Abstract
Acne in adult females is triggered mainly by hormones. Doxycycline is a reference treatment in acne. Spironolactone targets the androgen receptor of sebaceous glands and is prescribed off-label for female adult acne. This multicentre, controlled, randomized, double-blind prospective and parallel study assessed the efficacy of spironolactone compared with doxycycline in adult female acne. A total of 133 women with moderate acne were randomized to receive treatment with: (i) doxycycline and benzoyl peroxide for 3 months followed by a 3-month treatment with its placebo and benzoyl peroxide, or (ii) spironolactone and benzoyl peroxide for 6 months. Successfully treated patients continued with benzoyl peroxide or spironolactone alone for a further 6 months. Primary endpoints were treatment success at month 4 and month 6 with the AFAST score. At all visits, the ECLA score, lesion counts, local and systemic safety and quality of life were assessed. Spironolactone performed better at month 4 and showed a statistically significant better treatment success after 6 months than doxycycline (p = 0.007). Spironolactone was 1.37-times and 2.87-times more successful compared with doxycycline at respective time-points. AFAST and ECLA scores, as well as lesion counts always improved more with spironolactone. Patients’ quality of life was better with spironolactone at month 4 and month 6. Spironolactone was very well tolerated. This is the first study to show that, in female adults with moderate acne, treatment with spironolactone is significantly more successful than doxycycline and very well tolerated.
SIGNIFICANCE
This clinical study, conducted in 133 adult women with moderate acne, showed, for the first time, that treatment with spironolactone is significantly more effective than doxycycline, and very well tolerated.
Key words: acne, adult female acne, AFAST, doxycycline, quality of life, spironolactone,
Adult female acne differs from adolescent acne (1). In adolescents, acne presents with diffuse lesions, including non-inflammatory lesions, superficial inflammatory lesions and hyperseborrhoea on the face, while, in adult women, it presents more frequently with mild-to-moderate lesions and a few deep, inflammatory chronic cysts on the mandibular zone of the face (2). The prevalence of adult female acne is estimated at 12–54% (3).
The physiopathology of adult female acne is characterized by 2 specific features. First, a hormonal factor that triggers a premenstrual flare-up of acne lesions in more than 60% of women (4). This flare-up is efficiently controlled using either anti-androgens or 4th-generation oral contraceptives with a non-androgenic progestin. Secondly, a chronic activation of the innate immunity of the skin caused by resistant Cutibacterium acnes (C. acnes) strains in the pilosebaceous follicle, resulting from chronic courses of antibiotics (5).
In moderate-to-severe adult female acne, 4 different types of systemic treatments are approved, including: cyclins, which may potentially modify the cutaneous microbiome and cause bacterial resistance with a risk of non-clinical response; zinc salts, which mainly target inflammatory lesions and are considered to be less effective than cyclins; isotretinoin, which has been reported to be teratogenic, and a potential risk of depression (6); and anti-androgens, such as cyproterone acetate, which have recently been associated with meningioma and are therefore not recommended in acne (7, 8).
Doxycycline was the first authorised tetracycline derivate by the US Food and Drug Administration in 1967, and is still one of the most frequently prescribed antibiotics (9). It is a safe and effective treatment in patients with acne and is currently indicated in the treatment of moderate-to-severe acne (10). As is the case for all oral antibiotics, doxycycline should not be administered for more than 3 months (11, 12).
Spironolactone has been in therapeutic use for almost 60 years as a diuretic in elderly patients (13). At low doses (150 mg/day or less), it loses its antidiuretic activity, but blocks 5-alpha-reductase receptors of the sebaceous gland and inhibits production of luteinizing hormone (LH) at the pituitary level (14). Spironolactone is currently prescribed for off-label use in female adult acne due to its sebum-limiting effect. Several publications have reported the clinical benefit of spironolactone in women with acne (15, 16). Comparative studies are sparse and, to date, the clinical benefit of spironolactone in the treatment of moderate acne in adult females has not been compared in a blinded trial with that of a reference treatment of acne, such as systemic cyclins (11, 17).
The aim of this study was to demonstrate the effectiveness of spironolactone compared with doxycycline, the reference treatment in the management of adult female acne, in order to provide evidence that spironolactone is an efficient and safe alternative to doxycycline in adult female acne.
METHODS
This multicentre, controlled, randomized, double-blind prospective and parallel study was conducted at 7 sites in France between 31 January 2018 and 30 June 2022.
