Abstract
Müller glia, the main glial cell of the retina, are critical for neuronal and vascular homeostasis in the retina. During age-related macular degeneration (AMD) pathogenesis, Müller glial activation, remodeling, and migrations are reported in the areas of retinal pigment epithelial (RPE) degeneration, photoreceptor loss, and choroidal neovascularization (CNV) lesions. Despite this evidence indicating glial activation localized to the regions of AMD pathogenesis, it is unclear whether these glial responses contribute to AMD pathology or occur merely as a bystander effect. In this review, we summarize how Müller glia are affected in AMD retinas and share a prospect on how Müller glial stress might directly contribute to the pathogenesis of AMD. The goal of this review is to highlight the need for future studies investigating the Müller cell's role in AMD. This may lead to a better understanding of AMD pathology, including the conversion from dry to wet AMD, which has no effective therapy currently and may shed light on drug intolerance and resistance to current treatments.
Keywords: müller cells, age-related macular degeneration (AMD), macula, choroidal neovascularization (CNV), retinal pigment epithelial (RPE), dry to wet AMD conversion, retina, glia, gliosis
Age-related macular degeneration (AMD) is a major cause of irreversible vision loss among the elderly. There are two types of AMD: dry and wet (see the Fig.). Dry AMD progresses slowly and is recognized as the most common type of AMD. It is anatomically characterized by the thickening of Bruch's membrane, followed by drusen development, causing varying levels of vision impairment.1 In advanced or late stages of pathogenesis, dry AMD can lead to severe central vision loss, a condition called geographic atrophy (GA) in which pathological alterations include the loss of retinal pigment epithelial (RPE) cells and choriocapillaris dropout followed by photoreceptor degeneration.2 Wet or neovascular AMD is the leading cause of AMD-related blindness as it is the rapidly progressing form of the disease. In wet AMD, pathological angiogenesis (choroidal neovascularization, or CNV) and the recruitment of immune cells into the retina lead to the degeneration of photoreceptors.3 It has also been reported that some patients with dry or intermediate AMD rapidly develop CNV in the retina, leading to dry-to-wet AMD conversion. Conversion to wet AMD can cause rapid disease progression and sudden vision loss among these patients.4,5 All of these pathological changes in AMD occur in the central part of the posterior pole of the eye, consequently leading to central vision loss.
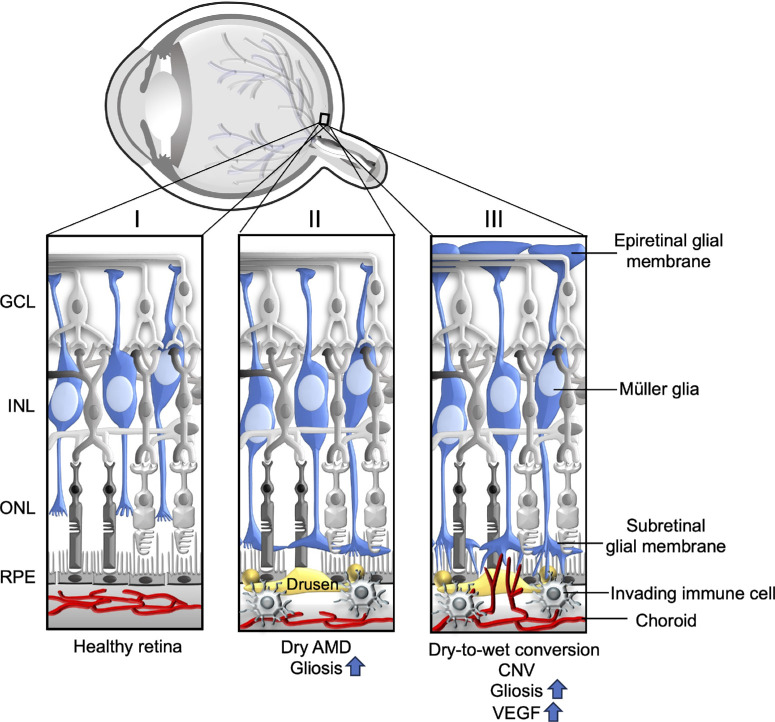
Figure.
Schematic representation of the Müller glial response in the healthy, dry, and late-stage or wet AMD retinas. (I) In healthy retinas, Müller glia span the entire thickness of the retina, forming the inner and outer limiting membranes. (II) In dry AMD retinas, activated Müller glia migrate to the outer retina, forming subretinal glial membranes; these GFAP and vimentin-positive glial membranes are explicitly found in the areas of RPE atrophy and choroidal vessel dropout. (III) In retinas with CNV or late-stage AMD, increased epiretinal glial membranes are reported in addition to lesion-specific activated subretinal Müller glial scars. These areas are also rich in recruited immune cells and microglia. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
The central vision loss in both forms of AMD is caused mainly by the dysfunction or degeneration of photoreceptors in the macula of the retina.6,7 Photoreceptors are the specialized retinal neurons that initiate phototransduction in the retina by converting photons of light into electrical signals. The two types of photoreceptors are rods and cones that are used for dim light vision and color vision, respectively. Their distribution, or the retinal mosaic, is highly specific for the human retina to enable the high-definition vision. The macula, the area of the retina that is responsible for central detailed vision, is cone-enriched and has the highest cone-to-rod ratio when compared to the peripheral retina.8,9 Within the macula, the cone density is highest in the centermost fovea and it decreases toward its periphery. The peripheral retina, the area of the retina outside the macula, is rod dominated.
The macula contains a milieu of different cell types normally working in cooperation to support photoreceptor survival and function, including RPE and choriocapillaris. Understanding how this supporting milieu contributes to photoreceptor degeneration during AMD pathogenesis is critical as it can improve our current knowledge of this disease mechanism. The RPE is required, among other functions, for the diurnal phagocytosis of photoreceptor outer segments, regeneration of 11-cis retinal, and providing nutritional support to photoreceptors.10 The choriocapillaris supplies nutritional and vascular support to the photoreceptors via the RPE. Impairment of each of these components and their respective functions have been reported in AMD.
