Abstract
Vutiglabridin, which affects the pharmacokinetics (PKs) of food, is currently under clinical development for the treatment of obesity. This study aimed to evaluate the effects of low‐ and high‐fat meals on PKs of vutiglabridin in healthy male subjects. A randomized, open‐label, single‐dose, three‐period, six‐sequence crossover study was conducted. The subjects received a single oral dose of vutiglabridin 480 mg in a fasted state, 30 min after the intake of a low‐fat meal (total 500–600 kcal, fat content 100–125 kcal) and high‐fat meal (total 800–1000 kcal, fat content 500–600 kcal), with a 21‐day washout period. Geometric mean ratios (GMRs) and 90% confidence intervals (CIs) for maximum plasma concentration (C max) and area under the plasma concentration‐time curve to the last measurable timepoint (AUClast) were calculated. After intake of low‐ and high‐fat meals, systemic exposure to vutiglabridin was increased, and the time to reach C max (T max) was delayed compared to that in the fasted state. The GMRs (90% CIs) of low‐fat meal to fasted state for C max and AUClast were 2.14 (1.76–2.60) and 2.15 (1.92–2.42), respectively, and those of high‐fat meal to fasted state were 3.07 (2.53–3.72) and 3.00 (2.67–3.37), respectively. The median T max was delayed by 1.5 h in both fed states compared with that in the fasted state. The study drug was well‐tolerated after administration in both the fed and fasted states. Food ingestion substantially increased the extent of oral vutiglabridin absorption in healthy subjects, and this enhancement increased with the fat content of the meal.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Vutiglabridin, a synthetic glabridin derivative, is under clinical development for the treatment of obesity. Single oral doses of vutiglabridin up to 720 mg were safe and well‐tolerated, and showed a biphasic distribution profile in a previous phase I study. The effect of food on the pharmacokinetics (PKs) of the drug was also observed with a single dose of vutiglabridin 240 mg.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study evaluated the effect of meal types containing different fat contents on the bioavailability of vutiglabridin.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Food ingestion substantially increased the extent of oral vutiglabridin absorption in healthy subjects and this enhancement increased with the fat content of the meal.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study provided the impact of food on the PKs of vutiglabridin at an effective dose, and to determine if dosage modification is necessary based on the varying meal types that increase systemic exposure.
INTRODUCTION
Obesity is the excessive accumulation of body fat due to an energy imbalance between the calories consumed and expended. Obesity has become a major public health issue that increases the risk of metabolic diseases and might lead to a reduced quality of life. 1 , 2 In addition to diet, therapy, and exercise, many different approaches to treat and control obesity have been suggested, such as drugs, dietary supplements, and bariatric surgery. Among these approaches, drug use (i.e., phentermine/topiramate, naltrexone/bupropion, liraglutide, and semaglutide) is regarded as the most effective option for weight loss by controlling appetite when patients fail to maintain body weight using only lifestyle modifications. 3 However, drugs that control appetite to restrict calorie intake may cause the loss of lean body and muscle mass, as well as total weight loss, which can increase the risk of sarcopenia and may disturb metabolic homeostasis. 4 , 5 , 6 , 7 Therefore, a treatment with multifunctional effects that enhances energy expenditure and reduces energy intake is needed. 7
Glabridin, extracted from Glycyrrhiza glabra L. (licorice) roots, is a prenylated isoflavonoid that has anti‐oxidative properties. 8 Glabridin has been shown to have other pharmacological functions in improving metabolic abnormalities, such as obesity, diabetes, and cardiovascular diseases. 9 , 10 The anti‐obesity effect of glabridin was reported that resulted in reducing body weight by ~25% in an obese mouse model and significantly decrease body fat mass in overweight human subjects. 11 , 12
