Abstract
18-Oxocortisol is the product of the metabolism of 11-deoxycortisol by the mitochondrial enzyme aldosterone synthase (CYP11B2). The traditional concept is that the CYP11B2 is exclusively expressed in zona glomerulosa cells and the 17α-hydroxylase (CYP17A1) enzyme, required to synthesize 11-deoxycortisol, is in the zona fasciculata of the human adrenal. It has been postulated that the substrate for 18-oxocortisol is either cortisol from the circulation or from zona fasciculata cells adjacent to the zona glomerulosa. P-glycoprotein, which is highly expressed in steroidogenic cells of the adrenal gland, efficiently expels cortisol from the cell. Double immunofluorescence staining for the CYP11B2 and CYP17A1 enzymes in 7 human adrenals demonstrated that a highly variable number of cells, from 0–33%, in different areas of the zona glomerulosa co-expressed both enzymes. In addition, there were a variable number of cells that exclusively expressed the CYP17A1 embedded within the zona glomerulosa surrounded by CYP11B2-expressing cells.
18-Oxocortisol in the media of human adrenocortical HAC15 cells was measured by ELISA after incubation with and without 10 nM of angiotensin II to stimulate CYP11B2 activity, with and without the 3β-hydroxysteroid dehydrogenase (HSD3B) inhibitor trilostane, and with variable amounts of cortisol or 11-deoxycortisol. Cortisol was a poor substrate, while 11-deoxycortisol was a significant substrate for the synthesis of 18-oxocortisol.
These data suggest that the biosynthesis of 18-oxocortisol in the human adrenal is likely catalyzed by co-expression of the two crucial enzymes CYP17A1 and CYP11B2 in a small proportion of cells within the zona glomerulosa. It is also possible that 11-deoxycortisol diffusing from cells expressing only CYP17A1 interspersed with cells expressing the CYP11B2 enzyme may be a paracrine substrate in the synthesis of 18-oxocortisol.
Keywords: 18-oxocortisol, adrenal steroids
Primary aldosteronism (PA) is the most common cause of secondary hypertension, affecting between 4–20% of patients with hypertension (1,2). PA is associated with a marked increase in cardiovascular morbidity (3) and mortality (4) compared to patients with essential hypertension. Among multiple etiologies of PA, the most common are an aldosterone-producing adenoma and bilateral zona glomerulosa hyperplasia (idiopathic hyperaldosteronism) in 30–70% of cases each, depending on the population. Rare causes of PA include unilateral adrenal zona glomerulosa hyperplasia, aldosterone-producing adrenocortical carcinoma (5), and familial PA. The latter comprise 1% or fewer patients with PA (6).
Aldosterone synthesis occurs in the zona glomerulosa of the adrenal through the action of multiple enzymes located in different subcellular compartments. Cholesterol from lipid droplets is hydrolyzed, then transported into the inner mitochondria membrane through the action of steroidogenic acute regulatory protein (StAR) where it is hydroxylated and cleaved to pregnenolone by the side chain cleavage enzyme (CYP11A1). Pregnenolone leaves the mitochondria and enters the endoplasmic reticulum where it is oxidized to progesterone by 3β-hydroxysteroid dehydrogenase-Δ4–5 isomerase type 2 (HSD3B2) (7), then converted to deoxycorticosterone by 21-hydroxylase (CYP21A2). Deoxycorticosterone enters the mitochondria where it is successively hydroxylated by the aldosterone synthase (CYP11B2) enzyme to form corticosterone,18-hydroxy corticosterone, a theoretical germinal diol which spontaneously dehydrates to aldosterone (8). In the normal adrenal gland CYP11B2 expression is limited to the zona glomerulosa, CYP11B1 to the zona fasciculata, along with CYP17A1 which is also active in the zona reticularis (8)(9). In the zona fasciculata, pregnenolone is converted in the endoplasmic reticulum into 17α-hydroxypregnenolone by the 17α-hydroxylase (CYP17A1), acted upon by the HSD3B2 to 17α-hydroxyprogesterone, then to 11-deoxycortisol by CYP21A2, which then is transferred to the mitochondria where it is acted upon by the 11β-hydroxylase (CYP11B1) and converted into cortisol (8).