The study complied with all local legal requirements for the conduct of clinical trials and conformed with Good Clinical Practice and the principles of the Declaration of Helsinki. The study received ethics committee approval from Comité de Protection des Personnes SUD OUEST and OUTRE MER on 3 October 2017 (Clinical Trial identifier NCT03334682 and EudraCT number: 2017-001392-22). The patients provided written informed consent to publication of their case details.
The study design is detailed in the recently published study protocol Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) (18). Hereafter, the main methods, as well as the main statistical analysis applied, are provided. The global scoring tool AFAST was used for clinical evaluation (19). The global score is composed of 2 scores. Score 1 assesses lesions on the entire face and score 2 assesses lesions in the mandibular regions. In addition, a count of inflammatory and non-inflammatory lesions was performed. Inclusion criteria were: female patients, aged above 20 years, with moderate acne (at least 10 inflammatory lesions and no more than 3 nodules according to the AFAST scoring tool). Patients had to be cyclin-treatment naïve or might have previously received oral cyclins for their acne, except during the 3 months preceding study participation. Moreover, patients had not received zinc salts or oral antibiotics for 30 days and oral isotretinoin for 6 months prior to inclusion. Patients with clinically confirmed polycystic ovary syndrome were excluded from the study.
The overall study duration was 12 months with a 6-month double-blind treatment period followed by a 6-month open-labelled maintenance period. The overall study design is shown in Fig. 1.
Fig. 1.
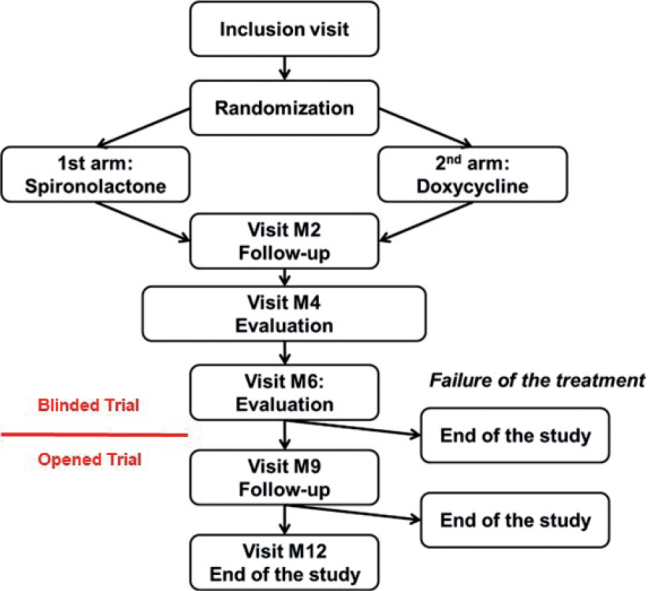
Study design. M: month.
Suitable patients were randomized in 1:1 (Ennov® Clinical, Paris, France) to receive either doxycycline 100 mg/day (Doxycycline Sandoz® 100 mg, Sandoz, Levallois-Perret, France) for 3 months followed by an oral placebo for 3 months, or spironolactone 150 mg/day (Spironolactone Arrow® 75 mg, Arrow Laboratory, Lyon, France) for 6 months. Randomization to treatment was stratified according to the different classes of contraception (combined oestrogen-progesterone, progesterone-only, and continuous or extended use pill) (20). As monotherapy with systemic antibiotics is not recommended, patients in both groups received benzoyl peroxide 5% (BPO, Cutacnyl® 5%, Galderma International, Courbevoie, France) in addition to their systemic treatment. Treatment blinding was removed for all patients after 6 months, in order to limit constraints for subjects in the doxycycline arm. If after 6 months the AFAST score 1 had decreased less than 2 points or if no decrease was observed compared with baseline, or if the AFAST score 2 was unchanged or higher than 1 compared with baseline, then subjects were withdrawn from the study and an alternative therapy was initiated (21). Patients whose acne improved at 6 months, and who were considered as “successfully treated” with doxycycline, continued treatment with BPO alone, while those who had received spironolactone continued spironolactone without BPO for a further 6 months.
Clinical assessments included Adult Female Acne Scoring Tool (AFAST) scores (1 and 2 at months 2, 4, 6, 9 and 12 visits from month 0, inflammatory and non-inflammatory lesion count and evolution of truncal acne using the ECLA scale at month 0, 2, 4, 6, 9 and 12, local and systemic safety, including laboratory blood analyses and adverse event (AE) assessments were made throughout the entire course of the study (22). Patients were asked to complete the Cardiff Acne Disability Index (CADI) and Euro Qol - 5D (EQ-5D) quality of life (QoL) questionnaires at all visits (23, 24).