Vutiglabridin (2‐(8,8‐dimethyl‐2,3,4,8,9,10‐hexahydropyrano[2,3‐f]chromen‐3‐yl‐5‐ethoxyphenol)) is a synthetic glabridin derivative that has been shown to improve on the low stability and bioavailability of glabridin and also has anti‐obesity effects by increasing energy expenditure. 13 According to a preclinical study, vutiglabridin caused dose‐dependent effects on obesity‐related parameters, including reduced body weight and increased energy expenditure, in a high‐fat diet‐induced obese C57BL/6J mouse model. 14
A previous first‐in‐human study concluded that single oral doses of vutiglabridin up to 720 mg were safe and well‐tolerated in healthy male subjects. 7 After a single oral administration of vutiglabridin, the maximum plasma concentration (C max) was reached within 1.5–3 h and showed a biphasic distribution profile. It increased at a rate lower than the dose proportionality owing to the saturation of the oral absorption process at 720 mg dose. The effect of food on the pharmacokinetics (PKs) of the drug was also observed with a single dose of vutiglabridin 240 mg. After the intake of a high‐fat meal (total ≥ 900 kcal, fat content ≥ 35%), the systemic exposure to vutiglabridin was increased by 2.5 times compared to the fasted state. However, the drug dosage used in that case (240 mg) would be too low for clinical use, expecting further evaluation of higher effective doses. Considering that patients with obesity could administer vutiglabridin under various meal conditions to manage calorie intake and that the lipophilicity of vutiglabridin could affect its oral absorption, the effect of meal types containing different fat contents on the bioavailability of vutiglabridin needs to be evaluated. Therefore, we conducted a consecutive food effect study to evaluate the effects of low‐ and high‐fat meals on PKs of vutiglabridin in healthy male subjects.
METHODS
Study introduction
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital and was conducted in accordance with the Declaration of Helsinki and Korean Good Clinical Practice (IRB no: H‐2005‐014‐1121). The trial was registered on the Clinical Research Information Service of Ministry of Health and Welfare of Republic of Korea (KCT0005208) on July 8, 2020, and on the ClinicalTrials.gov (NCT04733001) on February 1, 2021. The first subject was enrolled on July 12, 2020, and the last subject finished the schedule on February 10, 2021. Written informed consent was obtained from all participants prior to any study‐related procedures.
Study design and procedures
Healthy male subjects aged 19–50 years, with a body mass index (BMI) of 18–25 kg/m2 were enrolled in the study. The medical history, results of a physical examination, vital signs, 12‐lead electrocardiogram (ECG), and clinical laboratory tests were performed to determine the subjects' health status. Subjects with a history of hypersensitivity to drugs including licorice were excluded. Additionally, semen analysis was used to assess whether the subjects had testosterone levels less than 2.49 ng/mL or greater than 8.36 ng/mL, due to the reproductive toxicity observed in preclinical studies. Subjects with testosterone levels falling outside this range were also excluded.
This study used a randomized, open‐label, single‐dose, three‐period, six‐sequence crossover design (Figure 1). Subjects were randomly assigned to each treatment sequence. Subjects received a single oral dose of vutiglabridin 480 mg in the fasted state, 30 min after the intake of a low‐fat meal, and 30 min after the intake of a high‐fat meal, with a 21‐day washout period between the treatments. This washout period was chosen because it is more than five times the half‐life of vutiglabridin (~80 h). 7 The low‐fat meal contained 500–600 kcal with 100–125 kcal of fat content, and the high‐fat meal contained 900 kcal with 500–600 kcal of fat content. Subjects were asked to finish their meals within 20 min. These meal conditions were designed according to the US Food and Drug Administration guidelines for food effect studies. 15 , 16
FIGURE 1.
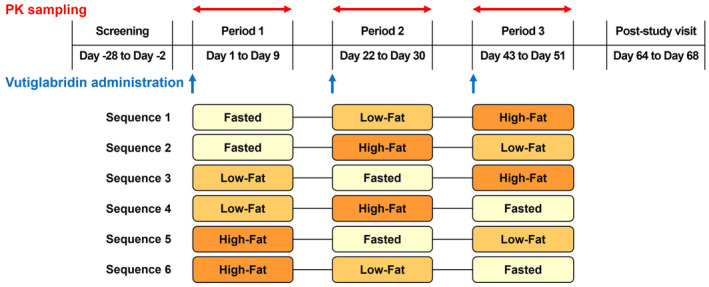
Study design. Fasted, vutiglabridin 480 mg administration in fasted state; Low‐Fat, vutiglabridin 480 mg administration after the intake of low‐fat meal (total 500–600 kcal, fat content 100–125 kcal); High‐Fat, vutiglabridin 480 mg administration after the intake of high‐fat meal (total 800–1000 kcal, fat content 500–600 kcal); PK, pharmacokinetic.
