Summary:
We have only recently started to appreciate the extent to which immune cell activation involves significant changes in cellular metabolism. We are now beginning to understand how commitment to specific metabolic pathways influences aspects of cellular biology that are the more usual focus of immunological studies, such as activation induced changes in gene transcription, post-transcriptional regulation of transcription, post translational modifications of proteins, cytokine secretion, etc. Here we focus on metabolic reprogramming in mononuclear phagocytes downstream of stimulation with inflammatory signals (such as LPS and IFNγ) versus alternative activation signals (IL-4), with an emphasis on work on dendritic cells and macrophages from our laboratory, and related studies from others. We cover aspects of glycolysis and its branching pathways (glycogen synthesis, pentose phosphate, serine synthesis, hexose synthesis and glycerol 3 phosphate shuttle), the tricarboxylic acid pathway, fatty acid synthesis and oxidation, and mitochondrial biology. Although our understanding of the metabolism of mononuclear phagocytes has progressed significantly over the last 10 years, major challenges remain, including understanding the effects of tissue residence on metabolic programming related to cellular activation, and the translatability of findings from mouse to human biology.
Keywords: Metabolism, immunometabolism, glycolysis, TCA cycle, mitochondria, inflammation, alternative activation, macrophages, dendritic cells, cytokines, LPS, IL-4
Introduction
All immune cells are characterized by their ability to respond to signals through pattern recognition receptors, antigen receptors and/or cytokine/growth factor receptors, and these signals can induce a shift from resting to activated states. Activation is associated with cell proliferation and/or the acquisition of effector functions that allow the cells to participate in immune responses. We now have detailed understanding of many of the molecular components of the core pathways that directly link receptors to transcription factors and thereby changes in gene expression. However, we have only recently begun to appreciate the extent to which immune cell activation requires metabolic reprogramming (1). We have become increasingly interested in this area over the last decade, and it is our work in this regard on mononuclear phagocytes, and work from others that has most directly impacted our approach, which will be the central theme of the discussion here. In particular, we will focus on dendritic cells (DCs), which sample the environment and are specialized in responding to danger signals within the sampled material to become activated to both participate in inflammation and, more importantly, to stimulate T cells, thereby bridging innate and adaptive immunity (2,3). Macrophages, like dendritic cells, sample the environment, and in doing so can become activated to make mediators, such as cytokines, that influence the biology of other cells. This process can be influenced not only by direct exposure to danger signals, but also by cytokine produced by other cell types, immune and non-immune. Physiologically they are broadly important, playing roles in the clearance of microbial infections and dead cells, initiating and resolving inflammation, and in tissue homeostasis (4).
Dendritic cells
Our initial interest in immune cell metabolism stemmed from the simple observation that tissue culture medium rapidly becomes acidified when DCs, differentiated from bone marrow progenitors by culture in granulocyte-macrophage-colony stimulating factor (GM-CSF), are activated by the danger signal bacterial lipopolysaccharide (LPS). Immunologists have correlated acidification of culture medium with successful in vitro proliferation of activated T cells for decades. Thompson and colleagues’ work (5,6), and that of others (7), showed that this reflected increased production of lactic acid by proliferating cells as a result of the cells committing to aerobic glycolysis (or Warburg metabolism), a pathway in which lactic acid is made from pyruvate, the terminal intermediate in the glycolysis pathway (Fig. 1). This pathway has unique characteristics, that will be discussed below, that allow it to support anabolism and thereby the increase in mass that is required prior to proliferation. However, we knew that DCs do not proliferate after LPS-activation, but rather change functionally by beginning to secrete large amounts of various cytokines and other mediators, and expressing surface molecules that allow them to follow chemokine gradients towards lymph nodes, and therein interact with and activate T cells (2,3). This prompted us to explore the underlying reasons why DCs engage Warburg metabolism after stimulation with Toll Like Receptor (TLR) agonists. Our hypothesis was that the engagement of glycolysis would be broadly consistent with the adoption, by activated DCs, of a secretory phenotype in which the production of a range of molecules destined for export is significantly increased.
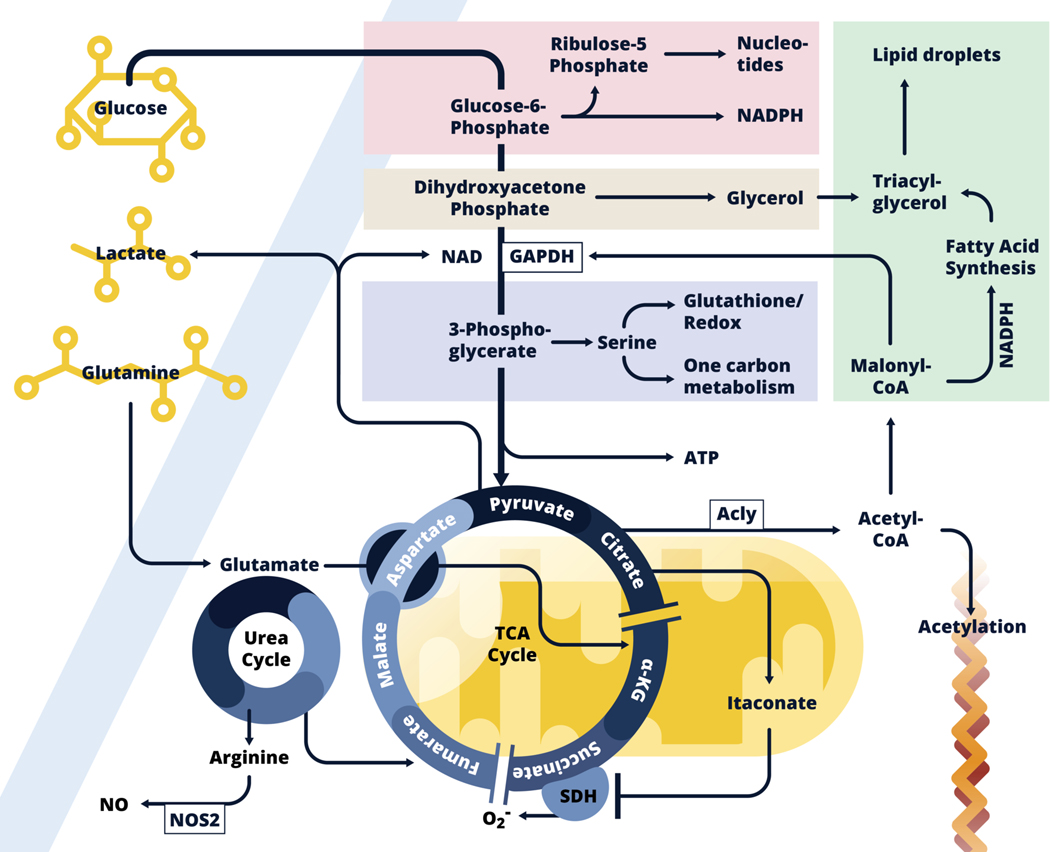
Figure 1:
Inflammatory dendritic cell and macrophage metabolism.
Glycolysis allows glucose carbon to be taken up and used not only to fuel the TCA cycle, but also to feed into the pentose phosphate pathway (Pink), the glycerol phosphate shuttle (Beige), and the serine synthesis pathway (Lavender), all of which are implicated in inflammatory activation. Core functions associated with these pathways are noted. Downstream in the glycolysis pathway, pyruvate is converted into lactate, which allows ATP generation in the absence of respiration, or enters into mitochondria (Yellow) where it is converted into acetyl-CoA which enters the TCA cycle. In inflammatory macrophages, expression of Idh, the enzyme that converts citrate to α-KG is suppressed, and citrate levels build and fuel both itaconate production within mitochondria, and following export into the cytoplasm, the production of acetyl-CoA which is used for fatty acid synthesis (FAS, Green), and as a donor for the acetylation of proteins, including histones. Fatty acids (synthesized and acquired) are used with glycerol for triacylglycerol synthesis and lipid droplet formation. Malonyl-CoA, an intermediate in the FAS pathway posttranslationally modifies GAPDH, the central enzyme in the glycolysis pathway, to minimize its RNA-binding properties thereby promoting the release and translation of Tnf mRNA. Succinate dehydrogenase (SDH), which catalyzes the conversion of succinate to fumarate, is inhibited by itaconate. Succinate accumulates and promotes inflammatory activation through reverse electron transport at Complex I to Complex II, with the accompanying generation of ROS. This leads to HIF1-α activation which promotes glycolysis. Itaconate also promotes activation of Nrf2, which allows parallel induction of anti-inflammatory effects (not shown). Induced Nos2 expression leads to the production of NO which results in the cessation of respiration due to the inhibition of components of the electron transport chain. Under these conditions, production of ATP by glycolysis is required for cell survival. Aspartate generated from glutamate, fuels the urea cycle in which nitrogens are donated to arginine, the substrate for NO production by NOS2. The urea cycle feeds the TCA cycle by donating carbons in the form of fumarate, which can generate malate, aspartate and eventually citrate. Glutamine also back-fills the TCA cycle at α-KG downstream.