Aldosterone excess in combination with a high salt intake results in significant elevation of blood pressure and cardiovascular damage. However, the correlation between the level of aldosterone and blood pressure is not tight; hypertension in PA can be very severe without having very high levels of aldosterone (10), suggesting that other, yet unidentified, adrenal steroids might participate in the pathological process. In 1982 Ulick extracted urine from patients with APA and, using GC-MS, identified 18-hydroxycortisol as a steroid excreted in significant quantities by patients with aldosterone-producing adenomas (11). However this steroid had no mineralocorticoid or glucocorticoid activity (12). By incubating 11-deoxycortisol with bullfrog adrenals, Ulick identified 18-oxocortisol (13), the product CYP11B2 use of 11-deoxycortisol as a substrate (Fig 1A) (13). 18-Oxocortisol has weak mineralocorticoid and hypertensinogenic activity (11,14,15). Patients with aldosterone-producing adenomas and those with glucocorticoid-suppressible aldosteronism have high plasma and urinary levels of 18-oxocortisol (16–20), a phenomenon that has been used to distinguish between the different causes of PA (17–19,21).
Fig 1A:

The action of the CYP11B2 on the conversion of 11-deoxycortisol into 18-oxocortisol is shown.
The site and mechanism for the synthesis of 18-oxocortisol in normal individuals is not clear. It has been assumed that the synthesis of its precursors, cortisol and 11-deoxycortisol does not occur in the zona glomerulosa where CYP11B2 is expressed. Blood flow to the adrenal gland is centripetal, from the capsule to the medulla, which limits zona glomerulosa access to the requisite precursors from the zona fasciculata, but the possibility still exists that in the adult human cortisol or 11-deoxycortisol may reach the cells expressing CYP11B2 by a paracrine mode. Therefore it was proposed that circulating cortisol reaches the zona glomerulosa where it is converted to 18-oxocortisol (22). Contrary to the distinct distribution of cells of the zona glomerulosa and zona fasciculata that occur in rodents and young children, zona fasciculata cells expressing the CYP11B1 extend into the subcapsular region of some areas of the adult human adrenal and surround zona glomerulosa cells expressing CYP11B2 (9). Herein, we tested the hypothesis that some subcapsular adrenal cells co-express CYP11B2 and CYP17A1 using multiplex immunofluorescence microscopy for CYP11B2 and CYP17A1. This proof-of-concept results from CYP11B2 / CYP17A1 multiplex immunofluorescence microscopy suggest that human adrenals have cells in the zona glomerulosa that co-express both enzymes and therefore directly produce 18-oxocortisol.
MATERIALS AND METHODS
Adrenals slides from archival samples were from 2 young (samples A027 1.8 yrs. old and A024, 10 yrs. old from reference (23)) and 5 adult individuals from the University of Michigan Gift of Life program and from Saitama Medical University International Medical Center approved by the Institutional Review Boards of both institutions (age range 26 to 56 yrs. deceased kidney donors).
Antibodies:
The CYP11B2 monoclonal antibody CYP11B2-41-13 is a mouse IgG1 isotype previously described and validated by us (9). The CYP17A1 mouse monoclonal antibody is an IgG2b isotype previously described by us (24). Validation of this antibody (CYP17A1 10-19-G6 IgG2bκ) was done by western blot with HAC15 cells (positive control) and HAC15 cells transduced with an all-in-one lentivirus CRIPR/Cas9 gRNA for the CYP17A1 (Genscript.com) demonstrating complete elimination of the band corresponding to the CYP17A1 (Fig 1B). Immunohistochemistry was done as previously described (9,25). Double immunofluorescence was performed from 5-micron sections of paraffin embedded samples of 7 normal human adrenals (from 3 young and 5 adult individuals). The slides were deparaffinized by progressive passage from xylene to graded alcohols and then subjected to antigen retrieval using a solution of EDTA 1 mM, diethanolamine 10 mM pH 9 in a steamer for 45 min. The slides were then blocked using Tris buffer 50 mM pH 7.4 with 5% normal goat serum and 0.5% SDS for 1 hr. A mixture of the two antibodies (CYP11B2-41-13 1/1,000 and CYP17A1-10-19-G6 1/4,000) were incubated in Tris Buffer 50 mM pH 7.4 with 5% goat serum and Tween-20 0.05% overnight. After washing 4 times with PBS, the slides were incubated with goat anti-mouse IgG1-Alexa Fluor 488 (JacksonImmunoresearch.com) and anti-mouse IgG2b-CF594 (Biotium.com) in the same buffer for 1 hr. After washing, the slides were incubated for a 30 min with DAPI, washed and mounted in DPX Mountant (Sigmaaldrich.com). Pictures were taken using a Nikon microscope with a fluorescent attachment. The pictures were pseudocolored using J-Image (https://imagej.nih.gov/ij/) and a composite prepared. Immunostaining was also done using a CYP17A1 rabbit polyclonal antibody (LifeSpanBiosciences.com LS-B14227-200). Using the adrenals from subjects having a continuous layer of CYP11B2 expressing zona glomerulosa cells, 4 different areas were randomly selected, and the number of total cells labeled with both CYP11B2 and CYP17A1 were counted and compared to the number of cells expressing only the CYP17A1. There was a very wide variation in the co-expression, from 0–33%, as well as cells where only one enzyme was expressed. In older patients 1–3 aldosterone-producing micronodules were similarly selected and counted as above.
