Abstract
Aims
The importance of the interaction between tumour cells and neutrophils has recently begun to emerge. However, the significance of tumour-infiltrating neutrophil (TIN) in colorectal carcinomas (CRCs) remains unclear. The aim of this study was to investigate the prognostic significance of TIN in CRCs.
Methods
CRCs were evaluated for TIN and were classified as neutrophil-rich (NR), neutrophil-intermediate (NI) and neutrophil-poor (NP) based on the presence of >15, 5–15 and <5 TIN per 100 tumour cells, respectively. Various clinicopathological parameters were recorded in each case including age, gender, histological grade, tumour, node, metastasis (TNM) stage, tumour location and DNA mismatch repair (MMR) status.
Results
Among the 348 CRC cases reviewed, 38 cases were NR, 43 cases were NI and 267 cases were NP. High TIN was associated with higher histological grade (p=0.0222), right-sided tumour location (p=0.0025), advanced TNM stage (p=0.0346) and higher rate of MMR-deficient CRCs (p=0.0027). Patients with NR CRCs had significantly poorer 5-year recurrence-free survival comparing to patients with NI or NP CRCs on Kaplan-Meier analysis (p=0.0001) and high TIN remained an independent risk factor with multivariate analysis (p=0.0137; HR: 1.930, 95% CI: 1.144 to 3.255). NR CRCs are more commonly seen in MMR-deficient than in MMR-proficient CRCs (p=0.0006). Patients with MMR-deficient NR CRCs showed similar 5-year recurrence-free survival compared with MMR-proficient NR CRCs.
Conclusions
Our findings reveal that high TIN confers poorer patient prognosis in both MMR-proficient and MMR-deficient CRCs.
INTRODUCTION
Colorectal carcinoma (CRC) is the third most commonly diagnosed cancer and the second-leading cause of cancer death worldwide.1 While the American Joint Committee on Cancer (AJCC) tumour, node, metastasis (TNM) staging remains the most frequently used prognostic tool, several tumour characteristics including DNA mismatch repair protein (MMR)/microsatellite instability (MSI) status, tumour subtypes, mutation status such as KRAS or BRAF mutations and immune microenvironment, have an independent impact on treatment and prognosis. Consequently, there is a need to evaluate various prognostic and predictive markers which will help to guide treatment decisions, even within each TNM stage.
With the advent of immune modulatory therapies, the inflammatory milieu within solid tumours has been increasingly recognised as an important feature.2 The type, density and location of tumour-infiltrating immune cells in the local microenvironment have been shown to be associated with the clinical outcome of several types of human cancers and affect all stages of tumour development.3 The infiltration of inflammatory cells and the altered expression of inflammatory mediators have been shown to be prognostic in CRC. For instance, the quantification of the adaptive immune response has been suggested as a prognostic factor, and a high density of tumour-infiltrating T lymphocytes has been associated with better survival.4–7 Evaluation of lymphocytic response to tumours has been considered important in the pathological evaluation of CRC, and also seems to correlate with the MMR status of the tumour. Even the innate immune response to cancers (macrophages, NK cells and neutrophils) has been shown to have prognostic significance. For example, tumour-associated macrophages seem to promote immunosuppression, tumour angiogenesis and progression, and have been associated with poor prognosis.8
Among the innate immune response, tumour-infiltrating neutrophils (TIN) are the least well studied in solid tumours.9 In many solid tumours, neutrophils have been shown to portend a worse prognosis.4 10–15 The role of TIN in CRCs remains controversial, with some studies showing high TIN associated with a worse prognosis,16–18 while others have found that this is a better predictor of patient survival.4 13 Most recently, Bui et al showed that TIN can induce non-homologous end-joining (NHEJ), allowing for survival and growth of CRC by means of increased DNA double-strand breaks burden and promotion of genomic instability by microRNA-dependent inhibition of homologous recombination repair.19 These findings might expand the therapeutic utilisation of small-molecule inhibitors targeting NHEJ from DNA damage repair-deficient pancreatic cancer20 to intratumoural neutrophil rich CRCs. Although the exact role of neutrophils still remains to be elucidated, it is speculated that TIN likely have an important role in CRC that require further studies. The goal of the study was to evaluate CRCs with TIN and investigate their clinicopathological correlations and impact on prognosis.
METHODS AND MATERIALS
A retrospective search of our pathology database from 2011 to 2017 identified 348 cases of resected CRCs. Clinical and histopathological data were gathered from the pathology reports and medical records. Clinical information such as age, gender and 5-year recurrence-free survival (RFS) status were collected from the medical records. Two pathologists (XZ and NP), who were blinded to the clinical data, reviewed all of the H&E-stained sections from each of the cases simultaneously. The following parameters were recorded: histological grade (low or high grade), TIN (rich, intermediate or poor), tumour location (left or right colon), tumour histological type (adenocarcinoma NOS, mucinous, micropapillary or medullary carcinoma), TNM stage (AJCC eighth edition) and MMR status (MSI by PCR or MMR loss by immunohistochemistry (IHC)). Tumours with MSI-high by PCR or MMR protein loss by IHC were classified as MMR-deficient (MMR-d). As to the TIN, only neutrophils infiltrating within the tumour epithelium or tumour cell nests were considered TIN. Tumours with neutrophils in the lumen associated with comedo-type necrosis (dirty necrosis/luminal neutrophils), in the tumour associated stroma, or areas immediately adjacent to necrosis, crypt rupture or mucosal ulcerations were excluded. The number of TIN was assessed in a semiquantitative manner using the mean value of 10 high power fields in a ×40 objective (magnification ×400; 0.08 mm2). Less than 5, 5–15 and greater than 15 neutrophils per 100 tumour cells were considered neutrophil-poor (NP), neutrophil-intermediate (NI) and neutrophil-rich (NR), respectively. This is similar to the criteria used in other studies investigating TIN in gastric and pancreatic carcinomas with minor modifications.10 21 Representative sections showing TIN by myeloperoxidase IHC were performed. Of note, the count of TIN was based on H&E-stained sections (figure 1A–D).