Glycolysis is able to support anabolism because intermediates in the core pathway are able to branch into accessory pathways that play critical roles in the synthesis of amino acids, nucleotides and fats (9). For example, glucose-6-phosphate can enter the pentose phosphate pathway (PPP), for the synthesis of nucleotides (Fig. 1). This pathway additionally generates NADPH which is important for redox balance and serves as an essential co-factor for fatty acid synthesis (FAS). Dihydroxyacetone phosphate is the precursor for glycerol-3 phosphate, and thereby triacylglycerol synthesis, through the glycerol phosphate shuttle, mediated by glycerol phosphate dehydrogenase (GPD1 or 2). This pathway generates NAD+, a required cofactor for GAPDH, as well as FADH2, which can fuel the electron transport chain (ETC) for respiration (discussed below), but is also used to generate glycerol for triacyglyceride synthesis (Fig. 1). Triacylglycerides, with cholesterol esters, are stored in lipid droplets, which accumulate in LPS-activated DCs (10), as well as in inflammatory macrophages (11,12). The activity of GAPDH, the central enzyme in the glycolysis pathway, results in the reduction of NAD+, and is a significant consideration in redox balance. Below GAPDH, the pathway generates ATP, but can also branch from 3-phosphoglycerate into the serine synthesis pathway. Serine is the basis for further amino acid synthesis, as well as mitochondrial one-carbon metabolism, which feeds nucleotide synthesis, the provision of methyl donors for protein and DNA methylation, and the synthesis of glutathione to maintain redox balance (Fig. 1). Finally, pyruvate can either be converted into lactate during Warburg metabolism, a process which requires NADH and regenerates NAD+ which supports GAPDH activity even in the absence of the generation of NAD+ by mitochondrial respiration (Fig. 1), or it can enter mitochondria via mitochondrial pyruvate carriers, where it is converted into acetyl coenzyme A (acetyl-CoA) which fuels the tricarboxylic acid (TCA) cycle, which can also be generated by fatty acid oxidation (FAO).
The TCA cycle generates the reducing equivalents NADH and FADH2. NADH is oxidized to NAD+ by complex I of the ETC (9). Complex II, succinate dehydrogenase (SDH), oxidizes the TCA cycle intermediate succinate to fumarate, generating FADH2. Electrons generated by complex I and II are passed through complex III to complex IV, which uses oxygen (O2) as final electron acceptor to generate H2O. Meanwhile, complex I, III and IV contribute to pumping protons (H+) across the mitochondrial membrane, to generate the mitochondrial membrane potential (Δψ), used by complex V to generate ATP (this is illustrated in figure 2, later in in this review). This entire process is referred to as oxidative phosphorylation (OXPHOS). Not all electrons will be efficiently transferred to complex IV and oxygen, which results in superoxide (O2-) production. O2- is converted to hydrogen peroxide (H2O2) in the mitochondrial matrix, which can be detoxified in the mitochondrial matrix to H2O. Inefficient electron transfer or decreased reduction capacity can result in increased superoxide and peroxide levels, which can in turn damage proteins, lipids and DNA. The use of pyruvate to make lactate is usually linked to cell growth in hypoxic conditions, where the pathway allows the production of ATP absent respiration. However, it is clear that other signals, including those delivered through activating immune receptors, allow the adoption of Warburg metabolism even when oxygen is not limiting.
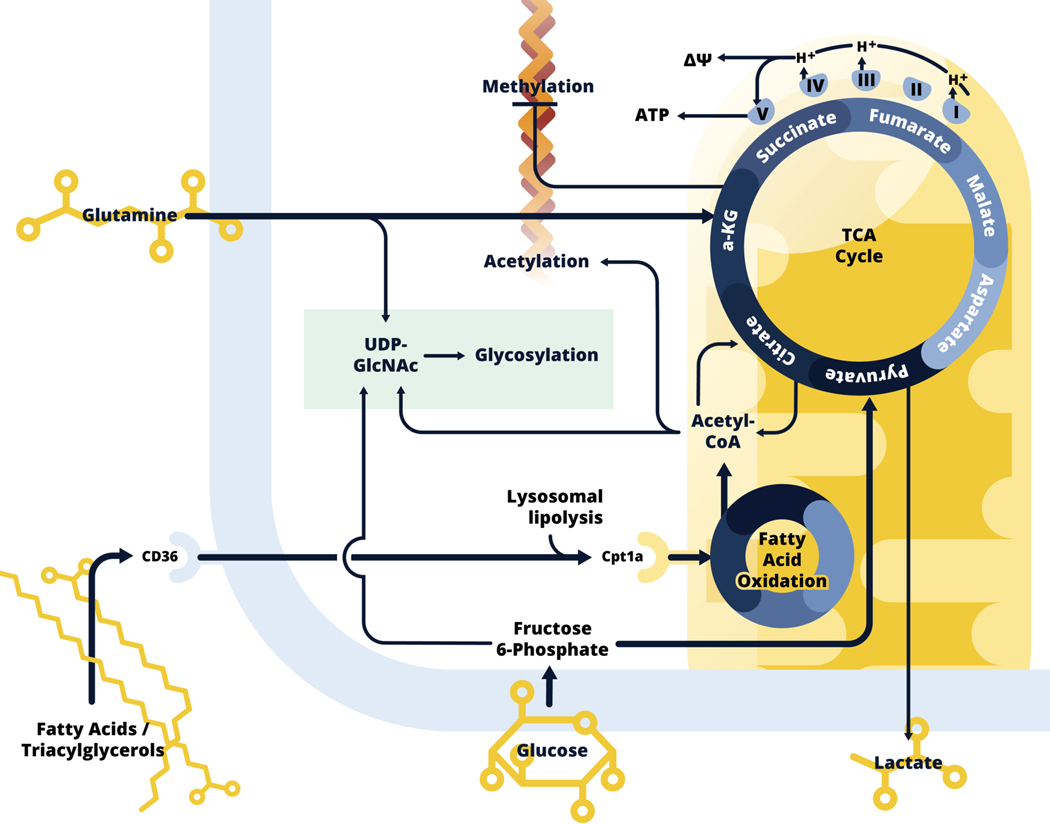
Figure 2.
Alternatively activated macrophage metabolism.
Alternatively activated macrophages use fatty acids, glutamine and glucose to fuel the TCA cycle, and respiration is generally increased in these cells. Exogenous fatty acids and triacylglycerols are taken up by CD36, and lysosomal lipolysis is used to generate fatty acids for fatty acid oxidation (FAO). Activated fatty acids enter the mitochondria, mediated by Cpt1a, to engage in fatty acid oxidation, which generates acetyl-CoA, to contribute to the TCA cycle. Glucose carbon is also critical for citrate synthesis. Unlike in inflammatory macrophages, Idh is expressed in alternatively activated macrophages, and in addition to being used for the production of acetyl-CoA, which is used for histone acetylation, citrate is converted to α-KG, which is also generated from glutamine by glutaminolysis. α-KG inhibits DNA methylases, and therefore the process of methylation, and in this way facilitates increased expression of genes and downstream processes associated with alternative activation. α-KG cycles through the TCA cycle by conversion into succinate, fumarate, malate, aspartate, and eventually back to citrate. These steps of the TCA cycle, as well as FAO, generate NADH and FADH<sub>2</sub> (not shown) which can donate electrons to Complex I and II of the electron transport chain (ETC), while complex I, III and IV pump protons into the intermembrane space, to generate mitochondrial membrane potential (ΔΨ), which provides the motive force for ATP generation by complex V. The cells integrate fructose 6-phosphate (Glc), glutamine (N) and acetyl-CoA (Ac) for emphasized synthesis of UDP-GlcNAc, a sugar donor for glycosylation (green).
We found that, as for T cells, increased acidification of tissue culture medium by activated DCs is due to increased production of lactic acid as a result of a commitment to Warburg metabolism (13). Glucose is critical for DC activation since pharmacologic inhibition of the pathway, or restriction of the amount of glucose available to cells in tissue culture, has a profound inhibitory effect on the production of proteins linked to activation, as well as on cellular survival, following exposure to TLR agonists (13). Resting DCs utilize FAO to fuel the TCA cycle, but this pathway, and indeed mitochondrial OXPHOS in general, are significantly inhibited 24 h after DCs have been stimulated with LPS, at which point the cells are primarily utilizing Warburg metabolism (13,14). Closer examination revealed that there is, in fact, a rapid increase in OXPHOS within hours of stimulation with LPS, that is followed by a decline in oxygen consumption after 6 h (14). This correlates with the induction of expression of nitric oxide synthase (Nos2), which uses arginine as a substrate for producing nitric oxide (NO), a molecule with anti-microbial properties and significant effects on mammalian cell biology, including the inhibition of respiration due to effects on the ETC (15). Being able to use aerobic glycolysis is a requirement for survival in cells that strongly express NOS2. Indeed, the profound decline in OXPHOS following stimulation of bone marrow derived DCs with LPS for greater than 6 h is not apparent in DCs recovered from lymphoid tissues and stimulated ex-vivo with TLR agonists, a finding consistent with the fact that these cells do not express NOS2. However, inflammatory monocyte-derived DCs induced in vivo by exposure to Listeria monocytogenes, do express NOS2 in response to LPS and also exhibit diminished respiration (14).
The finding that pharmacologic inhibition of NOS2 blocks the commitment to aerobic glycolysis but does not affect DC activation was initially at odds with observations that targeted inhibition of glycolysis has marked inhibitory effects on activation. Subsequent studies revealed very rapid (within minutes) metabolic responses to TLR agonists, measurable as an increased rate of extracellular acidification (ECAR), a mark of lactate release (10). This occurs in response to multiple different TLR agonists, and differs from the late stage commitment to Warburg metabolism in that it is apparent in DCs grown from bone marrow in FMS-like tyrosine kinase 3 ligand (Flt3L) as well as in GM-CSF, and in the absence of NO (10). Unbiased metabolomics support the view that glycolysis, the PPP and OXPHOS are all increased within hours of stimulation and 13C-glucose carbon tracing showed increased incorporation into TCA cycle citrate following activation. A more recent report showed that, in addition to extracellular glucose, DCs utilize the breakdown of stored intracellular glycogen, to release glucose to support this early increase in glycolysis that is important for activation (16). Citrate exported from mitochondria to the cytoplasm is converted back into acetyl-CoA, the primary substrate for FAS (10,13) (Fig. 1). Data, derived from inhibitor and genetic loss of function approaches targeting enzymes and transporters throughout the pathways of interest, support the view that FAS is important for DC activation because it allows the expansion of endoplasmic reticulum (ER) and Golgi, which are important for the changes in synthetic and secretory functions of these cells as they increase production of cytokines and other molecules destined for secretion or incorporation into the plasma membrane (10). It is important to point out that acetyl-CoA derived from citrate can also be used as an acetyl donor for the acetylation of proteins including histones, and that histone acetylation in the regulation of chromatin structure and therefore transcriptional potential is clearly important during myeloid cell activation (as discussed later herein). Moreover, acetyl-CoA derived from citrate can be used not only for FAS in the broad sense, but also for synthesis of the lipid mediator, PGE2 (10,17). PGE2 has multiple functions and can play roles in inflammation and in the resolution of inflammation, depending on context and is a major output of mononuclear phagocytes upon stimulation with TLR agonists, and it is of interest that its production is inhibited by the blockade of FAS (10,17). Of further significance, PGE2 has recently been shown to play an important role as a necessary autocrine stimulant for the production of pro-IL-1β by LPS-stimulated macrophages (discussed further below) (18). We found that the PPP plays an important role in these processes by supporting the production of NADPH, an important cofactor for FAS (Fig. 1). In addition, the PPP generates ribose for nucleotide synthesis (Fig. 1), which is critical for DNA and RNA synthesis with the latter presumably being important in the strong transcriptional response that results from TLR engagement. Our findings suggest that ATP derived from either glycolysis or OXPHOS is sufficient to provide bioenergetic support for DC activation, and reinforce the view that the importance of glycolysis as a core anabolic pathway is critical for DC activation and function.