Due to the different time to efficacy and optimal duration of treatment between doxycycline and spironolactone, the primary efficacy outcome was the best result at month 4 or month 6. The success rate was defined as the decrease in AFAST score 1 of at least 2 points compared with month 0, or to grade 0 if the severity score at baseline was 1, combined with a decrease in AFAST score of 1 point if the month 0 score was > 1, or 0 if the month 0 score was 1.
For sample size estimation purposes, the targeted difference in the percentage of success was set at 70% in the spironolactone group, compared with 50% success in the doxycycline group, indicating a relative increase in success equalling 40%. Assuming a type I error rate of 5% and at least 80% statistical power, the sample size required was a minimum of 91 subjects per group.
As spironolactone has a slow onset of action, treatment success might not have been optimal after 3 months, as the primary endpoint of the study was to confirm the efficacy of spironolactone compared with doxycycline using AFAST after 4 or 6 months of treatment (25). The comparison between the 2 arms was carried out using a Cochran–Mantel–Haenszel test, checking for class of contraception (4 strata). A modified intention-to-treat (mITT) and per protocol (PP) analysis was applied. The mITT was defined as the study population from which subjects were excluded who did not met the inclusion criteria and who were included but who never received treatment. All secondary objectives were expressed as percentages or as a status at a fixed time-point of follow-up. General linear mixed models were applied considering repeated measurements collected from the same participant during the follow-up. Variable selection for the multivariate model was based on the Akaike information criterion. Significance level was set at 5%.
RESULTS
A total of 133 adult female patients were included in this study. The mean age was somewhat higher in the doxycycline than in the spironolactone group (29.75 ± 7.90 years vs 27.52 ± 6.6 years, respectively). More subjects in the doxycycline group reported hyper-pilosity (12% vs 3% in the spironolactone group) and more subjects (68% vs 46% in the spironolactone group) reported a family history of acne. Detailed demographic data is shown in Table I.
Table I.
Demographic data at month 0
| Spironolactone group N = 65 | Doxycycline group N = 68 | |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 27.52 ± 6.6 | 29.75 ± 7.90 |
| Median (Min–Max) | 26 (20–49) | 27 (20–49) |
| Nibbling, n (%) | 44 (68) | 49 (72) |
| Frequency | 19 (39) | 21 (32) |
| Never | 35) (51) | 27 (42) |
| Rarely | 14 (26) | 15 (23) |
| Frequently | 0 | 2 (3) |
| Always | ||
| Premenstrual signs, n (%) | 36 (55) | 37 (54) |
| Hyper-pilosity, n (%) | 2 (3) | 8 (12) |
| Irregular menstruation, n (%) | 11 (17) | 4 (6) |
| Acne history, n (%) | ||
| Family | 16 (46) | 46 (68) |
| Father | 19 (41) | 13 (28) |
| Mother | 21 (46) | 20 (43) |
| Anti-mullerian hormone, n (%) | ||
| Significant | 1 (1) | 1 (2) |
| Not significant | 20 (31) | 9 (13) |
| Not applicable | 44 (68) | 58 (85) |
| Contraception, n (%) | ||
| Yes | 62 (95) | 65 (96) |
| Abstinence | 3 (5) | 4 (6) |
| 3rd and 4th generation oral contraception | 14 (22) | 13 (19) |
| Intra-uterine device, | 20 (31) | 19 (28) |
| Implant, 1st or 2nd generation oral contraception, intrauterine progesterone-diffusing device or any other hormonal contraception | 28 (43) | 32 (47) |
SD: standard deviation.
At month 0, the mean overall AFAST score was 2.7 ± 0.5 in both groups. The mean ECLA score on the trunk was 3.2 ± 2.3 in the spironolactone group and 2.9 ± 2.4 in the doxycycline group. The mean number of inflammatory lesions in the spironolactone group was 17.6 ± 5.6; and 20.3 ± 15.3 in the doxycycline group. Non-inflammatory lesion counts were 19.8 ± 14.5 in the spironolactone and 17.4 ± 9.3 in the doxycycline group. Detailed clinical data at month 0 are shown in Table II.
Table II.