Figure 1.
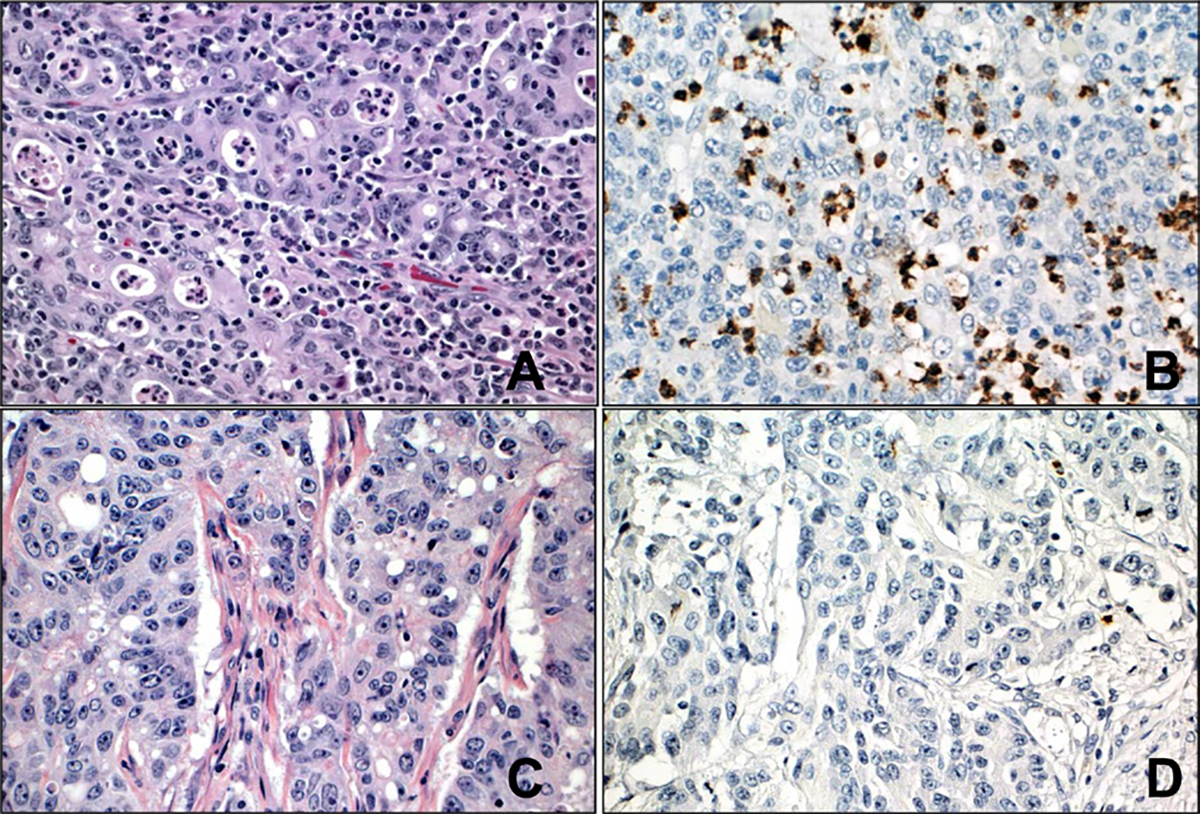
(A) Representative image of neutrophil-rich colorectal carcinoma (greater than 15 tumour-infiltrating neutrophils/100 tumour cells). (B) Representative image of immunohistochemical stain with myeloperoxidase highlighting tumour-infiltrating neutrophils in neutrophil-rich colorectal carcinoma. (C) Representative image of neutrophil-poor colorectal carcinoma (less than five tumour-infiltrating neutrophils/100 tumour cells). (D) Representative image of immunohistochemical stain with myeloperoxidase highlighting rare tumour-infiltrating neutrophils in neutrophil-poor colorectal carcinoma. (A, C: H&E, original magnification ×400; B, D: immunohistochemistry with 3,3-diaminobenzidine as chromogen, original magnification ×400).
Descriptive statistics were used to summarise the clinical and histological features. Continuous and categorical variables were compared by t-test and χ2 test, respectively. RFS was defined as the number of months from the date of surgical resection to recurrence. Both local and distant recurrence were included. For 5 years RFS, patients with recurrence within 5-year after surgical resection were censored at the date of recurrence, and patients without evidence of recurrence were censored at the date of last follow-up or at 5 years after the surgical resection, whichever was earlier. Recurrence free probabilities were calculated using Kaplan-Meier plot. The differences between groups were determined by log-rank test. Cox-proportional hazards models were used to evaluate the variable of clinical interest for the multivariate mode for RFS. P values less than 0.05 were considered statistically significant. All statistical tests were performed using GraphPad Software, Prism V.8.0 (San Diego, California, USA).