Another major supporting cell type within the retinal cellular milieu are the Müller glia, which are the focus of this review. Müller cells, the major macroglia of the retina, provide support to all retinal neurons, including photoreceptors. They occupy the entire neural retina from the central fovea to the far periphery. They are oriented radially and are thus in contact with all the cell types of the neural retina. Müller glial endfeet form the retina's inner and outer limiting membranes, providing structural and anatomic integrity to its cellular network. Müller glia are integral to the retina's homeostatic and metabolic support; they regulate ions, water, and bicarbonate transport,11 fuel exchange in the outer retina,12 contribute to retinoid metabolism,13 produce neurotrophic factors,14 and contribute to other functions. During development, Müller glial cells guide migrating cells, followed by their role in maintaining the layered retina throughout life.15 Finally, Müller glial cells have been reported to participate in phagocytosis during both development15 and retinal degenerative disease.16 Although little is known about the role of Müller cells and their contributions to AMD progression, current evidence indicates that AMD pathogenesis involves Müller cell activation and changes in signaling, followed by significant cell remodeling and migration.17–20 This suggests that Müller glial stress may be critical in contributing to AMD pathogenesis.
In this review, we have summarized the known facts about the critical roles of Müller glia in the healthy macula and changes observed in the retinas of patients with AMD and animal models of AMD-like pathology. As central vision loss in AMD mainly occurs due to photoreceptor degeneration and pathological CNV at the macula, we collected information on the significance of Müller glia in photoreceptor survival, and we finally have discussed prospects on how the pro-angiogenic and pro-inflammatory response from stressed Müller glia may impact AMD pathogenesis. This perspective review suggests that studies investigating the direct contribution of Müller glia to the development and progression of AMD are critical to better understanding the disease mechanism and improving therapeutic interventions. Müller glia in a healthy retina and disease are summarized in the Figure.
The key take-home messages from this review are based on the following premises, findings, and hypotheses:
-
(1)
Müller glia, the major macroglia of the retina, are radially oriented, span the entire width of the retina, and provide support to all retinal neurons.
-
(2)
In the macula, a cone photoreceptor-enriched area of the retina that is responsible for central detailed vision, Müller glia exhibit structural and metabolic adaptations different from peripherally located cells.
-
(3)
In AMD retinas, Müller glial activation, remodeling, and migrations are reported specific to the areas of RPE atrophy, CNV lesions, and photoreceptor loss.
-
(4)
Besides RPE cells, Müller glial cells preferentially express a subset of AMD-associated genes.
-
(5)
Based on their specialization, it is predicted that dysfunctional Müller glia contribute to AMD pathogenesis.
-
(6)
Identification of pathways and cell-cell interactions in which Müller glia are involved during AMD pathogenesis will improve the current understanding of disease mechanisms and may reveal novel therapeutic interventions for AMD.
Anatomy and functions of Müller glia in the macula
AMD pathology begins in the peripheral macula and spreads toward its center. Specifically, photoreceptor degeneration is reported to start in the parafovea, the macular region that surrounds the centermost fovea, and contains both rods and cones. Then, the degeneration spreads toward the foveal center or foveola, which contains cones only and is responsible for the highest visual acuity.6,21 The human retina includes two types of glial cells: macroglia, which include astrocytes and Müller glial cells, and microglia. Of these, Müller cells were reported as the only glia of the fovea, although a recent report suggests the presence of some astroglia in the human foveal pit.22 Müller cells in the macula are structurally adapted and morphologically distinct from Müller glia in other parts of the retina, allowing them to provide structural support to the parafovea and foveal cones. In the foveola, the ultrastructure of the Müller glia is described as an inverted cone-like structure, whereas they exhibit a z-shaped structure in the foveolar walls and parafovea region.23,24 These structural adaptations of Müller glia in the fovea and macula may also contribute to specialized optical functions facilitating light transmission through the cone-rich photoreceptors, enabling detailed central vision.22,24,25 It is thought that Müller glia function as optic fibers, guiding light to the photoreceptors through the inner retina without scattering.26 However, the optic guidance property of Müller cells in primate retina was disputed by a recent study.27
Müller cell density in the parafovea is five times higher than in the far peripheral non-macular retina28 (peak density of >30,000 cells/mm2 in the parafovea and approximately 6000 cells/mm2 in the far peripheral retina). Densely packed Müller glia and their structural adaptations could be critical to maintaining the avascular zone of the fovea29,30 and thus enabling an area of high visual acuity. In the monkey fovea, equal numbers of Müller glial trunks and cone photoreceptor terminals have been reported, with each Müller cell partially ensheathing two to three cone terminals. This 1:1 ratio of cone terminals to Müller glia emphasizes the importance of Müller glia and suggests that Müller cell pathology may detrimentally affect macular functions by disrupting the necessary ratio.31
Indeed, Müller glial pathology has been reported in multiple macular diseases. Müller cell degeneration and associated mitochondrial pathology in all retinal cell types within and near the areas of lesions are reported in macular telangiectasia,32 a rare form of macular degeneration culminating in central vision loss, which is typically characterized by RPE hyperplasia, subretinal neovascularization, photoreceptor loss, and central vision loss.33–35 In tractional macular disorders, disruption of the Müller glial cone structure in the foveola leads to the formation of epiretinal membranes (fibro-cellular semi-translucent avascular membrane made up of glial cells that appear at the inner limiting membrane or inner retinal surface,36 also referred to as epimacular membrane, surface-wrinkling retinopathy, cellophane maculopathy, and preretinal macular fibrosis) and macular holes.37 Additionally, Müller glial cell swelling, elevated inflammatory responses, and necrosis are reported in diabetic macular edema,38 a disease associated with hyperglycemia involving intraretinal fluid accumulation in the macular retina. As the Müller cell density is the highest in the parafovea, this may suggest a mechanism whereby Müller cell impairment at the macula can disrupt water homeostasis, contributing to macular edema.38
Metabolic Adaptations of Macular Müller Cells
The macula requires an efficient system of cooperating cells, including RPE, Müller glial cells, and photoreceptors, to facilitate metabolic energy flow and enable central vision. Glucose from the choroidal blood supply is transported via the RPE to photoreceptors, which rely on glycolysis as their primary source for ATP production due to the rapid drop in oxygen tension with increasing distance from the choroid.39 Photoreceptors deliver the byproduct of glycolysis, lactate, to Müller cells and RPE cells, both of which can effectively use lactate for mitochondrial oxidative phosphorylation to produce their ATP.40 This interdependent nature of the metabolic ecosystem can rapidly deteriorate due to insult and disease,41 and damage or pathology to one cell type in this ecosystem may lead to the dysregulation of the entire system. Relevant to this concept, recent studies have identified different Müller cell clusters with distinct metabolic signatures,42,43 suggesting that their metabolism across the retina may vary. These metabolic adaptations may be critical in protecting the retina from oxidative stress, a significant risk factor in the pathogenesis of AMD.44,45 Müller cells are the primary retinal source of antioxidants, such as glutathione and Nrf2, a major transcription factor that controls both basal and induced expression of genes dependent on antioxidant response elements in the retina.46–48 It has been reported that de novo serine synthesis is critical for Müller glia to balance oxidative stress in the retina.49 For example, when phosphoglycerate dehydrogenase (a rate-limiting enzyme in de novo serine metabolism) was inhibited by CBR-5884, cultured Müller cells exhibited increased cellular damage when exposed to oxidative stress. A comparison study performed with human primary Müller cells isolated from macular and peripheral retina indicated that macular Müller glia expresses more phosphoglycerate dehydrogenase than peripheral Müller glia.50 Macular Müller cells also exhibited increased reactive oxygen species (ROS) and glutathione levels compared to the peripheral Müller cells. They were found to be more susceptible to oxidative stress when phosphoglycerate dehydrogenase was inhibited. It has been reported that Nrf2 is an upstream regulator of serine biosynthesis; it controls the expression of key serine biosynthesis enzymes, including phosphoglycerate dehydrogenase, and supports the production of glutathione.51 This suggests that increased serine synthesis and glutathione levels in macular Müller glia could be driven by Müller glial Nrf2.