Fig 1B:

Representative western blot analysis of CYP17A1 in 5–20μg of protein extracts from the HAC15 cell as a control and HAC15 cells stably transduced with an all-in-one lentivirus with CRIPR/Cas9 with a gRNA for the CYP17A1 enzyme showing the disappearance of the signal for the CYP17A1 enzyme.
HAC15 incubations:
HAC15 cells were cultured in DMEM/F12 media (Sigmaaldrich.com) with Fetalgro 2.5% (RMBIO.com) in 96-well plates until confluent. The media was changed to DMEM/F12 without serum and the cells incubated overnight. On day 2 the cells were incubated in serum-free media + angiotensin II 10 nM to induce the CYP11B2 expression, except for 8 wells that were incubated with media only as unstimulated controls. On day 3, cells in the 8 control wells continued without angiotensin II, cells in 8 wells continued with angiotensin II 10 nM alone, and the rest of the wells were incubated in media with angiotensin II plus trilostane 30 μM for 30 min, followed by the addition of cortisol or 11-deoxycortisol (0, 1, 3, 10 and 30 μM) and incubated overnight. The supernatant was then collected and 18-oxocortisol measured by ELISA as previously described using a highly specific anti-18-oxocortisol monoclonal antibody (26).
RESULTS
The biosynthetic pathway for 11-deoxycortisol conversion to 18-oxocortisol by the CYP11B2 enzyme is shown in Fig 1A. Fig 1B is a representative western blot analysis for validation of the CYP17A1 monoclonal antibody in protein extracts from HAC15 cells and HAC15 cells stably transduced with an all-in-one lentivirus with CRIPR/Cas9 with a gRNA for the CYP17A1 enzyme showing the disappearance of immunoreactivity for the CYP17A1 enzyme. Immunohistochemistry visualized by DAB staining showed different patterns in different parts of the same adrenal with areas of CYP11B2-expressing cells scattered in a discontinuous pattern in the subcapsular region, as well as in tight clusters that have been called aldosterone-producing cell clusters (APCC) (27), recently designated aldosterone-producing micronodules (APM) by a consensus panel (28). Fig 2A shows IHC immunoreactivity with the CYP11B2 antibody showing two CYP11B2- clusters, one small and one large APM (29). Fig 2B is an adjacent section of IHC with the CYP17A1 antibody which shows CYP17A1 positive cells mingled within the CYP11B2-stained area.