RESULTS
Clinicopathological characteristics
A total of 348 resected CRC cases were identified, of which 38 cases (10.9%) were NR, 43 cases (12.4%) were NI and 267 cases (76.7%) were NP. The mean follow-up time was 3.8 years (median: 4 years, range: 0–9 years). The clinicpathological features of each group are shown in table 1. The average age at resection was 68.97, 67.26 and 63.95 years in patients with NR, NI and NP CRCs, respectively. There were no differences in patient age (p=0.0625) and gender distribution (p=0.7167) among the NR, NI and NP groups. However, higher TIN was significantly associated with high histological grade (p=0.0222), right-sided tumour location (p=0.0025), advanced TNM stage (p=0.0346) and with MMR-d status (p=0.0027) (table 1).
Table 1.
Clinicopathological characteristics of colorectal carcinoma patients
| N=348 | NR (N=38) | NI (N=43) | NP (N=267) | P value |
|---|---|---|---|---|
| Age (years) | 68.97±14.95 | 67.26±12.64 | 63.95±14.17 | 0.0625 |
| Sex | 0.7167 | |||
| Male | 16 (42.11%) | 22 (51.16%) | 126 (47.19%) | |
| Female | 22 (57.89%) | 21 (48.84%) | 141 (52.81%) | |
| Histological grade | 0.0222 | |||
| Low | 21 (55.26%) | 34 (79.07%) | 201 (75.28%) | |
| High | 17 (44.74%) | 9 (20.93%) | 66 (24.72%) | |
| Tumour location | 0.0025 | |||
| Left | 1 1 (28.95%) | 26 (60.47%) | 155 (58.05%) | |
| Right | 27 (71.05%) | 17 (39.53%) | 112 (41.95%) | |
| TNM stage | 0.0346 | |||
| I | 2 (5.26%) | 1 1 (25.58%) | 57 (21.35%) | |
| II | 16 (42.11%) | 9 (20.93%) | 100 (37.45%) | |
| III | 19 (50%) | 22 (51.16%) | 94 (35.21%) | |
| IV | 1 (2.63%) | 1 (2.33%) | 16 (5.99%) | |
| MMR status | 0.0027 | |||
| Proficient | 20 (52.63%) | 34 (79.07%) | 208 (77.90%) | |
| Deficient | 18 (47.37%) | 9 (20.93%% | 59 (22.10%) |
MMR, mismatch repair; NI, neutrophil-intermediate; NP, neutrophil-poor; NR, neutrophil-rich; TNM, tumour, node, metastasis.
NR CRCs are more commonly seen in MMR-d CRCs
Among the 348 CRC cases, 86 (24.7%) were MMR-d, of which 18 (20.93%) were NR; and 262 (75.3%) were MMR-proficient (MMR-p), of which 20 (7.63%) were NR. These data indicate that NR CRCs are more commonly seen in MMR-d CRCs (p=0.0006). Micropapillary carcinoma histology was more commonly seen in MMR-p NR CRCs (15/22 vs. 16/326, p<0.0001). On the contrary, medullary carcinoma histology was more often seen in MMR-d NR CRCs (8/18 vs. 5/68, p=0.0006). Comparing MMR-d NR (18 cases) with MMR-p NR (20 cases) CRCs, MMR-d NR CRCs were associated with older age (77.28±12.61 vs. 61.5±13.44 years, p=0.0007), female gender (16 of 18 cases vs. 8 of 20 cases, p=0.0018), right colon location (16 of 18 cases vs. 11 of 20 cases, p=0.0214) and lower TNM stage (p=0.0004). However, there was no significant difference in tumour grade between MMR-d and MMR-p NR CRCs (p=0.2032) (table 2). Patients with MMR-d NR CRCs showed a similar 5-year RFS to patients with MMR-p NR CRCs with a mean and median 5-year RFS in months of 15.0 and 10.5 (range: 2–35) and 15.5 and 20 (range: 1–45), respectively (p=0.5108, figure 2).
Table 2.
Comparison between MMR-d NR and MMR-p NR CRC patients
| MMR-d NR (N=18) | MMR-p NR (N=20) | P value | |
|---|---|---|---|
| Age (years) | 77.28±12.61 | 61.5±13.44 | 0.0007 |
| Sex | 0.0018 | ||
| Female | 16 (88.89%) | 8 (40%) | |
| Male | 2 (11.11%) | 12 (60%) | |
| Histological grade | 0.2032 | ||
| Low | 8 (44.44%) | 13 (65%) | |
| High | 10 (55.56%) | 7 (35%) | |
| Tumour location | 0.0214 | ||
| Left | 2 (11.11%) | 9 (45%) | |
| Right | 16 (88.89%) | 11 (55%) | |
| TNM stage | 0.0004 | ||
| I-II | 14 (77.78%) | 4 (20%) | |
| III-IV | 4 (22.22%) | 16 (80%) |
CRC, colorectal carcinoma; MMR, mismatch repair; MMR-d, MMR-deficlency; MMR-p, MMR-proficiency; NR, neutrophil-rich; TNM, tumour, node, metastasis.
Figure 2.
