Abstract
Most microorganisms live in conditions of nutrient limitation in their natural habitats. When exposed to these conditions they respond with physiological and morphological changes that enable them to survive. To obtain insights into the molecular mechanisms of this response a systematic genetic screen was performed to identify genes that when overexpressed can induce a starvation-like response in the yeast species Schizosaccharomyces pombe. One gene that meets these criteria, fnx1+, induces, transcriptionally correlates with, and is required for the entry into the quiescent G0 state that is normally induced by nitrogen starvation. fnx1+ encodes a protein with sequence similarity to the proton-driven plasma membrane transporters from the multidrug resistance group of the major facilitator superfamily of proteins. We propose that fnx1+ plays a role in the entry into G0, possibly by facilitating the release of a signaling substance into the environment as a means of cell-to-cell communication.
Starvation is the most fundamental stress in nature and as such is a driving force of evolution. When deprived of nutrients, microorganisms undergo dramatic changes in physiology and morphology that, arguably, enable them to survive. The standard conditions for performing the majority of laboratory experiments on unicellular organisms have been established based on a preference for the shortest possible generation time and therefore include a rich supply of nutrients in the incubation medium. This, however, is misrepresentative of the conditions of nutrient deprivation that microorganisms encounter for the predominant portion of their life span (27).
Yeast species have proven to be useful systems for research on a variety of biological problems. Several models for starvation have been studied in budding yeast cells. One of these is the stationary phase, the stage in the life span of a culture in normal medium during which there is no further increase in cell number (19, 53, 54). In standard medium preparations the limiting nutrient is the carbon source. True stationary phase sets in weeks after glucose is exhausted and cells shift from fermentation to oxidative carbon metabolism and is characterized by specific gene expression (4). Although yeast cells in stationary phase are starved of carbon, they are also subjected to other complex factors such as increased cell density, accumulation of secondary metabolites, and an increased rate of nutrient consumption. A simpler and better-controlled model for starvation is the abrupt removal of a certain nutritional component from an exponentially growing culture. This model has been studied, but not extensively, in budding yeast cells (28). One particular aspect of starvation that has been studied in great detail on the molecular level is amino acid starvation. A wealth of information has been acquired on the mechanisms by which budding yeast cells respond to deprivation of amino acids (20, 24, 36). It should be noted, however, that starvation for amino acids does not result in a quiescent cell cycle arrest but rather shifts the cell metabolism to a prototrophic mode.
Our goal was to obtain insight into the molecular mechanisms by which Schizosaccharomyces pombe cells respond to nutrient limitation. Several features of the starvation response have been documented in fission yeast: (i) reduction in cell size; (ii) acquired resistance to heat shock; (iii) growth arrest; and (iv) condensation of the chromatin (2, 5, 10, 13, 48). In addition, there are characteristics that are specific for the limiting nutrient. Cells starved of nitrogen arrest the cell cycle with a 1C DNA content and become competent for sexual interactions if cells of the opposite mating type are present. If the cells do not follow the sexual development pathway, they enter a G0 state in which they do not divide but do retain viability for prolonged periods of time (48). In contrast, cells starved of carbon arrest with a 2C DNA content, are not responsive to mating, and do not maintain long-term viability (5).
Although the physiological and morphological characteristics described above have long been known, there has been only one report of a systematic screen for proteins involved in the starvation response of fission yeast. This screen (61), which looked for mutants that failed to undergo size reduction in response to starvation by using gradient separation and microscopic observation, identified a positive regulator of cell cycle progression, nim1 (14). nim1 was originally reported to be the factor that accelerates mitosis relative to growth rate in response to starvation so that a smaller cell size can be achieved. Recently, however, this hypothesis has been disproved (3, 57), and the larger size of the nim1 mutant cells in starvation media is probably the result of their larger initial cell size during the exponential phase.
Several fission yeast proteins that may play a role in allowing cells to properly respond to nutrient limitation have been identified by investigators studying the general problems of cell cycle progression, meiosis, or second messengers (8, 9, 39, 45–47, 49, 50, 55, 58). One particularly relevant cell signaling pathway is the wak1-wis1-sty1 MAP kinase pathway. sty1, a protein kinase similar to the mammalian JNK/SAPK and p38/CSBP MAP kinases, is activated by a range of environmental stresses, including growth to saturation in YPD (50) or shift from growth in YE (rich medium) to Edinburgh minimal medium (EMM) (46). Together with its upstream activators wak1 and wis1, it forms a typical MAP kinase activation cascade (8, 45, 46). Downstream of this MAP kinase is the atf1 transcription factor (47, 50, 55), which is similar to the mammalian transcription factor ATF (18, 21, 63). atf1 is required for the expression of genes involved in the stress response, for example, gpd1, which is involved in the response to osmotic stress (39). It is conceivable that atf1 is responsible for implementing the transcriptional differentiation program in response to various environmental stresses; however, neither the wis1 signaling pathway nor the atf1 transcription factor are implicated in the transition to G0 in response to nitrogen starvation.
We undertook a systematic approach to identify proteins that regulate the transition from proliferative growth to starvation-induced differentiation by searching for genes which at an elevated expression level can induce the morphological and physiological changes characteristic of starved cells, even when in rich nutritional conditions. This approach was based on the expectation that ectopic overexpression of regulatory or signaling molecules in the starvation response pathway can mimic their activated state even in the absence of starvation. We describe here one of these genes, which is transcriptionally activated upon nitrogen starvation. Nitrogen starvation is required for cells to enter the long-term quiescent state, G0, while other forms of starvation, including growth to stationary phase, induce a differentiated state with much shorter survival period and which therefore cannot be termed a true G0 (48).
MATERIALS AND METHODS
Yeast strains and cell culture.