We explored how TLRs are coupled to metabolic pathways, and found that Akt, a kinase known from work on other cells to be critical for growth factor receptor driven increases in glycolysis, is essential for both early and late TLR agonist induced changes in glycolysis (10). TLR mediated Akt activation occurs via TANK-binding kinase 1 (TBK1) and I-kappa-B kinase (IKKε), and directs changes in metabolism by phosphorylating the enzyme that catalyzes the first step in glycolysis, Hexokinase II (HKII), thereby promoting its interaction with mitochondria, a process that increases its activity and thereby increases glycolytic flux. Over the same early time frame post stimulation that Akt is activated, 5’ AMP-activated protein kinase (AMPK) becomes transiently dephosphorylated. AMPK senses increased AMP/ATP ratios and usually promotes increased OXPHOS to promote ATP generation through catabolic processes such as mitochondrial FAO or glutamine (processes which will be discussed in greater detail below), and inhibits processes that energy demanding such as protein synthesis. Reduced AMPK phosphorylation plays an important role in DC activation since the inclusion of the AMPK-activator 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) in the stimulation cultures resulted in decreased activation as measured by IL-12 production and CD86 expression, by inhibiting the glycolytic switch (13). Moreover, loss of AMPK function resulted in exaggerated activation (13). Thus, we envisage DC activation as encompassing the temporally connected activation of a TBK-IKKε-Akt-HK axis, and the deactivation of AMPK to together promote anabolic processes linked to cellular activation (19).
The mammalian target of rapamycin mTOR pathway, which intersects with the Akt pathway, is linked to the promotion of glycolysis and related anabolic pathways. We found that loss of function of mTOR had no measurable effect on the early metabolic changes linked to DC activation, but had a profound effect on Nos2 expression and the commitment to aerobic glycolysis at later timepoints post-stimulation in GM-CSF DCs. We showed that inhibition of mTORC1 with rapamycin promotes the ability of DCs to activate T cells in vitro and to induce anti-tumor immunity in vivo (20), and postulated that this is due to increased DC survival linked to increased mitochondrial function in the absence of the inhibitory effect of NO on respiration (21). Hypoxia Inducible Factor 1 α (HIF-1α), which upregulates the glycolysis pathway under hypoxic conditions (22–24), is also playing a role in the response of DCs to danger signals, where it is necessary for increased glycolysis and DC activation even under normoxic conditions (25).
Macrophages: Inflammatory activation
In parallel with work on DCs there has been increased interest in changes in cellular metabolism linked to activation in other innate immune cells. Initial work indicated that inflammatory macrophages (those stimulated through TLRs) begin to use Warburg metabolism (26) and we now know that broad features of metabolic rewiring associated with activation in macrophages, characterized by increased flux of glucose into the PPP and into lactate in LPS-stimulated cells, echo those discussed above for DCs (27,28). In addition, macrophages stimulated with LPS plus interferon gamma (IFNγ), have greatly diminished OXPHOS, as is the case for LPS-stimulated DCs (12,29). A decline in OXPHOS is also apparent after stimulation with LPS alone, but is not as marked as in LPS-stimulated DCs (29,30).This reflects a requirement for IFNγ as well as LPS to promote maximal NO production in macrophages, but not DCs.
A broken TCA cycle
An integrative analysis of metabolism and transcription in inflammatory macrophages (cultured from bone marrow in colony stimulating factor-1, CSF-1), reinforced developing ideas of changes in metabolism associated with inflammatory activation, while also illuminating several previously unrecognized or underappreciated aspects of the metabolic changes that occur following stimulation (27). In particular, it focused attention on critical metabolic changes centered around citrate within the TCA cycle (reviewed recently in the broader context of metabolic reprogramming in inflammatory macrophages in (31)). It emphasized that although glucose carbon enters mitochondria and is used to produce citrate, a break occurs after this point in the TCA cycle, at least in part due to reduced expression of isocitrate dehydrogenase (Idh), that substantially diminishes the production of α-ketoglutarate (α-KG) from glucose carbon (Fig. 1). This favors the accumulation and export of citrate for FAS, which allows membrane synthesis (10), as well as lipid droplet development that, if inhibited, has marked effects in macrophage inflammatory potential (32) (Fig. 1). Moreover, the parallel induction of expression of aconitate decarboxylase 1 (encoded by Acod1, also referred to as Irg1) allows the use of (iso)citrate carbon, via aconitate, for high output synthesis of the metabolite itaconate (Fig. 1). This finding coalesced with a series of other papers which pointed to itaconate as a major inflammatory macrophage product (33,34), and initiated a search for the biological function of this metabolite. Initial work indicated that itaconate is an anti-microbial, able to stunt growth of multiple microorganisms by inhibiting bacterial enzymes isocitrate lyase and propionyl-CoA carboxylase (35–37), especially when glucose availability is limited (33,38). Further studies have indicated that it may serve important immune cell intrinsic functions, acting to inhibit succinate dehydrogenase (SDH) resulting in accumulation of succinate (39,40) (Fig. 1). Furthermore itaconate regulates the inflammatory potential of macrophages through a mechanism that involves the alkylation of multiple cysteines in Keap1 that result in it dissociating from, and thereby activating, nuclear factor erythroid-2-related factor 2 (Nrf2) (41). NRF2 induces the expression of anti-oxidant genes and represses pro-inflammatory gene transcription (41–43). These results are recapitulated by the addition of 4-octyl-itaconate or dimethyl-itaconate, derivatives believed to be more cell membrane-permeable than underivatized itaconate, to inflammatory cells (41,42). Work with dimethyl-itaconate has indicated that additional Nrf2-independent anti-inflammatory effects are mediated by inhibition of ΙκΒξ protein induction (42). Moreover, 4-octyl itaconate can induce the alkylation of cysteine 22 in GAPDH, thereby inhibiting its activity, glycolytic flux and inflammatory activation (44). These findings and others have generated considerable interest in the therapeutic potential of itaconate and in understanding, in greater detail, the biology of this fascinating metabolite (45).
A second TCA cycle break at the succinate to fumarate transition was also revealed by the integrative analysis (27). This break had been implied previously by work which had revealed a build-up of succinate (28) (Fig. 1), and discussed above others later showed that this was dependent on Acod1 expression and thus itaconate production (39,40). In LPS-stimulated macrophages this correlates with reverse electron transport from complex II to complex I, and the subsequent production of ROS at complex I, which serves to stabilize HIF-1α and thereby promote the commitment to glycolysis and production of the potent inflammatory mediator IL-1β (28). HIF-1α is essential for the induction of glycolytic metabolism and inflammatory macrophage activation (28,46). Pyruvate kinase M2 (PKM2), which converts phosphoenolpyruvic acid to pyruvate in glycolysis, plays a key role in stabilizing HIF-1α activity to promote glycolysis, succinate build up, and inflammatory activation, in part through its ability to exist in tetrameric vs. dimeric forms, with the latter being less enzymatically active but capable of playing a second role within the nucleus in complex with HIF-1α, to promote the expression of HIF-1α target genes (47). Intriguingly, the relationship between tetrameric and dimeric PKM2 depends on posttranslational modifications of the enzyme, by amongst other things, succinylation (48), which promotes dimerization. This suggests a self-promoting system in which succinate build up, promoted by PKM2 dimerization, promotes succinylation of PKM2 thereby reinforcing further succinate build up.
Findings support the view that in inflammatory macrophages glutamine carbon is used to refill the TCA cycle and produce succinate (27,41) (Fig. 1). However, despite clear evidence for a role for succinate in the inflammatory response (49), it is interesting that glutamine withdrawal did not affect expression of NOS2, a major mark of inflammatory activation in cells stimulated with LPS plus IFNγ (27). Apart from glutamine carbon, glutamine nitrogen can be utilized to fuel NO production, an integral part of inflammatory macrophage function. Here, the aspartate-arginosuccinate shunt is using glutamate and aspartate derived nitrogen to generate arginine for NO production and aspartate derived carbon to generate fumarate to fuel the TCA cycle (Fig. 1) (27). Inhibition of this process by targeting glutamic-oxaloacetic transaminase 1 (GOT1), the essential enzyme used to generate aspartate from the TCA cycle, is sufficient to block NO production, with coincident and anticipated increases in OXPHOS and decreases in ECAR. GOT1 inhibition unexpectedly also resulted in a marked reduction in interleukin-6 (IL-6) production (27). While the underlying reason for this is unclear, we postulate that it may reflect collateral reductions in cytosolic NAD+ levels due to inhibition of the role of GOT1 in the malate aspartate shuttle, which serves to promote redox balance between the nucleus and cytoplasm (9). In this case, reduced NAD+ would affect the NAD+-dependent activity of GAPDH, and thereby the role of glycolysis in the activation events that lead to IL-6 production.