Fig 2:
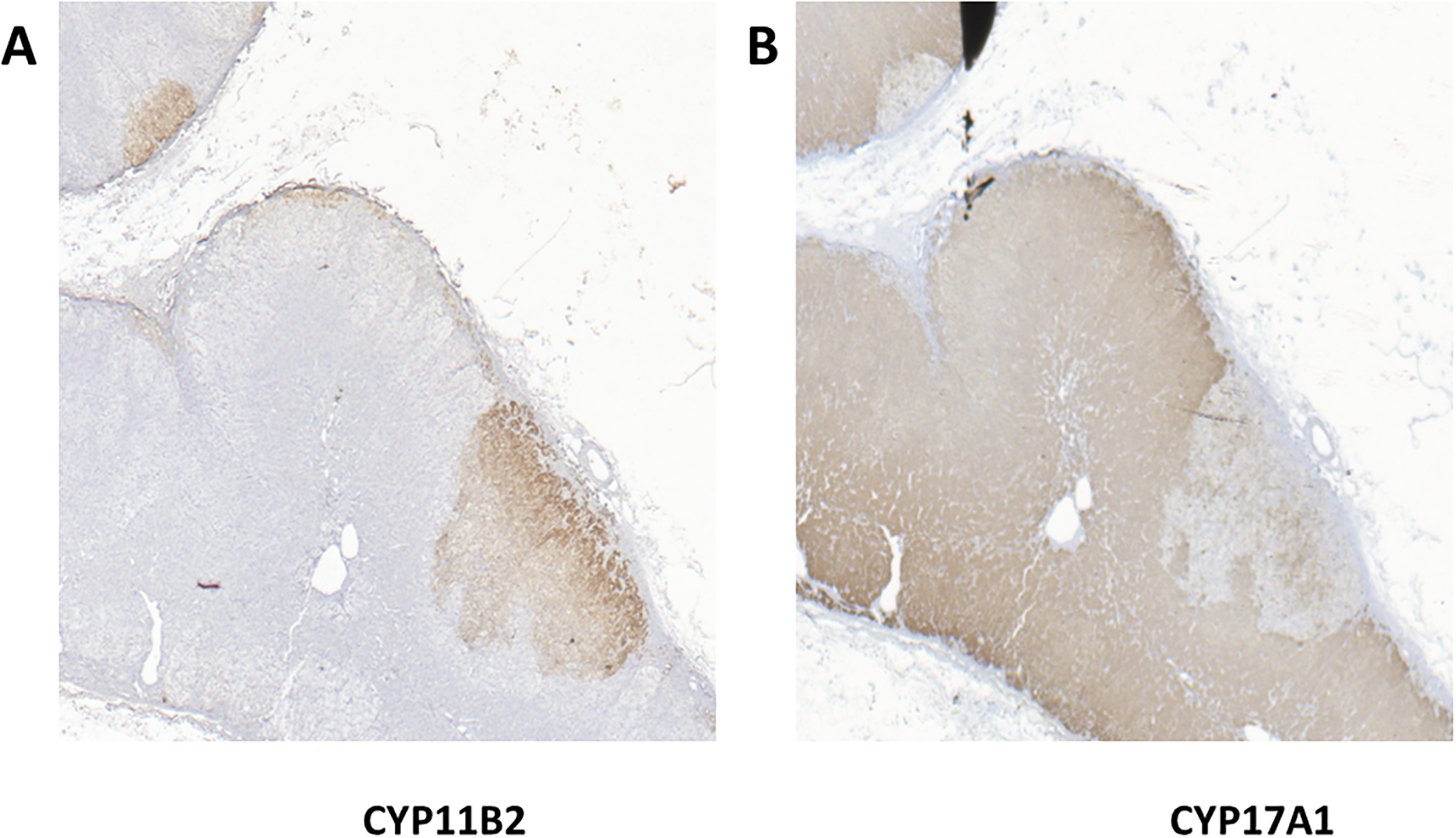
Adrenal immunohistochemistry for CYP11B2 and CYP17A1. Photomicrograph of two different aldosterone-producing micronodules incubated with the CYP11B2 (Panel A) or CYP17A1 antibodies (Panels B) suggesting some cells within the APM might be stained with both antibodies.
To determine whether the enzymes were in adjacent cells or co-expressed by the same cell, double immunofluorescent staining using the monoclonal and polyclonal antibodies was done. Fig 3 shows images of an adrenal from an adult showing an APM with DAPI (A), CYP11B2 (B), and CYP17A1 (C) monoclonal antibodies staining and the overlapping of the imagens (D) demonstrating scattered cells expressing either CYP11B2 or CYP17A1 intermixed in some areas of the APM. Fig 4 shows representative sections from 2 adrenals of young individuals in which the same CYP11B2 antibody and a different CYP17A1 polyclonal antibody were used for IHC. CYP11B2 immunoreactivity forms a continuous rim with CYP17A1 co-expression in some cells within the zona glomerulosa. The proportion of cells that co-expressed both enzymes varied significantly between 0–33% per high power field in different areas of the same adrenal. Cells with only CYP17A1 staining intermixed with the CYP11B2 stained cells varied between 0–26% per high power field within the same adrenal section. The adrenals were from individuals of different ages and sex, thus are too few to make statistical comparisons, nor were physiological modulators of adrenocortical function including sodium intake and stress known.
