Abstract
Objective
This meta-analysis aims to explore the potential link between vaccines and systemic lupus erythematosus (SLE).
Methods
We systematically searched PubMed, Cochrane Library, and Embase for observational studies from inception to September 3, 2023, using medical subject headings (MeSH) and keywords. Study quality was assessed using the NOS scale. Statistical analyses were conducted using STATA software (version 14.0). Publication bias was evaluated using funnel plots and Egger’s regression.
Results
The meta-analysis incorporated 17 studies, encompassing 45,067,349 individuals with follow-up periods ranging from 0.5 to 2 years. The pooled analysis revealed no significant association between vaccinations and an increased risk of SLE [OR = 1.14, 95% CI (0.86–1.52), I2 = 78.1%, P = 0.348]. Subgroup analyses indicated that HBV vaccination was significantly associated with an elevated risk of SLE [OR =2.11, 95% CI (1.11-4.00), I2 = 63.3%, P = 0.02], HPV vaccination was slightly associated with an increased risk of SLE [OR = 1.43, 95% CI (0.88–2.31), I2 = 72.4%, P = 0.148], influenza vaccination showed no association with an increased risk of SLE [OR = 0.96, 95% CI (0.82–1.12), I2 = 0.0%, P = 0.559], and COVID-19 vaccine was marginally associated with a decreased risk of SLE [OR = 0.44, 95% CI (0.18–1.21), I2 = 91.3%, P = 0.118].
Conclusions
This study suggests that vaccinations are not linked to an increased risk of SLE. Our meta-analysis results provide valuable insights, alleviating concerns about SLE risk post-vaccination and supporting further vaccine development efforts.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13075-024-03296-8.
Keywords: Vaccination, Systemic lupus erythematosus, Risk, Meta-analysis
Introduction
Systemic lupus erythematosus (SLE) is a prototypical autoimmune disease that affects multiple body systems and organs. It is characterized by loss of immune tolerance, sustained production of autoantibodies, hyperactive B cell and T cell responses and the breakdown of immune tolerance towards self-antigens [1, 2]. While the exact etiology of SLE remains incompletely understood, a complex interplay of genetic predisposition and environmental triggers is believed to be at the core. Notably, infectious agents, predominantly viruses, have been implicated in the initiation or exacerbation of SLE [3, 4]. Patients with SLE often experience immunosuppression, either spontaneously or as a consequence of treatment, rendering them more susceptible to infections [5, 6]. Hence, there is a compelling need for preventative vaccination.
Vaccines play an indispensable role in modern medicine and are meticulously designed to confer protection against viral, bacterial, or fungal infections in host organisms. Typically administered prophylactically, vaccines are a standard practice in safeguarding the health of the general population (children, adolescents and adults in good health) [7]. However, concerns regarding the potential link between infections and autoimmunity, as well as the risk of precipitating or exacerbating lupus following vaccination, have led to hesitancy in offering vaccinations to individuals with SLE [8, 9]. Vaccinations have been theorized as potential triggers for the onset of SLE, considering their role in eliciting antigen-specific immune responses [10].
It was reported an equivalent occurrence of autoimmune disorders in vaccinated and unvaccinated individuals, absence of clear association between HPV, HBV vaccines and autoimmune diseases, and the benefits of COVID-19 vaccination far outweigh the possible new-onset autoimmune diseases [11–14]. While an earlier review suggested a connection between vaccinations and an elevated risk of SLE [15], subsequent studies have yielded conflicting results, with some demonstrating no discernible impact on SLE risk [16–19]. Due to these contradictory findings, a definitive conclusion regarding the relationship between vaccinations and the risk of SLE has remained elusive. To gain a more comprehensive understanding of the association between vaccinations and the risk of SLE, we conducted this meta-analysis.
Methods
The meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [20]. The protocol was pre-registered in the International Prospective Register of Systematic Reviews (PROSPERO) platform, and the approval number is CRD42023460701.
Data sources
PubMed, Cochrane Library, and Embase were searched for studies published from database inception to September 3, 2023. There were no language restrictions, and the search strategy combined the use of Medical Subject Headings (MeSH) and keywords. The search terms included systemic lupus erythematosus, SLE, vaccination, and their variants. The details of the search strategy were shown in supplementary Tables 1–3.
Eligibility criteria
The observational studies were included on the basis of the following criteria: (1) cohort studies or case-control studies; (2) investigations of the association of vaccination and the risk of SLE. In this meta-analysis, any type of vaccine was included. Observational studies were excluded if they did not provide an odds ratio (OR) with corresponding 95% confidence interval (CI). If more than one study reported data from the same study, we included the study with the longest follow-up or the largest number of participants. This meta-analysis also excluded conference abstracts, study protocols, letter to editor, duplicate publications, and studies with no outcomes of interest.
Study selection
Study selection was performed by two reviewers (MJ Wang and HP Gu) who independently screened the literature based on the eligibility and exclusion criteria. Duplicate and irrelevant articles were first excluded according to their titles and abstracts. After that, the full texts of the potentially eligible articles were downloaded and read to identify all eligible studies. Any disagreements were resolved by the third reviewer (XL Li), who acted as an arbiter.
Data extraction
Data extraction was performed independently by the two above-mentioned reviewers (MJ Wang and HP Gu) who consulted he guidelines on data extraction for systematic reviews and meta-analysis [21]. We used predesigned forms for extracting data including the first author, year of publication, study type, sample size, follow-up years, age, diagnosis of SLE, vaccine type, observation time, and observation region. Disagreements were resolved by discussion with XL Li to reach a consensus.
Risk of bias
The Newcastle-Ottawa scale (NOS) was used to assess the study quality [22]. NOS include three aspects: selection, comparability, and exposure. Stars ranged from 0 to 9 points for the studies, four stars for selection of participants and measurement of exposure, two stars for comparability, and three stars for assessment of outcomes and adequacy of follow-up, with more stars indicating higher quality of study. Scores of 0–3, 4–6, and 7–9 were considered to indicated low, moderate, and high quality, respectively.
Evidence certainty
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was employed to access the overall certainty of evidence [23, 24]. By the GRADE system, the certainty of evidence derived from observational studies receives an initial grade of low quality. The quality of evidence from cohort studies can be improved at larger effect sizes (RR ≥ 2 or ≤ 0.5), dose-response gradients, or attenuation by plausible confounding after excluding various factors that could lead to downgrading [25]. Finally, the evidence of outcomes can be graded as high, moderate, low, or very low [26].