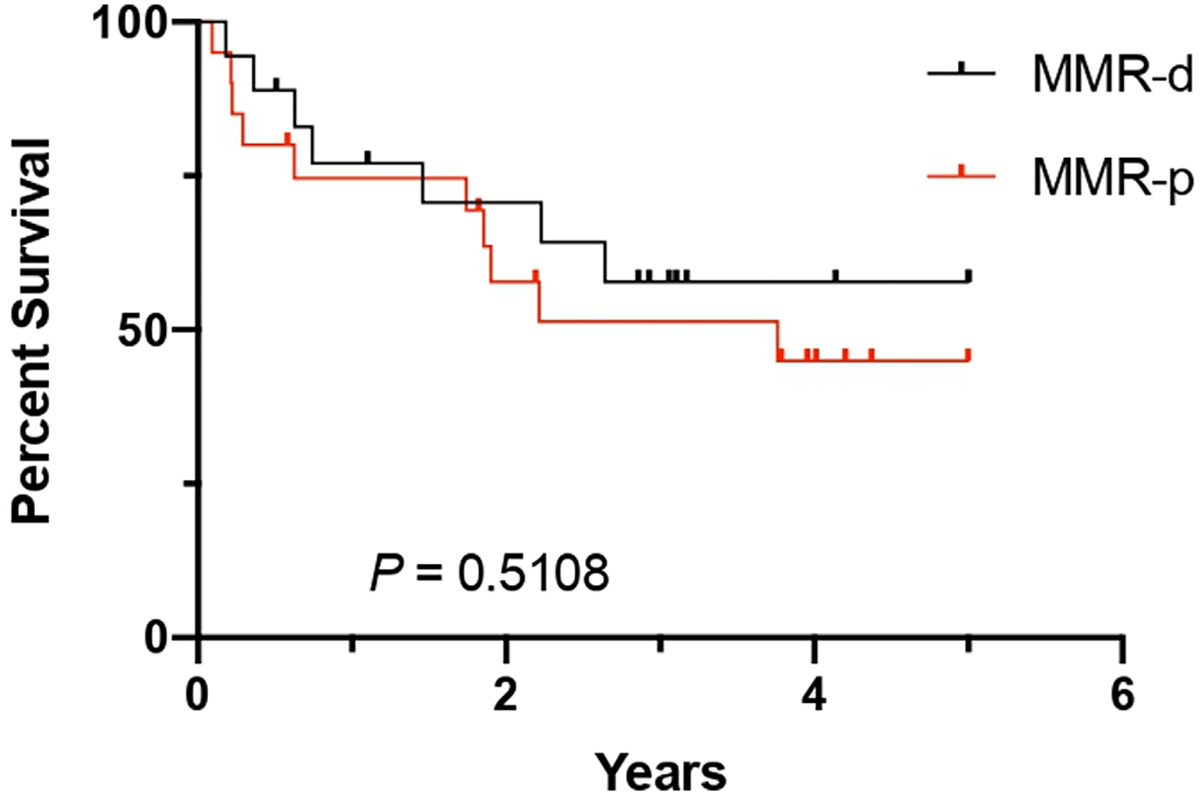
5-year recurrence-free survival in patients with neutrophil-rich mismatch repair protein-deficient (MMR-d) and MMR-proficient (MMR-p) colorectal carcinoma.
Comparing MMR-d NR CRCs (18 cases) with MMR-d NI/NP CRCs (68 cases), MMR-d NR CRCs were associated with older age (77.28±12.61 vs. 67.18±15.23 years, p=0.0114) and female gender (16 of 18 cases vs. 41 of 68 cases, p=0.0225) and presented with higher tumour grade (p=0.0385). However, there were no significant differences in tumour location (p=0.2495) and TNM stage (p=0.8796) (table 3).
Table 3.
Comparison between MMR-d NR and MMR-d NI/NP CRC patients
| MMR-d NR (N=18) | MMR-d NI/NP (N=68) | P value | |
|---|---|---|---|
| Age (yars) | 77.28±12.61 | 67.18±15.23 | 0.0114 |
| Sex | 0.0225 | ||
| Female | 16 (88.89%) | 41 (60.29%) | |
| Male | 2 (11.11%) | 27 (39.71%) | |
| Histological grade | 0.0385 | ||
| Low | 8 (44.44%) | 48 (70.59%) | |
| High | 10 (55.56%) | 20 (29.41%) | |
| Tumour location | 0.2495 | ||
| Left | 2 (11.11%) | 16 (23.53%) | |
| Right | 16 (88.89%) | 52 (76.47%) | |
| TNM stage | 0.8796 | ||
| I-II | 14 (77.78%) | 54 (79.41%) | |
| III-IV | 4 (22.22%) | 14 (20.59%) |
CRC, colorectal carcinoma; MMR, mismatch repair; MMR-d, MMR-deficiency; MMR-p, MMR-proficiency; NI, neutrophil-intermediate; NP, neutrophil-poor; NR, neutrophil-rich; TNM, tumour, node, metastasis.
Patients with NR CRCs show poor 5-year RFS
Patients with NR CRCs showed a poorer 5-year RFS compared with patients with NI or NP CRCs (p=0.0001); however, there was no difference in 5-year RFS between the NI and NP groups (p=0.2014) (figure 3A). The mean and median 5-year RFS for NR, NI and NP in months were 15.3 and 13 (range: 1–45), 24.8 and 26 (range: 1–56) and 18.2 and 14 (range: 0–57), respectively. We then combined NI and NP CRC cases as one group and compared the 5-year RFS with NR CRC cases. Using this two-tier system, NR and NI/NP, patients with NR CRCs continued to demonstrate poorer 5-year RFS compared with patients with NI/NP CRCs with a mean and median 5-year RFS in months of 15.3 and 13 (range: 1–45) and 19.1 and 16 (0–57), respectively (p<0.0001, figure 3B).