Overall, cultured primary human macular Müller cells have increased glycolysis, glycolytic, and glycolytic reserve capacity compared to peripheral Müller glia, suggesting a glycolytic adaptation to meet increased energy needs at the macula. In contrast, the oxygen consumption rate, assessing mitochondrial ATP production, varied minimally between macular and peripheral Müller cells. Although basal mitochondrial respiration was elevated in macular Müller cells, no difference in spare respiratory capacity or ATP production was observed in macular compared to the peripheral Müller cells.50 Given these results, glycolytic adaptations and increased serine synthesis in macular Müller glia might be a compensatory mechanism to buffer the increased neuronal activity and thus oxidative stress in the macula.
Role of Müller Glia in Photoreceptor Survival
Interaction with Müller glial cells is critical for photoreceptor survival. Müller glia facilitate photoreceptor synapse formation with secondary neurons in the inner nuclear layer during development (reviewed in Ref. 15). Müller cell-derived pigment epithelium-derived factor and permissive glycans (presumably localized on Müller membranes) promote photoreceptor outer segment (OS) assembly.52 Consistently, in a mouse model of retinitis pigmentosa, a retinal disease which is characterized primarily by early rod followed by cone photoreceptor degeneration, upregulation of genes responsible for photoreceptor OS maintenance was reported in the Müller glia/astroglia cell clusters,53 suggesting a compensatory glial response during disease driven photoreceptor degeneration. Müller glia express specialized glutamate transporters (glutamate/L‐aspartate transporter) and contribute to the termination of retinal photoreceptor synaptic activity by uptake and clearance of glutamate. In addition, Müller glia supply neurotransmitter precursors such as glutamine for glutamate synthesis to photoreceptors (reviewed in Ref. 54). As indicated above, Müller cells regulate glucose metabolism and protect photoreceptors from excess oxidative stress by providing antioxidants (glutathione and Nrf2). Müller glia also regulate retinal blood flow, extracellular pH, and water homeostasis, and they release neurotrophic factors, growth factors, and chemokines to provide a pro-survival microenvironment in the retina. Specifically, Müller cells maintain the K+, glutamate, and water balance in the retina utilizing the K+ channel Kir4.1, the glutamate/L‐aspartate transporter (GLAST), and aquaporin 4 (AQP4), respectively.55,56 Functional inactivation of Kir4.1 or aquaporin 4 disrupts potassium and water balance within the retinal tissue, leading to neurodegeneration and edema,56 and reduction in GLAST compromises retinal function.57 Finally, we refer the readers to a review article examining the metabolic relationship among RPE, Müller cells, and photoreceptors, with a focus on lactate shuttling, which we will not discuss in detail here.58
In addition to providing metabolic support for the photoreceptors, Müller glia offer structural support. In the fovea, which contains mainly Müller glia and cone photoreceptors, specialized Müller processes ensheath the unmyelinated axons of photoreceptors to form the Henle fiber layer. Elastic Müller glia provide a softer embedding for the comparatively harder photoreceptors, protecting them from mechanical stress.11,59
A transgenic mouse model with conditional Müller cell ablation exhibited impaired rod and cone b-waves and photoreceptor degeneration along with blood-retina-barrier breakdown and intraretinal neovascularization,60 suggesting that Müller glia are critical for proper structure and function of the photoreceptors. Similarly, in eyes with macular telangiectasia, the loss of Müller glia at the macula has been associated with photoreceptor outer segment loss,32 emphasizing the necessity of Müller glia for photoreceptor maintenance. Additionally, in a diabetic mouse model, it has been shown that disruption of VEGFR2 signaling, specifically in the Müller glia, causes Müller glial degeneration, leading to photoreceptor and inner retinal neuron degeneration, suggesting that Müller glia VEGFR2 is needed for photoreceptor and neuronal survival.61 Müller glia's interaction with other glial cells may also lead to damaging effects on photoreceptors. For instance, microglia and Müller glial interaction has been reported as a mediator in light-induced photoreceptor degeneration, and their interaction has been shown to regulate growth factors in degenerating retinas. Specifically, microglia-derived NGF, BDNF, and CNTF can indirectly regulate photoreceptor cell apoptosis by modulating GDNF and bFGF production and release from Müller glia, both of which are neuroprotective for photoreceptors.14 Collectively, these reports highlight the critical roles Müller glial cells play in photoreceptor maintenance and survival and suggest that Müller glial damage/dysfunction can be an upstream insult signal in photoreceptor degeneration.
Role of Müller Glia in the Pathogenesis of AMD
In addition to the RPE, Müller glia cells have been identified as a predictive cell type for AMD based on their expression of AMD-related genes. Single-cell RNA sequencing of human retinas demonstrated that retinal glial cells (Müller cells and astrocytes) express increased levels of AMD-associated genes, such as VEGFA, COL4A3, TIMP3, and HTRA1 compared to other retinal cell types.43 Moreover, in situ hybridization results indicated colocalization of the AMD susceptibility genes CFI and TIMP3 with APOE in human Müller glia.43 In support of this, another single-cell RNA seq study reported a significant presence of AMD risk genes HTRA1, APOE, and CFI in primate Müller cells.62 These results challenge the existing dogma that the expression of AMD risk genes is associated only with RPE and photoreceptors as they reveal an association of Müller glia with AMD genetic risk. Additionally, a single-cell transcriptome analysis from human macular samples indicates the prevalence of a subcluster of Müller cells with reduced mitochondrial (mt) DNA42 in samples from patients with early AMD. These changes in mtDNA in Müller cells are similar to those in the RPE, where reduced mt DNA copy number and increased mt DNA mutational load have been strongly associated with AMD.63
Overall, the presence of AMD-associated markers in Müller cells and the Müller cells’ critical roles in maintaining the macula and photoreceptors highlight that Müller glial dysfunction, in addition to RPE dysfunction (which is a well-reported hallmark of AMD pathogenesis), may have roles in driving AMD progression. Likewise, these observations suggest that therapies targeting Müller glia may be beneficial in preventing or mitigating AMD. In the following parts of this review, we have included the current understanding of Müller glial response during dry and wet AMD, as well as added a perspective on how stressed Müller glia might contribute to AMD pathogenesis, overall highlighting the need for future studies in this area.