Post-translational modifications driven by metabolism
The realization that itaconate can alkylate cysteines and thereby alter protein function (41) is a recent discovery in an area of growing importance, the ability of metabolites to post-translationally modify proteins. This encompasses the critical role of regulated histone methylation and acetylation in the epigenetic control of gene expression (discussed for macrophage activation in (50,51)). Increases in the flux of glucose carbon into the TCA cycle early after inflammatory activation results in increased levels of acetyl-CoA that are used for histone acetylation (Fig. 1), to promote gene expression of pro-inflammatory cytokines. This is promoted by TLR-driven increases in the activity of ATP Citrate Lyase (Acly) (52,53) (Fig. 1). Interestingly, the process becomes attenuated over prolonged stimulation, due to a reduction of the entry of glucose carbon into the TCA cycle due its diversion, through the conversion of DHAP into G3P by glycerol-3-phosphate dehydrogenase (GPD2), into the glycerol phosphate shuttle, a pathway that feeds glycerol synthesis (53) (Fig. 1). Serine biosynthesis is also increased as a result of inflammatory activation and is critical for IL-β production because the pathway intersects with one-carbon metabolism, also increased in LPS-activated cells, to produce S-adenosyl methionine, which is needed as a donor for histone tri-methylation at lysine 36 (54), an activating mark (Fig. 1). Synthesized serine is also used for glycine and thereby glutathione production, which is essential for the ability of activated cells to make IL-1β (55) (Fig. 1). The role of metabolic reprogramming to accentuate changes in histone acetylation and methylation is also critical for the establishment of trained immunity. Here, activation by, for example, the fungal carbohydrate danger signal β-glucan confers the ability of the innate immune system, and in particular monocyte-derived macrophages, to subsequently respond in an enhanced way to the same or distinct inflammatory signals, thereby conferring on the innate immune system a degree of memory and the potential to clear infection more quickly after a priming event. The nature of the priming signal has been shown to be an mTORC1-and HIF-1α-mediated increase in glycolysis and one carbon metabolism leading to epigenetic changes at target loci, that include changes in histone acetylation and methylation that maintain genes in open, more transcriptionally permissive, chromatin (56–58).
Malonyl-CoA is an intermediate in FAS, which has recently been shown to play an additional role in the post-translational malonylation of K213 in the active site of GAPDH (Fig. 1). This is important because GAPDH is an RNA-binding protein, within the active site of which mRNAs bind via their 3’UTRs. This both prevents mRNA translation and limits GAPDH activity. In T cells, this moonlighting activity of GAPDH activity was established to be a critical factor in the importance of upregulated glycolysis for activation, since engagement of GAPDH in glycolysis effectively diminishes its ability to sequester cytokine mRNAs, thereby permitting maximal translation and cytokine production (59). In a parallel fashion, Tnf mRNA is bound to GAPDH in resting macrophages, and LPS-stimulated GAPDH malonylation mediates both the release of the cytokine coding mRNA, thereby allowing translation, and simultaneously favors enhanced glycolysis to support subsequent steps in activation including the production of IL-1β (60).
NAD+: Maintaining glycolysis
Because of the importance of glycolysis in inflammatory activation, and the central role of GAPDH in this pathway, there has been considerable interest in further understanding the biology of this enzyme in the context of immune cell activation. NAD+ is an essential cofactor for GAPDH and other enzymes involved in anabolic pathways, and in respiring cells is usually in sufficient supply to fulfill this role, since redox balance is maintained by, amongst other things, the mitochondrial redox shuttles (the malate aspartate shuttle and the glycerol phosphate shuttle), which exchange NADH and NAD+ equivalents across the mitochondrial membrane (9). To some extent, reductions in the TCA cycle and respiration in inflammatory macrophages and associated changes in redox balance are compensated in so far as GAPDH activity is concerned by the conversion of pyruvate to lactate, which is linked to the oxidation of NADH (Fig. 1). Nevertheless, it was recognized for some time that NAD+ levels decline during inflammatory activation. Recent reports showed that there is a significant contribution of de novo NAD+ synthesis from tryptophan via the kynurenine pathway to the NAD+ pool in resting macrophages (61). This plays an important role as a substrate for the activity of sirtuin 3 (SIRT 3), which promotes respiration by deacetylating critical lysines in Complex I of the ETC. Following activation with LPS, tryptophan catabolism is increased in human monocyte-derived macrophages, with associated increases in the levels of intermediates in the kynurenine pathway, but at the same time, expression of quinolate phosphoribosyltransferase (QPRT), the critical enzyme involved in de novo NAD+ synthesis, is down-regulated. This contributes to decreased OXPHOS due to increased Complex I acetylation, and the accumulation of succinate, which is linked to inflammation (as discussed above). In these cells, approaches for increasing NAD+ were sufficient to restore respiration and suppress the inflammatory function of the LPS-stimulated cells (61). In mouse macrophages (27), and in human monocyte-derived macrophages (61), inflammatory activation leads to significant consumption of NAD+ by poly ADP-ribose polymerases (PARPs). In mouse inflammatory macrophages, PARPs are activated as part of the response initiated by oxidative DNA damage occurring as a result of increased ROS production at Complex III (29), reiterating the important role of ROS in macrophage metabolic adaptations (28,41,62), as well as to anti-microbial functions (63). As part of the response to reduced NAD+ levels and the loss of the redox balancing power of the TCA cycle, inflammatory mouse macrophages upregulate expression of nicotinamide phsophoribosyltransferase (NAMPT) to salvage NAD+ from nicotinamide, which is produced when NAD+ is consumed by PARPs. The importance of this pathway in inflammatory macrophages is illustrated by the fact that FK866, an inhibitor of NAMPT, results in reductions in NAD+, glycolysis and inflammatory activation, which can be reversed by the addition of nicotinamide mononucleotide, the NAD+ salvage pathway intermediate downstream of NAMPT (29).
We would argue that the ability of FK866 to regulate inflammatory activation reflects the inhibitory effects of NAD+ depletion on GAPDH. Direct targeting of GAPDH by koningic acid (heptelidic acid), which covalently modifies the active site cysteine, results in similar effects on activation as those observed using FK866 (29). Moreover, there is a recent realization that dimethylfumarte, a drug used extensively for the treatment of psoriasis, a disease caused by Th17 cells, which are highly glycolytic, functions by succinating the GAPDH active site cysteine and thereby inhibiting enzymatic activity, with suppressive effects on the inflammatory capacity of macrophages and Th17 cells (64). The ability of dimethylfumarate, which is more cell permeable and electrophilic derivative of the TCA cycle intermediate fumarate, to inhibit GAPDH may indicate inherent regulatory properties of bona fide fumarate on glycolysis.
Macrophages: Alternative activation
A particularly interesting aspect of macrophage biology is the ability of these cells to assume different functions in response to the receipt of distinct signals. The cells express many different types of receptors that allow them to respond to a range of signals emanating from external as well as cell-internal environments, and the transcriptional response initiated by these different receptor types can include overlapping or largely distinct gene sets (65). Therefore, the potential for functional diversity within the macrophage response is large, and the issue of whether or not metabolism is fine-tuned and distinct to support each activation state is largely unexplored with most work having focused on inflammatory macrophages, e.g., those stimulated by TLR agonists.
An exception to the focus on inflammatory macrophages has been the interest in “alternatively activated” macrophages. This in itself is a complex area since what constitutes an alternatively activated macrophage has been the subject of discussion (66). However, we have focused on IL-4 (which shares a signaling receptor with IL-13)-driven activation, since this was how alternatively activated macrophages were first described (67), and moreover is consistent with the ongoing interest in the laboratory on the mechanism of action of IL-4 during helminth infections, where it is a defining cytokine produced by Th2 cells, innate lymphoid cells 2 (ILC2s), eosinophils and other cells associated with type 2 immunity (68). There is considerable interest in the function of alternatively activated macrophages, as they are implicated in important processes, such as immunity against helminths, wound healing, and adipose tissue homeostasis (4). However, it is also true that there is a less clear view of the effector mechanisms that delineate alternatively activated macrophage function. For inflammatory macrophages, the production of cytokines such as TNF and IL-1β are clearly linked to important functions in health and disease in humans that are recapitulated in mice. However, for alternatively activated macrophages, specific links between gene expression and function are less clear. Exceptions to this include resistin-like molecule-alpha (Retlna/RELMα), which is implicated in wound healing (48) and metabolic homeostasis (69), chitinase-like 3 (Chil3/YM-1), which regulates neutrophil infiltration of damaged tissue (70) and tissue repair (71) and arginase 1 (ARG1), which can effectively deplete environmental arginine, thereby limiting the access of other cells to this important amino acid and in so doing exert a level of control of activation in these cells (4,72).