Statistical analysis
The adjusted odds ratios (OR) and their corresponding 95% confidence intervals (CI) from each trial were utilized to evaluate the relationship between vaccination and the risk of SLE. Heterogeneity was assessed using the χ2-test and I2-values. A fixed-effects model was applied when the P-value exceeded 0.1, and the I2 statistic was less than 50%. In cases where I2 surpassed 50%, signifying substantial heterogeneity, a random-effects model was employed. To ensure the robustness of the overall results, a sensitivity analysis was conducted. Publication bias was evaluated by visually inspecting the funnel plot and statistically testing it using Egger’s regression. Subgroup analyses were carried out based on geographical region, length of observation, and study design. All statistical analyses were conducted using Stata statistical software version 14.0, developed by Stata Corp in College Station, Texas, USA, for added precision and reliability.
Results
Literature search
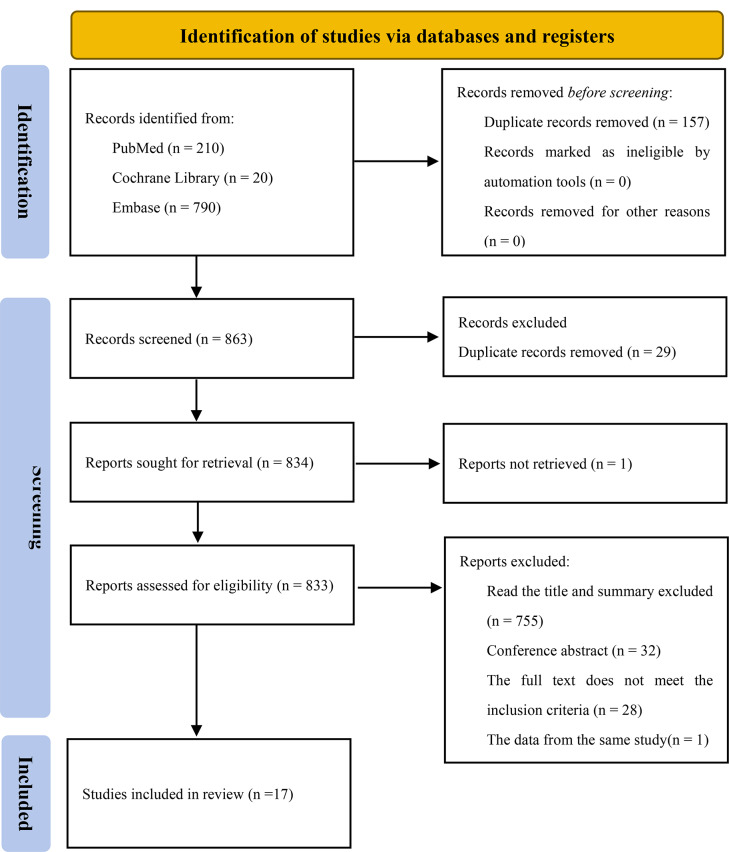
A total of 1020 records were collected through the search. After title and abstract screening 46 articles were considered potentially relevant. Seventeen studies were included after full text review, which reported the incidence of SLE on follow-up. The selection process is presented in Fig. 1.
Fig. 1.
Studies screening process
Study characteristics
This meta-analysis encompassed 17 studies, enrolling a total of 45,067,349 individuals, with a publication date up to September 3, 2023. Of these studies, ten were cohort investigations, and the remaining seven were case-control studies. The average follow-up duration across these studies varied from 0.5 to 2 years, and most studies consistently adhered to well-defined diagnostic criteria for SLE. While the adjusted estimates were available for nearly all studies, it’s worth noting that there were slight variations in the adjusted confounders employed. The primary characteristics of the studies included in this analysis are detailed in Table 1, and the excluded studies were shown in supplementary Table 4.
Table 1.
Characteristics of the included studies
| Author | Year | Country | Study type | Study size | Follow-up | Age (years) | Observation time | Diagnosis of SLE | Vaccine type | NOS scores |
|---|---|---|---|---|---|---|---|---|---|---|
| Ju HJ [16] | 2023 | Korea | Cohort study | 3,838,120 vaccination, 3,834,804 historical |
(100.7 ± 90.3), (100.7 ± 88.5) days |
(45.7 ± 18.7), (44.8 ± 18.7) | NA | ICD-10 | COVID-19 | 5 |
| Peng K [27] | 2023 | Hong Kong | Cohort study | 4,197,188 population | NA | 54.09 ± 18.08, 54.25 ± 18.33 | 28 days | ICD-9 | COVID-19 | 7 |
| Skufca J [17] | 2018 | Finland | Cohort study | 134,615 vaccinated, 105,990 unvaccinated | 365 days | (11–15) | NA | ICD-10 | HPV | 6 |
| Hviid A [18] | 2018 | Danish and Swedish | Cohort study | 319,298 vaccinated, 16,067,162 unvaccinated | NA | 18–44 | NA | ICD-10 | HPV | 5 |
| Miranda S [19] | 2017 | French | Cohort study | 1,393,167 vaccination, 4,746,593 unvaccinated | NA | 13–16 | (20 ± 11) months | ICD-10 | HPV | 5 |
| Geier DA [28] | 2017 | USA | Case-control study |
48,816 cases, 21,998 controls |
3–37 days | 6–39 | NA | SLE (VAERS code: 10,042,945) | HPV | 6 |
| Bardenheier BH [29] | 2016 | USA | Case-control study | 39 cases,117 controls | 1 year | most in the 18- to 24- year-old range | 3 years | ICD-9 | AVA | 7 |
| Lai YC [30] | 2015 | USA | Case-control study | 80 cases, 151 controls | NA | NA | NA | SLE (VAERS code: 10,042,945) | Zoster | 5 |
| Grimaldi-Bensouda L [31] | 2014 | France and Canada | Case-control study | 105 cases, 712 controls | 24 months | (32.9 ± 12.6), (34.8 ± 13.8) | 12 or 24 months | The 1997 ACR criteria | Any vaccine (including influenza, DTPP) | 7 |
| Persson I [32] | 2014 | Sweden | Cohort study | 3,347,467 vaccination, 2,497,572 unvaccination | 27 months | 1->80 | 45 days | ICD-10 | Influenza | 9 |
| Zou Y [33] | 2014 | China | Case-control study | 471 cases identified from 1,253,832 individuals | NA | (39.08 ± 11.03), (39.05 ± 10.96) | NA | The 1997 ACR criteria | HBV | 7 |