The S. pombe strains used were a haploid strain (h− leu1-32 ura4-D18), a diploid strain (h−/h+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-m210/ade6-m216), an ste11 mutant strain (h90 aff1 ura4-D18) (49), an atf1 deletion strain (h− atf1::ura4) (50), a cyr1 deletion strain (h− ade6-M216 leu1-32 ura4-D18 cyr::ura4) (31, 60), a pde1 mutant strain (h90 ade6-M216 leu1-32 cgs2-2) (32, 58), and a cdc2 mutant strain (h− leu1-32 cdc2-33) (38), all of which are derived from strains 972 and 975 (26). A cDNA library (a generous gift from C. Norbury, B. Edgar, and P. Nurse) in which expression is controlled by the thiamine-repressible promoter nmt1 (33) was used for the fnx screen. Transformation was performed either by electroporation or by a lithium acetate protocol (37, 40). Cells were cultured in EMM (37) at 25 or 30°C in a gyratory water bath or on EMM agar plates. In the experiments in which the nmt1 promoter was repressed, 5 μg of thiamine per ml of EMM was added. Crosses and sporulation of diploids were performed in ME sporulation medium (37). The following starvation media were used: EMM containing no nitrogen source (EMM-N), EMM containing 5 g of glucose per liter instead of 20 g per liter (EMM low glucose), and EMM containing 10 mg of Na2HPO4 and 1 g of sodium acetate per liter instead of 3 g of phtallate and 2.2 g of Na2HPO4 per liter (EMM low phosphate).
Northern blot analysis.
Total RNA was prepared from cells incubated in EMM or starvation media with the RNeasy kit from QIAGEN. RNA was quantified by UV spectrophotometry, and equal amounts were separated by a 1.2% agarose gel containing formaldehyde. The RNA was transferred to a nylon membrane and hybridized with fnx1+ or actin (34) probes by standard methods. The hybridization signal was quantified with a PhosphorImager system from Molecular Dynamics.
DNA manipulations.
fnx1+ 1.1-kb partial cDNA insert isolated from the fnx screen was used as a probe for hybridization to an ordered S. pombe cosmid filter library (22, 25, 62), obtained from the Resource Center/Primary Database of the German Human Genome Project (RZDP), Max Planck Institute for Molecular Genetics, Berlin, Germany (http://www.rzdp.de/). Five positive cosmids were identified (ICRFc60E0633D, ICRFc60D1021D, ICRFc60B0923D, ICRFc60B1125D, and ICRFc60B1129E) and obtained from the RZDP. Restriction endonuclease and Southern blot analyses revealed a 3.2-kb HindIII fragment containing the entire open reading frame (ORF) that was subcloned into pBluescript(KS−) and sequenced. The 400-bp HindIII/EcoRI fragment, containing the start codon, was subcloned into pBluescript(KS−) and used as a PCR template with an oligonucleotide primer, 5′-AGTCTAGACATATGGTCGATCAGGTTAATTT-3′, which introduced an NdeI site at the start codon of fnx1+. The PCR product was digested with NdeI and EcoRI and subcloned together with the EcoRI/BamHI 3′ fragment from the cDNA into NdeI/BamHI sites of the pREP1 expression vector in which transcription is under the control of the nmt1 promoter (33). Green fluorescent protein (GFP) was expressed from the nmt1 promoter using the pGFP41 vector (6).
Sequence analysis of fnx1+.
The amino acid sequence of fnx1 was used as input for a BLAST search (1) (http://www.ncbi.nlm.nih.gov/cgi-bin/BLAST/). Fourteen putative transmembrane domains were identified by using the TMpred software developed by the Bioinformatics Group at the Swiss Institute for Experimental Cancer Research (ISREC) and publicly available at http://ulrec.unil.ch/software/TMPRED_form.html. Signature motifs for the major facilitator superfamily (42) were identified by visual inspection.
Construction of a Δfnx1 strain.
A 1.2-kb EcoRI/EcoRV fragment containing the entire fnx1+ ORF was removed from the 3.2-kb HindIII fragment and replaced by an 1.8-kb EcoRI/HincII insert containing the ura4+ gene. A 2.7-kb HindIII/HincII fragment from this construct was gel purified and used as a deletion construct to transform wild-type diploid cells. Eight stable ura+ transformants were isolated. Southern blot analysis was performed and identified six fnx1+ deletion heterozygous mutants which were sporulated. Additional Southern blot analysis was performed on the Δfnx1 haploid strains that were obtained through the sporulation of the heterozygous diploid to confirm the deletion. The Δfnx1 haploid strain was crossed with a wild-type strain to eliminate the leu1 and ade6 mutations.
Survival of Δfnx1 cells in medium lacking nitrogen.
Wild-type cells and Δfnx1 cells were grown in EMM to 2 × 106 cells/ml, washed, transferred to EMM-N, and then incubated for 21 days at 32°C with constant shaking. Two independent experiments were performed with triplicate plating for efficiency of plating determination.
Cell density experiments.
Wild-type and Δfnx1 cells (2 × 107/ml and 10-fold serial dilutions) were incubated for 6 days in EMM-N. Cells were then either spotted in 10-fold serial dilutions from each independent culture EMM plates or subjected to heat shock treatment for 20 min at 50°C and then spotted onto EMM as the untreated cultures.
Fluorescence microscopy.
Cells were stained with DAPI (4′,6-diamidino-2-phenylindole) to visualize the DNA in fixed cells (37) and were observed and photographed with a Zeiss Axioscop fluorescence microscope.
Flow cytometry analysis.
The cells were fixed in ethanol, and flow cytometry analysis was performed with a Coulter XL-MCL flow cytometer (Coulter) as previously described (43). Aggregated samples were briefly sonicated before measurement.
GenBank accession number.
The GenBank accession number for the fnx1 sequence is AF029304.
RESULTS
Screen for fnx genes.
We transformed S. pombe cells with a cDNA library in the REP3X expression vector in which transcription is controlled by the thiamine-repressible nmt1 promoter (15, 33). In three independent experiments we obtained a total of approximately 300,000 transformants, which were inoculated into liquid medium that contained no thiamine so that the promoter driving the library was derepressed. The inoculation was at low density (6 × 105 cells/ml) in order to ensure an ample supply of nutrients in the medium. After incubation for 18 h, an adequate time for induction of the nmt1 promoter (15), we subjected the cells to a heat shock of 48°C for 40 min. Under these conditions less than 0.5% of growing wild-type cells survived. Any transformants that were in the starvation state due to the introduced cDNA would have a higher chance of survival. We then plated the cells onto solid medium containing thiamine to repress the promoter and allow the cells to resume growth. They were next replicated onto plates which would again induce transcription of the cDNA, and the clones that did not grow were selected for further study. Since the first selection step was based on survival, we anticipated that the chances of finding toxic genes in the second, negative selection step were small. However, in order to further minimize this possibility, in two of the experiments we used the cdc2-33 (38) mutant as the starting strain. At the restrictive temperature of 36°C, cdc2 mutant cells arrest the cell cycle but continue to grow and elongate until they lose viability. However, when cdc2 mutants cells are starved at 36°C, they arrest both growth and cell cycle progression and therefore remain viable (52). We took advantage of this fact and modified the screening protocol for the second and third independent experiments by using cdc2-33 cells instead of wild-type cells and by including an additional 12-h incubation at 36°C after the heat shock step as a positive selection for cdc2-33 cells which could survive this treatment if they were in a starvation-like growth arrest due to the cDNA overexpression.