Integration of various metabolic pathways
Whereas flux through the TCA cycle and OXPHOS are significantly diminished as a result of inflammatory activation, the opposite is true during alternative activation (27). This reflects increased usage of three major fuels for the TCA cycle, glucose, glutamine and fatty acids (27,73) (Fig. 2), and inhibition of pathways that use these fuels have been shown to affect alternative activation. These three fuels contribute together to a metabolic pathway that is significantly upregulated as a result of alternative activation, the hexosamine biosynthesis pathway (27) (Fig. 2). This pathway emerges from fructose-6-phosphate and integrates metabolic signals from nucleotide synthesis (UDP), glycolysis (Glc), glutamine metabolism (N) and acetyl-CoA (Ac), which can be derived from, for example, FAO or glucose oxidation (Fig. 2). Grossly, hexosamine biosynthesis appears to be of particular importance due to its ability to provide UDP-GlcNAc for N-glycosylation, since tunicamycin, an inhibitor of this process, can block aspects of IL-4 driven activation (27). However, it is possible that increased emphasis on hexosamine biosynthesis reflects additional aspects of the biology of alternatively activated macrophages that remain to be determined. Increased use of glucose following IL-4 stimulation is important for alternative activation (27,73,74), since both 2-DG, which blocks very early steps in glycolysis, and UK5099, a selective inhibitor of the mitochondrial pyruvate channel, lead to diminished expression of alternative activation marks (74,75). Alternative macrophage activation in vitro, and during helminth infection, is dependent on mTOR, with evidence for roles for both mTORC1 (73) and mTORC2 (74,76). We found that mTORC2 is responsible for phosphoinositide 3-kinase/Akt-dependent induction of glycolysis in alternatively activated macrophages, a function that it also serves in other cells such as brown adipocytes (77) and in glioblastoma cells (78). In this case, the upstream signal for mTORC2 activation is CSF-1, an important macrophage growth and survival factor, and interferon regulatory factor-4 (IRF4) -dependent changes in glucose metabolism downstream of this pathway synergize with IL-4 induced signaling through STAT6 to support alternative activation (74).
An important requirement for glucose carbon that has entered the TCA cycle is for the ACLY-dependent synthesis of acetyl-CoA as a donor for the activating acetylation of histones associated with genes that are targets of IL-4 signaling (73). Glutamine also plays an important role in the epigenetic regulation of IL-4 induced gene expression through the production of αKG, by glutaminolysis, which acts to promote the jumonji domain containing 3 (Jmjd3)-dependent demethylation of the repressive H3K27me3 mark at IL-4-responsive loci, and thereby promote expression of genes associated with alternative activation (79) (Fig. 2). An interesting subtlety here is that the ratio of αKG to succinate is telling, with a high ratio being important for alternative activation, and a low ratio predisposing towards inflammatory activation. In the setting of alternative activation, glutaminolysis is also linked strongly to increased FAO and the development of spare respiratory capacity that are hallmarks of alternatively activated macrophages. An intriguing development here is the finding that the nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ), which is most usually thought of as regulating the expression of genes that control fatty acid metabolism including FAO (80,81), has recently been shown to be critical for glutaminolysis in IL-4-stimulated macrophages (82). In the absence of PPARγ, macrophages fail to become alternatively activated and begin to make itaconate and take on other features of inflammatory macrophages. Indeed, early work showed that PPARγ can attenuate expression of macrophage inflammatory programmes (83–85). Functionally, IL-4 stimulates PPARγ expression via STAT6, and PPARγ promotes glutaminolysis, and the increased OXPHOS that is a mark of alternative activation. PPARγ has been known for some time to be important for alternative activation (86–88), and it is now apparent that deletion of PPARγ and removal of glutamine effectively phenocopy each other in terms of their effect on IL-4 driven activation (82). Consistent with the observed function of PPARγ, overexpression of PPARγ coactivator 1β (PGC1β), a transcriptional coactivator that promotes mitochondrial biogenesis and the expression of OXPHOS related genes by interacting with PPARγ downstream of AMPK activation, is able to suppress inflammatory activation and promote the expression of some marks of alternative activation, such as Arginase 1 (89). Broadly consistent with this, PGC1β-deficient macrophages display increased inflammation (89,90).
The etomoxir controversy - a role for FAO?
Work by Chawla was the first to show that etomoxir, a drug that inhibits carnitine palmitoyl transferase 1 (CPT1), was able to block aspects of alternative activation (89). Cpt1 facilitates long chain fatty acid transport through the outer mitochondrial membrane by the production of acyl-carnitines which are then transported through the carnitine-acylcarnitine transporter, SLC25A20. Transfer of fatty acids across the inner membrane into the matrix is then mediate by Cpt2 through a process that is coupled to the removal of carnitine (91). Within the matrix, fatty acids can be used to fuel FAO. This work therefore linked increased mitochondrial activity and FAO specifically in the IL-4 driven activation process (89). Expanding on this work, we also observing increased respiration in IL-4 activated macrophages, and etomoxir-sensitive expression of alternative activation marks (12). In these studies, the data indicated that FAO is fueled by a process that involves lysosomal lipolysis of triacylglycerides acquired via CD36; inhibition of this pathway, by varied loss of function approaches to diminish lysosomal acid lipase, Cpt1a or CD36 activity, resulted in diminished expression of IL-4 induced genes. As a component of these studies, increased mitochondrial respiration, and enhanced spare respiratory capacity, marks of alternative activation in bone marrow derived macrophages stimulated with IL-4 in vitro, were shown to also be characteristics of peritoneal macrophages in mice mounting a strong type 2 response to infection with the parasitic helminth Heligmosomoides polygyrus. Moreover, these metabolic parameters, as well as protective immunity to the parasite, were found to be significantly diminished by intraperitoneal injection of the lipase inhibitor tetrahydrolipistatin (12).
Taken together, these data support the view that FAO is integral to alternative activation. Nevertheless, precisely how FAO would support the expression of IL-4-induced genes remains unresolved. Moreover, a role for FAO in alternative activation has been questioned in light of results from the use of Cpt1a-deficient macrophages from LysM-Cre x Cpt1af/f mice, which were found to not phenocopy etomoxir-treated macrophages, since in response to IL-4 they continue to be able to express a range of genes that are considered markers of alternative activation (92). Similarly, LysM-Cre x Cpt2f/f macrophages alternatively activated (93–95). Moreover, despite the fact that their ability to synthesize long chain acyl-carnitines, and oxidize exogenous palmitate, is significantly reduced, Cpt1a-deficient macrophages continue to be susceptible to the inhibitory effects of etomoxir, at the concentrations used in the work described above, on the expression of genes associated with IL-4-driven alternative activation, and on oxygen consumption rate and spare respiratory capacity (93,95,96). In these studies, the specificity of etomoxir as a Cpt1 inhibitor was shown to be dose-dependent and apparent in cells treated with 3 μM of the drug, and off-target effects, most prominently the dysregulation of the free CoA pool, were observed at concentrations of the drug that had been used to demonstrate an effect on alternative activation (>100 μM) (96). Here, a metabolomics approach identified increased levels of pantothenate, the precursor in CoA synthesis, alongside reduced levels of CoA itself, as features of alternatively activated macrophages treated with high doses of etomoxir, vs. the lower dose which was shown to be capable of inhibiting FAO. The addition of exogeneous CoA was able to recover alternative activation in the presence of high dose etomoxir, suggesting that CoA deficiency is the critical high dose-etomoxir induced perturbation that results in the suppression of alternative activation. Moreover, exogenous CoA was able to boost IL-4-driven alternate activation in the absence of etomoxir. The hypothesized mechanism of action of etomoxir in the inhibition of alternative activation is that it depletes free CoA through the formation of etomoxiryl-CoA. CoA is critically required for a range of important cellular processes including, but not limited to FAS, protein acetylation, the oxidation of pyruvate, and for FAO itself. Moreover, apart from acetyl CoA, FAO also provides macrophages with NADH and FADH2, which can be utilized to fuel the ETC and generate ATP, but at this time which of these is affected by etomoxir to inhibit alternative activation is unclear. It should be noted that, even at high concentrations etomoxir does not affect the expression of genes linked to IL-4-driven activation in human macrophages (97), although here it is important to bear in mind that IL-4 target genes in mouse and human macrophages overlap to only a small degree (30). As of now, considerable confusion exists around this topic, and a role for FAO and the Cpt1a/Cpt2 system in alternative macrophage activation remains to be resolved.
While Cpt1a/Cpt2 per se are not an absolute requirement for alternative activation, it remains clear that increased FAO is a mark of IL-4 driven activation (12,73,74,89,93), and it is of interest that this pathway has been implicated as being important in situations where macrophages are overloaded with fats, for example as in atherosclerosis, where the absence of CPT1a leads to more severe disease (95), and in efferocytosis, a physiological function of alternatively activated macrophages in which these cells are required to handle the lipid load delivered by phagocytosis (98). Moreover, Cpt1a, and indeed OXPHOS itself, have inherently anti-inflammatory effects, in suppressing the potential of macrophages to make inflammatory cytokines (99), which is a property related to alternative activation. Thus, the elucidation of the regulation of FAO, and of its biological roles in macrophages, remains of interest.
It seems feasible that in the absence of FAO, IL-4-activated macrophages could increase usage of alternative fuels to support alternative activation. Detailed analyses of glucose and glutamine metabolism in Cpt1a−/− macrophages are lacking, but would be informative in terms of understanding how differential fuel usage to support the TCA cycle might be important in these cells. The potential for the adaptive use of different fuels to maintain OXPHOS as being inherent to alternative activation would be compatible with the finding that OXPHOS is critical for this process (30,75,89,100,101). However, a counterargument to this is provided by the finding that alternative activation has been reported to occur in the presence of rotenone, which blocks OXPHOS by inhibiting ETC complex I (92). Nevertheless, as we have learned from 13C-tracing studies in inflammatory macrophages, a lack of measurable OXPHOS does not equate to a complete lack of flux into the TCA cycle (27), and a deeper understanding of how mitochondrial metabolism is regulated during alternative activation is likely to be important.
An important role for mitochondrial metabolism
Support for an important role for mitochondria in IL-4 mediated macrophage activation emerged unexpectedly from recent studies on polyamine synthesis. The polyamine spermidine, derived from arginine, serves as a substrate for hypusination of eukaryotic initiation factor 5 (eIF5A). Targeted inhibition of this process was found to have profound effects on mitochondrial biology, resulting in reduced flux through the TCA cycle, diminished expression of ETC components, and reduced OXPHOS. In IL-4 stimulated macrophages, targeted inhibition of hypusination, or of ETC chain components, resulted in reduced expression of alternative activation marks such as CD206, CD301, RELMα and ARG1, both in vitro and in vivo (100). In contrast, inhibition of hypusination had no effect on inflammatory macrophage activation, a result that is consistent with the view that mitochondrial biology is repurposed in inflammatory cells to support inflammatory mediator production rather than respiration (31), while the latter is essential for alternative activation.