| Angelo MG [34] | 2014 | 40 countries | Cohort study | 31,173 HPV, 24,241 controls | NA | 8–72 | 30 days (day 0–29) | NA | HPV | 6 |
| Arnheim-Dahlström L [35] | 2013 | Denmark, Sweden | Cohort study | 230,005 vaccinated, 2,374,231 unvaccinated | 180 days | 12.8 (2.7) | NA | ICD-10 | HPV | 8 |
| Chao C [36] | 2012 | USA | Cohort study | 117,761 vaccinated, 412,151 unvaccinated | 180 days | (9–26) | NA | ICD-9 | HPV | 6 |
| Verstraeten T [37] | 2008 | USA | Cohort study | 36,744 cases, 31,768 controls | 1.8 years | 10–72 | NA | NA | HPV, HSV, HBV | 6 |
| GEIER DA [38] | 2005 | USA | Case-control study | 47 cases,782 controls | NA | 24–39 | (3–19) days | Costart Code | HBV | 6 |
| Cooper GS [39] | 2002 | USA | Case-control study | 265 cases, 355 controls | NA | 15–81 | 13 months | The 1997 ACR criteria | HBV | 7 |
(ICD: international classification of diseases, COVID-19: coronavirus disease 2019, HPV: human papillomavirus, HBV: hepatitis B virus, HSV: herpes simplex virus, NA: not applicable, DTPP: diphtheria, tetanus, pertussis, poliomyelitis)
Quality assessment
According to NOS criteria, the average score was 6.35 of all included observational studies, and the score for each trail was 5 or above, indicating that all studies were of moderate, or high quality in this meta-analysis. The scores of the included studies are shown in Table 1. The details were specified in supplementary Table 5.
The different type of vaccine and the risk of SLE
There were seven studies of human papillomavirus (HPV) vaccine [17–19, 28, 34–36], four studies of hepatitis B virus (HBV) vaccine [33, 37–39], two influenza vaccine [31, 32], two coronavirus disease 2019 (COVID-19) vaccine [16, 27], one zoster vaccine [30], one AVA vaccine [29], one mentioned HPV, HSV and HBV vaccine [37], one mentioned any vaccines (including influenza, DTPP) [31], and one no mentioned the type of vaccine [39] explored the association between a history of vaccination and the risk of SLE [31, 37, 39]. The pooling analysis showed that a history of HBV vaccination is significant associated with an increased risk of SLE [OR = 2.11, 95% CI (1.11-4.00), I2 = 63.3%, P = 0.02], HPV vaccination was slightly associated with an increased risk of SLE [OR = 1.43, 95% CI (0.88–2.31), I2 = 72.4%, P = 0.148], influenza vaccination was no associated with an increased risk of SLE [OR = 0.96, 95% CI (0.82–1.12), I2 = 0.0%, P = 0.559], COVID-19 vaccine marginally significant associated with an decreased risk of SLE [OR = 0.44, 95% CI (0.18–1.21), I2 = 91.3%, P = 0.118].
Any vaccine and risk of SLE
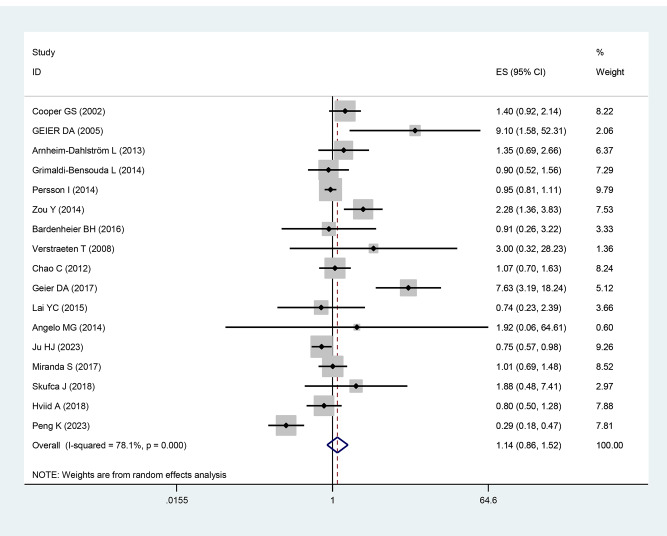
Seventeen studies explored the association between a history of vaccination and the risk of SLE. The pooling analysis showed that a history of vaccinations was not markedly associated with an increased risk of SLE [OR = 1.14, 95% CI (0.86–1.52), I2 = 78.1%, P = 0.348, Fig. 2]. Sensitivity analysis showed that none of the individual studies reversed the pooled-effect size, which means that the results are robust (supplementary Fig. S1, and supplementary Table 6).
Fig. 2.
Meta-analysis of the risk of SLE associated with any vaccine
Subgroup analysis
In the subgroup analysis, a history of vaccination in North America, Europe and Asia suggested a marginally significant association between vaccinations and risk of SLE [OR = 1.87, 95% CI (0.99–3.52), I2 = 73.2%, P = 0.053; OR = 0.96, 95% CI (0.84–1.10), I2 = 0.0%, P = 0.551; and OR = 0.79, 95% CI (0.30–2.07), I2 = 93.9%, P = 0.628, respectively] (Table 2). There was no significant association between vaccination and the risk of SLE at any of length of observations (Table 2). In the subgroup analyses by study design, meta-analysis of case-control studies showed a significant association between vaccinations and increased risk of SLE [OR = 1.84, 95% CI (1.03–3.31), I2 = 75.9%, P = 0.041], whereas meta-analysis of cohort studies suggested a slightly significant association between vaccinations and risk of SLE [OR = 0.85, 95% CI (0.65–1.11), I2 = 68.3%, P = 0.243] (Table 2).
Table 2.