Plasma drug concentration analysis
Serial blood samples were obtained at 0 (predose), 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, 36, 48, 72, 96, 144, and 192 h after dosing to evaluate PK characteristics. Approximately 6 mL of blood was collected from each sampling point in heparinized tubes. The plasma obtained by centrifugation at 1910 g for 10 min at 4°C was then aliquoted into Eppendorf tubes and stored at −70°C until analysis.
Vutiglabridin is a racemic mixture containing a 1:1 ratio of (R)‐ and (S)‐forms. The total plasma concentration of vutiglabridin was calculated by adding the concentrations of (R)‐ and (S)‐vutiglabridin. Plasma concentrations of vutiglabridin were determined by liquid chromatography with tandem mass spectrometry using a Waters Xevo TQ‐S mass spectrometer (Waters). Lower limit of quantification was 1.0 ng/mL for (R)‐vutiglabridin and 2.0 ng/mL for (S)‐vutiglabridin. The methods used for the bioanalysis of plasma concentrations were the same as those used in a previous phase I study. 7
Pharmacokinetic assessments
The PK assessment was conducted in subjects who completed the study with quantifiable blood concentrations of vutiglabridin. PK parameters were estimated by noncompartmental methods using Phoenix WinNonlin (version 8.3; Certara). The primary PK parameters were C max and area under the plasma concentration‐time curve to the last measurable timepoint (AUClast), calculated by the linear‐up/log‐down trapezoidal method. The secondary PK parameters were the AUC from time 0 to 24 h and AUC extrapolated to infinity, time to reach C max (T max), terminal elimination half‐life, clearance, and volume of distribution.
Safety and tolerability assessments
Safety and tolerability were evaluated for all subjects who received at least one dose of the study drug throughout the study period. These included monitoring adverse events (AEs), vital signs, physical examinations, 12‐lead ECG, clinical laboratory tests, and semen analysis. Semen analysis was performed to evaluate possible reproductive toxicity and included the evaluation of semen volume, pH, number of white blood cells, sperm number, concentration, motility, and morphology.
Statistical analysis
SAS (version 9.4, SAS Institute) was used for statistical analysis. PK for the log transformed C max and AUClast, the geometric mean ratios (GMRs), and 90% confidence intervals (CIs) were calculated from the analysis of variance model. The sequence, period, and treatment were included as fixed effects and subjects nested within the sequence were included as a random effect in the model.
RESULTS
Study population
Twelve healthy male subjects were randomized into six sequences and included in the PK and safety analyses. Demographic characteristics were similar among the sequences. The mean ± standard deviation of age, height, weight, and BMI across all subjects were 31.0 ± 4.1 years, 175.3 ± 2.2 cm, 73.5 ± 7.0 kg, and 23.9 ± 2.2 kg/m2, respectively (Tables S1–S3).
The effect of food on the PK of vutiglabridin
After administering vutiglabridin with low‐ and high‐fat meals, systemic exposure to vutiglabridin increased and T max was delayed compared to that in the fasted state (Figure 2). The GMRs (90% CIs) of low‐fat meal to fasted state for C max and AUClast were 2.14 (1.76–2.60) and 2.15 (1.92–2.42), respectively, and those of high‐fat meal to fasted state were 3.07 (2.53–3.72) and 3.00 (2.67–3.37), respectively (Table 1). The median T max was delayed by 1.5 h in both fed states compared with that in the fasted state. The individual C max and AUClast of vutiglabridin increased as fat content increased (Figure 3).
FIGURE 2.
