A, Tagawa T, Ikuta T, Kuroki T. Cholesterol sulfate, a second messenger for the η isoform of protein kinase C, inhibits promotional phase in mouse skin carcinogenesis. Cancer Res. 1995;55:4865–4869. [PubMed] [Google Scholar]
- 5.Chida K, Yamada S, Kato N, Kuroki T. Phosphorylations of Mr 34,000 and 40,000 proteins by protein kinase C in mouse epidermis in vivo. Cancer Res. 1990;48:4018–4023. [PubMed] [Google Scholar]
- 6.Chida K, Tsunenaga M, Kasahara K, Kohno Y, Kuroki T. Regulation of creatine phosphokinase B activity by protein kinase C. Biochem Biophys Res Commun. 1990;173:346–350. doi: 10.1016/s0006-291x(05)81063-0. [DOI] [PubMed] [Google Scholar]
- 7.Chida K, Kasahara K, Tsunenaga M, Kohno Y, Yamada S, Ohmi S, Kuroki T. Purification and identification of creatine phosphokinase B as a substrate of protein kinase C in mouse skin in vivo. Biochem Biophys Res Commun. 1990;173:351–357. doi: 10.1016/s0006-291x(05)81064-2. [DOI] [PubMed] [Google Scholar]
- 8.Datto M B, Li Y, Panus J F, Howe D J, Xiong Y, Wang X F. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning M F, Kazanietz M G, Blumberg P M, Yuspa S H. Cholesterol sulfate activates multiple protein kinase C isoenzymes and induces granular cell differentiation in cultured murine keratinocytes. Cell Growth Differ. 1995;6:1619–1626. [PubMed] [Google Scholar]
- 10.Dlugosz A A, Yuspa S H. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J Cell Biol. 1993;120:217–225. doi: 10.1083/jcb.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard J M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frevert E U, Kahn B B. Protein kinase C isoforms ε, η, δ and ζ in murine adipocytes: expression, subcellular localization and tissue-specific regulation in insulin-resistant states. Biochem J. 1996;316:865–871. doi: 10.1042/bj3160865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. . (Review.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodnight J, Kazanietz M G, Blumberg P M, Mushinski J F, Mischak H. The cDNA sequence, expression pattern and protein characteristics of mouse protein kinase C-ζ. Gene. 1992;122:305–311. doi: 10.1016/0378-1119(92)90219-f. [DOI] [PubMed] [Google Scholar]
- 15.Goodnight J A, Mischak H, Kolch W, Mushinski J F. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem. 1995;270:9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- 16.Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1α,25-dihydroxyvitamin D3. Endocrinology. 1983;113:1950–1957. doi: 10.1210/endo-113-6-1950. [DOI] [PubMed] [Google Scholar]
- 17.Ikuta T, Chida K, Tajima O, Matsuura Y, Iwamori M, Ueda Y, Mizuno K, Ohno S, Kuroki T. Cholesterol sulfate, a novel activator for the η isoform of protein kinase C. Cell Growth Differ. 1994;5:943–947. [PubMed] [Google Scholar]
- 18.Jiang C K, Connolly D, Blumenberg M. Comparison of methods for transfection of human epidermal keratinocytes. J Investig Dermatol. 1991;97:969–973. doi: 10.1111/1523-1747.ep12491889. [DOI] [PubMed] [Google Scholar]
- 19.Kanegae Y, Lee G, Sato Y, Tanaka M, Nakai M, Sakaki T, Sugano S, Saito I. Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res. 1995;23:3816–3821. doi: 10.1093/nar/23.19.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanegae Y, Makimura M, Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn J Med Sci Biol. 1994;47:157–166. doi: 10.7883/yoken1952.47.157. [DOI] [PubMed] [Google Scholar]
- 21.Kartasova T, Darwiche N, Kohno Y, Koizumi H, Osada S, Huh N, Lichti U, Steinert P M, Kuroki T. Sequence and expression patterns of mouse SPR1: correlation of expression with epithelial function. J Investig Dermatol. 1996;106:294–304. doi: 10.1111/1523-1747.ep12340741. [DOI] [PubMed] [Google Scholar]
- 22.Kasahara K, Chida K, Tsunenaga M, Kohno Y, Ikuta T, Kuroki T. Identification of lamin B2 as a substrate of protein kinase C in BALB/MK-2 mouse keratinocytes. J Biol Chem. 1991;266:20018–20023. [PubMed] [Google Scholar]
- 23.Kawabe, S., T. Ikuta, M. Ohba, K. Chida, E. Ueda, K. Yamanishi, and T. Kuroki. Cholesterol sulfate activates transcription of transglutaminase 1 gene in normal human keratinocytes. J. Investig. Dermatol., in press. [DOI] [PubMed]
- 24.Lee E, Yuspa S H. Changes in inositol phosphate metabolism are associated with terminal differentiation and neoplasia in mouse keratinocytes. Carcinogenesis. 1991;12:1651–1658. doi: 10.1093/carcin/12.9.1651. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y S, Dlugosz A A, McKay R, Dean N M, Yuspa S H. Definition by specific antisense oligonucleotides of a role for protein kinase C α in expression of differentiation markers in normal and neoplastic mouse epidermal keratinocytes. Mol Carcinog. 1997;18:44–53. [PubMed] [Google Scholar]
- 26.Macfarlane D E, Manzel L. Activation of β-isozyme of protein kinase C (PKC β) is necessary and sufficient for phorbol ester-induced differentiation of HL-60 promyelocytes. Studies with PKC β-defective PET mutant. J Biol Chem. 1994;269:4327–4331. [PubMed] [Google Scholar]