Fig 3:

Adrenal immunofluorescence for CYP11B2 and CYP17A1 using both monoclonal antibodies. Multiplex images for CYP11B2, CYP17A1 and DAPI for different areas of a zona glomerulosa aldosterone-producing micronodule from an adult individual with some cells co-staining with the CYP11B2 monoclonal and CYP17A1 polyclonal antibodies.
Fig 4:

Adrenal immunofluorescence for CYP11B2 and CYP17A1. Multiplex photomicrographs of different areas of zona glomerulosa from 2 individuals with continuous CYP11B2 zona glomerulosa staining (Panels A and B) with some cells co-staining with the CYP11B2 monoclonal and alternative CYP17A1 polyclonal antibodies. The areas show widely different co-expression patterns.
To determine the efficiency of 18-oxocortisol generation from exogenous precursors, we incubated HAC15 cells preincubated with vehicle or angiotensin II to stimulate CYP11B2 transcription and with the 3β-hydroxysteroid dehydrogenase-Δ4–5 isomerase inhibitor trilostane (30) 30 μM to inhibit the early pathway of steroidogenesis and then incubated with different concentrations of cortisol or 11-deoxycortisol. Fig 5 shows that the endogenous biosynthesis of 18-oxocortisol in cells stimulated with angiotensin II was greater than in cells only incubated with angiotensin II, trilostane and 1–30 μM cortisol. Cells incubated with the 11-deoxycortisol produced greater quantities of 18-oxocortisol than cells incubated with the same concentrations of cortisol.
Fig 5:

18-oxocortisol production of HAC15 cells incubated with vehicle (control) or 10 nM angiotensin II (AII) in the first two columns and angiotensin II plus 30μM trilostane and different concentrations of cortisol or 11-deoxycortisol.
DISCUSSION
18-Oxocortisol has been called hybrid steroid, as its synthesis requires the action of both CYP17A1 and CYP11B2. This is accomplished through the successive hydroxylation of the suboptimal substrate 11-deoxycortisol by the CYP11B2 enzyme to cortisol, 18-hydroxycortisol, and finally, through what is believed to be an additional hydroxylation to generate a theoretical derivative having a germinal diol at the 18 position, which undergoes immediate spontaneous dehydration to 18-oxocortisol (31) (13), as shown in Fig 1A.
The biosynthesis of 18-oxocortisol in the normal adrenal has been difficult to explain as traditional concepts have been that the two enzymes required for its synthesis were exclusively expressed in different adrenal zones with the CYP11B2 in the zona glomerulosa and the CYP17A1 in the zona fasciculata. To explain this synthesis cortisol was administered to dexamethasone-suppressed individuals with normal adrenal function and to adrenal insufficient individuals, and 18-oxocortisol measured in the urine (22). Dexamethasone-suppressed normal individuals administered cortisol 20 mg twice in one day had a significant increase in urine 18-oxocortisol compared to their control levels (22). However, this did not prove that this is the mechanism by which normal 18-oxocortisol is synthesized because the concentration of endogenous cortisol reaching the adrenal from the circulation is much smaller than that produced by the twice daily administration of a pulse of cortisol approximately 4 times greater than the daily production rate of cortisol. In addition, due to cortisol binding by CBG (corticosteroid binding globulin), the amount of free cortisol resulting from an oral bolus administration reaches a peak that would be significantly higher than the average concentration of physiologic free cortisol reaching the adrenal. Moreover, the adrenal expresses high levels of the p-glycoprotein or multiple drug resistant protein that more efficiently pumps more hydrophilic steroids including cortisol from the cell to the interstitial space (32); cortisol is preferentially excluded from adrenocortical cells, unless overwhelmed by high concentrations such as those that might be produced by surrounding cells. In HAC15 cells in which CYP11B2 was transcriptionally increased with angiotensin II and the early steroid pathway inhibited with trilostane, incubation with cortisol showed a dose responsive increase in the synthesis of 18-oxocortisol, but high concentrations of cortisol were required to match the rate of 18-oxocortisol produced by cells treated with angiotensin II alone, as shown in Fig 5. 11-Deoxcortisol, the initial substrate for CYP11B2, is less polar, thus less effectively excluded by the p-glycoprotein (33), and was transformed into 18-oxocortisol more efficiently than cortisol. Circulating 11-deoxycortisol is much lower than cortisol, thus is unlikely to be a significant substrate. Moreover, the circulation within the adrenal is centripetal and neither precursor is likely to reach high enough concentrations to overcome the influence of the p-glycoprotein pumping the steroids out of the cells (32).