Disease data at month 0
| Spironolactone group N = 65 | Doxycycline group N = 68 | |
|---|---|---|
| AFAST Score Global | ||
| Mean ± SD | 2.71 ± 0.50 | 2.70 ± 0.50 |
| Median (Min–Max) | 3 (2–4) | 3 (2–4) |
| AFAST Score 1 (GEA score) Mean ± SD | 2.7 ± 0.5 | 2.7 ± 0.5 |
| Median (Min–Max) | 3 (2–4) | 3 (2–4) |
| AFAST 2 Score (submandibular) Mean ± SD | 2.0 ± 0.7 | 1.9 ± 0.7 |
| Median (Min–Max) | 2 (0–3) | 2 (0–3) |
| ECLA score | ||
| Mean ± SD | 3.2 ± 2.3 | 2.9 ± 2.4 |
| Median | 3 (0–11) | 2 (0–11) |
| Median (Min–Max) | ||
| Inflammatory lesion count (n) | ||
| Mean ± SD | 17.6 ± 5.6 | 20.3 ± 15.3 |
| Median (Min–Max) | 17 (10–33) | 16 (10–109) |
| Non-inflammatory lesion count (n) | ||
| Mean ± SD | 19.8 ± 14.5 | 17.4 ± 9.3 |
| Median (Min–Max) | 17 (1–100) | 17 (0–47) |
| CADI score (0–15) | ||
| Mean ± SD | 6.24 ± 2.64 | 6.32 ± 2.65 |
| Median (Min–Max) | 6.5 (1–14) | 6.32 (2–13) |
| EQ-5D score (1–5) | ||
| Mean ± SD | 5.7 ± 0.8 | 5.7 ± 0.9 |
| Median (Min–Max) | 6 (5–8) | 5.5 (5–8) |
SD: standard deviation; AFAST: Adult Female Acne Scoring Tool; ECLA: Échelle de Cotation des Lésions d’Acné or Acne Lesion Score Scale; CADI: Cardiff Acne Disability Index; EQ-5D: Euro Qol - 5D.
Fig. 2 provides detailed information about the patient populations and disposition.
Fig. 2.
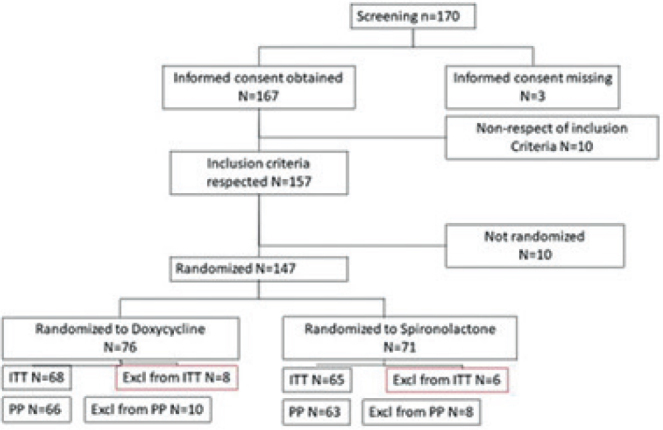
Patient disposition. ITT: Intent-to-treat; PP: per protocol ; Excl: excluded.
The primary efficacy criterion was treatment success, based on AFAST scores at month 4 and month 6.
When using the Mantel-Haenzel (MH) χ2 test on the mITT population, the odds ratio (OR) was in favour of spironolactone, with an OR of 1.37 and a 95% confidence interval (95% CI) of 0.60–3.12 (p = 0.579) at month 4, and an OR of 2.87 and a 95% CI of 1.38–5.99 (p = 0.007) at month 6.
Treatment with spironolactone was 1.37-times and 2.87-times more successful compared with treatment with doxycycline at month 4 and month 6, respectively.
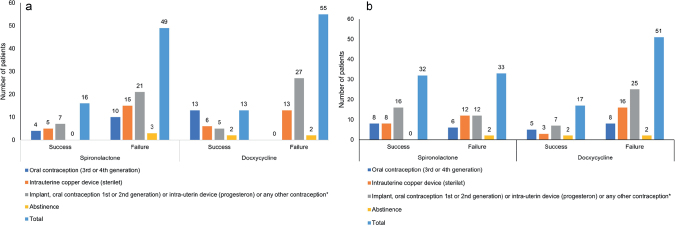
As the randomization to treatment was stratified on contraception, a MH χ2 test was performed on the mITT population. The results showed that, for all contraception methods, treatment success was significantly (p = 0.0013, OR 3.40; 95% CI 1.66–6.98) superior with spironolactone (62%) than with doxycycline (32%).
Treatment success, according to the different means of contraception at month 4 and month 6, is shown in Fig. 3.
Fig. 3.
Treatment success at Month 4 and Month 6 according to contraceptive means and overall. (a) Treatment success at Month 4. (b) Treatment success at Month 6.