Figure 3.
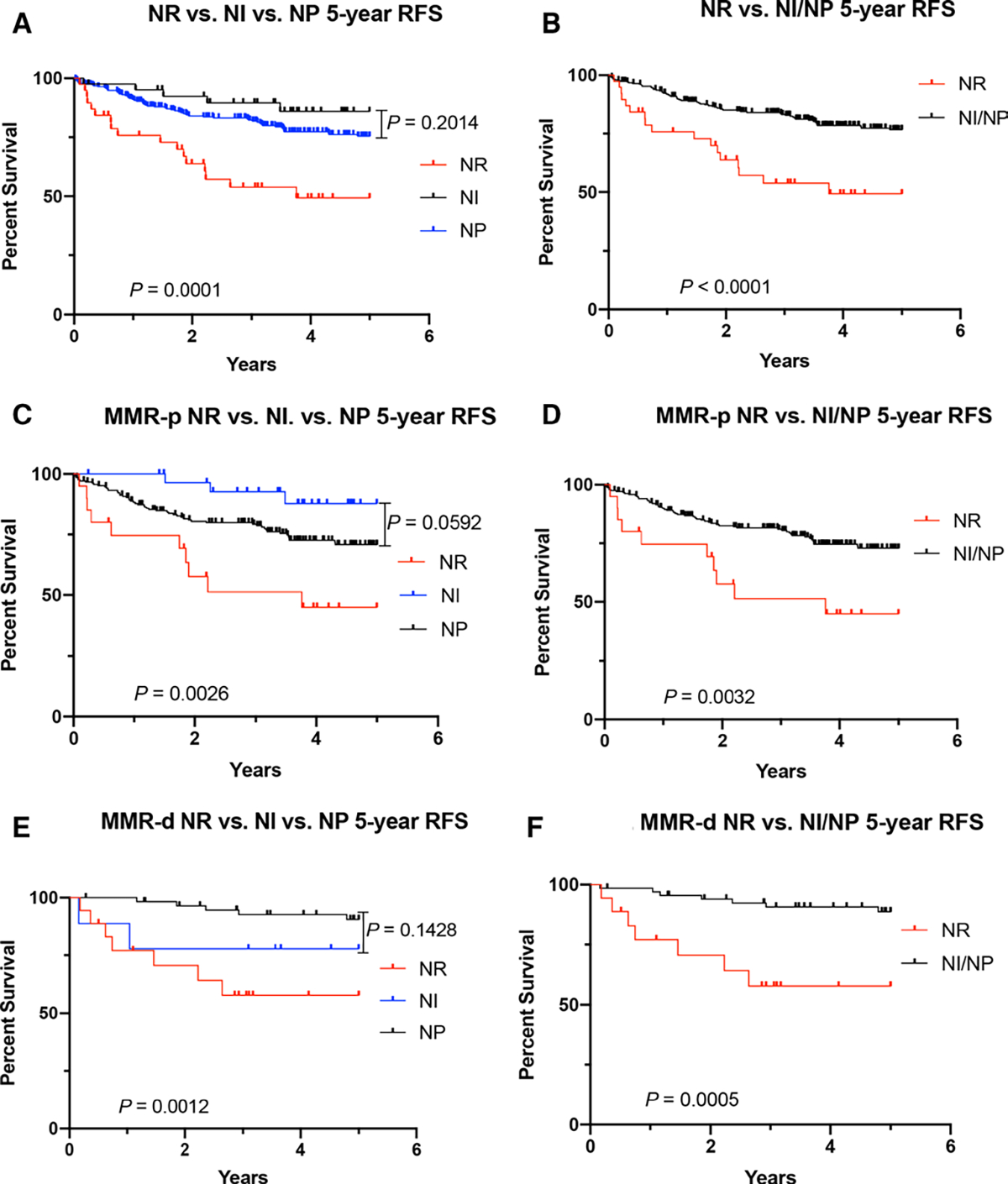
(A) 5-year RFS in patients with NR, NI and NP colorectal carcinoma. (B) Comparison of 5-year RFS in patients with NR and NI/NP colorectal carcinoma. (C) 5-year RFS in patients with NR, NI and NP MMR-proficient colorectal carcinoma. (D) Comparison of 5-year RFS in patients with NR and NI/NP MMR-proficient colorectal carcinoma. (E) 5-year RFS in patients with NR, NI and NP MMR-deficient colorectal carcinoma. (F) Comparison of 5-year RFS in patients with NR and NI/NP MMR-deficient colorectal carcinoma. MMR-d, MMR-deficient; MMR-p, MMR-proficient; NI, neutrophil-intermediate; NP, neutrophil-poor; NR, neutrophil-rich; RFS, recurrence-free survival.