Double immunofluorescence of normal adult human adrenal glands revealed that the majority of cells expressing the CYP11B2 in the subcapsular area were not stained by the CYP17A1 antibody (Fig 3A), however a small proportion of CYP11B2 positive cells (0–33%) in APM and in young individuals with a continuous CYP11B2 positive zona glomerulosa expressed both enzymes. These cells are most likely responsible for the synthesis of of 18-oxocortisol in the circulation with normal adrenals. The contribution of circulating steroid substrates to the adrenal zona glomerulosa cell is probably minimal, however a paracrine secretion of 11-deoxycortisol or cortisol remains a lesser possibility. One adrenal with continuous CYP11B2 staining had a much larger number of cells co-expressing the CYP11B2, figure 4, lower right panel. We speculate that this individual for whom we do not have clinical information might have been exposed to high stress, as we have shown that chronic ACTH increases the excretion of 18-oxocortisol and decreases the excretion of aldosterone (34)
Patients with an aldosterone-producing adenoma, especially those with KCNJ5 mutations (21), and patients with Familial Hyperaldosteronism 1 (16) and 3 produce large quantities of 18-oxocortisol (35,36). Co-expression of CYP11B2 and CYP17A1 has been shown to be frequent by immunofluorescence in aldosterone-producing adenomas (37).
In conclusion:
We demonstrated that the delineation between the zonas glomerulosa and fasciculata and the steroidogenic capacities of their cells in normal human adrenal glands are not as sharp as once assumed. 18-oxocortisol, a product of the enzymes CYP17A1 and CYP11B2 is produced in a limited number of human adrenal zona glomerulosa cells that co-express both enzymes and less likely in cells expressing only CYP11B2 acting upon 11-deoxycortisol diffusing from adjacent fasciculata cells expressing CYP17A1 embedded within the zona glomerulosa. Cortisol is highly unlikely to be a substrate for the CYP11B2 due to the strong expression of p-glycoprotein which preferentially extrudes it from these cells.
Supplementary Material
Highlights: In the normal human adult adrenal gland.
Circulating cortisol is an unlikely substrate for the synthesis of 18-oxocortisol
CYP11B2 and CYP17A1 are co-expressed in a few cells of the zona glomerulosa
Cells expressing only CYP17A1 may also be embedded within the zona glomerulosa
11-Deoxycortisol might be a paracrine substrate for the synthesis of 18-oxocortisol
Acknowledgements:
Research reported in this publication was supported by National Heart, Lung and Blood Institute grant R01 HL144847 (CEGS), the National Institute of General Medical Sciences grant U54 GM115428 (CEGS), Department of Veteran Affairs BX00468 (CEGS) and the National Institute of Diabetes and Digestive and Kidney grant R01 DK43140 (WER). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: Nothing to disclose.