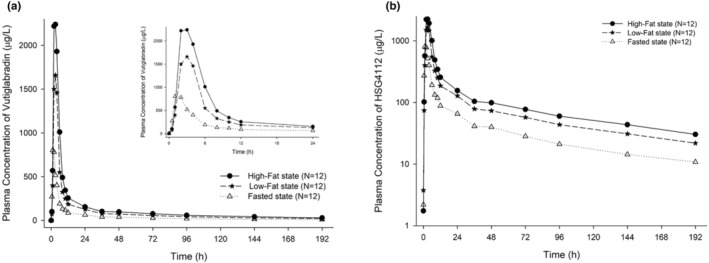
Mean plasma concentration‐time profiles of vutiglabridin after a single oral administration in high‐fat, low‐fat and fasted states. (a) linear scale (plasma concentration‐time profiles until 24 h are inserted as inset figures), (b) semi‐log scale.
TABLE 1.
Pharmacokinetic parameters of vutiglabridin after a single oral administration in low‐fat, high‐fat and fasted states.
| Parameter | Low‐fat (N = 12) | High‐fat (N = 12) | Fasted (N = 12) | GMR a (90% CI) Low‐fat/fasted | GMR b (90% CI) High‐fat/fasted |
|---|---|---|---|---|---|
| C max (μg/L) | 1880 ± 565 | 2700 ± 880 | 961 ± 539 | 2.1417 (1.7646–2.5994) | 3.0661 (2.5263–3.7213) |
| AUClast (h∙μg/L) | 17,700 ± 4400 | 24,700 ± 6750 | 8410 ± 2970 | 2.1514 (1.9165–2.4152) | 2.9991 (2.6716–3.3667) |
| AUC0–24 (h∙μg/L) | 9810 ± 2410 | 14,000 ± 3410 | 4470 ± 1880 | – | – |
| AUCinf (h∙μg/L) | 20,800 ± 5430 | 29,000 ± 9130 | 9760 ± 3280 | – | – |
| T max (h) | 3.0 [2.0–4.0] | 3.0 [2.0–4.0] | 1.5 [1.0–4.0] | – | – |
| t 1/2 (h) | 93.5 ± 28.2 | 93.5 ± 18.5 | 85.2 ± 13.8 | – | – |
| CL/F (L/h) | 24.5 ± 6.2 | 17.7 ± 4.0 | 53.8 ± 15.7 | – | – |
| V d/F (h) | 3320 ± 1450 | 2330 ± 524 | 6610 ± 2200 | – | – |
Note: Data are presented as mean ± standard deviation, except for T max, which is presented as median [minimum − maximum].
Abbreviations: AUC0‐24, area under the concentration‐time curve from 0 to 24 hours; AUCinf, AUC from 0 to infinity; AUClast, area under the concentration‐time curve from 0 to last measurable timepoint; CI, confidence interval; CL/F, total apparent clearance; C max, maximum plasma concentration; GMR, geometric mean ratio; t 1/2, half‐life; T max, time to reach C max; Vd, volume of distribution.
GMR of low‐fat meal to fasted state.
GMR of high‐fat meal to fasted state.
FIGURE 3.
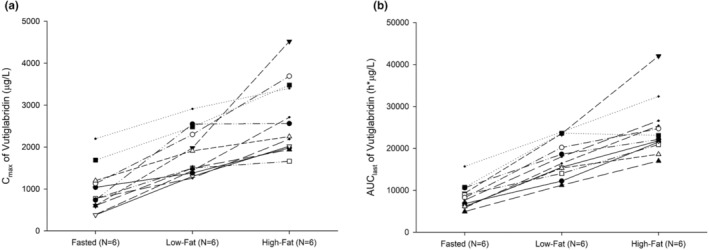
Individual pharmacokinetic parameters of vutiglabridin in high‐fat, low‐fat and fasted states. (a) C max, (b) AUClast. C max, maximum plasma concentration; AUClast, area under concentration‐time curve from 0 to last measurable timepoint.
Safety and tolerability
All AEs were mild, and all subjects recovered without sequelae (Table S3). A total of seven AEs were reported in five of the 12 subjects (41.7%). Under the conditions of fasting, low‐fat meal intake, and high‐fat meal intake, three AEs (in 2 subjects), one AE (in 1 subject), and three AEs (in 3 subjects) occurred, respectively (Table S2). Five adverse drug reactions (ADRs) in three subjects (25.0%), consisting of gastrointestinal and nervous system disorders (nausea and headache), were assessed as related to vutiglabridin (Table S3). These findings were also observed in a previous study. 7 There were no clinically significant changes observed in vital signs, physical examinations, 12‐lead ECG, clinical laboratory tests, and semen analysis.