This 5-year RFS remained statistically significant in both MMR-p cases (p=0.0026) and MMR-d cases (p=0.0012) (figure 3C,E). Similar to the entire cohort, there was no statistical significance between NI and NP groups in both MMR-p and MMR-d cases (p=0.0592 and p=0.1428, respectively). Using the two-tier system, NR and NI/NP, patients with NR CRCs showed a poorer 5-year RFS compared to patients with NI/NP CRCs in both MMR-p (mean and median 5-year RFS in months of 15.6 and 20 (range: 1–45) and 18.6 and 16 (range: 0–56), respectively) (p=0.0032) and MMR-d (mean and median 5-year RFS in months of 15 and 10.5 (range: 2–35), and 24.1 and 22 (range: 1–57), respectively) groups (p=0.0005, figure 3D,F).
Other variables associated with a 5-year RFS on univariate analyses included patient age, tumour histological grade, tumour stage, TIN and MMR status (table 4). On multivariate analysis, patient’s age (>65 years), high tumour grade, NR CRC, MMR-p and higher TNM stage were associated with a poor 5-year RFS (table 4). These findings demonstrated that NR CRC is an independent risk factor for poorer 5-year RFS.
Table 4.
Univariate and multivariate analyses of prognostic factors for 5-year recurrence-free survival of colorectal carcinoma patients
| Variables | N=348 | Univariate Analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | ||
| Age at diagnosis (years) | |||||
| <65 | 175 | ||||
| ≥65 | 173 | <0.0001 | 1.856 | 1.257 to 2.741 | 0.0019 |
| Sex | |||||
| Female | 184 | ||||
| Male | 164 | 0.2144 | |||
| Histological grade | |||||
| Low | 256 | ||||
| High | 92 | <0.0001 | 1.804 | 1.186 to 2.746 | 0.0058 |
| Tumour location | |||||
| Left | 192 | ||||
| Right | 156 | 0.7654 | |||
| TNM stage | |||||
| I-II | 195 | ||||
| III-IV | 153 | <0.0001 | 1.869 | 1.217 to 2.870 | 0.0043 |
| MMR status | |||||
| Deficient | 86 | ||||
| Proficient | 262 | 0.0451 | 2.277 | 1.288 to 4.026 | 0.0047 |
| TINs | |||||
| NI/NP | 310 | ||||
| NR | 38 | 0.0008 | 1.930 | 1.144 to 3.255 | 0.0137 |
MMR, mismatch repair; NI, neutrophil-intermediate; NP, neutrophil-poor; NR, neutrophil-rich; TIN, tumour-infiltrating neutrophils; TNM, tumour, node, metastasis.
DISCUSSION
Inflammatory cells are an essential component of the tumour microenvironment. Of notable interest are neutrophils, which have long been overlooked. Evidence has now accumulated that TIN can represent an essential constituent of cancer-related inflammation.15 22 23 Functional studies show that cancer cells and/or other immune cells within the tumour microenvironment modulate neutrophils to infiltrate the tumour tissue and to acquire tumour-promoting activities including angiogenesis, migration, invasion, mutagenesis and immunosuppression.23 24 Recent studies have shown that neutrophil extracellular traps, which consist of chromatin DNA filaments coated with granule proteins, released by neutrophils can promote colon cancer liver metastases25 and interfere with cytolytic cytotoxic T lymphocytes and NK cell interaction with tumour cells.26 The neutrophil rich environment within tumours as seen in many solid tumours, including CRCs, has been shown to be associated with at least a 66% increased risk of tumour recurrence, metastasis or death.9 Furthermore, TIN has been shown to be the greatest indicator of poor outcome among multiple immune cell populations across 39 different cancer types from the Cancer Genome Atlas data.27 A recent study demonstrated that both high peripheral blood neutrophil-to-lymphocyte ratio and the presence of poorly differentiated clusters significantly affect survival in patients with stage I and II CRCs, and an increased rate of poorly differentiated cluster positivity in patients with a high neutrophil-to-lymphocyte ratio was observed.28 Studies have shown that micropapillary carcinoma represents the same biological phenomenon as poorly differentiated clusters,29 and micropapillary carcinoma overlaps substantially with PDC morphologically.30 31 Thus, considering micropapillary pattern as poorly differentiated clusters have been proposed in CRCs.32 In our current study, we showed that patients with NR CRCs had a worse 5-year RFS. Higher TIN was significantly associated with higher tumour histological grade and micropapillary carcinoma histology, both of which are known to be poor prognostic histological features in CRCs.33 34
To the best of our knowledge, this is the first study to evaluate the clinico-pathological significance of TIN in MMR-d CRCs. Our study revealed that NR CRCs were more frequently seen in MMR-d than MMR-p CRCs. Patients with MMR-d NR CRCs showed similar poor 5-year RFS as that of MMR-p NR CRCs, even though MMR-d CRCs showed lower TNM stage. These findings further suggest that NR CRC is a subtype of CRC with poorer prognosis in both MMR-p and MMR-d CRCs. Patients with MMR-d CRC have shown a relatively better prognosis compared with patients with MMR-p CRC, which is also confirmed in our present study. The insertion/deletion mutations in coding microsatellites induced by MMR deficiency have contributed to the high immunogenicity of MMR-d/MSI-high tumours including CRCs. This high immunogenicity often manifests as a dense infiltration of lymphocytes, which is the hallmark of MSI-high tumours. The high immunogenicity and dense intratumoural