Six of the surviving strains that morphologically resembled wild-type starved cells and showed a significantly higher degree of heat shock resistance and growth inhibition were selected for further characterization. The cDNAs from the six clones were isolated and reintroduced into wild-type cells to retest for heat shock resistance and growth inhibition. The clones isolated from the screen were named fnx for facilitated nutritional exit from the proliferative cycle and for the mythical bird Phoenix that rose from its ashes because these clones were isolated after heat-shock treatment. The six cDNAs represented five different genes. Here we describe the characterization of fnx1+, which was the only gene represented by two cDNA transformants among the final six.
Overexpression phenotype of fnx1+.
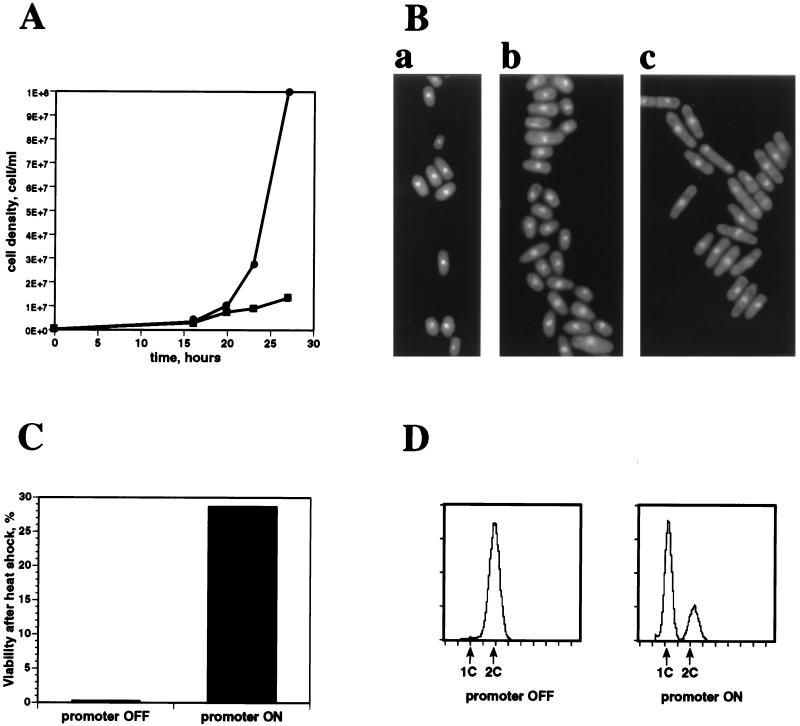
We analyzed fnx1+-overexpressing cells with respect to the physiological markers of the starvation response that had been previously characterized. Since the original cDNA was truncated at the 5′ end we reconstructed the whole ORF in the pREP1 vector (see Materials and Methods). The phenotypes of overexpression of the full ORF and the truncated cDNA were identical. In conditions allowing cDNA expression, fnx1+ transformants displayed a growth arrest when compared to cells in which the promoter was repressed (Fig. 1A). fnx1+ transformants had the characteristic morphology of starved S. pombe cells (Fig. 1Ba): a short and rounded shape (Fig. 1Bb) and a bright appearance under phase microscopy (data not shown), which was dependent on derepression of the promoter (compare Fig. 1Bb with 1Bc). fnx1+-overexpressing cells were highly resistant to heat shock of 48°C (Fig. 1C), as could have been predicted by the screening strategy. A fluorescence-activated cell sorter analysis of fnx1+-overexpressing cells (Fig. 1D, promoter ON) showed that the population was enriched for cells with 1C DNA content, a characteristic of wild-type cells starved of nitrogen but not of growing wild-type cells, wild-type cells starved of carbon (5), or fnx1+ transformants in which the promoter was repressed (Fig. 1D, promoter OFF). Taken together, these observations show that fnx1+ overexpression in cells in rich medium can cause a response similar to that of wild-type cells to starvation.
FIG. 1.
Phenotypes of cells overexpressing fnx1 in EMM. (A) Growth curve of cells with derepressed (■) or repressed (•) expression from pREP1-fnx1 showing the growth inhibition upon fnx1+ overexpression. (B) Cells stained with DAPI: a, wild-type cells starved of nitrogen; b, pREP1-fnx1 transformed cells with derepressed promoter; c, pREP1-fnx1 transformed cells with repressed promoter. (C) Heat shock resistance of pREP1-fnx1+ transformed cells with repressed (promoter OFF) or derepressed (promoter ON) fnx1+ overexpression measured as efficiency of plating before and after treatment of a culture of 2 × 106 cells/ml at 48°C for 20 min. (D) Flow cytometric analysis of the DNA content of pREP1-fnx1 transformed cells with repressed (promoter OFF) or derepressed (promoter ON) fnx1+ overexpression shows the presence of a prominent 1C DNA peak when the promoter is induced.
fnx1+ is transcriptionally activated upon nitrogen starvation.
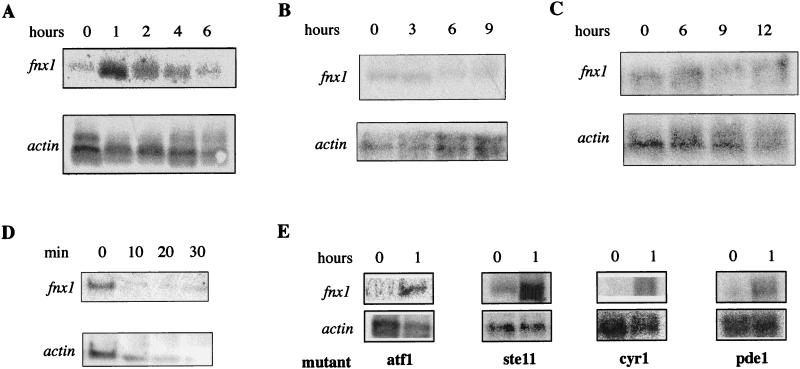
To address the question of whether this response was direct or physiologically relevant, we investigated whether fnx1+ was transcriptionally activated in wild-type cells by starvation. A Northern blot analysis with fnx1+ cDNA as a probe was performed on RNA from wild-type cells at different times after a shift to media lacking several different basic nutrients. It revealed that the level of fnx1+ RNA increased sharply soon after the shift to medium lacking nitrogen (Fig. 2A). We quantified the signal and determined that fnx1+ RNA increased sevenfold after 1 h of incubation in medium lacking nitrogen. This demonstrated that the RNA level and presumably the protein level and activity of fnx1 correlated with the transfer of the cells into nitrogen starvation conditions. fnx1 RNA did not increase after a shift to medium lacking either carbon or phosphorus (Fig. 2B and C).