DISCUSSION
This study was conducted to evaluate the effects of low‐ and high‐fat meals on the PKs of vutiglabridin. In contrast to a previous exploratory study, which was conducted to determine the presence of the food effect, 7 in this consecutive study, we quantified the effect of food on the PK of vutiglabridin by asking subjects to consume meals with different fat contents. It is important to evaluate the effect of low‐fat meals separately from that of high‐fat meals, because the magnitude of fat content can affect the PKs of lipophilic drugs by altering their solubility. Additionally, low‐fat meals are recommended for long‐term weight management and are expected to be consumed in obese populations. 17 Therefore, our results can be applied in actual clinical environments, where obese patients are likely to consume low‐fat meals to reduce their calorie intake.
The dose level for this study was determined by considering the anticipated efficacy of the drug, which is higher than the dose administered in the previous food effect study. 7 However, to address safety concerns related to increased systemic exposure of vutiglabridin in the presence of high‐fat meals, the selected dose level is still lower than the maximum tolerated dose observed in a previous single ascending dose study. 7 A single 480 mg dose of vutiglabridin was selected as a safe and effective dose for evaluating the effect of low‐ and high‐fat meals.
The determination of the sample size did not follow a formal statistical calculation due to the exploratory nature of the study. However, the present study included a minimum of 12 subjects in adherence to food effect bioavailability study guidelines 16 to thoroughly evaluate the impact of food on the drug's PKs. This aligns with the recommended threshold necessary for a statistical assessment of food effects on drug behavior, as outlined in the guidelines.
Food can affect drug absorption by decreasing the process of gastric emptying, raising gastrointestinal pH, increasing hepatic blood flow, or physically interacting with the drug. 18 , 19 Fat contents can increase biliary secretion, leading to increased micelle solubility and can further delay gastric emptying. 20 In this study, the systemic exposure of vutiglabridin increased threefold under high‐fat meal condition. Because of the lipophilicity of vutiglabridin, the fat content resulted in increased drug solubility and extended absorption time to high exposure. 18 , 20 , 21 Additionally, the exposure increased twofold even in the low‐fat meal condition compared to the fasted state. These results indicate that the effect of food on the PKs of vutiglabridin and the increase in systemic exposure depend on the fat content of the meals. Therefore, dose adjustments should be considered when planning further clinical studies or practical applications of this drug, as the target exposure at an effective dosage can be changed by meal conditions. Food delays the absorption of vutiglabridin, which in the present study, resulted in a delay in T max under both low‐ and high‐fat meal conditions.
Vutiglabridin shows high lipophilicity and limited solubility, which is consistent with Biopharmaceutics Classification System (BCS) Class II. The increase in systemic exposure of vutiglabridin under fed conditions aligns with behaviors commonly seen in BCS Class II drugs. In these instances, food, particularly high‐fat meals, can enhance solubility and delay gastric emptying, leading to increased drug absorption and systemic exposure, consistent with the observed food effects in this study.
A single oral dose of vutiglabridin 480 mg was safe and well‐tolerated in both low‐ and high‐fat meal‐fed states, even though drug exposure was increased by food intake. The incidence of AEs did not increase as meal conditions changed; this dose‐independent occurrence of AEs was also recorded in a previous study. 7 Moreover, the ADRs observed in the present study were consistent with those previously reported. 7 The previous study also reported mean values of C max and AUClast after a single dose of 720 mg in fasted state of 1030 μg/L and 13,700 h∙μg/L, respectively. 7 The systemic exposure observed at a single dose of 480 mg in fed states in the present study was higher than that of the 720 mg single dose in fasted state and was safe and well‐tolerated. This suggests that future studies can be considered at higher doses if the target exposure for achieving efficacy is not attained at the current dose.