The multivariate model using a regression logistic was applied to identify all factors associated with treatment success and assessed at month 0. The results confirmed results from the MH test (p = 0.0007; OR 3.90; 95% CI 1.77–8.58). An almost significant association observed regarding family acne history and AFAST 1 (p = 0.05; OR 0.438; 95% CI 0.19–1.02). Analysis of the PP data confirmed the mITT results.
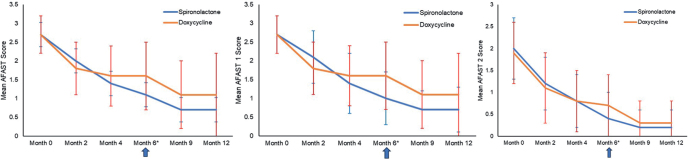
The overall AFAST score, as well as the AFAST 1 and AFAST 2 scores, decreased progressively over time in both groups; the decrease was more important with spironolactone than with doxycycline (Fig. 4). For AFAST 1, the mean delta at month 4 from month 0 was 1.3 for spironolactone and 1.1 for doxycycline. At month 6, the mean delta was 1.7 and 1.1 for spironolactone and doxycycline, respectively. The mean delta from month 0 for AFAST 2 for spironolactone was 1.2 and 1.6 at month 4 and month 6, respectively, and for doxycycline 1.1 and 1.2 at month 4 and month 6, respectively.
Fig. 4.
Evolution of the Adult Female Acne SCoring Tool (AFAST) score. (a) Global AFAST score, (b) AFAST 1 (Global Evaluation of Acne; GEA) score and (c) AFAST 2 (submandibular) score. *Evaluation of treatment outcome (success or failure).
The mean ECLA score for the trunk (Fig. 5), as well as the mean inflammatory and non-inflammatory lesion count (Fig. 6), decreased continuously in both groups starting at month 2 until month 12, with a more important decrease in the spironolactone group.
Fig. 5.
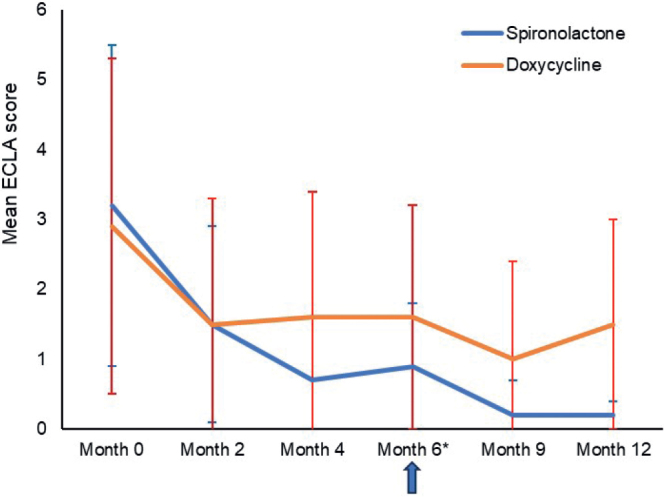
Evolution of the Échelle de Cotation des Lésions d’Acné or. Acne Lesion Score Scale (ECLA) score over time. *Evaluation of treatment outcome (success or failure).
Fig. 6.
Evolution of lesion count over time. (a) Inflammatory lesion count, (b) non-inflammatory lesion count. *Evaluation of treatment outcome (success or failure).
At month 4, the mean delta from month 0 for the inflammatory lesion count was 12.7 lesions with spironolactone and 13.0 with doxycycline. At month 6, the mean deltas were 14.4 and 12.9 with spironolactone and doxycycline, respectively.
At month 4, the mean delta from month 0 for the non-inflammatory lesion was 9.2 with spironolactone and 5.6 with doxycycline. At month 6, mean deltas were 12.2 and 6.2 with spironolactone and doxycycline, respectively.
The QoL assessed with CADI was similar in both groups at baseline (spironolactone: 6.24 ± 2.64 points; doxycycline: 6.32 ± 2.65 points) until month 4 and then started to improve more with spironolactone (2.56 ± 2.38 points) than with doxycycline (3.44 ± 2.44) from month 6. Details are shown in Fig. 7.
Fig. 7.