FIG. 2.
Increase in the relative amount of fnx1 RNA upon nitrogen starvation. Northern blot analysis of wild-type cells in nitrogen-free medium (A), medium lacking carbon (B), and medium lacking phosphorus (C). The different time scales for each medium reflect the corresponding differences in the rates at which cells arrest proliferation. (D) Northern blot analysis of wild-type cells heat shocked at 48°C for the indicated times. Since actin RNA decreases in response to heat shock, the loading control in this case is the amount of RNA determined spectrophotometrically. (E) Increase of fnx1 RNA level after a 1-h incubation in medium lacking nitrogen in cells mutated in genes known to function in stress-response pathways.
Since we knew from the design of the screen that fnx1 overexpression enabled cells to survive a 48°C heat shock, we tested whether it was transcriptionally activated by this treatment. We performed Northern blot analysis on RNA isolated from wild-type growing cells subjected to a heat shock of 48°C. Heat shock led to decrease of the level of fnx1+, as well as of actin RNA (Fig. 2D).
To address the question of whether the transcriptional activation of fnx1 was mediated through known pathways of stress-induced transcription, we monitored the level of fnx1 RNA by Northern blot analysis on RNA isolated from ste11 and atf1 mutant strains. ste11 is a transcription factor required for the transcription of genes required for the mating program that in fission yeast requires nitrogen starvation (49). atf1 is a transcription factor that is required for the transcription of genes responsive to osmotic and oxidative stress (50). Also, we tested whether fnx1+ expression was under the control of the cyclic AMP (cAMP) system (59) by using mutants in the adenylate cyclase, cyr1 (31, 60), and in the cAMP phosphodiesterase, pde1 (32, 58). fnx1+ transcription was found to be activated by nitrogen starvation in strains carrying mutations in ste11, atf1, cyr1, or pde1, suggesting that its transcription is under the control of a novel pathway (Fig. 2E).
fnx1+ is required for long-term survival of cells in G0.
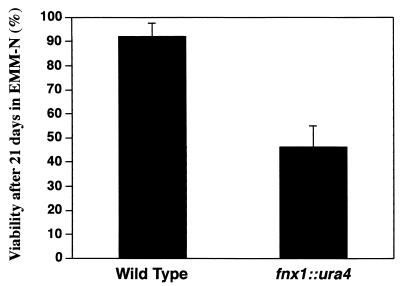
Since fnx1+ overexpression was capable of causing a starvation-like response even in rich media and since its RNA level increased in response to nitrogen starvation, we next asked whether fnx1+ was also required for the changes that cells undergo in response to nitrogen depletion. For this purpose, we constructed an fnx1 null strain. A diploid strain in which one copy of the fnx1+ gene was replaced by the selectable ura4+ marker was sporulated and yielded four viable haploid spores that segregated 2:2 for uracil auxotrophy. The ura+ haploid strain was confirmed to be an fnx1+ deletion mutant by Southern blot analysis. fnx1 null mutants (Δfnx1) showed no apparent phenotype with regard to growth rate, cell size, or morphology (data not shown). We asked whether the Δfnx1 mutants were capable of following the two developmental fates triggered by nitrogen starvation: sexual differentiation and entry into a quiescent G0 state. No mating defects were observed with Δfnx1 cells, indicating that fnx1+ is not required for the sexual differentiation pathway (data not shown). To test the second possibility, Δfnx1 and wild-type cells were grown in EMM to mid-exponential phase and transferred to EMM-N at the same cell density. The initial responses to nitrogen starvation were similar in both cultures. Cells arrested the cell cycle with a 1C DNA content, reduced their size, and became heat shock resistant within 8 h without an immediate decrease in viability. However, whereas wild-type cells retained a viability of close to 100% in this dormant state for more than 3 weeks, as previously reported (48), Δfnx1 cells were found to be only 50% viable after 3 weeks of incubation (Fig. 3). This decreased viability in later stages of starvation of Δfnx1 cells demonstrated that fnx1+ was required for the full and efficient implementation of the differentiation program that was initially induced almost immediately after the shift to nitrogen starvation conditions.
FIG. 3.
Survival of wild-type and Δfnx1 cells in medium lacking nitrogen after 21 days. The results represent the viability relative to that at day 1. The results are the averages of two independent experiments, and the viability for each of them was measured in triplicate. The error bars represent one standard deviation from the average of the two independent experiments.
fnx1 is a MDR-MFS transporter.