A limitation of this study was that only healthy male subjects were included. Preclinical studies, conducted in rats and dogs following multiple oral doses, have shown that the PKs of vutiglabridin is not significantly affected by sex. However, it is important to evaluate the extent to which food intake may alter exposure in women. Physiological differences between men and women, such as organ blood flow, body composition, and endogenous hormonal fluctuations may affect the PKs of vutiglabridin, particularly in terms of volume of distribution and elimination considering the lipophilicity of the drug. 22 Therefore, it is essential to investigate the PKs in women, who account for a large proportion of the obese population. In obese patients, the pharmacological effect may be altered due to a larger volume of drug distribution. Vutiglabridin is a lipophilic drug with a biphasic distribution and has a high affinity for adipose tissue, which in obese patients may result in an increased volume of distribution. 23 This may lead to a prolonged half‐life of the drug. A future trial performed in obese patients to evaluate the effect of weight loss through pharmacodynamic markers would allow the examination of clinically relevant changes in exposure to vutiglabridin under different meal conditions. Therefore, to assess the impact of food on the PKs of vutiglabridin at an effective dose, and to determine if dosage modification is necessary based on the varying meal types that increase systemic exposure, further clinical studies in obese patients are needed. Moreover, the observed moderate variability in PK parameters of vutiglabridin, with coefficient of variations ranging from 25% to 35% in AUClast (Table 1), 18 supports the importance of conducting subsequent studies in a larger sample size.
CONCLUSION
This study investigated the effects of low‐ and high‐fat meals on the PKs of vutiglabridin in healthy male subjects. Food ingestion substantially increased the extent of oral vutiglabridin absorption in healthy subjects and this enhancement increased with the fat content of the meal. A single oral dose of vutiglabridin 480 mg in fed states was safe and well‐tolerated, regardless of meal type.
AUTHOR CONTRIBUTIONS
H.J.W., D.Y.Y., J.S.O., J.Y.C., and K.S.Y. wrote the manuscript. S.M.L., D.Y.Y., and I.J.J. designed the research. S.M.L., D.Y.Y., and I.J.J. performed the research. H.J.W., S.M.L., D.Y.Y., and I.J.J. analyzed the data. S.K.Y. provided new reagents/analytical tools.
FUNDING INFORMATION
This study was sponsored by Glaceum Inc., Suwon, Republic of Korea.
CONFLICT OF INTEREST STATEMENT
S.‐K.Y. is a current employee of Glaceum Inc., and hold stocks/shares. Glaceum Inc. provided funding for this research and holds Patent US9783551B2, which grants intellectual properties (IPs) for the synthesis and use of the compounds in the article. All other authors declared no competing interests for this work.
Supporting information
Tables S1–S3
ACKNOWLEDGMENTS
The clinical study was conducted at the Clinical Trials Center, Seoul National University Hospital. H.W. received a scholarship from the BK21 FOUR educational program. The author’s affiliations are listed as of the time this study was conducted.
Won H, Yoon DY, Lee S, et al. Effects of meal type on the bioavailability of vutiglabridin, a novel anti‐obesity agent, in healthy subjects. Clin Transl Sci. 2024;17:e13744. doi: 10.1111/cts.13744
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to confidentiality restrictions.