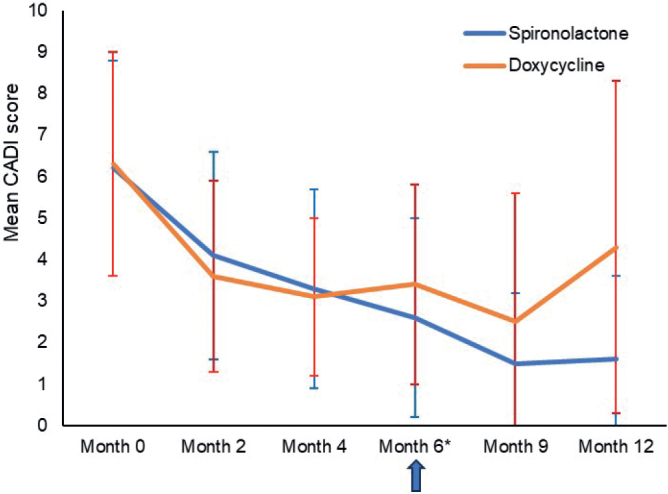
Evolution of Cardiff Acne Disability Index (CADI) score over time. *Evaluation of treatment outcome (success or failure).
The EQ-5D score remained almost unchanged in both groups (baseline: spironolactone: 5.8 ± 0.8 points; doxycycline: 5.7 ± 0.9 points), with no relevant change during the study (month 6: spironolactone: 5.5 ± 0.8 points; doxycycline: 5.8 ± 0.7 points and month 12: spironolactone: 5.6 ± 0.8 points; doxycycline: 5.5 ± 1.0 points).
Overall, 309 adverse events (AEs) were reported in 118 patients (50 patients who received doxycycline and 68 who received spironolactone) during the study. Of those AEs, 189 (61.2%) occurred in the spironolactone group and 120 (38.8%) in the doxycycline group; all but 3 AEs were mild or moderate in intensity. The 3 non-serious but severe AEs reported with spironolactone were pain in the upper abdomen, gastric reflux, and breast discomfort. Six AEs were considered serious, 4 (dyskinesia, gastroenteritis, hand/foot cramp and dizziness with vestibular damage) in the spironolactone and 2 (Staphylococcus aureus over-infection and papillary carcinoma of the right thyroid section) in the doxycycline group; none was considered to be related to the studied treatment. All AEs were transient and resolved during the conduct of the study without discontinuing study treatment. Twenty-three non-serious AEs, related to spironolactone, were reported; 9 events of dysmenorrhea combined with irregular menstrual cycles, 9 events of vaginal bleeding and intermenstrual bleeding, and 5 events of upper abdominal pain. None led to withdrawal of the patients from the study.
Laboratory evaluations did not show any clinically abnormal values. Moreover, no cases of hyperkalemia were observed in patients who received spironolactone.
DISCUSSION
To the best of our knowledge, this is the first double-blind clinical study to compare the effectiveness of spironolactone taken for 6 months with that of doxycycline administered for 3 months (according to its labelling) followed by its placebo (for 3 months), after 4 and 6 months in women with moderate-to-severe acne using AFAST.
Treatment outcome was superior with spironolactone compared with doxycycline, with an OR of 1.37 (95% CI 0.60–3.12; p = 0.579) and an OR of 1.371 (95% CI of 1.37–5.99; p = 0.007) at month 4 and month 6, respectively, with spironolactone being 1.37-times and 2.87-times more successful compared with doxycycline at month 4 and month 6, respectively.
ECLA confirmed that, for truncal acne, after 4 months no further treatment benefit was observed with doxycycline, while treatment outcome continued to improve until month 6 with spironolactone, as reported by Bienenfeld et al. (26). After this period, patients who reported treatment success in the group previously treated with doxycycline and its placebo for 3 months each continued to improve when on BPO alone. However, this improvement remained less important compared with that observed in patients who continued their spironolactone treatment for a further 6 months. Results for AFAST and ECLA were paralleled for inflammatory and non-inflammatory lesion counts, showing a similar evolution over time.
This study shows that the onset of efficacy of doxycycline is faster than that of spironolactone, with an improvement in acne lesions within the first 2 months of treatment. Conversely, treatment with spironolactone results in a clinical benefit after only 2–4 months of treatment. However, treatment outcome with spironolactone continues to improve after the first 4 months of treatment, which is not observed with doxycycline.
Overall safety with spironolactone and doxycycline was good. All spironolactone-related AEs resolved during the course of the study, with no treatment withdrawal. No treatment-related serious AE was reported.
The observed tolerance to spironolactone was similar to that reported in the literature (27). No patient for whom AEs were reported also reported hyperkalemia. These results confirm results published by Wang et al. in 2020 (28).