Bibliography
- 1.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017;69(14):1811–1820. [DOI] [PubMed] [Google Scholar]
- 2.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The Unrecognized Prevalence of Primary Aldosteronism: A Cross-sectional Study. Ann Intern Med. 2020;173(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41–50. [DOI] [PubMed] [Google Scholar]
- 4.Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M, Endres S. Observational Study Mortality in Treated Primary Aldosteronism: The German Conn’s Registry. Hypertension. 2012;60(3):618–624. [DOI] [PubMed] [Google Scholar]
- 5.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr., The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 6.Boulkroun S, Fernandes-Rosa FL, Zennaro MC. Old and new genes in primary aldosteronism. Best Pract Res Clin Endocrinol Metab. 2020:101375. [DOI] [PubMed] [Google Scholar]
- 7.Cherradi N, Rossier MF, Vallotton MB, Timberg R, Friedberg I, Orly J, Wang XJ, Stocco DM, Capponi AM. Submitochondrial distribution of three key steroidogenic proteins (steroidogenic acute regulatory protein and cytochrome P450scc and 3β-hydroxysteroid dehydrogenase isomerase enzymes) upon stimulation by intracellular calcium in adrenal glomerulosa cells. J Biol Chem. 1997;272:7899–7907. [DOI] [PubMed] [Google Scholar]
- 8.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1–2):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi GP, Gioco F, Fassina A, Gomez-Sanchez CE. Normoaldosteronemic aldosterone-producing adenoma: immunochemical characterization and diagnostic implications. J Hypertens. 2015;33(12):2546–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulick S, Land M, Chu MD. 18-Oxocortisol, a naturally occuring mineralocorticoid agonist. Endocrinology. 1983;113:2320–2322. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Sanchez EP, Gomez-Sanchez CE, Smith JS, Ferris MW, Foecking MF. Receptor binding and biological activity of 18-hydroxycortisol. Endocrinology. 1984;115:462–466. [DOI] [PubMed] [Google Scholar]
- 13.Ulick S, Chu MD, Land M. Biosynthesis of 18-oxocortisol by aldosterone producing adrenal tissue. J Biol Chem. 1983;258:5498–5502. [PubMed] [Google Scholar]
- 14.Gomez-Sanchez CE, Gomez-Sanchez EP, Smith JS, Ferris MW, Foecking MF. Receptor binding and biological activity of 18-oxo-cortisol. Endocrinology. 1985;116:6–10. [DOI] [PubMed] [Google Scholar]
- 15.Hall CE, Gomez-Sanchez CE, Hungerford S. Hypertensive potency of 18-oxocortisol in the rat. Hypertension. 1986;8:317–322. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Sanchez CE, Montgomery M, Ganguly A, Holland OB, Gomez-Sanchez EP, Grim CE, Weinberger MH. Elevated urinary excretion of 18-oxocortisol in glucocorticoid suppressible aldosteronism. J Clin Endocrinol Metab. 1984;59:1022–1024. [DOI] [PubMed] [Google Scholar]
- 17.Gordon RD, Hamlet SM, Tunny TJ, Gomez-Sanchez CE, Jayasinghe LS. Distinguishing aldosterone-producing adenoma from other forms of hyperaldosteronism and lateralizing the tumour pre-operatively. Clinical & Experimental Pharmacology & Physiology. 1986;13(4):325–328. [DOI] [PubMed] [Google Scholar]
- 18.Mulatero P, Morra di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, Rabbia F, Terzolo M, Gomez-Sanchez EP, Gomez-Sanchez CE, Veglio F. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab. 2012;97(3):881–889. [DOI] [PubMed] [Google Scholar]
- 19.Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Takase K, Seiji K, Sasamoto H, Honma S, Okuyama M, Yamashita K, Gomez-Sanchez CE, Rainey WE, Arai Y, Sasano H, Nakamura Y, Ito S. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65(5):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulick S, Blumenfeld JD, Atlas SA, Wang JZ, Vaughan ED. The unique steroidogenesis of the aldosteronoma in the differential diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 1993;76:873–878. [DOI] [PubMed] [Google Scholar]