In work to understand the role of mitochondrial biology in alternative activation, we became interested in the possibility that mitochondrial Δψ could be important, since in recent work Δψ was shown to be critical for the production of mitochondrial ROS and cellular proliferation, whereas in comparison the TCA cycle was critical for histone acetylation (102). Moreover, changes in Δψ have been linked to different cell fates, with, for example, lower Δψ being a mark of stemness and longevity in CD8+ T cells (103). As described above, potential across the inner mitochondrial membrane is established as the ETC exports protons from the mitochondrial matrix into the intermembrane space, and provides the motive force for ATP synthesis via complex V. The magnitude of Δψ is an indirect measure of TCA cycle activity and the associated availability of NADH and FADH2 to fuel the ETC. We found that the inflammatory lipid mediator PGE2, is able to dissipate Δψ due to its ability to regulate expression of genes encoding components of the malate aspartate shuttle (104). Mitochondrial shuttles are active in cells that are respiring strongly, such as alternatively activated macrophages, and facilitate redox balance by allowing the regeneration of NADH to fuel the ETC through a process that also provides NAD+ to support glycolytic flux. In this way, alternative activation although dependent on glucose and respiration, does not require the de novo NAD+ synthesis pathway which is essential for inflammatory macrophage activation, as discussed above (29). In IL-4 activated macrophages, reduced Δψm due to PGE2 co-stimulation results in a loss of spare respiratory capacity and a reduction in the expression of some alternative activation markers, such as Retnla. This effect was found to be due to the ability of Δψ to regulate the transcriptional activity of ETV1. The data identified ETV1 as a transcription factor that can modulate alternative activation in response to changes in mitochondrial biology, a pathway that is currently under further investigation.
Future prospects
There have been significant recent advances in our understanding of the nature and significance of the metabolic changes that occur as mononuclear phagocytes, and other immune cells, become activated. It is likely that lessons being learned about links between metabolism and immune cell function and fate will have broader significance in terms of understanding how cell biology in general is tuned by metabolism. However, in the study of mononuclear phagocytes there are several distinct challenges for the near future.
Most of the studies discussed in this review have been performed in vitro on mouse bone marrow derived macrophages or DCs, or human monocyte-derived macrophages. These cells provide tractable and important model systems, and in the case of human monocyte derived macrophages are likely to strongly reflect the types of cells that are engaged in, for example, acute inflammatory conditions. However, these cells represent the tip of the iceberg of the broad population of distinct types of mononuclear phagocytes which exist as embedded residents of non-immune as well as immune tissues (Fig. 3). The biology of these tissue-resident (including tumor-resident) cells reflects their exposure to the local metabolic environments and signals, which are likely to have an impact on activation outcome (105,106), and this complexity is not captured in studies of bone marrow or monocyte-derived cells. Some progress has been made on studying immunometabolism in accessible tissue macrophage populations such as, for example, peritoneal macrophages and alveolar macrophages, but in general our view of tissue-resident cells and how they respond metabolically to activation in vivo is less than optimal at this time (107) (Fig. 3). It is also true that most work on the metabolism of dendritic cells and macrophages has involved the use of defined stimuli such as LPS or IL-4, whereas in vivo we should expect complexity to be provided by the way that cells integrate multiple signals to enact a spectrum of activation states (65). Increased emphasis on studying immune cell metabolism in vivo, or in more sophisticated culture systems such as those provided by organoids (107,108), is therefore warranted. In this general context, the application of advances in single-cell metabolomics (109) and spatial metabolomics (110–113) has the potential to greatly improve the resolution of future studies.
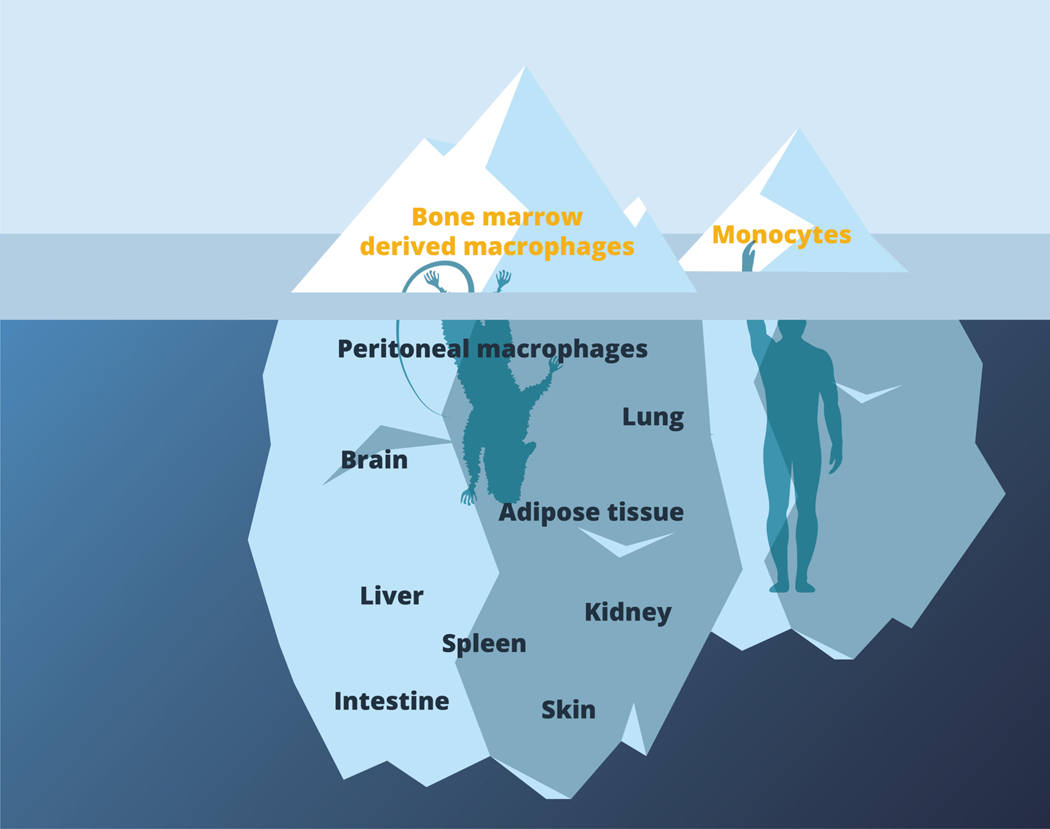
Figure 3.
Bone marrow and monocyte-derived macrophages are the tip of mononuclear phagocyte iceberg.
Most work to date has been performed using mouse bone marrow derived macrophages or human monocyte derived macrophages. These provide important, tractable primary cell populations that can be considered to be representative of macrophages that derive from monocytes in acute inflammatory settings. However, there is a broad range of tissue-resident macrophages that remain relatively uncharacterized in terms of their baseline and adaptive metabolic states. These are represented within the body of the iceberg beneath the surface. Future work will need to explore metabolism in these cells during health and disease, and to confirm the relatedness of findings from mice and humans.
Future work will also need to provide a clear picture of the extent to which the regulation of metabolism to support cellular activation is similar or distinct in mouse (in which most of the work has been done) versus human mononuclear phagocytes. At this time we know, for example, that inflammatory macrophage from both produce TNF, IL-1β and IL-6, and exhibit broadly similar metabolic characteristics including increased glycolytic activity, diminished OXPHOS, the production of itaconate, increased oxidative stress, and the depletion of intracellular NADPH and NAD+ after LPS stimulation (30,41,114). However, human monocyte-derived macrophages do not express NOS2 or make NO after LPS stimulation (115–119), and therefore will not experience the pseudo-hypoxic conditions that inflammatory mouse macrophages are subjected to. Moreover, the subtleties of NAD+ synthesis may also differ between inflammatory macrophages from the two species (29,61). Nevertheless, the exciting, overarching message is that metabolic tuning is critical for immune cell activation and that targeted interruption of implicated pathways can alter outcome. This has opened the door for the development of therapeutic approaches to manipulate immune cell-intrinsic metabolic pathways in the context of disease treatment (120).
Acknowledgements
The authors thank Johan Friden for the graphics, Angela Castoldi for reading the review and providing helpful comments, and Erika Pearce for support. The laboratory is supported by funding from the Max Planck Society, the NIH (AI110481) and the DFG (FOR 2599, CIBSS EXC-2189 and CRC 1160).
Footnotes
Conflict of interest
EJP is a founder of Rheos Medicines.
