Fig. 3. Scope and selectivity.
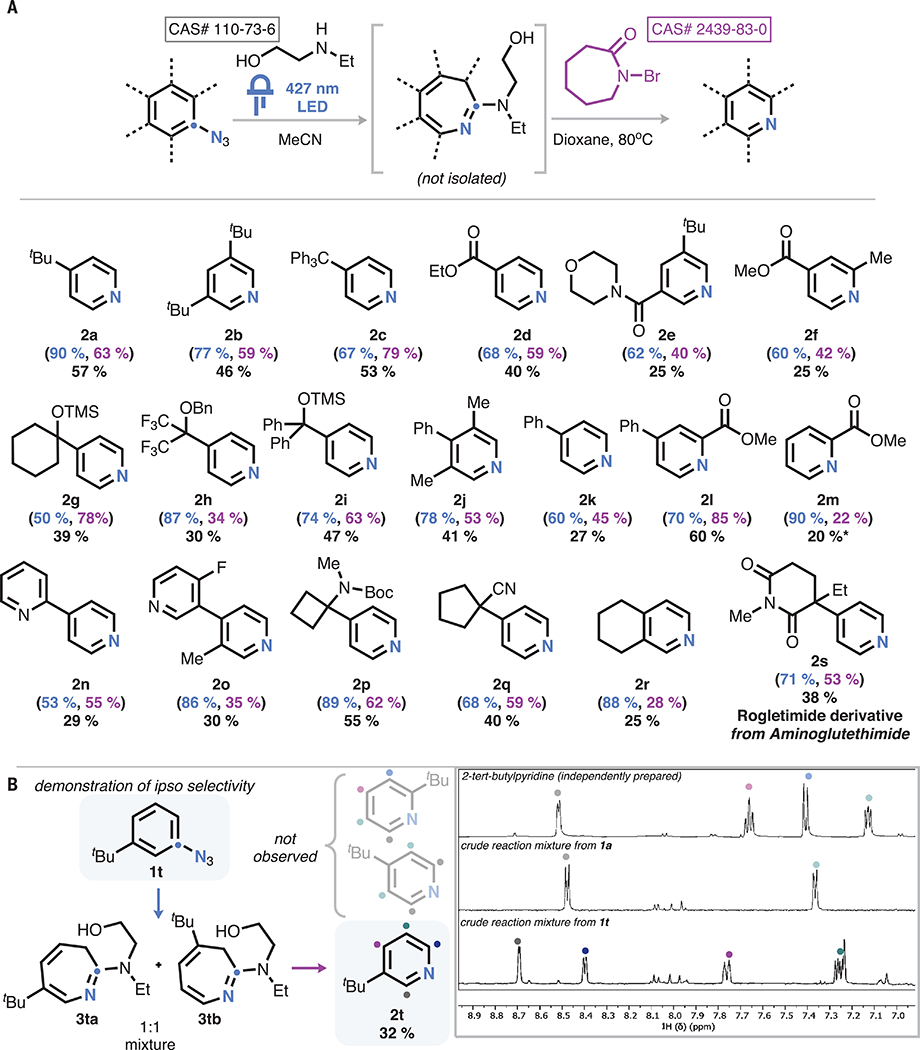
(A) Scope of the reaction. Standard conditions: (i) 0.3 mmol aryl azide, 0.3 mmol ethylaminoethanol, 3 ml MeCN, and 427-nm light-emitting diode (LED); (ii) 0.3 mmol DBU, 3 ml dioxane heated to 80°C, and 0.6 mmol N-bromocaprolactam added dropwise at 80°C. Isolated yield of pyridine from aryl azide (black). Proton NMR (1H-NMR) yield of photolysis (blue) and interpolated yield of pyridine based on azepine yield (purple), shown in parentheses. Asterisk indicates NMR yield. (B) Demonstration that nonsymmetric substrates form only one pyridine isomer.