Müller Glial Activation and Remodeling in AMD
Activation of Müller glia or “gliosis” has been reported in many retinal diseases. Whereas low-grade glial cell activation may contribute to the survival of stressed neurons, massive activation of Müller glia can be detrimental.64 In the degenerating retina, either impairment of Müller glia or their overactivation can contribute to increased tissue stiffness, impaired antioxidant supply, upregulated intermediate filaments, increased complement activation, monocyte recruitment, and edema, as well as glial scar formation (reviewed in Ref. 65). Briefly, Müller glial scars are based on cellular hypertrophy, proliferation, and migration of the Müller glia that encapsulate degenerating photoreceptors and neurons to separate them from the healthy retinal areas. However, increased tissue stiffness resulting from the upregulation of intermediate filaments in Müller glia can lead to neuronal damage, and glial scars can lead to epiretinal membranes, as has been shown in retinal detachment, diabetic retinopathy, and AMD (reviewed in Ref. 65).
During AMD pathogenesis, which typically begins as early or dry AMD and then progresses to severe dry AMD (GA) or wet (neovascular) AMD, Müller glial activation, remodeling, and migration occurs explicitly in the areas of RPE atrophy, photoreceptor loss, and CNV lesions (see the Fig.).17,18,66 However, it remains unclear whether Müller glial stress/activation occurs in response to pathology or directly contributes to it.67 In healthy retinas, bases of photoreceptors and Müller cells form adherens junctions to create the external limiting membrane (ELM). This structure provides stability and integrity for the retina's cellular organization and anatomy. However, in the eyes of patients with GA, Müller cells form dense subretinal membranes posterior to the external limiting membrane (see the Fig.).17,66 These GFAP and vimentin-positive (markers of Müller glial activation or gliosis) glial membranes are observed directly above the areas of RPE degeneration, suggesting a strong association between activated Müller glial migration and RPE pathology during AMD. Gliotic Müller cells are also reported above soft drusens.68 Corroborating these observations in human retinas, similar findings are reported in a light-induced rat model of photoreceptor degeneration, which demonstrates rod photoreceptor degeneration followed by cone degeneration.6,69 In this model, the retinal areas of photoreceptor loss were occupied with activated GFAP positive Müller glia70 confirming the occurrence of glial stress during photoreceptor degeneration. Thus, across multiple species, there is a strong correlation between Müller cell stress/activation localization and areas of RPE/photoreceptor stress.
In contrast to subretinal glial membranes observed in GA, increased presence of epiretinal glial membranes found on the vitreal side of the inner limiting membrane are reported in patients with neovascular AMD (see the Fig.).20 These epiretinal glial membranes are common in aged human retinas, and increased presence of age-related preretinal membranes has been associated with the severity of neovascular AMD. These glial membranes found between the inner limiting membrane and vitreous in AMD retinas are formed by Müller cells and astroglia components.71 This glial migration toward the vitreous could be triggered by retinal ischemia in AMD,71 causing the Müller glia to migrate to receive nutritional supply from the vitreous. Epiretinal glial membranes could lead to complications such as retinal tractions and may even interfere with access of currently existing intravitreal therapies of AMD to the target tissues.
Additionally, an association has been identified between microglia and epiretinal glial membranes in the retinas of patients with neovascular AMD. Numerous Iba1-positive microglial cells have been found in the glial membranes’ areas, suggesting Müller glial-microglia interaction in AMD pathogenesis.20 The specific Müller glia-microglia interaction has been investigated in a light-induced photoreceptor degeneration rat model,70 and the resulting data suggests that Müller cell activation can coordinate microglial migration to the damaged photoreceptors. Similarly, suppression of the cytokine Ccl2 in the Müller glia reduced both microglial recruitment to the areas of injury and photoreceptor loss during photoreceptor degeneration.72 Furthermore, it has been shown that microglia secreted IL-1β can upregulate chemokine expressions in Müller cells during photo-oxidative retinal damage.73 This bidirectional communication between Müller cells and microglia is critical in shaping overall injury responses in the retina.74 It may suggest a crucial role of Müller glia-microglia interaction in AMD.
A recent study examining human GA retinas further confirmed19 the presence of subretinal glial membranes in the regions of choroidal and RPE atrophies. Similarly, the outgrowth of Müller cell processes toward the choroid and migration of retinal neurons alongside these remodeled Müller cell processes was reported in Wistar rats, which exhibit age-related photoreceptor and RPE degeneration75 as well as in patients with non-neovascular AMD. Displaced neurons were reported to form ectopic synapses as part of the remodeling process.76 These results suggest that Müller glial migration is associated with the survival of displaced neurons and might contribute to ectopic synapse formation in the outer retina during degeneration. This is in line with observations indicating the outgrowth of Müller glia toward the subretinal space in experimental retinal detachment.77
In summary, Müller cell activation and remodeling, which has been demonstrated to occur during AMD, may affect cells in direct contact with Müller cells, such as photoreceptors, or may affect the cells in the vicinity of Müller cells, such as the RPE and choroid, indirectly, via the uptake/release of factors. We hypothesize that activated Müller glia that migrate to the subretinal space may contribute to RPE cell death in GA via recruiting microglia or by secreting pro-inflammatory factors locally. In addition, activated Müller glia may also increase the availability of pro-angiogenic factors, such as VEGF locally in the subretinal space, causing a disruption in RPE barrier function and triggering pathological CNV (see the Fig.).