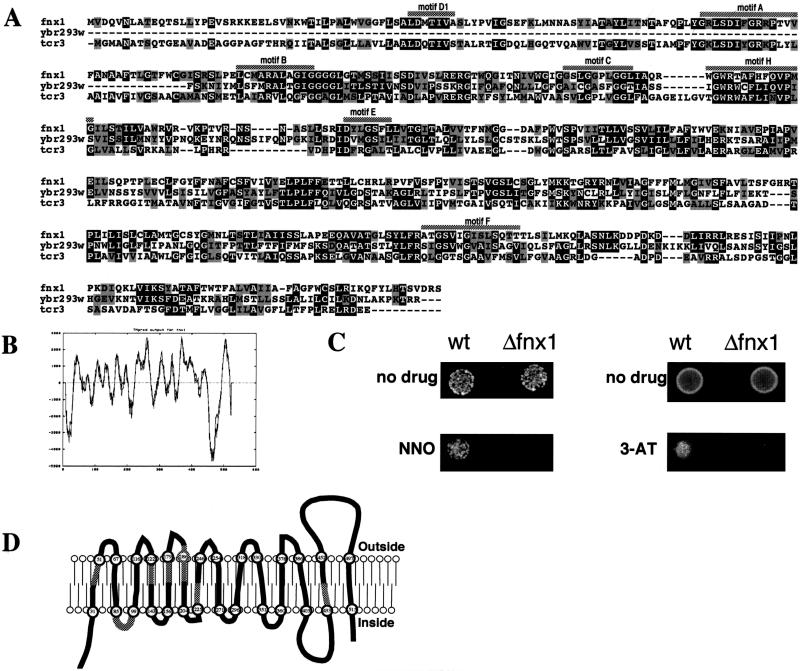
Sequence analysis of fnx1+ revealed that it belonged to the multidrug resistance (MDR) group of the major facilitator superfamily (MFS) of proteins (42). The proteins of this group are transmembrane transporters that reside in the plasma membrane and use the electrochemical proton gradient for active efflux of substrate. Based on a BLAST search of the GenBank database, only proteins from this family were identified to have sequence similarity to fnx1. Figure 4A shows a sequence alignment with (i) the ORF that had the highest BLAST score, ybr293w from Saccharomyces cerevisiae, which has been classified in cluster II of the subgroup by computer analysis of the budding yeast genome (16), and (ii) the tcrC gene product of Streptomyces aureofaciens, which has been shown to be required for tetracycline resistance (7). We identified in fnx1 all of the specific “signature” motifs found in MFS proteins (these are underlined in Fig. 4A). Using the TMpred software (the output is shown on Fig. 4B), we identified 14 putative transmembrane domains that are schematically shown in Fig. 4D. The number and the topology of the predicted transmembrane domains are conserved between fnx1 and the other members of the group, for one of which they have been mapped biochemically (41). The order of the specific signature motifs and their positions relative to the predicted transmembrane structure of the protein are also conserved.
FIG. 4.
Sequence analysis of fnx1. (A) A sequence alignment between fnx1, a closely related ORF from S. cerevisiae Ybr293w (16), and the tcrC gene product of Streptomyces aureofaciens (7) was generated by using the Clustal algorithm (51). Black boxes represent identities, and gray boxes represent conservative substitutions. The MFS-MDR signature motifs (42) are marked with shaded bars above the sequence. (B) Output of the TMpred program for fnx1. The graph plots the probability of a transmembrane domain with the amino acid position. (C) Sensitivity of Δfnx1 cells to 3-amino-1,2,4-triazole and 4-nitroquinoline N-oxide. On the left, 5 × 102 wild-type or Δfnx1 cells were spotted onto EMM or EMM containing 1 μM 4-nitroquinoline N-oxide. On the right, 5 × 104 wild-type or Δfnx1 cells were spotted onto EMM or EMM containing 25 mM 3-amino-1,2,4-triazole. (D) Schematic representation of the transmembrane regions of fnx1 as predicted with TMpred. The numbers in the circles denote amino acid positions. The shaded areas represent the position of the respective MFS-MDR signature motifs.
Most MDR-MFS proteins have been identified based on their ability to confer resistance to toxic drugs. It is not known, however, what their physiological substrates are, and such proteins have not previously been implicated in the starvation response of budding or fission yeast.
To test experimentally whether fnx1 can function as an active efflux transporter, we tested the sensitivity of wild-type and Δfnx1 cells to several drugs that have been shown to be substrates of cluster II MFS-MDR proteins (16). The drugs tested were: crystal violet, a substrate for SGE (11, 12, 16); 3-amino-1,2,4-triazole and 4-nitroquinoline N-oxide substrates for SNQ1/ATR1 (17, 23); and carbonyl cyanide m-chlorophenylhydrazone, a substrate for the bacterial emrA (29). We found that Δfnx1 cells were more sensitive to 25 mM 3-amino-1,2,4-triazole and 1 μM 4-nitroquinoline N-oxide than were wild-type cells (Fig. 4D). Δfnx1 and wild-type cells had no difference in sensitivity in the range from 0 to the maximum concentration for which there was cell growth to both crystal violet (0 to 1 μg/ml) and to carbonyl cyanide m-chlorophenylhydrazone (0 to 100 μM) (data not shown). These results indicated that fnx1 was required for the efflux of toxic drugs from the cell in a substrate-specific manner. The substrate specificity of fnx1 is similar to that of ATR1/SNQ1 from budding yeast; however, the amino acid sequence of ATR1/SNQ1 is not as similar to that of fnx1 as the sequence of the yet uncharacterized S. cerevisiae ORF ybr293w (Fig. 4A).
Cell density-dependent survival of fnx1 null cells.
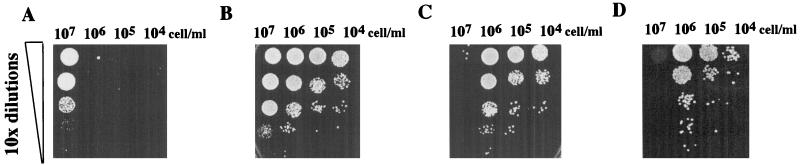
If we consider the sequence similarity of fnx1 to the MFS-MDR proteins, the simplest explanation of its action is that it facilitates the release of a substance into the medium that signals the initiation of the starvation response. Since there have been no reports of cell-to-cell communication in response to starvation in any yeast species, we tested wild-type cells for evidence of this type of interaction by starving them of nitrogen at cell densities ranging from 2 × 104 to 2 × 107 cells/ml. Surprisingly, we found that the cells at 2 × 107 cells/ml were morphologically and physiologically distinct from the cells incubated at lower cell densities. They were smaller, appeared darker in the phase microscope, and took longer to reenter the cell cycle than cells incubated at lower densities. Also, cells incubated at 2 × 107 cells/ml were resistant to a severe heat shock of 50°C for 20 min, while cells at lower cell densities were sensitive to this treatment (Fig. 5A). In contrast to wild-type cells (Fig. 5B), Δfnx1 cells at 2 × 107 cells/ml were not able to enter this distinct differentiated state. After just 6 days of incubation in nitrogen starvation medium, Δfnx1 cells at 2 × 107 cells/ml completely lost their viability (Fig. 5C). After 21 days of incubation in this medium, Δfnx1 cells lost viability at the low cell concentrations as well, which is consistent with our earlier observations (Fig. 3). Based on these experiments, we propose that there are two components of nitrogen starvation-induced differentiation: the first is based only on the response of an individual cell, and the second, which is characterized by heat shock resistance at higher temperature, is dependent on cell density and requires fnx1. One possible explanation for this requirement would be the fnx1-facilitated transport of a signaling molecule out of the cell as a means of cell-to-cell communication. To test this model, we incubated Δfnx1 cells together with wild-type cells at 2 × 107 cells/ml but found that the viability of the Δfnx1 cells was not rescued (Fig. 5D). Furthermore, wild-type cells did not undergo growth arrest in the presence of fnx1+-overexpressing cells. This was demonstrated by coincubating fnx1+-overexpressing cells with wild-type cells that could be identified microscopically by expression of GFP (6). The number of wild-type cells increased exponentially regardless of the number of fnx1+-overexpressing cells at the start of the incubation period (Table 1).