- 27.Marte B M, Meyer T, Stabel S, Standke G J, Jaken S, Fabbro D, Hynes N E. Protein kinase C and mammary cell differentiation: involvement of protein kinase C α in the induction of β-casein expression. Cell Growth Differ. 1994;5:239–247. [PubMed] [Google Scholar]
- 28.Mehrel T, Hohl D, Rothnagel J A, Longley M A, Bundman D, Cheng C, Lichti U, Bisher M E, Steven A C, Steinert P M, Yuspa S H, Roop D R. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 29.Missero C, Calautti E, Eckner R, Chin J, Tsai L H, Livingston D M, Dotto G P. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuno K, Kubo K, Saido T C, Akita Y, Osada S, Kuroki T, Ohno S, Suzuki K. Structure and properties of a ubiquitously expressed protein kinase C, nPKC δ. Eur J Biochem. 1991;202:931–940. doi: 10.1111/j.1432-1033.1991.tb16453.x. [DOI] [PubMed] [Google Scholar]
- 32.Murakami A, Chida K, Suzuki Y, Kikuchi H, Imajoh-Ohmi S, Kuroki T. Absence of down-regulation and translocation of the η isoform of protein kinase C in normal human keratinocytes. J Investig Dermatol. 1996;106:790–794. doi: 10.1111/1523-1747.ep12346391. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi H, Brewer K A, Exton J H. Activation of the ζ isozyme of protein kinase C by phosphatidylinositol 3,4,5-triphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 34.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. . (Review.) [DOI] [PubMed] [Google Scholar]
- 35.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 36.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;15:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 37.Ohno S, Kawasaki H, Konno Y, Inagaki M, Hidaka H, Suzuki K. A fourth type of rabbit protein kinase C. Biochemistry. 1988;27:2083–2087. doi: 10.1021/bi00406a040. [DOI] [PubMed] [Google Scholar]
- 38.Ohno S, Konno Y, Akita Y, Yano A, Suzuki K. A point mutation at the putative ATP-binding site of protein kinase C alpha abolishes the kinase activity and renders it down-regulation-insensitive. A molecular link between autophosphorylation and down-regulation. J Biol Chem. 1990;265:6296–6300. [PubMed] [Google Scholar]
- 39.Osada S, Mizuno K, Saido T C, Akita Y, Suzuki K, Kuroki T, Ohno S. A phorbol ester receptor/protein kinase, nPKC η, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990;265:22434–22440. [PubMed] [Google Scholar]
- 40.Osada S, Mizuno K, Saido T C, Suzuki K, Kuroki T, Ohno S. A new member of the protein kinase C family, nPKC θ, predominantly expressed in skeletal muscle. Mol Cell Biol. 1992;12:3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osada S, Hashimoto Y, Nomura S, Kohno Y, Chida K, Tajima O, Kubo K, Akimoto K, Koizumi H, Kitamura Y, Suzuki K, Ohno S, Kuroki T. Predominant expression of nPKC η, a Ca2+-independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth Differ. 1993;4:167–175. [PubMed] [Google Scholar]
- 42.Pessino A, Passalacqua M, Sparatore B, Patrone M, Melloni E, Pontremoli S. Antisense oligodeoxynucleotide inhibition of δ protein kinase C expression accelerates induced differentiation of murine erythroleukaemia cells. Biochem J. 1995;312:549–554. doi: 10.1042/bj3120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito I, Oya Y, Yamamoto K, Yuasa T, Shimojo H. Construction of nondefective adenovirus type 5 bearing a 2.8-kilobase hepatitis B virus DNA near the right end of its genome. J Virol. 1985;54:711–719. doi: 10.1128/jvi.54.3.711-719.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroeder W T, Thacher S M, Stewart-Galetka S, Annarella M, Chema D, Siciliano M J, Davies P J, Tang H Y, Sowa B A, Duvic M. Type I keratinocyte transglutaminase: expression in human skin and psoriasis. J Investig Dermatol. 1992;99:27–34. doi: 10.1111/1523-1747.ep12611394. [DOI] [PubMed] [Google Scholar]
- 45.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thacher S M, Rice R H. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985;40:685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- 47.Tsunenaga M, Kohno Y, Horii I, Yasumoto S, Huh N H, Tachikawa T, Yoshiki S, Kuroki T. Growth and differentiation properties of normal and transformed human keratinocytes in organotypic culture. Jpn J Cancer Res. 1994;85:238–244. doi: 10.1111/j.1349-7006.1994.tb02088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueda E, Ohno S, Kuroki T, Livneh E, Yamada K, Yamanishi K, Yasuno H. The η isoform of protein kinase C mediates transcriptional activation of the human transglutaminase 1 gene. J Biol Chem. 1996;271:9790–9794. doi: 10.1074/jbc.271.16.9790. [DOI] [PubMed] [Google Scholar]
- 49.Yamanishi K, Liew F M, Konishi K, Yasuno H, Doi H, Hirano J, Fukushima S. Molecular cloning of human epidermal transglutaminase cDNA from keratinocytes in culture. Biochem Biophys Res Commun. 1991;175:906–913. doi: 10.1016/0006-291x(91)91651-r. [DOI] [PubMed] [Google Scholar]
- 50.Yuspa S H, Ben T, Hennings H, Lichti U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982;42:2344–2349. [PubMed] [Google Scholar]
- 51.Yuspa S H, Kilkenny A E, Steinert P M, Roop D R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]