References
- 1.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3(12):984–93. [DOI] [PubMed] [Google Scholar]
- 3.Joffre O, Nolte MA, Spörri R, Sousa CRE. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227(1):234–47. [DOI] [PubMed] [Google Scholar]
- 4.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007. Aug;27(2):173–8. [DOI] [PubMed] [Google Scholar]
- 6.Frauwirth KA, Riley JL, Harris MH, Parry R V., Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–77. [DOI] [PubMed] [Google Scholar]
- 7.Hume DA, Radik JL, Ferber E, Weidemann MJ. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978;174(3):703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amati L, Pepe M, Elena Passeri M, Letizia Mastronardi M, Jirillo E, Covelli V. Toll-Like Receptor Signaling Mechanisms Involved in Dendritic Cell Activation: Potential Therapeutic Control of T Cell Polarization. Curr Pharm Des. 2006;12(32):4247–54. [DOI] [PubMed] [Google Scholar]
- 9.Chandel NS. Navigating Metabolism. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2015. 248 p. [Google Scholar]
- 10.Everts B, Amiel E, Huang SCC, Smith AM, Chang C-H, Lam WY, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15(4):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YL, Morales-Rosado J, Ray J, Myers TG, Kho T, Lu M, et al. Toll-like receptor agonists promote prolonged triglyceride storage in macrophages. J Biol Chem. 2014. Jan 31;289(5):3001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SC, Everts B, Ivanova Y, Sullivan DO, Nascimento M, Smith AM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15(August):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everts B, Amiel E, Van Der Windt GJW, Freitas TC, Chott R, Yarasheski KE, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120(7):1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuehr DJ, Nathan CF. Nitric oxide: A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169(5):1543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thwe PM, Pelgrom L, Cooper R, Beauchamp S, Reisz JA, D’Alessandro A, et al. Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses. Cell Metab. 2017. Sep 5;26(3):558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013;440(1):105–11. [DOI] [PubMed] [Google Scholar]
- 18.Zasłona Z, Pålsson-McDermott EM, Menon D, Haneklaus M, Flis E, Prendeville H, et al. The Induction of Pro–IL-1β by Lipopolysaccharide Requires Endogenous Prostaglandin E2 Production. J Immunol. 2017;198(9):3558–64. [DOI] [PubMed] [Google Scholar]
- 19.Pearce EJ, Everts B. Dendritic cell metabolism. Vol. 15, Nature Reviews Immunology. Nature Publishing Group; 2015. p. 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amiel E, Everts B, Freitas TC, King IL, Curtis JD, Pearce EL, et al. Inhibition of Mechanistic Target of Rapamycin Promotes Dendritic Cell Activation and Enhances Therapeutic Autologous Vaccination in Mice. J Immunol. 2012;189(5):2151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amiel E, Everts B, Fritz D, Beauchamp S, Ge B, Pearce EL, et al. Mechanistic Target of Rapamycin Inhibition Extends Cellular Lifespan in Dendritic Cells by Preserving Mitochondrial Function. J Immunol. 2014;193(6):2821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddle SR, Ahmad A, Ahmad S, Deeb SS, Malkki M, Schneider BK, et al. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am J Physiol - Lung Cell Mol Physiol. 2000. Feb;278(2 22–2):407–16. [DOI] [PubMed] [Google Scholar]
- 23.Obach M, Navarro-Sabaté À, Caro J, Kong X, Duran J, Gómez M, et al. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem. 2004. Dec 17;279(51):53562–70. [DOI] [PubMed] [Google Scholar]
- 24.Semenza GL, Jiang B-H, Leung SW, Passantino R, Concordet J- P, Maire P, et al. Hypoxia Response Elements in the Aldolase A, Enolase 1, and Lactate Dehydrogenase A Gene Promoters Contain Essential Binding Sites for Hypoxia-inducible Factor 1*. J Biol Chem. 1996;271(51):32529–37. [DOI] [PubMed] [Google Scholar]
- 25.Bhandari T, Olson J, Johnson RS, Nizet V. HIF-1α Influences Myeloid Cell Antigen Presentation and Response to Subcutaneous OVA Vaccination. J Mol Med. 2013;91(10):1199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Prados J-C, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–14. [DOI] [PubMed] [Google Scholar]
- 27.Jha AK, Huang SCC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–30. [DOI] [PubMed] [Google Scholar]
- 28.Tannahill GM, Curtis AM, Adamik J, Palsson-Mcdermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron AM, Castoldi A, Sanin DE, Flachsmann LJ, Field CS, Puleston DJ, et al. Inflammatory macrophage dependence on NAD + salvage is a consequence of reactive oxygen species–mediated DNA damage. Nat Immunol. 2019;20(4):420–32. [DOI] [PubMed] [Google Scholar]
- 30.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016;17(3):684–96. [DOI] [PubMed] [Google Scholar]
- 31.Ryan DG, O’Neill LAJ. Krebs Cycle Reborn in Macrophage Immunometabolism. Annu Rev Immunol. 2020. Apr 26;38(1):annurev-immunol-081619–104850. [DOI] [PubMed] [Google Scholar]
- 32.Castoldi A, Monteiro LB, Bakker N van T, Sanin DE, Rana N, Corrado M, et al. Triacylglycerol synthesis enhances macrophage inflammatory function. bioRxiv. 2020. Feb 5; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A. 2013;110(19):7820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strelko CL, Lu W, Dufort FJ, Seyfried TN, Chiles TC, Rabinowitz JD, et al. Itaconic acid is a mammalian metabolite induced during macrophage activation. J Am Chem Soc. 2011. Oct 19;133(41):16386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rittenhouse JW, McFadden BA. Inhibition of isocitrate lyase from Pseudomonas indigofera by itaconate. Arch Biochem Biophys. 1974;163(1):79–86. [DOI] [PubMed] [Google Scholar]
- 36.Rao GR, McFadden BA. Isocitrate lyase from Pseudomonas indigofera. IV. Specificity and inhibition. Arch Biochem Biophys. 1965;112(2):294–303. [DOI] [PubMed] [Google Scholar]
- 37.Berg IA, Filatova L V., Ivanovsky RN. Inhibition of acetate and propionate assimilation by itaconate via propionyl-CoA carboxylase in isocitrate lyase-negative purple bacterium Rhodospirillum rubrum. FEMS Microbiol Lett. 2002;216(1):49–54. [DOI] [PubMed] [Google Scholar]
- 38.Naujoks J, Tabeling C, Dill BD, Hoffmann C, Brown AS, Kunze M, et al. IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLoS Pathog. 2016;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016;24(1):158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J Biol Chem. 2016. Jul 1;291(27):14274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bambouskova M, Gorvel L, Lampropoulou V, Sergushichev A, Loginicheva E, Johnson K, et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 2018. Apr 26;556(7702):501–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016. May 23;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao ST, Han C, Xu DQ, Fu XW, Wang JS, Kong LY. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun. 2019. Dec 1;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Neill LAJ, Artyomov MN. Itaconate: the poster child of metabolic reprogramming in macrophage function. Vol. 19, Nature Reviews Immunology. Nature Publishing Group; 2019. p. 273–81. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Liu H, Lian G, Zhang SY, Wang X, Jiang C. HIF1 α -Induced Glycolysis Metabolism Is Essential to the Activation of Inflammatory Macrophages. Cell Rep. 2017;3:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palsson-Mcdermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates hif-1α activity and il-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21(1):65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, et al. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1β Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep. 2017;19(11):2331–44. [DOI] [PubMed] [Google Scholar]
- 49.Murphy MP, O’Neill LAJ. Krebs Cycle Reimagined: The Emerging Roles of Succinate and Itaconate as Signal Transducers. Vol. 174, Cell. Cell Press; 2018. p. 780–4. [DOI] [PubMed] [Google Scholar]
- 50.Schultze JL. Chromatin Remodeling in Monocyte and Macrophage Activation. In: Advances in Protein Chemistry and Structural Biology. Academic Press Inc.; 2017. p. 1–15. [DOI] [PubMed] [Google Scholar]
- 51.Verberk SG, de Goede KE, Van den Bossche J. Metabolic-epigenetic crosstalk in macrophage activation: an updated view. Epigenomics. 2019. May;11(7):719–21. [DOI] [PubMed] [Google Scholar]
- 52.Lauterbach MA, Hanke JE, Serefidou M, Mangan MSJ, Kolbe CC, Hess T, et al. Toll-like Receptor Signaling Rewires Macrophage Metabolism and Promotes Histone Acetylation via ATP-Citrate Lyase. Immunity. 2019;51(6):997–1011. [DOI] [PubMed] [Google Scholar]
- 53.Langston PK, Nambu A, Jung J, Shibata M, Aksoylar HI, Lei J, et al. Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat Immunol. 2019. Sep 1;20(9):1186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu W, Wang Z, Zhang K, Chi Z, Xu T, Jiang D, et al. One-Carbon Metabolism Supports S-Adenosylmethionine and Histone Methylation to Drive Inflammatory Macrophages. Mol Cell. 2019;75(6):1147–60. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez AE, Ducker GS, Billingham LK, Martinez CA, Mainolfi N, Suri V, et al. Serine Metabolism Supports Macrophage IL-1β Production. Cell Metab. 2019;29(4):1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. MTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (80- ). 2014;345(6204). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science (80- ). 2014;345(6204):121086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arts RJW, Novakovic B, ter Horst R, Carvalho A, Bekkering S, Lachmandas E, et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016;24(6):807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang CH, Curtis JD, Maggi LB, Faubert B, Villarino A V., O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013. Jun 6;153(6):1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galván-Peña S, Carroll RG, Newman C, Hinchy EC, Palsson-McDermott E, Robinson EK, et al. Malonylation of GAPDH is an inflammatory signal in macrophages. Nat Commun. 2019;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019. Jan 1;20(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167(2):457–470.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signaling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kornberg MD, Bhargava P, Kim PM, Putluri V, Snowman AM, Putluri N, et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Nature. 2018;360(6387):449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xue J, Schmidt S V., Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity. 2014. Feb 20;40(2):274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J Exp Med. 1992. Jul 1;176(1):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris NL, Loke P. Recent Advances in Type-2-Cell-Mediated Immunity: Insights from Helminth Infection. Immunity. 2017. Dec 19;47(6):1024–36. [DOI] [PubMed] [Google Scholar]