The number of systemic AEs was higher in the spironolactone group than in the doxycycline group after 6 months of treatment. This may be because treatment with doxycycline was stopped after 3 months, while patients on spironolactone continued their treatment for a further 3-month period. The majority of spironolactone-related AEs were mild to moderate events of irregular menstruation and did not lead to withdrawal of the patients from treatment. When analysing spironolactone-related AEs in depth, 23 mild or moderate non-serious AEs were reported. All of these resolved during the study and none led to the withdrawal of the patient from the study.
While the number of AEs was higher with spironolactone than with doxycycline, the low number of spironolactone-related AEs may be due to the fact that this study did not include patients with clinically confirmed polycystic ovary syndrome, who frequently require higher doses of spironolactone, thus increasing the risk of AEs related to spironolactone (29). This may be considered a bias of the current study and further research may have to be conducted to confirm these findings.
In 2010, St Jean et al. reported that, in patients not responding to treatment with oral isotretinoin, treatment with spironolactone improved facial acne in 50% and truncal acne in 37.5% of the patients. Thus, spironolactone may not only be a valuable and safe alternative to doxycycline, but also to oral isotretinoin (30).
Quality of life improved in both treatment groups until month 4, and continued to improve in patients treated with spironolactone, although it worsened after month 9 in those patients who received BPO alone after month 6, showing that a continued improvement in acne also improves the patient’s quality of life.
Current international guidelines recommend treatment of moderate-to-severe acne with oral antibiotics, such as doxycycline, in combined use with topical retinoids and or BPO (11, 17).
To date, spironolactone has not received market approval to treat female acne, even in the USA where it has been prescribed off-label to treat acne for more than 40 years. This benefit is supported by numerous clinical studies, and spironolactone is proposed more and increasingly re frequently as an off-label alternative to antibiotics if the latter are contraindicated or if treatment with antibiotics is not successful (15).
In conclusion, while further studies may be necessary to confirm these findings, spironolactone deserves its authorisation as an efficacious and safe alternative to oral cyclins, and potentially to isotretinoin, in the treatment of female acne; even though a direct comparison with oral isotretinoin remains to be conducted.
ACKNOWLEDGEMENTS
The authors acknowledge the writing support of Karl Patrick Göritz, Scientific and Medical Writing Services, France.
This study was supported by a grant from the French Ministry of Health (PHRC-16-0290).
Data are available upon reasonable request from the corresponding author.
Clinical Trial Identifier. NCT05486910.
The study received ethics committee approval from CPP SUD OUEST and OUTRE MER on 3 October 2017 (Clinical Trial identifier NCT03334682 and EudraCT number: 2017-001392-22).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Preneau S, Dreno B. Female acne – a different subtype of teenager acne? J Eur Acad Dermatol Venereol 2012; 26: 277–282. [DOI] [PubMed] [Google Scholar]
- 2.Dreno B, Thiboutot D, Layton AM, Berson D, Perez M, Kang S. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol 2015; 29: 1096–1106. [DOI] [PubMed] [Google Scholar]
- 3.Branisteanu DE, Toader MP, Porumb EA, Serban IL, Pinzariu AC, Branisteanu CI, et al. Adult female acne: Clinical and therapeutic particularities (Review). Exp Ther Med 2022; 23: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol 2001; 15: 541–545. [DOI] [PubMed] [Google Scholar]
- 5.Dréno B, Dagnelie MA, Khammari A, Corvec S. The skin microbiome: a new actor in inflammatory acne. Am J Clin Dermatol 2020; 21: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreno B, Thiboutot D, Gollnick H, Bettoli V, Kang S, Leyden JJ, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol 2014; 24: 330–334. [DOI] [PubMed] [Google Scholar]
- 7.Castellanos Lorduy HJ, Pérez Cely HC, Casadiego Rincón EJ, Henao Riveros SC, Colorado CL. Cutibacterium acnes tetracycline resistance profile in patients with acne vulgaris, in a Colombian Dermatologic Center. Actas Dermosifiliogr (Engl Ed) 2021. May 19: S0001-7310(21)00191-5 [DOI] [PubMed] [Google Scholar]