REFERENCES
- 1. Kyle TK, Dhurandhar EJ, Allison DB. Regarding obesity as a disease: evolving policies and their implications. Endocrinol Metab Clin N Am. 2016;45(3):511‐520. doi: 10.1016/j.ecl.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288‐298. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 3. Sombra LRS, Anastasopoulou C. Pharmacologic therapy for obesity. StatPearls. StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- 4. Gill LE, Bartels SJ, Batsis JA. Weight management in older adults. Curr Obes Rep. 2015;4(3):379‐388. doi: 10.1007/s13679-015-0161-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12(7):487‐491. doi: 10.1007/BF02982710 [DOI] [PubMed] [Google Scholar]
- 6. Cava E, Yeat NC, Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8(3):511‐519. doi: 10.3945/an.116.014506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Na JY, Yoon DY, Yoo H, et al. Safety, tolerability, pharmacokinetic, and pharmacodynamic characteristics of vutiglabridin: a first‐in‐class, first‐in‐human study. Clin Transl Sci. 2022;15(11):2744‐2757. doi: 10.1111/cts.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahn J, Lee H, Jang J, Kim S, Ha T. Anti‐obesity effects of glabridin‐rich supercritical carbon dioxide extract of licorice in high‐fat‐fed obese mice. Food Chem Toxicol. 2013;51:439‐445. doi: 10.1016/j.fct.2012.08.048 [DOI] [PubMed] [Google Scholar]
- 9. Li C‐x, Li T‐h, Zhu M, Lai J, Wu Z. Pharmacological properties of glabridin (a flavonoid extracted from licorice): a comprehensive review. J Funct Foods. 2021;85:104638. doi: 10.1016/j.jff.2021.104638 [DOI] [Google Scholar]
- 10. Nakagawa K, Kishida H, Arai N, Nishiyama T, Mae T. Licorice flavonoids suppress abdominal fat accumulation and increase in blood glucose level in obese diabetic KK‐A(y) mice. Biol Pharm Bull. 2004;27(11):1775‐1778. doi: 10.1248/bpb.27.1775 [DOI] [PubMed] [Google Scholar]
- 11. Lee JW, Choe SS, Jang H, et al. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J Lipid Res. 2012;53(7):1277‐1286. doi: 10.1194/jlr.M022897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tominaga Y, Nakagawa K, Mae T, et al. Licorice flavonoid oil reduces total body fat and visceral fat in overweight subjects: a randomized, double‐blind, placebo‐controlled study. Obes Res Clin Pract. 2009;3(3):169‐178. doi: 10.1016/j.orcp.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 13. Bae IY, Choi MS, Ji YS, Yoo SK, Kim K, Yoo HH. Species differences in stereoselective pharmacokinetics of HSG4112, a new anti‐obesity agent. Pharmaceutics. 2020;12(2):127. doi: 10.3390/pharmaceutics12020127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi LS, Jo IG, Kang KS, et al. Discovery and preclinical efficacy of HSG4112, a synthetic structural analog of glabridin, for the treatment of obesity. Int J Obes. 2021;45(1):130‐142. doi: 10.1038/s41366-020-00686-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration . Assessing the Effects of Food on Drugs in INDs and NDAs—Clinical Pharmacology Considerations. 2022. https://www.fda.gov/media/121313/download. June 2022
- 16. US Food and Drug Administration . Food Effect Bioavailability and Fed Bioequivalence Studies. 2002. https://www.fda.gov/files/drugs/published/Food‐Effect‐Bioavailability‐and‐Fed‐Bioequivalence‐Studies.pdf. January 2020
- 17. Pirozzo S, Summerbell C, Cameron C, Glasziou P. Advice on low‐fat diets for obesity. Cochrane Database Syst Rev. 2002;2(2):CD003640. doi: 10.1002/14651858.Cd003640 [DOI] [PubMed] [Google Scholar]
- 18. Malcolm Rowland TNT. Rowland and Tozer's Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. Vol 199. 5th ed. Wolters Kluwer; 2019. [Google Scholar]
- 19. Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37(3):213‐255. doi: 10.2165/00003088-199937030-00003 [DOI] [PubMed] [Google Scholar]
- 20. Devriese LA, Koch KM, Mergui‐Roelvink M, et al. Effects of low‐fat and high‐fat meals on steady‐state pharmacokinetics of lapatinib in patients with advanced solid tumours. Investig New Drugs. 2014;32(3):481‐488. doi: 10.1007/s10637-013-0055-4 [DOI] [PubMed] [Google Scholar]
- 21. Omachi F, Kaneko M, Iijima R, Watanabe M, Itagaki F. Relationship between the effects of food on the pharmacokinetics of oral antineoplastic drugs and their physicochemical properties. J Pharm Health Care Sci. 2019;5(1):26. doi: 10.1186/s40780-019-0155-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143‐157. doi: 10.2165/00003088-200948030-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruno CD, Harmatz JS, Duan SX, Zhang Q, Chow CR, Greenblatt DJ. Effect of lipophilicity on drug distribution and elimination: influence of obesity. Br J Clin Pharmacol. 2021;87(8):3197‐3205. doi: 10.1111/bcp.14735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to confidentiality restrictions.