Subgroup analysis for the risk of vaccination with SLE
| Subgroups | Included studies | OR (95% CI) |
Heterogeneity | |
|---|---|---|---|---|
| I 2 (%) | P-value | |||
| Region | ||||
| North America | 7 | 1.87 (0.99, 3.52) | 73.2 | 0.053 |
| Europe | 7 | 0.96 (0.84, 1.10) | 0.0 | 0.551 |
| Asia | 3 | 0.79 (0.30, 2.07) | 93.9 | 0.628 |
| Observation time | ||||
| ≥ 180 days | 8 | 1.09 (0.89, 1.33) | 0.0 | 0.408 |
| < 180 days | 8 | 1.13 (0.68, 1.87) | 87.3 | 0.634 |
| Study type | ||||
| Case-control study | 7 | 1.84 (1.03, 3.31) | 75.9 | 0.041 |
| Cohort study | 10 | 0.85 (0.65, 1.11) | 68.3 | 0.243 |
Evidence certainty
The GRADE level of evidence is very low for the risk of SLE with any vaccine. The GRADE level of evidence is very low for the risk of SLE with HPV vaccine and HBV vaccine, and is low for the risk of SLE with influenza, and COVID-19 vaccine. The GRADE level of evidence is very low for the risk of SLE in case-control study and cohort study. The GRADE level of evidence is very low for the risk of SLE in North America and Asia, and is low for the risk of SLE in Europe. The GRADE level of evidence is low for the risk of SLE in observation time ≥ 180 days, and is very low for the risk of SLE in observation time < 180 days. GRADE evidence certainty for the outcomes is shown in Table 3.
Table 3.
GRADE certainty of evidence
| Outcome | Exposure | Study numbers | GRADE | Evidence quality | ||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | ||||
| SLE | Any vaccine | 17 | 0 | -1a | 0 | 0 | 0 | Very Low |
| SLE | HPV vaccine | 7 | 0 | -1a | 0 | 0 | 0 | Very Low |
| SLE | HBV vaccine | 4 | 0 | -1a | 0 | 0 | 0 | Very Low |
| SLE | Influenza vaccine | 2 | 0 | 0 | 0 | 0 | 0 | Low |
| SLE | COVID-19 vaccine | 2 | 0 | -1a | 0 | 0 | 0 | Low |
| SLE | Case-control study | 7 | 0 | -1a | 0 | 0 | 0 | Very Low |
| SLE | Cohort study | 10 | 0 | -1a | 0 | 0 | 0 | Very Low |
| SLE | North America | 7 | 0 | -1a | 0 | 0 | 0 | Very Low |
| SLE | Europe | 7 | 0 | 0 | 0 | 0 | 0 | Low |
| SLE | Asia | 3 | 0 | -1a | 0 | 0 | 0 | Very Low |
| SLE | Observation time ≥ 180 days | 8 | 0 | 0 | 0 | 0 | 0 | Low |
| SLE | Observation time < 180 days | 8 | 0 | -1a | 0 | 0 | 0 | Very Low |
SLE: systemic lupus erythematosus, Explanations: a. high heterogeneity
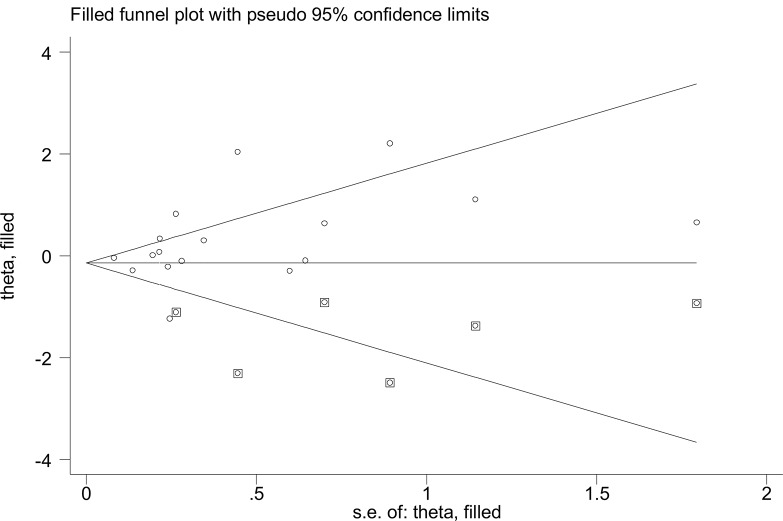
Publication bias
A visual inspection of the funnel plot showed a significant publication bias in the outcome of vaccination and risk of SLE. Correction was implemented using the trim and fill method. After inferring possible missing studies, the corrected odds ratio for vaccination associated with the risk of SLE was found to be 0.87 (90% CI 0.64–1.18, P = 0.361, Fig. 3), indicating vaccination is not associated with increased risk of SLE. The trim and fill method demonstrated a roughly symmetrical distribution of the funnel plot, thus exhibiting less susceptibility to publication bias and greater credibility.
Fig. 3.
Publication bias of the risk of SLE associated with any vaccine
Discussion
Main findings
Our meta-analysis encompassed 17 studies involving 45,067,349 individuals, providing a comprehensive assessment of the relationship between vaccinations and risk of SLE. We observed no significant increase in the risk of SLE among those who were vaccinated compared to non-vaccinated controls, suggesting that vaccination may not be an independent risk factor for SLE. Subgroup analyses, including history of HPV, influenza and COVID-19 vaccination, vaccination in North American, Europe and Asia, and cohort studies, revealed no substantial association between vaccinations and an increased risk of SLE. However, the meta-analysis of HBV vaccination, and case-control studies indicated a significant connection between vaccinations and risk of SLE, respectively.
Interpretation of findings
A previous review included 12 studies investigated those vaccinations significantly increased the risk of SLE [15]. The result was still consistent at short vaccinated time, excluding high risk of bias studies, studies without receiving funds from pharmacemeutical companies, and outcomes of short vaccinated. In the subgroup analyses, showed a significant association between vaccinations and increased risk of SLE in case-control studies, while suggested a marginally significant association between vaccinations and increased risk of SLE in cohort studies. In the included 12 studies, there were five studies mentioned HPV vaccine [28, 34–37], two of influenza vaccine [31, 32], and four mentioned HBV vaccination [33, 37–39], and subgroup analyses showed HBV vaccination was significantly linked to an increased risk of SLE, whereas HPV vaccine and influenza vaccine slightly related to the risk of SLE.