Secretory Role of Müller Glia in Health and Disease
Current reports indicate that Müller glia are critical for the structural and functional properties of the macula, suggesting that stressed Müller glia in the areas of CNV lesions and RPE atrophy may contribute to the pathogenesis of AMD. As a major secretory cell type in the retina, Müller cells secrete several trophic factors and inflammatory cytokines, such as PEDF, TSP-1, VEGF, GDNF, MMP-2, and -9, IL-1β, Tnf-α, and IL-6, which can contribute to or mitigate neovascularization and other retinal pathologies.78 Normal levels of TSP1 and VEGF secreted by Müller glia are critical for maintaining the endothelial cell tight junctions and blood-retina barrier (BRB) functions in a healthy retina. However, under pathological conditions, Müller glia reduce PEDF and increase VEGF expression and secretion, causing barrier function disruption and neovascularization.79 In addition, Müller glia also express proteases, such as MMP-9 and HTRA1,43 which are major contributing factors in extracellular matrix turnover and the pathogenesis of AMD.80–82 Increased plasma levels of MMP-9 have been associated with neovascular AMD,83 and genetic polymorphisms near MMP-9 were explicitly linked to CNV in a large genomewide association study (GWAS).84 These findings suggest that Müller cells may also play a critical role in extracellular matrix remodeling in AMD, a hypothesis that warrants further studies.
During bright light exposure, microglial activation triggers Müller glia to secrete neurotrophic factors such as GDNF, facilitating photoreceptor survival. In addition to directly influencing neuronal survival, Müller glial-derived factors may affect other retinal cell types, such as microglia (as discussed above). In cocultures of retinal microglia and Müller glia, interactions increased secretion of proinflammatory factors from Müller glia, leading to microglial activation with a positive feedback loop system. Suppressing Ccl2, a potent chemokine for monocytes, in Müller glia, reduced microglial recruitment and photoreceptor death in the retinas of light-induced rat model of photoreceptor degeneration.72 Activated microglia cocultured with Müller glia also increase IL-1β and IL-6 secretion from Müller glia.85
Another study reported that Müller glia-derived Tnf-α can induce mitophagy and apoptosis in the RPE via the EGFR/p38/NF-κB/p62 pathway under high glucose conditions.86 Further, increased Müller glial MCP1 expression has been reported in retinal detachment, leading to macrophage and microglial recruitment and photoreceptor cell death.87 This represents a possible mechanism that may also be involved in AMD, although these studies have not yet been performed. Together, these reports suggest that stressed Müller glia may contribute to AMD pathogenesis through overactive pro-angiogenic and inflammatory responses.
Müller Glial Remodeling in CNV and Conversion to Wet AMD
The pro-angiogenic role of Müller glia under pathologic conditions and their ability to interact with other cells as a major secretory cell in the retina (as mentioned above) suggest that stressed Müller glia may contribute to the induction of CNV. The reports from animal models and patients with AMD discussed below emphasize this hypothesis.
In a rat model of AMD-like pathology, induced with subretinal injections of human lipid hydroperoxide, a component found in the submacular region in patients with AMD, early RPE atrophy was reported, followed by photoreceptor and inner retinal degeneration, and finally CNV. In this model, Müller gliosis based on GFAP expression occurred 5 days after injection and increased in severity with time, peaking 20 days post-subretinal injection.88 By 12 to 20 days post-injection, activated Müller glia formed subretinal glial membranes posterior to the atrophic RPE and photoreceptors. Similarly, the senescence-accelerated OXYS rat model of AMD exhibited increased GFAP expression in Müller glia as the RPE atrophy progressed.89 Together, these studies suggest that RPE pathology begins as an early event before significant gliosis, and that massive gliosis is associated with severe or neovascular AMD. In these models, Müller gliosis occurred before detectable neovascularization but worsened as the CNV lesions grew, suggesting that activated Müller cells may contribute to neovascular AMD or conversion to wet AMD pathogenesis.
Pathological neovascularization in AMD is mainly mediated via excess VEGF; angiogenesis is initiated by VEGF binding to VEGFR2 receptors on endothelial cells, activating downstream angiogenic signaling pathways, and leading to capillary angiogenesis. Whereas RPE-derived VEGF is critical for maintaining the choroid vasculature,90 excess production and secretion of RPE-derived VEGF can lead to pathological angiogenesis under disease conditions.91,92 Müller cells represent another major source of VEGF in the retina,93,94 and they also express VEGFR2, which is critical for photoreceptor survival.61 Excess VEGF secretion from the Müller glia has been reported to cause RPE barrier dysfunction and neovascularization.95 Notably, VEGF provided by the scar-forming Müller glia and the physical scar itself may interfere with the availability and efficacy of anti-VEGF therapies in AMD. In addition, disrupted photoreceptor synaptic function mediated by Müller cell activation overlying the CNV has been proposed as another possible cause of incomplete response to anti-VEGF therapy or suboptimal vision recovery in some patients.96 Specifically, local Müller glia activation may cause disrupted expression of two critical components of synaptic transmission, synaptic vesicle protein 2 and vesicular glutamate transporter 1, in the outer plexiform layer overlying the CNV.96 This hypothesis is supported by data obtained from the laser-induced CNV mouse model, in which photoreceptor synaptic dysfunction and reduction of ERG b-waves occur quickly after the laser lesion, suggesting the contribution of Müller glial disruption as an early event in the CNV model contributing to photoreceptor death.96,97
Additional support that Müller glia-derived VEGF likely contributes to retinal neovascularization98,99 comes from data in diabetic retinopathy. Conditions of increased oxidative stress and ischemia can increase the levels of Müller glial VEGF.100 As oxidative stress and ischemia are major contributing factors in AMD, upregulated Müller glial VEGF may also contribute to pathological neovascularization in AMD. Macular Müller cells are more susceptible to oxidative stress than those derived from the periphery, and the presence of AMD may further exacerbate their stress response.
Several other factors indicate a role for Müller glia in neovascular AMD. First, in addition to the typical increase in GFAP expression observed in AMD progression, hypercitrullination of GFAP in Müller glial endfeet has been associated with CNV lesions in mouse models and in maculas of patients with exudative AMD.18 The enzyme responsible for citrullination, peptidyl arginine deiminase-4 (PAD4), localizes to the Müller glial endfeet in the areas of injury in a lesion-specific manner. Glial cell-specific PAD4 deficiency mitigated citrullinated GFAP increase after injury, suggesting that glial PAD4 is critical for the citrullination of GFAP. The study offers two additional possible mechanisms for pathology: (1) citrullination might alter GFAP filament dynamics and stability, thereby affecting Müller glial function; and (2) the formation of anti-citrullinated GFAP antibodies, which trigger complement activation, may contribute to inflammation during AMD pathogenesis. Finally, in selected patients with late-stage GA, subretinal Müller glial membranes were observed above the areas of atrophic RPE and choroidal atrophy, together with the formation of either non-exudative or exudative CNV,19 suggesting that these subretinal Müller glial membranes may have a role in the conversion from dry to wet AMD, perhaps by the excess secretion of VEGF. We hypothesize that Müller glia-derived VEGF may exert a paracrine response initiating pathological CNV, leading to dry-to-wet AMD conversion (see the Fig.).