FIG. 5.
Cell density-dependent modes of the response to nitrogen starvation. Cells (2 × 107/ml and 10-fold serial dilutions of wild-type and Δfnx1 cells) were incubated for 6 days in medium lacking nitrogen and spotted onto the top row of the plates with 10-fold serial dilutions of each independent culture spotted underneath. (A) Wild-type cells after heat shock treatment. Only the culture at 2 × 107 cells/ml was able to survive. (B) Wild-type cells not subjected to heat shock. The smaller size of the colonies at the cell density of 2 × 107 cells/ml reflects the longer recovery time after starvation. (C) Δfnx1 cells not subjected to heat shock. The culture at 2 × 107 cells/ml contained no viable cells. (D) Mixture of equal numbers of wild-type ura4-D18 (ura−) and Δfnx1 (ura+) cells not subjected to heat shock. Only Δfnx1 cells are able to grow on the EMM (without uracil) plate used for determination of viability. The presence of fnx1+ cells did not rescue the loss of viability of fnx1 null cells.
TABLE 1.
Cells overexpressing fnx1+ do not inhibit the growth of wild-type cellsa
| GFP/fnx1 ratio | Cell density (cells/ml) at:
|
% GFP-expressing cells at 24 h | ||
|---|---|---|---|---|
| 0 h | 13 h | 24 h | ||
| 1:1 | 1.8 × 106 | 7.2 × 106 | 5.3 × 107 | 97 |
| 1:10 | 1.3 × 106 | 2.4 × 106 | 8.6 × 106 | 56 |
Cells overexpressing fnx1+ from the REP1-fnx1+ plasmid and cells expressing GFP from the pGFP41 plasmid were mixed in a 1:1 or a 10:1 ratio and incubated in conditions that derepress the promoter. By 24 h the GFP-expressing cells overgrew the fnx1+-expressing cells.
DISCUSSION
Unicellular organisms respond to starvation by undergoing a cellular differentiation program that includes morphological and physiological changes that arguably enable them to survive adverse conditions. There are several possible approaches for the identification and characterization of gene products involved in such a process: (i) look for proteins with sequence similarities to characterized components of the pathway in other systems; (ii) look for loss of function mutants that are unable to perform a specific cellular function; or (iii) look for genes that at an elevated dosage and/or expression level can induce a particular cellular function. We used the third approach to look for proteins involved in the starvation response of S. pombe.
We screened a cDNA library for genes that when overexpressed can induce the starvation response even in rich nutrient conditions. The screen and the subsequent testing for physiological relevance identified fnx1 as a protein that has a function in the nitrogen starvation-induced transition to a quiescent G0 state. fnx1+ overexpression induces a starvation-like response in rich medium causing cells to arrest cell cycle progression with a 1C DNA content, which is characteristic of cells starved of nitrogen. The fnx1+ RNA level is increased in response to nitrogen starvation but not in response to starvation of other nutrients or entry into stationary phase. The increase of fnx1 RNA level 1 h after a shift to nitrogen starvation medium coincides temporally with the growth arrest and cell differentiation in response to nitrogen starvation. fnx1 is also required for maintaining the long-term viability of cells (Fig. 3) that is a feature of the nitrogen depletion-induced G0 state (48). Even though the loss of viability of fnx1 null mutants is manifested after long-term incubation, we think it stems from the failure of the cells to differentiate properly when first shifted to medium lacking nitrogen, which normally induces a burst of fnx1 transcription. Although fnx1 RNA can be detected at a very low level in wild-type growing cells, we do not think that fnx1 has a critical function in growing conditions since Δfnx1 cells display a normal growth rate and morphology in complete medium.
Based on sequence similarity, topology of the predicted transmembrane domains, and the presence and relative positions of several signature motifs, fnx1 is a member of the MDR-MFS group of transporters. MDR proteins facilitate the efflux of toxic drugs from cells; however, their physiological substrates have not been identified. We experimentally confirmed that fnx1 has the properties of an MFS-MDR transporter by demonstrating that Δfnx1 cells are more sensitive than wild-type cells to 3-amino-1,2,4-triazole and 4-nitroquinoline N-oxide. Members of one MDR family, that of the ABC transporters which require ATP hydrolysis, are involved in cell specialization in Dictyostelium discoideum (44) and in mating pheromone release in S. cerevisiae (35). fnx1 is the first member of the MFS-MDR family to be implicated in the starvation response of a microbial species.
Since MFS-MDR proteins usually reside in the plasma membrane and facilitate the efflux of a substance from the cell (42) one possible mode of action of fnx1 would be through the release of a compound into the medium to signal the onset of G0. In testing this hypothesis we discovered a cell density-dependent component of nitrogen starvation-induced G0. Compared to wild-type cells starved at low cell densities, those starved at a high cell density display physiologically different properties, including higher heat shock resistance and longer recovery time. In contrast, fnx1 deletion mutants are severely defective in surviving under this high-cell-density starvation condition. Although this cell density dependence is suggestive of cell-to-cell communication mediated by fnx1, we were not able to observe any effects of cells overexpressing fnx1+ on the growth of wild-type cells in coculture or of wild-type starved cells on the viability of cells deleted for fnx1+. This means that if fnx1 is involved in the efflux of a compound from the cell into the environment, providing this compound in trans is not sufficient to induce the starvation response. This suggests the possibility that the starvation response requires the cell to eliminate such a compound from its cytoplasm, thereby creating a steeper concentration gradient across the plasma membrane.
Since fnx1 is an MDR protein, it is formally possible that its function is to eliminate a toxic substrate from nitrogen-starved cells. Since we have not identified the physiological substrate of the fnx1 transporter we have not experimentally ruled out this possibility. However, this model does not explain our observation that overexpression of fnx1 in wild-type nonstarved cells caused a phenotype similar to that of wild-type cells starved of nitrogen (Fig. 1).