The polling analysis showed no obvious association between vaccinations and the risk of SLE in our study, which differed from the previous review. The previous study included 12 studies with 8,732,085 participants [28–39], while our study included 17 studies with 45,067,349 individuals. We added five studies with an increase of 36,335,264 people providing a comprehensive assessment of the relationship between vaccinations and risk of SLE [16–19, 27]. In the added five studies, three mentioned the HPV vaccine [17–19] and two mentioned the COVID-19 vaccine [16, 27], with all cohort studies. The conclusions about HBV vaccine, HPV vaccine and influenza vaccine were consistent with the previous review, because the same included studies of HBV and influenza vaccine, and the additional three studies on HPV vaccine also support the conclusions of the previous study. There was no association between vaccinations and the risk of SLE was confirmed in different regions, whether it’s in North America, Europe or Asia. The prior review showed at an increased SLE risk with short vaccination times in five included trails but did not a specify time frame [28, 32, 35, 36, 38]. Interestingly, in our study, we found no significant association between the timing of vaccination, whether within or beyond 180 days, and an increased risk of SLE. Furthermore, in our subgroup analyses, case-control studies also demonstrated a significant association between vaccinations and an elevated SLE risk with the same included studies, while cohort studies indicated a marginally significant association.
The both studies showed that HBV vaccination was significantly associated with increased risk of SLE, because the both studies included the same trails. It has been reported that hepatitis B is contribute to onset or exacerbation of autoimmune disorders via molecular mimicry [40, 41]. Whereas, it has been proven the safety and efficacy in preventing HBV infection [42, 43], and ruled out any confirmed evidence that HBV vaccine causes autoimmune disorders [44]. HBV vaccine contains yeast, aluminium, thimerosal, hepatitis B surface antigen epitopes, and adjuvants, which may result in hepatitis B vaccine being associated with autoimmune diseases among susceptible adult vaccine recipients [44]. HBV vaccination induces autoimmunity and the possible mechanisms seem to be molecular mimicry, the production of particular autoantibodies, and the role of certain vaccine adjuvants. HBV vaccine accelerates SLE-like disease in an autoimmune genetically prone mouse model, the level of anti-dsDNA antibodies and resulted in early onset of proteinuria, and might increase risk of autoimmunity in genetically susceptible individuals [45, 46]. The result showed that HBV vaccination was significantly associated with increased risk of SLE, however, the included studies were observed 1–2 decades ago, with the development of vaccine technology, new clinical trials are needed to provide new evidence.
In the two studies, the most of observational studies have been on the HPV vaccine, HPV vaccine was demonstrated to be safe, well-tolerated, and highly efficacious in preventing persistent infections and cervical diseases among young women [47]. The epidemiological and molecular data suggest that the vast molecular homology between viral peptides and human proteins may involvement of HPV infection in the pathogenesis of SLE [48]. While, the adjuvants component of the HPV vaccine have been hypothesized to induce abnormal activation of the immune system. This may be resolve by advances in vaccine design and preparation processes, as well as improvements in adjuvants [49]. There is a female predominance for SLE, and occurs in middle-aged adults in all nationalities. There are significant similar in HPV vaccination populations and ages. The previous review and our study showed that HPV vaccination not related to increased risk of SLE, which is a benefit for females.
Viral infection is believed to modify the induction and development of autoimmune diseases, and triggered mechanisms including molecular mimicry, bystander activation, and immortalisation of infected B cells [50, 51]. The COVID-19 infectious disease was first emerged in 2019, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Multiple autoantibodies and cytokine have been detected among SARS-CoV-2 infected patients including anti-CCP antibodies, anti-nuclear antibodies, majorly interleukin, tumour necrosis factor-α, and SARS-CoV-2 infection also diminished and dysfunctional T-regulatory cells [52–55]. Although widespread COVID-19 vaccine has reduced disease severity and mortality, vaccine-related adverse events such as autoimmune and autoinflammatory diseases have been documented, however these reports were case reports and there are no systematic large sample observational studies [56–60]. There was a study showed COVID-19 vaccination had a significant protective effect among all patients with SLE with COVID-19 [61], a non-causal association between of COVID-19 and risk of SLE [62], and COVID-19 vaccination reduced the risk of COVID-19-associated autoimmune diseases [16, 27]. In this study, the pooled findings showed that COVID-19 vaccination no associate with the risk of SLE. It seem that COVID-19 vaccine reduced the risk of autoimmune diseases by reducing viral infection. However, with only two studies, more cases and longer follow-up time clinical studies need to provide in the future to confirm our results.
The epidemiology of SLE is distributed unequally among geographical regions. SLE occurs more frequently in high-income countries. The USA, Poland, and Barbados had the highest SLE incidence. The United Arab Emirates, Barbados and Brazil had the highest SLE prevalence [63]. A previous systematic review showed that the highest incidence and prevalence of SLE were possibly in North America, and Asian people to have intermediate incidence and prevalence of SLE [64], for differences in gene environment interactions. Therefore, we employed the subgroup analysis by study design to observe the relationship between SLE and vaccination, and to observe whether vaccination is a potential pathogenesis of the different SLE incidence in different regions. However, in subgroup analysis showed that no obvious increased SLE risk associated with vaccine was observed in North America, Europe and Asia.