lymphocytic infiltrate are associated with favourable response to treatment with immune checkpoint inhibitors in MSI-high tumours including CRCs.35 36 However, immunotherapy with immune checkpoint inhibitors only works in MSI-high CRCs, and even within this subgroup, the response rate is only around 35%.37–40 More recent data have demonstrated that there is close interaction between TIN and immunosuppression in the tumour microenvironment. Of note, the activity of 5-fluorouracil, one of the most widely used chemotherapeutic agents for the treatment of CRC, is at least in part mediated by its selective induction of myeloid cell apoptosis to enhance antitumour cytotoxic T lymphocyte activity.41 TIN can release a host of molecules from their granules and cytoplasm including various cytokines, chemokines, reactive oxygen species (ROS), arginase-1 (ARG1) and ligands of immune checkpoints, which are capable of suppressing innate and adaptive immune cell function to promote tumour growth, progression and metastasis.42 Neutrophil-derived ROS can inhibit intratumoural T cell and NK cell activities.43 44 The ARG1 produced by TIN reduces extracellular L-arginine in the tumour microenvironment, which controls T cell metabolism and causes subsequent T cell dysfunction and immune suppression of T cell-mediated antitumour immunity.45 46 The expression of PD-L1 in TIN can drive immune checkpoint engagement and T cell exhaustion, which may be responsible for neutrophil-mediated immune suppression.24 These findings may explain why certain patients even with high tumour-infiltrating lymphocytes still have a poor prognosis and/or have minimal response to immune checkpoint inhibitor therapies. In our current study, NR CRCs are more frequently seen in MMR-d CRCs with high tumour-infiltrating lymphocytes and medullary carcinoma histology. There was no difference in tumour-infiltrating lymphocytes in CRCs with high or intermediate/poor tumour TIN. We speculate that the higher number of TIN in MMR-d CRCs can suppress the antitumoural effects of intratumoural lymphocytes and result in worse prognosis in MMR-d NR CRCs compared with MMR-d NI/NP CRCs.
Our study demonstrates some limitations. The cohort, particularly the NR CRC group, is small due to the rarity of NR CRC cases. A larger sample size may be needed to further investigate the role of TIN in MMR-d tumours. A TIN threshold in CRCs has not been well established in the literature, and hence a threshold of >15 TIN per 100 tumour cells was taken as an arbitrary value in the current study based on prior studies on other solid tumours. This study only considered intratumoural infiltrating neutrophils without consideration of stromal neutrophils, since most prior studies showed that only TIN in the epithelial cells, but not stromal neutrophils, portend an adverse outcome in solid tumours.15 47 The peripheral blood neutrophil-to-lymphocyte ratio was not available in this study. The correlation between neutrophil-to-lymphocyte ratio and TIN status could not be evaluated. Additionally, this study is retrospective in nature and no cases had been treated with immune checkpoint inhibitors. The therapeutic response of immune checkpoint inhibitors on MMR-d CRC with or without TIN could not be evaluated. Further prospective studies are needed to validate the data generated by this analysis.
In conclusion, we evaluated the presence of TIN using >15 neutrophils per 100 tumour cells as the threshold for NR CRCs, which were more commonly seen in MMR-d CRCs. When comparing NR with NI/NP CRCs, high TIN was significantly associated with higher histological grade, advanced TNM stage, and poorer 5-year RFS. Similarly, patients with MMR-d NR CRCs showed similar 5-year RFS compared with MMR-p NR CRCs. Our findings reveal that TIN may be an additional helpful histological parameter to indicate poorer prognosis in both MMR-p and MMR-d CRCs. The significance of TIN in mediating the effects of CRC therapies, especially MMR-d CRCs receiving immune checkpoint inhibitor therapy, is an emerging area of research, which requires further evaluation under larger studies. Nonetheless, the incorporation of tumour-infiltrating neutrophils into current prognostic models and possible therapeutic manipulation of the neutrophil lineage in CRCs based on established and validated clinicopathological features continue to remain a priority.
Take home messages.
Colorectal carcinomas (CRCs) with high tumour-infiltrating neutrophil (TIN) was associated with high histological grade, right-sided tumour location, advanced tumour, node, metastasis (TNM) stage and mismatch repair protein (MMR)-deficient status.
Patients with neutrophil-rich (NR) CRCs had poor 5-year recurrence-free survival comparing to patients with neutrophil-intermediate or neutrophil-poor CRCs, and high TIN is an independent risk factor on multivariate analyses.
NR CRCs are more commonly seen in MMR-deficient than in MMR-proficient CRCs. Patients with MMR-deficient NR CRCs showed similar 5-year recurrence-free survival compared with MMR-proficient NR CRCs.
Acknowledgements
The authors would like to thank Dr. Iván A. González for his critical review and further data analysis for this study.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests None declared.