Another intriguing possibility is that, in addition to the efflux of a certain organic compound, fnx1 also facilitates the codirectional transport of water, which has been shown for some other transporters with similar transmembrane topology (30, 56). Since water influx into the cell is required for exponential growth, it is possible that fnx1-facilitated outflow of water from the cell is responsible for triggering the nitrogen starvation-induced differentiation.
ACKNOWLEDGMENTS
This work was supported by grant MCB-9513714 from the National Science Foundation.
We thank Adam Kuspa, Richard Atkinson, Sandra S. Salus, and Janos Demeter for their valuable comments and suggestions and Maureen McLeod, Susan Forsburg, and Tony Carr for providing us with S. pombe strains and vectors.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bedard D, Singer R. The nature of G0 in yeast. In: Waechter D E, Baserga R, editors. Genetic expression in the cell cycle. Orlando, Fla: Academic Press, Inc.; 1982. pp. 245–268. [Google Scholar]
- 3.Belenguer P, Pelloquin L, Oustrin M L, Ducommun B. Role of the fission yeast nim1 protein kinase in the cell cycle response to nutritional signals. Biochem Biophys Res Commun. 1997;232:204–208. doi: 10.1006/bbrc.1997.6253. [DOI] [PubMed] [Google Scholar]
- 4.Braun E L, Fuge E K, Padilla P A, Werner-Washburne M. A stationary-phase gene in Saccharomyces cerevisiae is a member of a novel, highly conserved gene family. J Bacteriol. 1996;178:6865–6872. doi: 10.1128/jb.178.23.6865-6872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello G, Rodgers L, Beach D. Fission yeast enters the stationary phase G0 state from either mitotic G1 or G2. Curr Genet. 1986;11:119–125. [Google Scholar]
- 6.Craven, R., D. J. F. Griffiths, K. S. Sheldrick, R. E. Randall, I. M. Hagan, and A. M. Carr. Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Submitted for publication. [DOI] [PubMed]
- 7.Dairi T, Alsaka T, Katsumata R, Hasegawa M. A self-defense gene homologous to tetracycline effluxing gene essential for antibiotic production in Streptomyces aureofaciens. Biosci Biotechnol Biochem. 1995;59:1835–1841. doi: 10.1271/bbb.59.1835. [DOI] [PubMed] [Google Scholar]
- 8.Degols G, Shiozaki K, Russell P. Activation and regulation of the spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devoti J, Seydoux G, Beach D, McLeod M. Interaction between ran1+ protein kinase and cAMP dependent protein kinase as negative regulators of fission yeast meiosis. EMBO J. 1991;10:3759–3786. doi: 10.1002/j.1460-2075.1991.tb04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egel R. Meiosis in fission yeast. In: Nasim A, Young P, Johnston B F, editors. Molecular biology of the fission yeast. New York, N.Y: Academic Press, Inc.; 1989. pp. 31–73. [Google Scholar]
- 11.Ehrenhofer-Murray A E, Seitz M U, Sengstag C. The Sge1 protein of Saccharomyces cerevisiae is a membrane-associated multidrug transporter. Yeast. 1998;14:49–65. doi: 10.1002/(SICI)1097-0061(19980115)14:1<49::AID-YEA199>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Ehrenhofer-Murray A E, Wurgler F E, Sengstag C. The Saccharomyces cerevisiae SGE1 gene product: a novel drug-resistance protein within the major facilitator superfamily. Mol Gen Genet. 1994;244:287–294. doi: 10.1007/BF00285456. [DOI] [PubMed] [Google Scholar]
- 13.Fantes P, Nurse P. Control of size at division in fission yeast by a growth modulated size control over nuclear division. Exp Cell Res. 1977;107:377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- 14.Feilotter H, Nurse P, Young P G. Genetic and molecular analysis of cdr1/nim1 in Schizosaccharomyces pombe. Genetics. 1991;127:309–318. doi: 10.1093/genetics/127.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsburg S L. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2966. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goffeau A, Park J, Paulsen I, Jonniaux J-L, Dinh T, Mordant P, Saier M H., Jr Multidrug-resistant transport proteins in yeast: complete inventory and phylogenetic characterization of yeast open reading frames within the major facilitator superfamily. Yeast. 1997;13:43–54. doi: 10.1002/(SICI)1097-0061(199701)13:1<43::AID-YEA56>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Gompel-Klein P, Brendel M. Allelism of SNQ1 and ATR1, genes of the yeast Saccharomyces cerevisiae required for controlling sensitivity to 4-nitroquinoline-N-oxide and aminotriazole. Curr Genet. 1990;18:93–96. doi: 10.1007/BF00321122. [DOI] [PubMed] [Google Scholar]
- 18.Habener J F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990;4:1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- 19.Harder W, Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- 20.Hinnebusch A G, Fink G R. Positive regulation in the general amino acid control of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1983;80:5374–5378. doi: 10.1073/pnas.80.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeffler J P. Structure/function relationships of CREB/ATF proteins. J Invest Dermatol. 1992;98:21S–28S. doi: 10.1111/1523-1747.ep12462126. [DOI] [PubMed] [Google Scholar]
- 22.Hoheisel J D, Maier E, Mott R, McCarthy L, Grigoriev A V, Schalkwyk L C, Nizetic D, Francis F, Lehrach H. High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S. pombe. Cell. 1993;73:109–120. doi: 10.1016/0092-8674(93)90164-l. [DOI] [PubMed] [Google Scholar]
- 23.Kanazawa S, Driscoll M, Struhl K. ATR1, a Saccharomyces cerevisiae gene encoding a transmembrane protein required for aminotriazole resistance. Mol Cell Biol. 1988;8:664–673. doi: 10.1128/mcb.8.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelker H C, Pogo A O. The stringent and relaxed phenomena in Saccharomyces cerevisiae. J Biol Chem. 1980;255:1526–1535. [PubMed] [Google Scholar]
- 25.Lehrach H, Drmanac R, Hoheisel J D, Larin Z, Lennon G. Hybridization fingerprinting in genome mapping and sequencing. In: Davies K E, Tilghman S M, editors. Genome analysis. I. Genetic and physical mapping. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. pp. 39–81. [Google Scholar]
- 26.Leupold U. Genetic methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:169–177. [Google Scholar]
- 27.Lewis D L, Gatie D K. The ecology of quiescent microbes. ASM News. 1991;57:27–32. [Google Scholar]