Studies have explored the link between vaccines and autoimmune diseases, revealing that microbial elements resembling human proteins might not trigger an immune response due to our immune systemic inherent tolerance mechanisms [65]. However, when these elements are encountered in the presence of impaired tolerance, autoimmunity can be induced. Factors like genetics and the environment play a role in modulating immune tolerance. Only a minority of vaccinated individuals develop autoimmune phenomena, indicating a genetic predisposition to vaccine-induced autoimmunity. Vaccines can activate the adaptive immune response to provide protection but might also stimulate hyperinflammatory conditions and produce specific autoantibodies, leading to adverse events. These reactions result from the interplay between the vaccinated personal susceptibility and vaccine components. Immune cross-reactivity, where certain vaccine components resemble specific human proteins, can cause the immune system to attack similar proteins in susceptible individuals, leading to autoimmune diseases-a phenomenon known as molecular mimicry [66]. In the case of HBV, influenza, and HPV vaccines, suspicions arise regarding their potential to trigger autoimmunity through molecular mimicry induced by viral particle vaccination [40, 67]. mRNA vaccines, such as the COVID-19 mRNA vaccine, act as both antigen and adjuvant, recognized by endosomal Toll-like receptors and cytosolic inflammasome components, thereby triggering inflammation and immunoreaction [68]. Studies have shown that the side effects of COVID-19 vaccines result from a temporary increase in IFN-I production alongside the induction of an immune response [69]. Adjuvants, which are compounds used to boost the immune response, can potentially disrupt immune tolerance. Vaccine adjuvants may enhance vaccine immunogenicity by activating the NLR pyrin domain-containing 3 (NLRP3) inflammasome [70]. The NLRP3 inflammasome plays a crucial role in both the innate and adaptive immune systems and is linked to various autoimmune diseases, including rheumatoid arthritis and systemic lupus erythematosus (SLE) [71]. IgE-mediated reactions, linked to polyethylene glycols as a known culprit, could be responsible for anaphylactic reactions following COVID-19 vaccination [72, 73]. Other components such as the buffering/oxidation inhibitor histidine and the non-ionic surfactant polysorbate 80 may also play a role in anaphylaxis or severe hypersensitivity reactions after vaccination [74]. Advancements in vaccine technology, increased attention to vaccine side effects and mechanisms, and the optimization of vaccination strategies may help explain why vaccination has not been associated with an increased risk of SLE.
Implications and limitations
The main strength of our meta-analysis lies in the inclusion of 17 relevant observational studies, ensuring a robust evaluation of the association between vaccinations and the risk of SLE. With a substantial pooled sample size, we were able to effectively assess this relationship. Our findings suggest that vaccination may not pose a significant risk for SLE. Notably, we focused exclusively on cohort and case-control studies, effectively managing various confounding factors and enhancing the reliability of our conclusions. While our meta-analysis did not involve covariate analysis, the cohort studies we included adequately controlled for confounders, minimizing bias and ensuring the reliability of our findings.
Several limitations of the meta-analysis should be considered. First, subgroup analyses based on vaccine type would have been underpowered, the main reasons being limited vaccine studies. Additional well-designed studies with larger samples about various vaccine types are required to examine the association between vaccination and SLE. Second, the vaccination exposure window and follow-up time were inconsistent across the included studies. Third, all studies included in the meta-analysis were conducted in North America, Europe, and Asia, no studies were from African countries. Therefore, the findings of this meta-analysis cannot be generalised to African populations, additional observational studies from African countries are needed to provide epidemiological evidence for the influence of vaccinations on the risk of SLE in Africans.
Conclusions
This meta-analysis indicates that vaccination may not be significantly associated with an increased risk of SLE. However, for HBV vaccination, it shows a potential association with a risk of SLE. Our meta-analysis results provide valuable insights, alleviating concerns about SLE risk post-vaccination and supporting further vaccine development efforts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Supplementary Fig. S1. Sensitivity analysis of the risk of SLE caused by any vaccine
Supplementary Material 2: Supplementary Tables, Supplementary Table 1-3: Details of the Literature Search Strategy, Supplementary Table 4: Details of the excluded studies, Supplementary Table 5: Details of the NOS, Supplementary Table 6: Sensitivity analyses
Acknowledgements
Not applicable.
Abbreviations
- SLE
systemic lupus erythematosus
- PRISMA
preferred reporting items for systematic reviews and meta-analyses
- PROSPERO
international prospective register of systematic reviews
- MeSH
medical subject headings
- OR
odds ratio
- CI
confidence interval
- NOS
Newcastle-Ottawa scale
- ICD
international classification of diseases
- COVID-19
coronavirus disease 2019
- HPV
human papillomavirus
- HBV
hepatitis B virus
- HSV
herpes simplex virus
- DTPP
diphtheria,tetanus,pertussis,poliomyelitis
- NLRP3
NLR pyrin domain containing 3
Author contributions
ZJ X and CP W: conceived the study. MJ W and HP G: collected the data and drafted the manuscript. XL L, YQ Z and L H revised the manuscript and language. HC L conducted the subgroup analysis, and edit the manuscript. All authors have read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No.82374395, 81973829), and Zhejiang Province Scientific Research Project of Traditional Chinese Medicine (No.2021ZA063).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meijiao Wang and Huanpeng Gu contributed equally to this article as co-first authors.
Contributor Information
Zhijun Xie, Email: xzj575@163.com.
Chengping Wen, Email: chengpw2010@126.com.
References
- 1.Kiriakidou M, Ching CL. Systemic Lupus Erythematosus. Ann Intern Med. 2020;172(11):ITC81–ITC96. doi: 10.7326/AITC202006020. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Boulougoura A, Endo Y, Tsokos GC. Abnormalities of T cells in systemic lupus erythematosus: new insights in pathogenesis and therapeutic strategies. J Autoimmun. 2022;132:102870. doi: 10.1016/j.jaut.2022.102870. [DOI] [PubMed] [Google Scholar]