- 21.Tezuka Y, Yamazaki Y, Kitada M, Morimoto R, Kudo M, Seiji K, Takase K, Kawasaki Y, Mitsuzuka K, Ito A, Nishikawa J, Asai N, Nakamura Y, Gomez-Sanchez CE, Ito S, Dezawa M, Sasano H, Satoh F. 18-Oxocortisol Synthesis in Aldosterone-Producing Adrenocortical Adenoma and Significance of KCNJ5 Mutation Status. Hypertension. 2019;73(6):1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freel EM, Shakerdi LA, Friel EC, Wallace AM, Davies E, Fraser R, Connell JM. Studies on the origin of circulating 18-hydroxycortisol and 18-oxocortisol in normal human subjects. J Clin Endocrinol Metab. 2004;89(9):4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimoto K, Seki T, Hayashi Y, Mikami S, Al-Eyd G, Nakagawa K, Morita S, Kosaka T, Oya M, Mitani F, Suematsu M, Kabe Y, Mukai K. Human Adrenocortical Remodeling Leading to Aldosterone-Producing Cell Cluster Generation. Int J Endocrinol. 2016;2016:7834356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida T, Nishimoto K, Fukumura Y, Asahina M, Goto H, Kawano Y, Shimizu F, Tsujimura A, Seki T, Mukai K, Kabe Y, Suematsu M, Gomez-Sanchez CE, Yao T, Horie S, Watada H. Disorganized Steroidogenesis in Adrenocortical Carcinoma, a Case Study. Endocr Pathol. 2017;28(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura Y, Maekawa T, Felizola SJ, Satoh F, Qi X, Velarde-Miranda C, Plonczynski MW, Ise K, Kikuchi K, Rainey WE, Gomez-Sanchez EP, Gomez-Sanchez CE, Sasano H. Adrenal CYP11B1/2 expression in primary aldosteronism: Immunohistochemical analysis using novel monoclonal antibodies. Mol Cell Endocrinol. 2014;392(1–2):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morra di Cella S, Veglio F, Mulatero P, Christensen V, Aycock K, Zhu Z, Gomez-Sanchez EP, Gomez-Sanchez CE. A time-resolved fluoroimmunoassay for 18-oxocortisol and 18-hydroxycortisol. Development of a monoclonal antibody to 18-oxocortisol. J Steroid Biochem Mol Biol. 2002;82(1):83–88. [DOI] [PubMed] [Google Scholar]
- 27.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95(5):2296–2305. [DOI] [PubMed] [Google Scholar]
- 28.Williams TA, Gomez-Sanchez CE, Rainey WE, Giordano TJ, Lam AK, Marker A, Mete O, Yamazaki Y, Zerbini MCN, Beuschlein F, Satoh F, Burrello J, Schneider H, Lenders JWM, Mulatero P, Castellano I, Knosel T, Papotti M, Saeger W, Sasano H, Reincke M. International Histopathology Consensus for Unilateral Primary Aldosteronism. J Clin Endocrinol Metab. 2021;106(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, Plouin PF, Lalli E, Jeunemaitre X, Benecke A, Meatchi T, Zennaro MC. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010;56(5):885–892. [DOI] [PubMed] [Google Scholar]
- 30.Thomas JL, Mack VL, Glow JA, Moshkelani D, Terrell JR, Bucholtz KM. Structure/function of the inhibition of human 3beta-hydroxysteroid dehydrogenase type 1 and type 2 by trilostane. J Steroid Biochem Mol Biol. 2008;111(1–2):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kojima I, Inano H, Tamaoki B. The final steps of aldosterone biosynthesis is catalyzed by an NADPH-dependent and molecular oxygen-requiring enzyme. Biochem Biophys Res Commun. 1982;106:617–624. [DOI] [PubMed] [Google Scholar]
- 32.Bello-Reuss E, Ernest S, Holland OB, Hellmich MR. Role of multidrug resistance P-glycoprotein in the secretion of aldosterone by human adrenal NCI-H295 cells. American Journal of Physiology - Cell Physiology. 2000;278(6):1256–1265. [DOI] [PubMed] [Google Scholar]
- 33.Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R. Human p-glycoprotein transports cortisol, aldosterone and dexamethasone, but not progesterone. J Biol Chem. 1992;267:24248–24252. [PubMed] [Google Scholar]
- 34.Gomez-Sanchez CE, Clore JN, Estep HL, Watlington CO. Effect of chronic adrenocorticotropin stimulation on the excretion of 18-hydroxycortisol and 18-oxocortisol. J Clin Endocrinol Metab. 1988;67:322–326. [DOI] [PubMed] [Google Scholar]
- 35.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93(8):3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura Y, Kitada M, Satoh F, Maekawa T, Morimoto R, Yamazaki Y, Ise K, Gomez-Sanchez CE, Ito S, Arai Y, Dezawa M, Sasano H. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17. Mol Cell Endocrinol. 2016;422:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