- 28.Lillie S H, Pringle J R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980;143:1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomovskaya O, Lewis K. Emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci USA. 1992;89:8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loo D D, Zeuthen T, Chandy G, Wright E M. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda T, Mochizuki N, Yamamoto M. Adenylyl cyclase is dispensable for vegetative cell growth in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1990;87:7814–7818. doi: 10.1073/pnas.87.20.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matviw H, Li J, Young D. The Schizosaccharomyces pombe pde1/cgs2 gene encodes a cyclic AMP phosphodiesterase. Biochem Biophys Res Commun. 1993;194:79–82. doi: 10.1006/bbrc.1993.1787. [DOI] [PubMed] [Google Scholar]
- 33.Maundrell K. nmt1 of fission yeast. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- 34.Mertins P, Gallwitz D. A single intronless actin gene in the fission yeast Schizosaccharomyces pombe: nucleotide sequence and transcripts formed in homologous and heterologous yeast. Nucleic Acids Res. 1987;15:7369–7379. doi: 10.1093/nar/15.18.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaelis S. STE6, the yeast a-factor transporter (review) Semin Cell Biol. 1993;4:17–27. doi: 10.1006/scel.1993.1003. [DOI] [PubMed] [Google Scholar]
- 36.Moehle C M, Hinnebusch A G. Association of RAP1 binding sites with stringent control of ribosomal protein gene transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2723–2735. doi: 10.1128/mcb.11.5.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 38.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 39.Ohmiya R, Aiba H, Yamada H, Mizuno T. Clarification of the promoter structure of the osmoregulated gpd1(+) gene encoding an isozyme of NADH-dependent glycerol-3-phosphate dehydrogenase in fission yeast. Biosci Biotechnol Biochem. 1997;61:553–555. doi: 10.1271/bbb.61.553. [DOI] [PubMed] [Google Scholar]
- 40.Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulsen I T, Brown M H, Littlejohn T G, Mitchell B A, Skurray R A. Molecular characterization of the multidrug resistance proteins QacA and QacB: membrane topology and identification of residues involved in specificity for divalent cations. Proc Natl Acad Sci USA. 1996;93:3630–3635. doi: 10.1073/pnas.93.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sazer S, Sherwood S. Mitochondrial growth and DNA synthesis occur in the absence of nuclear DNA replication in fission yeast. J Cell Sci. 1990;97:509–516. doi: 10.1242/jcs.97.3.509. [DOI] [PubMed] [Google Scholar]
- 44.Shaulsky G, Kuspa A, Loomis W F. A multidrug resistance transporter/serine protease gene is required for prestalk specialization in Dictyostelium. Genes Dev. 1995;9:1111–1122. doi: 10.1101/gad.9.9.1111. [DOI] [PubMed] [Google Scholar]
- 45.Shieh J C, Wilkinson M G, Buck V, Morgan B A, Makino K, Millar J. The mcs4 response regulator coordinately controls the stress-activated wak1-wis1-sty1 map kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- 46.Shiozaki K, Russell P. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- 47.Shiozaki K, Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by spc1 kinase through atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- 48.Su S S Y, Tanaka Y, Samejima I, Tanaka K, Yanagida M. A nitrogen starvation-induced dormant G(0) state in fission yeast: the establishment from uncommitted G(1) state and its delay for return to proliferation. J Cell Sci. 1996;109:1347–1357. doi: 10.1242/jcs.109.6.1347. [DOI] [PubMed] [Google Scholar]
- 49.Sugimoto A, Lino Y, Maeda T, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 1991;5:1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- 50.Takeda T, Toda T, Kominami K I, Kohnosu A, Yanagida M, Jones N. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei W, Nurse P, Broek D. Yeast cells can enter a quiescent state through either G1, S, G2 or M phase of the cell cycle. Cancer Res. 1993;53:1867–1870. [PubMed] [Google Scholar]
- 53.Werner-Washburne M, Braun E, Johnston G C, Singer R A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993;57:383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Werner-Washburne M, Braun E L, Crawford M E, Peck V M. Stationary phase in Saccharomyces cerevisiae. Mol Microbiol. 1996;19:1159–1166. doi: 10.1111/j.1365-2958.1996.tb02461.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilkinson M G, Samuels M, Takeda T, Toone W M, Shieh J C, Toda T, Millar J, Jones N. The atf1 transcription factor is a target for the sty1 stress-activated map kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- 56.Wright E M, Loo D D, Turk E, Hirayama B A. Sodium cotransporters. Curr Opin Cell Biol. 1996;8:468–473. doi: 10.1016/s0955-0674(96)80022-6. [DOI] [PubMed] [Google Scholar]
- 57.Wu L, Russell P. nim1 kinase promotes mitosis by inactivating wee1 tyrosine kinase. Nature. 1993;363:738–741. doi: 10.1038/363738a0. [DOI] [PubMed] [Google Scholar]
- 58.Wu S, McLeod M. The sak1+ gene of Schizosaccharomyces pombe encodes an RFX family DNA-binding protein that positively regulates cAMP-dependent protein kinase-mediated exit from the mitotic cell cycle. Mol Cell Biol. 1995;15:1479–1488. doi: 10.1128/mcb.15.3.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto M. Regulation of meiosis in fission yeast. Cell Struct Funct. 1996;21:431–436. doi: 10.1247/csf.21.431. [DOI] [PubMed] [Google Scholar]
- 60.Young D, Riggs M, Field J, Vojtek A, Broek D, Wigler M. The adenylyl cyclase gene from Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1989;86:7989–7993. doi: 10.1073/pnas.86.20.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young P G, Fantes P A. Schizosaccharomyces pombe mutants affected in their division response to starvation. J Cell Sci. 1987;88:295–304. doi: 10.1242/jcs.88.3.295. [DOI] [PubMed] [Google Scholar]
- 62.Zehetner G, Lehrach H. The Reference Library System: sharing biological material and experimental data. Nature. 1994;367:489–491. doi: 10.1038/367489a0. [DOI] [PubMed] [Google Scholar]
- 63.Ziff E B. Transcription factors: a new family gathers at the cAMP response site. Trends Genet. 1990;6:69–72. doi: 10.1016/0168-9525(90)90081-g. [DOI] [PubMed] [Google Scholar]