- 3.Rigante D, Mazzoni MB, Esposito S. The cryptic interplay between systemic lupus erythematosus and infections. Autoimmun Rev. 2014;13(2):96–102. doi: 10.1016/j.autrev.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Tsokos GC. Gut viruses in the pathogenesis of systemic lupus erythematosus. Sci Bull. 2023;68(7):664–5. doi: 10.1016/j.scib.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 5.He J, Li ZG. Dilemma of immunosuppression and infection risk in systemic lupus erythematosus. Rheumatology. 2023;62(Supp1):i22–9. doi: 10.1093/rheumatology/keac678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illescas-Montes R, Corona-Castro CC, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ. Infectious processes and systemic lupus erythematosus. Immunology. 2019;158(3):153–60. doi: 10.1111/imm.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gualano MR, Olivero E, Voglino G, et al. Knowledge, attitudes and beliefs towards compulsory vaccination: a systematic review. Hum Vacc Immunother. 2019;15(4):918–31. doi: 10.1080/21645515.2018.1564437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millet A, Decaux O, Perlat A, Grosbois B, Jego P. Systemic lupus erythematosus and vaccination. Eur J Intern Med. 2009;20(3):236–41. doi: 10.1016/j.ejim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Mok CC, Chan KL, Tse SM. Hesitancy for SARS-CoV-2 vaccines and post-vaccination flares in patients with systemic lupus erythematosus. Vaccine. 2022;40(41):5959–64. doi: 10.1016/j.vaccine.2022.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks CG, Santos ADE, Barbhaiya M, Costenbader KH. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Cl Rh. 2017;31(3):306–20. doi: 10.1016/j.berh.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petráš M, Lesná IK, Dáňová J. Can vaccination trigger Autoimmune disorders? A Meta-analysis. LID – 10.3390/vaccines9080821 [doi] LID – 821. Vaccines (Basel) 2021;9(8):821. doi: 10.3390/vaccines9080821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang HY, Shi YD, Zhang X, et al. Human papillomavirus vaccination and the risk of autoimmune disorders: a systematic review and meta-analysis. Vaccine. 2019;37(23):3031–9. doi: 10.1016/j.vaccine.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 13.Guo M, Liu XX, Chen XM, Li QG. Insights into new-onset autoimmune diseases after COVID-19 vaccination. Autoimmun Rev 2023;22(7). [DOI] [PMC free article] [PubMed]
- 14.JP S. Immune-mediated myelitis following hepatitis B vaccination. Autoimmun Rev 2012;12(2):144–9. [DOI] [PubMed]
- 15.Wang B, Shao XQ, Wang D, Xu DH, Zhang JA. Vaccinations and risk of systemic lupus erythematosus and rheumatoid arthritis: a systematic review and meta-analysis. Autoimmun Rev. 2017;16(7):756–65. doi: 10.1016/j.autrev.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Ju HJ, Lee JY, Han JH, Lee JH, Bae JM, Lee S. Risk of autoimmune skin and connective tissue disorders after mRNA-based COVID-19 vaccination. J Am Acad Dermatol. 2023;89(4):685–93. doi: 10.1016/j.jaad.2023.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skufca J, Ollgren J, Artama M, Ruokokoski E, Nohynek H, Palmu AA. The association of adverse events with bivalent human papilloma virus vaccination: a nationwide register-based cohort study in Finland. Vaccine. 2018;36(39):5926–33. doi: 10.1016/j.vaccine.2018.06.074. [DOI] [PubMed] [Google Scholar]
- 18.Hviid A, Svanström H, Scheller NM, Grönlund O, Pasternak B, Arnheim-Dahlström L. Human papillomavirus vaccination of adult women and risk of autoimmune and neurological diseases. J Intern Med. 2018;283(2):154–65. doi: 10.1111/joim.12694. [DOI] [PubMed] [Google Scholar]
- 19.Miranda S, Chaignot C, Collin C, Dray-Spira R, Weill A. M. Z. Human papillomavirus vaccination and risk of autoimmune diseases: a large cohort study of over 2 million young girls in France. Vaccine. 2017;35(36):4761–8. doi: 10.1016/j.vaccine.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Page MA-O, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor KS, Mahtani KR, Aronson JK. Summarising good practice guidelines for data extraction for systematic reviews and meta-analysis. Bmj Evid-Based Med. 2021;26(3):88–90. doi: 10.1136/bmjebm-2020-111651. [DOI] [PubMed] [Google Scholar]
- 22.GA Wells BS, D O’Connell J, Peterson V, Welch M, Losos P, Tugwell. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyse. https://www.ohrica/programs/clinical_epidemiology/oxfordasp. 2023.
- 23.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–6. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Li XL, Huang L, Tang YJ, Hu XM, Wen CP. Gout and risk of dementia, Alzheimer’s disease or vascular dementia: a meta-epidemiology study. Front Aging Neurosci. 2023;15:1051809. doi: 10.3389/fnagi.2023.1051809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng K, Li X, Yang DL, et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population based cohort study. Eclinicalmedicine. 2023;63:102154. doi: 10.1016/j.eclinm.2023.102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geier DA, Geier MR. Quadrivalent human papillomavirus vaccine and autoimmune adverse events: a case-control assessment of the vaccine adverse event reporting system (VAERS) database. Immunol Res. 2017;65(1):46–54. doi: 10.1007/s12026-016-8815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardenheier BH, Duffy J, Duderstadt SK, et al. Anthrax Vaccine and the risk of rheumatoid arthritis and systemic Lupus Erythematosus in the U.S. military: a case-control study. Mil Med. 2016;181(10):1348–56. doi: 10.7205/MILMED-D-15-00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai Yc Fau . Yew YW, Yew YW. Severe autoimmune adverse events post herpes zoster vaccine: a case-control study of adverse events in a National Database. J Drugs Dermatol. 2015;14(7):681–4. [PubMed] [Google Scholar]
- 31.Grimaldi-Bensouda L, Le Guern V, Kone-Paut I, et al. The risk of systemic Lupus Erythematosus Associated with vaccines An International Case-Control Study. Arthritis Rheumatol. 2014;66(6):1559–67. doi: 10.1002/art.38429. [DOI] [PubMed] [Google Scholar]
- 32.Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with pandemrix: a population- and registry-based cohort study with over 2 years of follow-up. J Intern Med. 2014;275(2):172–90. doi: 10.1111/joim.12150. [DOI] [PubMed] [Google Scholar]
- 33.Zou YF, Feng CC, Zhu JM, et al. Prevalence of systemic lupus erythematosus and risk factors in rural areas of Anhui Province. Rheumatol Int. 2014;34(3):347–56. doi: 10.1007/s00296-013-2902-1. [DOI] [PubMed] [Google Scholar]
- 34.Angelo MG, David MP, Zima J, et al. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidem Dr S. 2014;23(5):466–79. doi: 10.1002/pds.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnheim-Dahlström L, Pasternak B, Fau - Svanström H, Svanström H, Fau - Sparén P, Sparén P, Fau - Hviid A, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013;347:f5906. doi: 10.1136/bmj.f5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271(2):193–203. doi: 10.1111/j.1365-2796.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- 37.Verstraeten T, Descamps D, David MP, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008;26(51):6630–8. doi: 10.1016/j.vaccine.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 38.Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38(4):295–301. doi: 10.1080/08916930500144484. [DOI] [PubMed] [Google Scholar]