- 8.Leclerc-Mercier S, Buisson V, Dreno B. New regulations for oral contraceptive prescription in France in 2013: what is the impact on adult female acne? Eur J Dermatol 2016; 26: 345–349. [DOI] [PubMed] [Google Scholar]
- 9.Barbieri JS, Spaccarelli N, Margolis DJ, James WD. Approaches to limit systemic antibiotic use in acne: Systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol 2019; 80: 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore A, Ling M, Bucko A, Manna V, Rueda MJ. Efficacy and safety of subantimicrobial dose, modified-release doxycycline 40 mg versus doxycycline 100 mg versus placebo for the treatment of inflammatory lesions in moderate and severe acne: a randomized, double-blinded, controlled study. J Drugs Dermatol 2015; 14: 581–586. [PubMed] [Google Scholar]
- 11.Nast A, Dreno B, Bettoli V, Bukvic Mokos Z, Degitz K, Dressler C, et al. European evidence-based (S3) guideline for the treatment of acne – update 2016 – short version. J Eur Acad Dermatol Venereol 2016; 30: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 12.Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016; 74: 945–973.e33. [DOI] [PubMed] [Google Scholar]
- 13.[No authors listed] Spironolactone. Brit Med J 1967; 2: 751.6025984 [Google Scholar]
- 14.Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human facial sebocytes and acts antagonistically to testosterone and 5 alpha-dihydrotestosterone in vitro. J Invest Dermatol 1993; 100: 660–662. [DOI] [PubMed] [Google Scholar]
- 15.Han JJ, Faletsky A, Barbieri JS, Mostaghimi A. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther 2021; 11: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmina E, Dreno B, Lucky WA, Agak WG, Dokras A, Kim JJ, et al. Female Adult acne and androgen excess: a report from the Multidisciplinary Androgen Excess and PCOS Committee. J Endocr Soc 2022; 6: bvac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016; 74: 945–973.e33. [DOI] [PubMed] [Google Scholar]
- 18.Poinas A, Lemoigne M, Le Naour S, Nguyen JM, Schirr-Bonnans S, Riche VP, et al. FASCE, the benefit of spironolactone for treating acne in women: study protocol for a randomized double-blind trial. Trials 2020; 21: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auffret N, Claudel JP, Leccia MT, Poli F, Farhi D, Dréno B. AFAST – Adult Female Acne Scoring Tool: an easy-to-use tool for scoring acne in adult females. J Eur Acad Dermatol Venereol 2016; 30: 824–828. [DOI] [PubMed] [Google Scholar]
- 20.Cooper DB, Patel P, Mahdy H. Oral contraceptive pills. Treasure Island, FL: StatPearls Publishing LLC, 2023. [PubMed] [Google Scholar]
- 21.Dreno B, Poli F, Pawin H, Beylot C, Faure M, Chivot M, et al. Development and evaluation of a Global Acne Severity Scale (GEA Scale) suitable for France and Europe. J Eur Acad Dermatol Venereol 2011; 25: 43–48. [DOI] [PubMed] [Google Scholar]
- 22.Dreno B, Bodokh I, Chivot M, Daniel F, Humbert P, Poli F, et al. La grille ECLA : un système de cotation de l’acné pour la pratique quotidienne du dermatologue. Ann Dermatol Venereol 1999; 126: 136–41. [PubMed] [Google Scholar]
- 23.Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol 1992; 17: 1–3. [DOI] [PubMed] [Google Scholar]
- 24.Balestroni G, Bertolotti G. [EuroQol-5D (EQ-5D): an instrument for measuring quality of life]. Monaldi Arch Chest Dis 2012; 78: 155–159 (in Italian). [DOI] [PubMed] [Google Scholar]
- 25.Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol 2017; 18: 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bienenfeld A, Nagler AR, Orlow SJ. Oral antibacterial therapy for acne vulgaris: an evidence-based review. Am J Clin Dermatol 2017; 18: 469–490. [DOI] [PubMed] [Google Scholar]
- 27.Isvy-Joubert A, Nguyen JM, Gaultier A, Saint-Jean M, Le Moigne M, Boisrobert E, et al. Adult female acne treated with spironolactone: a retrospective data review of 70 cases. Eur J Dermatol 2017; 27: 393–398. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Lipner SR. Retrospective analysis of adverse events with spironolactone in females reported to the United States Food and Drug Administration. Int J Womens Dermatol 2020; 6: 272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu P, Elman SA, Abudu B, Beckles A, Salian P, Yanes DA, et al. High-dose spironolactone for acne in patients with polycystic ovarian syndrome: a single-institution retrospective study. J Am Acad Dermatol 2021; 85: 740–741. [DOI] [PubMed] [Google Scholar]
- 30.Saint-Jean M, Ballanger F, Nguyen JM, Khammari A, Dreno B. Importance of spironolactone in the treatment of acne in adult women. J Eur Acad Dermatol Venereol 2011; 25: 1480–1481. [DOI] [PubMed] [Google Scholar]