Summary and Perspective
Structurally and metabolically adapted Müller glia at the macula are critical for preserving high visual acuity and central vision in humans. During AMD pathogenesis, Müller glia undergo significant remodeling, which includes glial activation and migration followed by glial membrane formations in the subretinal and inner retinal areas (see the Fig.). In AMD retinas, these glial stress responses are reported specific to the areas of pathology, such as RPE atrophy, CNV lesions, and photoreceptor loss, suggesting an association between Müller glial stress and AMD pathology. However, little is understood about the role of Müller glial stress in AMD pathogenesis. The evidence presented in this review indicates that stressed Müller glia may directly contribute to the pathogenesis of AMD or exacerbate the severity of the disease. Müller cells express several AMD-associated genes, including HTRA1, MMP-9, and CFI, and they are reported as a predictive cell type for AMD pathogenesis, suggesting that altered expression of these genes in the Müller glia may contribute to AMD. Thus, studying cellular responses in genetically characterized Müller cells carrying these gene polymorphisms may provide critical information about the genetic aspects of AMD. Pro-inflammatory and pro-angiogenic responses reported in Müller glia under pathological conditions may also be involved in AMD. Identifying Müller-glia-involved pathways in AMD will improve the current understanding of AMD disease mechanisms and may lead to better therapeutic interventions. We hope this perspective review emphasizes the critical need to investigate the role of Müller glia in AMD pathogenesis.
Acknowledgments
The authors would like to thank Navneet Ammal Kaidery, PhD, Department of Pediatrics (MUSC), for help creating the figure for this manuscript.
Funding for this project was provided in part by the National Institutes of Health (NIH) R01EY030072, the Department of Veterans Affairs IK6 BX004858, RX000444 and BX003050 and the South Carolina SmartState Endowment.
Disclosure: S. Navneet, None; K. Wilson, None; B. Rohrer, None
References
- 1. Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M.. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013; 54: Orsf68–Orsf80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C.. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017; 37: 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ambati J, Atkinson JP, Gelfand BD.. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013; 13: 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heier JS, Brown DM, Shah SP, et al.. Intravitreal aflibercept injection vs sham as prophylaxis against conversion to exudative age-related macular degeneration in high-risk eyes: a randomized clinical trial. JAMA Ophthalmol. 2021; 139: 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt-Erfurth U, Waldstein SM, Klimscha S, et al.. Prediction of individual disease conversion in early AMD using artificial intelligence. Invest Ophthalmol Vis Sci. 2018; 59: 3199–3208. [DOI] [PubMed] [Google Scholar]
- 6. Curcio CA, Medeiros NE, Millican CL.. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996; 37: 1236–1249. [PubMed] [Google Scholar]
- 7. Pfau M, von der Emde L, de Sisternes L, et al.. Progression of photoreceptor degeneration in geographic atrophy secondary to age-related macular degeneration. JAMA Ophthalmol. 2020; 138: 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curcio CA, Sloan KR, Kalina RE, Hendrickson AE.. Human photoreceptor topography. J Comp Neurol. 1990; 292: 497–523. [DOI] [PubMed] [Google Scholar]
- 9. Hussey KA, Hadyniak SE, Johnston RJ Jr. Patterning and development of photoreceptors in the human retina. Front Cell Dev Biol. 2022; 10: 878350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85: 845–881. [DOI] [PubMed] [Google Scholar]
- 11. Reichenbach A, Bringmann A.. New functions of Müller cells. Glia. 2013; 61: 651–678. [DOI] [PubMed] [Google Scholar]
- 12. Poitry-Yamate CL, Poitry S, Tsacopoulos M.. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995; 15: 5179–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xue Y, Sato S, Razafsky D, et al.. The role of retinol dehydrogenase 10 in the cone visual cycle. Sci Rep. 2017; 7: 2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harada T, Harada C, Kohsaka S, et al.. Microglia-Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002; 22: 9228–9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tworig JM, Feller MB.. Müller glia in retinal development: from specification to circuit integration. Front Neural Circuits. 2021; 15: 815923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakami S, Imanishi Y, Palczewski K.. Müller glia phagocytose dead photoreceptor cells in a mouse model of retinal degenerative disease. FASEB J. 2019; 33: 3680–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards MM, McLeod DS, Bhutto IA, Grebe R, Duffy M, Lutty GA.. Subretinal glial membranes in eyes with geographic atrophy. Invest Ophthalmol Vis Sci. 2017; 58: 1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palko SI, Saba NJ, Mullane E, et al.. Compartmentalized citrullination in Müller glial endfeet during retinal degeneration. Proc Natl Acad Sci USA. 2022; 119: e2121875119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edwards MM, McLeod DS, Shen M, et al.. Clinicopathologic findings in three siblings with geographic atrophy. Invest Ophthalmol Vis Sci. 2023; 64: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edwards MM, McLeod DS, Bhutto IA, Villalonga MB, Seddon JM, Lutty GA.. Idiopathic preretinal glia in aging and age-related macular degeneration. Exp Eye Res. 2016; 150: 44–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdin AD, Devenijn M, Fulga R, Langenbucher A, Seitz B, Kaymak H.. Prevalence of geographic atrophy in advanced age-related macular degeneration (AMD) in daily practice. J Clin Med. 2023; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delaunay K, Khamsy L, Kowalczuk L, et al.. Glial cells of the human fovea. Mol Vis. 2020; 26: 235–245. [PMC free article] [PubMed] [Google Scholar]
- 23. Reichenbach A, Bringmann A.. Glia of the human retina. Glia. 2020; 68: 768–796. [DOI] [PubMed] [Google Scholar]
- 24. Syrbe S, Kuhrt H, Gärtner U, et al.. Müller glial cells of the primate foveola: an electron microscopical study. Exp Eye Res. 2018; 167: 110–117. [DOI] [PubMed] [Google Scholar]
- 25. Bringmann A, Syrbe S, Görner K, et al.. The primate fovea: structure, function and development. Prog Retin Eye Res. 2018; 66: 49–84. [DOI] [PubMed] [Google Scholar]
- 26. Franze K, Grosche J, Skatchkov SN, et al.. Müller cells are living optical fibers in the vertebrate retina. Proc Natl Acade Sci USA. 2007; 104: 8287–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirschfeld K. Do Müller cells act as optical fibers in the primate retina? Invest Ophthalmol Vis Sci. 2019; 60: 345–348. [DOI] [PubMed] [Google Scholar]