- 39.Cooper GS, Dooley Ma Fau - Treadwell EL, Treadwell El Fau - St Clair EW, St Clair Ew Fau - Gilkeson GS, Gilkeson GS. Risk factors for development of systemic lupus erythematosus: allergies, infections, and family history. J Clin Epidemiol 2002;55(10):982–989. [DOI] [PubMed]
- 40.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–94. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wraith DC, Goldman M. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(939):1659–66. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 42.Das S, Ramakrishnan K, Behera SK, Ganesapandian M, Xavier AS, Selvarajan S. Hepatitis B Vaccine and Immunoglobulin: key concepts. J Clin Transl Hepato. 2019;7(2):165–71. doi: 10.14218/JCTH.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Lello FA, Martínez AP, Flichman DM. Insights into induction of the immune response by the hepatitis B vaccine. World J Gastroenterol. 2022;28(31):4249–62. doi: 10.3748/wjg.v28.i31.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geier MR, Geier DA, AC Z A review of hepatitis B vaccination. Expert Opin Drug Saf. 2003;2(2):113–22. doi: 10.1517/14740338.2.2.113. [DOI] [PubMed] [Google Scholar]
- 45.Agmon-Levin N, Arango MT, Kivity S, et al. Immunization with hepatitis B vaccine accelerates SLE-like disease in a murine model. J Autoimmun. 2014;54:21–32. doi: 10.1016/j.jaut.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Ravel G, Christ M, Horand F. Autoimmunity, environmental exposure and vaccination: is there a link? Toxicology. 2004;196(3):211–6. doi: 10.1016/j.tox.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Lu BB, Kumar A, Castellsagué X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic Review & Meta-Analysis. Bmc Infect Dis. 2011;11:13. doi: 10.1186/1471-2334-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segal Y, Calabrò M, Kanduc D, Shoenfeld Y. Human papilloma virus and lupus: the virus, the vaccine and the disease. Curr Opin Rheumatol. 2017;29(4):331–42. doi: 10.1097/BOR.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 49.Mariani L, Venuti A. HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future. J Transl Med. 2010;8:105. doi: 10.1186/1479-5876-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gracia-Ramos AE, Martin-Nares E. New Onset of Autoimmune diseases following COVID-19 diagnosis. LID – 10.3390/cells10123592 [doi] LID – 3592. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO. Viruses and autoimmunity: a review on the potential Interaction and Molecular mechanisms. LID – 10.3390/v11080762 [doi] LID – 762. Viruses. 2019;11(8):762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halpert G, Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev 2020;19(12). [DOI] [PMC free article] [PubMed]
- 53.Hu B, Huang S. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–6. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montazersaheb S, Khatibi SMH, Hejazi MS et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 2022;19(1). [DOI] [PMC free article] [PubMed]
- 55.Mobasheri L, Nasirpour MH, Masoumi E, Azarnaminy AF, Jafari M. SARS-CoV-2 triggering autoimmune diseases. Cytokine. 2022;154:155873. doi: 10.1016/j.cyto.2022.155873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez Y, Rojas M, Beltrán S, et al. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J Autoimmun. 2022;132:102898. doi: 10.1016/j.jaut.2022.102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HJ, Jung M, Lim BJ, Han SH. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int. 2022;101(4):826–8. doi: 10.1016/j.kint.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zavala-Miranda MF, González-Ibarra SG, Pérez-Arias AA, Uribe-Uribe NO, Mejia-Vilet JM. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021;100(6):1340–1. doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen E, Feldmann R, Pirker R, Jochmann J, Breier F. New onset of systemic lupus erythematosus following COVID-19 mRNA vaccination. J Eur Acad Dermatol Venereol. 2024;38(1):e6–e7. doi: 10.1111/jdv.19513. [DOI] [PubMed] [Google Scholar]
- 60.Tuschen K, Bräsen JH, Schmitz J, Vischedyk M, Weidemann A. Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int. 2021;100(4):941–4. doi: 10.1016/j.kint.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calabrese C, Atefi G, Evans KA, Moynihan M, Palmer L, SJ W. Risk factors for severe COVID-19 among patients with systemic lupus erythematosus: a real-world analysis of a large representative US administrative claims database, 2020–2021. RMD Open. 2023;9(3):e003250. doi: 10.1136/rmdopen-2023-003250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang FX, Zhang N, Meng RG, et al. Non-causal association of COVID-19 with systemic lupus erythematosus: evidence from a bidirectional mendelian randomization. J Infect. 2023;86(4):E87–E90. doi: 10.1016/j.jinf.2023.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian JR, Zhang DY, Yao X, Huang YQ, Lu QJ. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis. 2023;82(3):351–6. doi: 10.1136/ard-2022-223035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang WY. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology. 2017;56(11):1945–61. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- 65.Kanduc D. Peptide cross-reactivity: the original sin of vaccines. Front Biosci (Schol Ed) 2012;4(4):1393–401. doi: 10.2741/s341. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, Xu ZW, Wang P, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 67.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–7. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sprent J. COVID-19 vaccine side effects: the positives about feeling bad. Sci Immunol. 2021;6(60):eabj9256. doi: 10.1126/sciimmunol.abj9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reinke S, Thakur A, Gartlan C, Bezbradica JS. Inflammasome-mediated immunogenicity of clinical and experimental vaccine adjuvants. Vaccines (Basel) 2020;8(3):554. doi: 10.3390/vaccines8030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Z, Guo J, Bi L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed Pharmacother. 2020;130:110542. doi: 10.1016/j.biopha.2020.110542. [DOI] [PubMed] [Google Scholar]
- 72.Krantz MS, Liu Y, Phillips EJ., Jr COVID-19 vaccine anaphylaxis: PEG or not? Allergy. 2021;76(6):1934–7. doi: 10.1111/all.14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cabanillas B, Akdis CA. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2021;76(6):1617–8. doi: 10.1111/all.14711. [DOI] [PubMed] [Google Scholar]
- 74.Borgsteede SD, Geersing TH, Tempels-Pavlica Z. Other excipients than PEG might cause serious hypersensitivity reactions in COVID-19 vaccines. Allergy. 2021;76(6):1941–2. doi: 10.1111/all.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary Fig. S1. Sensitivity analysis of the risk of SLE caused by any vaccine
Supplementary Material 2: Supplementary Tables, Supplementary Table 1-3: Details of the Literature Search Strategy, Supplementary Table 4: Details of the excluded studies, Supplementary Table 5: Details of the NOS, Supplementary Table 6: Sensitivity analyses
Data Availability Statement
No datasets were generated or analysed during the current study.