- 69.Kumamoto Y, Camporez JPG, Jurczak MJ, Shanabrough M, Horvath T, Shulman GI, et al. CD301b+ Mononuclear Phagocytes Maintain Positive Energy Balance through Secretion of Resistin-like Molecule Alpha. Immunity. 2016. Sep 20;45(3):583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sutherland TE, Logan N, Rückerl D, Humbles AA, Allan SM, Papayannopoulos V, et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol. 2014. Nov 18;15(12):1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutherland TE, Rückerl D, Logan N, Duncan S, Wynn TA, Allen JE. Ym1 induces RELMα and rescues IL-4Rα deficiency in lung repair during nematode infection. PLoS Pathog. 2018;14(11):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, et al. Arginase-1–Expressing Macrophages Suppress Th2 Cytokine–Driven Inflammation and Fibrosis. Kazura JW, editor. PLoS Pathog. 2009. Apr 10;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Covarrubias AJ, Aksoylar HI, Yu J, Snyder NW, Worth AJ, Iyer SS, et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife. 2016;5(e11612). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang SCC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, et al. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity. 2016;45(4):817–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, et al. Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation. Cell Metab. 2018;28(3):463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hallowell RW, Collins SL, Craig JM, Zhang Y, Oh M, Illei PB, et al. MTORC2 signalling regulates M2 macrophage differentiation in response to helminth infection and adaptive thermogenesis. Nat Commun. 2017. Jan 27;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Albert V, Svensson K, Shimobayashi M, Colombi M, Muñoz S, Jimenez V, et al. mTORC2 sustains thermogenesis via Akt-induced glucose uptake and glycolysis in brown adipose tissue. EMBO Mol Med. 2016. Mar 1;8(3):232–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masui K, Cavenee WK, Mischel PS. mTORC2 and Metabolic Reprogramming in GBM: at the Interface of Genetics and Environment. Brain Pathol. 2015. Nov;25(6):755–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, et al. Α-Ketoglutarate Orchestrates Macrophage Activation Through Metabolic and Epigenetic Reprogramming. Nat Immunol. 2017;18(9):985–94. [DOI] [PubMed] [Google Scholar]
- 80.Dreyer C, Keller H, Mahfoudi A, Laudet V, Krey G, Wahli W. Positive regulation of the peroxisomal β-oxidation pathway by fatty acids through activation of peroxisome proliferator-activated receptors (PPAR). Biol Cell. 1993;77(C):67–76. [DOI] [PubMed] [Google Scholar]
- 81.Kersten S, Desvergne B, Wahli W. Roles of PPARS in health and disease. Nature. 2000;405(6785):421–4. [DOI] [PubMed] [Google Scholar]
- 82.Nelson VL, Nguyen HCB, Garcìa-Cañaveras JC, Briggs ER, Ho WY, DiSpirito JR, et al. PPARγ is a nexus controlling alternative activation of macrophages via glutamine metabolism. Genes Dev. 2018;32(15–16):1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ricote M, Li AC, Willson TM, Kelly CJK, Glass CK. The peroxisome proliferator activated receptor-g is a negative regulator of macrophage activation. Nat Lett. 1998;391(1):79–82. [DOI] [PubMed] [Google Scholar]
- 84.Jiang C, Ting A, Seed B. PPARgamma agonists inhibit production of monocyte inflammatory cytokine. Nature. 1998;391(6662):82–6. [DOI] [PubMed] [Google Scholar]
- 85.Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–52. [DOI] [PubMed] [Google Scholar]
- 86.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–7. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen MTA, Chen A, Lu WJ, Fan WQ, Li PP, Oh DY, et al. Regulation of chemokine and chemokine receptor expression by PPARγ in adipocytes and macrophages. PLoS One. 2012;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Odegaard JI, Ricardo-gonzalez RR, Goforth MH, Christine R, Subramanian V, Mukundan L, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7(1):48–52. [DOI] [PubMed] [Google Scholar]
- 91.Knottnerus SJG, Bleeker JC, Wüst RCI, Ferdinandusse S, IJlst L, Wijburg FA, et al. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev Endocr Metab Disord. 2018;19(1):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Divakaruni AS, Hsieh WY, Minarrieta L, Duong TN, Kim KKO, Desousa BR, et al. Etomoxir Inhibits Macrophage Polarization by Disrupting CoA Homeostasis. Cell Metab. 2018;28(3):490–503.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nomura M, Liu J, Rovira II, Gonzalez-Hurtado E, Lee J, Wolfgang MJ, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17(3):216–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez-Hurtado E, Lee J, Choi J, Selen Alpergin ES, Collins SL, Horton MR, et al. Loss of macrophage fatty acid oxidation does not potentiate systemic metabolic dysfunction. Am J Physiol - Endocrinol Metab. 2017;312(5):E381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nomura M, Liu J, Yu ZX, Yamazaki T, Yan Y, Kawagishi H, et al. Macrophage fatty acid oxidation inhibits atherosclerosis progression. J Mol Cell Cardiol. 2019;127:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Divakaruni AS, Hsieh WY, Minarrieta L, Duong TN, Kim KKO, Desousa BR, et al. Etomoxir Inhibits Macrophage Polarization by Disrupting CoA Homeostasis. Cell Metab. 2018;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Namgaladze D, Brüne B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim Biophys Acta - Mol Cell Biol Lipids. 2014;1841(9):1329–35. [DOI] [PubMed] [Google Scholar]
- 98.Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, et al. Efferocytosis Fuels Requirements of Fatty Acid Oxidation and the Electron Transport Chain to Polarize Macrophages for Tissue Repair. Cell Metab. 2019;29(2):443–456.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malandrino MI, Fucho R, Weber M, Calderon-Dominguez M, Mir JF, Valcarcel L, et al. Enhanced fatty acid oxidation in adipocytes and macrophages reduces lipid-induced triglyceride accumulation and inflammation. Am J Physiol Endocrinol Metab. 2015. May 1;308(9):E756–69. [DOI] [PubMed] [Google Scholar]
- 100.Puleston DJ, Buck MD, Klein Geltink RI, Kyle RL, Caputa G, O’Sullivan D, et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019;30(2):352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15(6):813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martínez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, et al. TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol Cell. 2016. Jan 21;61(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sukumar M, Liu J, Mehta GU, Patel SJ, Roychoudhuri R, Crompton JG, et al. Mitochondrial Membrane Potential Identifies Cells with Enhanced Stemness for Cellular Therapy. Cell Metab. 2016. Jan 12;23(1):63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanin DE, Matsushita M, Klein Geltink RI, Grzes KM, van Teijlingen Bakker N, Corrado M, et al. Mitochondrial Membrane Potential Regulates Nuclear Gene Expression in Macrophages Exposed to Prostaglandin E2. Immunity. 2018;49(6):1021–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Artyomov MN, Sergushichev A, Schilling JD. Integrating immunometabolism and macrophage diversity. Semin Immunol. 2016. Oct 1;28(5):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van den Bossche J, Saraber DL. Metabolic regulation of macrophages in tissues. Cell Immunol. 2018. Aug 1;330:54–9. [DOI] [PubMed] [Google Scholar]
- 107.Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell. 2018. Dec 13;175(7):1972–1988.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caputa G, Castoldi A, Pearce EJ. Metabolic adaptations of tissue-resident immune cells. Nat Immunol. 2019;20(7):793–801. [DOI] [PubMed] [Google Scholar]
- 109.Duncan KD, Fyrestam J, Lanekoff I. Advances in mass spectrometry based single-cell metabolomics. Analyst. 2019;144(3):782–93. [DOI] [PubMed] [Google Scholar]
- 110.Rappez L, Stadler M, Triana S, Phapale P, Heikenwalder M, Alexandrov T. Spatial single-cell profiling of intracellular metabolomes in situ Keywords. bioRxiv. 2019;510222. [Google Scholar]
- 111.Geier BK, Sogin E, Michellod D, Janda M, Kompauer M, Spengler B, et al. Spatial metabolomics of in situ, host-microbe interactions. bioRxiv. 2019;555045. [DOI] [PubMed] [Google Scholar]
- 112.Melnik A V, Vázquez-Baeza Y, Aksenov AA, Hyde E, McAvoy AC, Wang M, et al. Molecular and Microbial Microenvironments in Chronically Diseased Lungs Associated with Cystic Fibrosis. mSystems. 2019;4(5):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Geier B, Sogin EM, Michellod D, Janda M, Kompauer M, Spengler B, et al. Spatial metabolomics of in situ host–microbe interactions at the micrometre scale. Nat Microbiol. 2020. Feb 3;1–3. [DOI] [PubMed] [Google Scholar]
- 114.Duroux-Richard I, Roubert C, Ammari M, Présumey J, Grün JR, Häupl T, et al. MiR-125b controls monocyte adaptation to inflammation through mitochondrial metabolism and dynamics. Blood. 2016;128(26):3125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee M, Rey K, Besler K, Wang C, Choy J. Immunobiology of Nitric Oxide and Regulation of Inducible Nitric Oxide Synthase. In: Results Probl Cell Differ. 2017. p. 181–207. [DOI] [PubMed] [Google Scholar]
- 116.Ralph AP, Yeo TW, Salome CM, Waramori G, Pontororing GJ, Kenangalem E, et al. Impaired pulmonary nitric oxide bioavailability in pulmonary tuberculosis: Association with disease severity and delayed mycobacterial clearance with treatment. J Infect Dis. 2013;208(4):616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MacMicking J, Xie Q, Nathan C. Nitric Oxide and Macrophage Function. Annu Rev Immunol. 1997;15(1):323–50. [DOI] [PubMed] [Google Scholar]
- 118.Murray HW, Teitelbaum RF. L-Arginine-Dependent Reactive Nitrogen Intermediates and the Antimicrobial Effect of Activated Human Mononuclear Phagocytes. J Infect Dis. 1992;165(3):513–7. [DOI] [PubMed] [Google Scholar]
- 119.Cameron ML, Granger DL, Weinberg JB, Kozumbo WJ, Koren HS. Human alveolar and peritoneal macrophages mediate fungistasis independently of L-arginine oxidation to nitrite or nitrate. Am Rev Respir Dis. 1990;142(6):1313–9. [DOI] [PubMed] [Google Scholar]
- 120.Mazumdar C, Driggers EM, Turka LA. The Untapped Opportunity and Challenge of Immunometabolism: A New Paradigm for Drug Discovery. Vol. 31, Cell metabolism. NLM (Medline); 2020. p. 26–34. [DOI] [PubMed] [Google Scholar]