- 28. Distler C, Dreher Z.. Glia cells of the monkey retina–II. Müller cells. Vision Res. 1996; 36: 2381–2394. [DOI] [PubMed] [Google Scholar]
- 29. Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J.. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012; 53: 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Provis JM, Dubis AM, Maddess T, Carroll J.. Adaptation of the central retina for high acuity vision: cones, the fovea and the avascular zone. Prog Retin Eye Res. 2013; 35: 63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burris C, Klug K, Ngo IT, Sterling P, Schein S.. How Müller glial cells in macaque fovea coat and isolate the synaptic terminals of cone photoreceptors. J Comp Neurol. 2002; 453: 100–111. [DOI] [PubMed] [Google Scholar]
- 32. Zucker CL, Bernstein PS, Schalek RL, Lichtman JW, Dowling JE.. A connectomics approach to understanding a retinal disease. Proc Natl Acad Sci USA. 2020; 117: 18780–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kedarisetti KC, Narayanan R, Stewart MW, Reddy Gurram N, Khanani AM. Macular telangiectasia type 2: a comprehensive review. Clin Ophthalmol. 2022; 16: 3297–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Powner MB, Gillies MC, Zhu M, Vevis K, Hunyor AP, Fruttiger M.. Loss of Müller's cells and photoreceptors in macular telangiectasia type 2. Ophthalmology. 2013; 120: 2344–2352. [DOI] [PubMed] [Google Scholar]
- 35. Powner MB, Gillies MC, Tretiach M, et al.. Perifoveal Müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010; 117: 2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fung AT, Galvin J, Tran T.. Epiretinal membrane: a review. Clin Exp Ophthalmol. 2021; 49: 289–308. [DOI] [PubMed] [Google Scholar]
- 37. Bringmann A, Unterlauft JD, Barth T, Wiedemann R, Rehak M, Wiedemann P.. Müller cells and astrocytes in tractional macular disorders. Prog Retin Eye Res. 2022; 86: 100977. [DOI] [PubMed] [Google Scholar]
- 38. Lai D, Wu Y, Shao C, Qiu Q.. The role of Müller cells in diabetic macular edema. Invest Ophthalmol Vis Sci. 2023; 64: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winkler BS, Arnold MJ, Brassell MA, Puro DG.. Energy metabolism in human retinal Müller cells. Invest Ophthalmol Vis Sci. 2000; 41: 3183–3190. [PMC free article] [PubMed] [Google Scholar]
- 40. Kanow MA, Giarmarco MM, Jankowski CS, et al.. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife. 2017; 6: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pfeiffer RL, Marc RE, Jones BW.. Müller cell metabolic signatures: evolutionary conservation and disruption in disease. Trends Endocrinol Metab. 2020; 31: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu B, He J, Zhong L, et al.. Single-cell transcriptome reveals diversity of Müller cells with different metabolic-mitochondrial signatures in normal and degenerated macula. Front Neurosci. 2022; 16: 1079498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Menon M, Mohammadi S, Davila-Velderrain J, et al.. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat Commun. 2019; 10: 4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruan Y, Jiang S, Gericke A.. Age-related macular degeneration: role of oxidative stress and blood vessels. Int J Mol Sci. 2021; 22: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jabbehdari S, Handa JT.. Oxidative stress as a therapeutic target for the prevention and treatment of early age-related macular degeneration. Surv Ophthalmol. 2021; 66: 423–440. [DOI] [PubMed] [Google Scholar]
- 46. Navneet S, Zhao J, Wang J, et al.. Hyperhomocysteinemia-induced death of retinal ganglion cells: the role of Müller glial cells and NRF2. Redox Biol. 2019; 24: 101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Navneet S, Cui X, Zhao J, et al.. Excess homocysteine upregulates the NRF2-antioxidant pathway in retinal Müller glial cells. Exp Eye Res. 2019; 178: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu Z, Wei Y, Gong J, et al.. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014; 57: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang T, Gillies MC, Madigan MC, et al.. Disruption of de novo serine synthesis in Müller cells induced mitochondrial dysfunction and aggravated oxidative damage. Mol Neurobiol. 2018; 55: 7025–7037. [DOI] [PubMed] [Google Scholar]
- 50. Zhang T, Zhu L, Madigan MC, et al.. Human macular Müller cells rely more on serine biosynthesis to combat oxidative stress than those from the periphery. Elife. 2019; 8: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. DeNicola GM, Chen PH, Mullarky E, et al.. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet. 2015; 47: 1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X, Iannaccone A, Jablonski MM.. Contribution of Müller cells toward the regulation of photoreceptor outer segment assembly. Neuron Glia Biol. 2004; 1: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tomita Y, Qiu C, Bull E, et al.. Müller glial responses compensate for degenerating photoreceptors in retinitis pigmentosa. Exp Mol Med. 2021; 53: 1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bringmann A, Unterlauft JD, Barth T, Wiedemann R, Rehak M, Wiedemann P.. Foveal configurations with disappearance of the foveal pit in eyes with macular pucker: presumed role of Müller cells in the formation of foveal herniation. Exp Eye Res. 2021; 207: 108604. [DOI] [PubMed] [Google Scholar]
- 55. Li X, Lv J, Li J, Ren X. Kir4.1 may represent a novel therapeutic target for diabetic retinopathy (review). Exp Ther Med. 2021; 22: 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bringmann A, Wiedemann P.. Müller glial cells in retinal disease. Ophthalmologica. 2012; 227: 1–19. [DOI] [PubMed] [Google Scholar]
- 57. Barnett NL, Pow DV.. Antisense knockdown of GLAST, a glial glutamate transporter, compromises retinal function. Invest Ophthalmol Vis Sci. 2000; 41: 585–591. [PubMed] [Google Scholar]
- 58. Hurley JB, Lindsay KJ, Du J.. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J Neurosci Res. 2015; 93: 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu YB, Franze K, Seifert G, et al.. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci USA. 2006; 103: 17759–17764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shen W, Fruttiger M, Zhu L, et al.. Conditional Müller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci. 2012; 32: 15715–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fu S, Dong S, Zhu M, et al.. Müller glia are a major cellular source of survival signals for retinal neurons in diabetes. Diabetes. 2015; 64: 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yan W, Peng YR, van Zyl T, et al.. Cell atlas of the human fovea and peripheral retina. Sci Rep. 2020; 10: 9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA.. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010; 51: 5470–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014; 15: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cuenca N, Fernández-Sánchez L, Campello L, et al.. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014; 43: 17–75. [DOI] [PubMed] [Google Scholar]