Ethics approval The present study was approved by the Institutional Review Board of Yale University (No. 2000028621).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2.Donskov F Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol 2013;23:200–7. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 4.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 6.Terzić J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101–14. [DOI] [PubMed] [Google Scholar]
- 7.Dahlin AM, Henriksson ML, Van Guelpen B, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol 2011;24:671–82. [DOI] [PubMed] [Google Scholar]
- 8.Erreni M, Mantovani A, Allavena P. Tumor-Associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron 2011;4:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen M, Hu P, Donskov F, et al. Tumor-Associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One 2014;9:e98259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caruso RA, Bellocco R, Pagano M, et al. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Mod Pathol 2002;15:831–7. [DOI] [PubMed] [Google Scholar]
- 11.Carus A, Ladekarl M, Hager H, et al. Tumor-Associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer 2013;81:130–7. [DOI] [PubMed] [Google Scholar]
- 12.Jensen HK, Donskov F, Marcussen N, et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 2009;27:4709–17. [DOI] [PubMed] [Google Scholar]
- 13.Droeser RA, Hirt C, Eppenberger-Castori S, et al. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One 2013;8:e1050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roncucci L, Mora E, Mariani F, et al. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2008;17:2291–7. [DOI] [PubMed] [Google Scholar]
- 15.Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol 2019;16:601–20. [DOI] [PubMed] [Google Scholar]
- 16.Wikberg ML, Ling A, Li X, et al. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol 2017;68:193–202. [DOI] [PubMed] [Google Scholar]
- 17.Rao H-L, Chen J-W, Li M, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One 2012;7:e30806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H-L, Zhou X, Xue Y-F, et al. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J Gastroenterol 2012;18:3303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bui TM, Butin-Israeli V, Wiesolek HL, et al. Neutrophils alter DNA repair landscape to impact survival and shape distinct therapeutic phenotypes of colorectal cancer. Gastroenterology 2021;161:225–38. [DOI] [PubMed] [Google Scholar]
- 20.Gout J, Perkhofer L, Morawe M, et al. Synergistic targeting and resistance to PARP inhibition in DNA damage repair-deficient pancreatic cancer. Gut 2021;70:743–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid MD, Basturk O, Thirabanjasak D, et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol 2011;24:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ocana A, Nieto-Jiménez C, Pandiella A, et al. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer 2017;16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol 2013;23:141–8. [DOI] [PubMed] [Google Scholar]
- 24.Tazzyman S, Niaz H, Murdoch C. Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Semin Cancer Biol 2013;23:149–58. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Liu Q, Zhang X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020;583:133–8. [DOI] [PubMed] [Google Scholar]
- 26.Álvaro Teijeira, Garasa S, Gato M, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 2020;52:856–71. [DOI] [PubMed] [Google Scholar]
- 27.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turri G, Barresi V, Valdegamberi A, et al. Clinical significance of preoperative inflammatory markers in prediction of prognosis in node-negative colon cancer: correlation between neutrophil-to-lymphocyte ratio and poorly differentiated clusters. Biomedicines 2021;9. doi: 10.3390/biomedicines9010094. [Epub ahead of print: 19 Jan 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong M, Kim JW, Shin MK, et al. Poorly differentiated clusters in colorectal adenocarcinomas share biological similarities with Micropapillary patterns as well as tumor buds. J Korean Med Sci 2017;32:1595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno H, Kajiwara Y, Shimazaki H, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol 2012;36:193–201. [DOI] [PubMed] [Google Scholar]
- 31.Shivji S, Conner JR, Barresi V, et al. Poorly differentiated clusters in colorectal cancer: a current review and implications for future practice. Histopathology 2020;77:351–68. [DOI] [PubMed] [Google Scholar]
- 32.Barresi V, Branca G, Vitarelli E, et al. Micropapillary pattern and poorly differentiated clusters represent the same biological phenomenon in colorectal cancer: a proposal for a change in terminology. Am J Clin Pathol 2014;142:375–83. [DOI] [PubMed] [Google Scholar]
- 33.Johncilla M, Chen Z, Sweeney J, et al. Tumor grade is prognostically relevant among mismatch repair deficient colorectal carcinomas. Am J Surg Pathol 2018;42:1686–92. [DOI] [PubMed] [Google Scholar]
- 34.Lee HJ, Eom D-W, Kang GH, et al. Colorectal micropapillary carcinomas are associated with poor prognosis and enriched in markers of stem cells. Mod Pathol 2013;26:1123–31. [DOI] [PubMed] [Google Scholar]
- 35.Le DT, Uram JN, Wang H, et al. Pd-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bever KM, Le DT. An expanding role for immunotherapy in colorectal cancer. J Natl Compr Canc Netw 2017;15:401–10. [DOI] [PubMed] [Google Scholar]
- 37.Mendonça Gorgulho C, Krishnamurthy A, Lanzi A, et al. Gutting it out: developing effective immunotherapies for patients with colorectal cancer. J Immunother 2021;44:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sclafani F Pd-1 inhibition in metastatic dMMR/MSI-H colorectal cancer. Lancet Oncol 2017;18:1141–2. [DOI] [PubMed] [Google Scholar]
- 40.Almquist DR, Ahn DH, Bekaii-Saab TS. The role of immune checkpoint inhibitors in colorectal adenocarcinoma. BioDrugs 2020;34:349–62. [DOI] [PubMed] [Google Scholar]
- 41.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010;70:3052–61. [DOI] [PubMed] [Google Scholar]
- 42.Jaillon S, Ponzetta A, Di Mitri D, et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer 2020;20:485–503. [DOI] [PubMed] [Google Scholar]
- 43.OuYang L-Y, Wu X-J, Ye S-B, et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J Transl Med 2015;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Hag A, Clark RA. Down-regulation of human natural killer activity against tumors by the neutrophil myeloperoxidase system and hydrogen peroxide. J Immunol 1984;133:3291–7. [PubMed] [Google Scholar]
- 45.Munder M, Schneider H, Luckner C, et al. Suppression of T-cell functions by human granulocyte arginase. Blood 2006;108:1627–34. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 2004;64:5839–49. [DOI] [PubMed] [Google Scholar]
- 47.Rakaee M, Busund L-T, Paulsen E-E, et al. Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget 2016;7:72184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article.