- 66. Wu KH, Madigan MC, Billson FA, Penfold PL.. Differential expression of GFAP in early v late AMD: a quantitative analysis. Br J Ophthalmol. 2003; 87: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Telegina DV, Kozhevnikova OS, Kolosova NG.. Changes in retinal glial cells with age and during development of age-related macular degeneration. Biochemistry (Mosc). 2018; 83: 1009–1017. [DOI] [PubMed] [Google Scholar]
- 68. Johnson PT, Lewis GP, Talaga KC, et al.. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci. 2003; 44: 4481–4488. [DOI] [PubMed] [Google Scholar]
- 69. Jackson GR, Owsley C, Curcio CA.. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002; 1: 381–396. [DOI] [PubMed] [Google Scholar]
- 70. Di Pierdomenico J, Martínez-Vacas A, Hernández-Muñoz D, et al.. Coordinated Intervention of microglial and Müller cells in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2020; 61: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramírez JM, Ramírez AI, Salazar JJ, de Hoz R, Triviño A.. Changes of astrocytes in retinal ageing and age-related macular degeneration. Exp Eye Res. 2001; 73: 601–615. [DOI] [PubMed] [Google Scholar]
- 72. Rutar M, Natoli R, Provis JM.. Small interfering RNA-mediated suppression of Ccl2 in Müller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration. J Neuroinflammation. 2012; 9: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Natoli R, Fernando N, Madigan M, et al.. Microglia-derived IL-1β promotes chemokine expression by Müller cells and RPE in focal retinal degeneration. Mol Neurodegener. 2017; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang M, Wong WT.. Microglia-Müller cell interactions in the retina. Adv Exp Med Biol. 2014; 801: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sullivan R, Penfold P, Pow DV.. Neuronal migration and glial remodeling in degenerating retinas of aged rats and in nonneovascular AMD. Invest Ophthalmol Vis Sci. 2003; 44: 856–865. [DOI] [PubMed] [Google Scholar]
- 76. Pfeiffer RL, Marc RE, Jones BW.. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog Retin Eye Res. 2020; 74: 100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lewis GP, Chapin EA, Luna G, Linberg KA, Fisher SK.. The fate of Müller's glia following experimental retinal detachment: nuclear migration, cell division, and subretinal glial scar formation. Mol Vis. 2010; 16: 1361–1372. [PMC free article] [PubMed] [Google Scholar]
- 78. Araújo RS, Santos DF, Silva GA.. The role of the retinal pigment epithelium and Müller cells secretome in neovascular retinal pathologies. Biochimie. 2018; 155: 104–108. [DOI] [PubMed] [Google Scholar]
- 79. Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A.. PEDF derived from glial Müller cells: a possible regulator of retinal angiogenesis. Exp Cell Res. 2004; 299: 68–78. [DOI] [PubMed] [Google Scholar]
- 80. Navneet S, Rohrer B.. Elastin turnover in ocular diseases: a special focus on age-related macular degeneration. Exp Eye Res. 2022; 222: 109164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Navneet S, Brandon C, Simpson K, Rohrer B.. Exploring the therapeutic potential of elastase inhibition in age-related macular degeneration in mouse and human. Cells. 2023; 12: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Limb GA, Daniels JT, Pleass R, Charteris DG, Luthert PJ, Khaw PT.. Differential expression of matrix metalloproteinases 2 and 9 by glial Müller cells: response to soluble and extracellular matrix-bound tumor necrosis factor-alpha. Am J Pathol. 2002; 160: 1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lauwen S, Lefeber DJ, Fauser S, Hoyng CB, den Hollander AI.. Increased pro-MMP9 plasma levels are associated with neovascular age-related macular degeneration and with the risk allele of rs142450006 near MMP9. Mol Vis. 2021; 27: 142–150. [PMC free article] [PubMed] [Google Scholar]
- 84. Fritsche LG, Igl W, Bailey JN, et al.. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016; 48: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang M, Ma W, Zhao L, Fariss RN, Wong WT.. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation. 2011; 8: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu Y, Li L, Pan N, et al.. TNF-α released from retinal Müller cells aggravates retinal pigment epithelium cell apoptosis by upregulating mitophagy during diabetic retinopathy. Biochem Biophys Res Commun. 2021; 561: 143–150. [DOI] [PubMed] [Google Scholar]
- 87. Nakazawa T, Hisatomi T, Nakazawa C, et al.. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci USA. 2007; 104: 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kim SY, Kambhampati SP, Bhutto IA, McLeod DS, Lutty GA, Kannan RM.. Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model. Exp Eye Res. 2021; 203: 108391. [DOI] [PubMed] [Google Scholar]
- 89. Telegina DV, Kozhevnikova OS, Bayborodin SI, Kolosova NG.. Contributions of age-related alterations of the retinal pigment epithelium and of glia to the AMD-like pathology in OXYS rats. Sci Rep. 2017; 7: 41533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA. 2009; 106: 18751–18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO.. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003; 22: 1–29. [DOI] [PubMed] [Google Scholar]
- 92. D'Amore PA. Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci. 1994; 35: 3974–3979. [PubMed] [Google Scholar]
- 93. Saint-Geniez M, Maharaj AS, Walshe TE, et al.. Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS One. 2008; 3: e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Le YZ. VEGF production and signaling in Müller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vision Res. 2017; 139: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rodrigues M, Xin X, Jee K, et al.. VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013; 62: 3863–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mettu PS, Allingham MJ, Cousins SW.. Incomplete response to anti-VEGF therapy in neovascular AMD: exploring disease mechanisms and therapeutic opportunities. Prog Retin Eye Res. 2021; 82: 100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Caicedo A, Espinosa-Heidmann DG, Hamasaki D, Pina Y, Cousins SW.. Photoreceptor synapses degenerate early in experimental choroidal neovascularization. J Comp Neurol. 2005; 483: 263–277. [DOI] [PubMed] [Google Scholar]
- 98. Bai Y, Ma JX, Guo J, et al.. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009; 219: 446–454. [DOI] [PubMed] [Google Scholar]
- 99. Wang JJ, Zhu M, Le YZ.. Functions of Müller cell-derived vascular endothelial growth factor in diabetic retinopathy. World J Diabetes. 2015; 6: 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rossino MG, Lulli M, Amato R, Cammalleri M, Monte MD, Casini G.. Oxidative stress induces a VEGF autocrine loop in the retina: relevance for diabetic retinopathy. Cells. 2020; 9: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]