Abstract
Purpose
The pediatric population, especially under-five children, is highly susceptible to malaria and accounts for 76 % of global malaria deaths according to the World Malaria Report 2022. The purpose of this manuscript is to discuss the various factors involved in the susceptibility of the pediatric population to Malaria and the importance of this age group for malaria elimination.
Methodology
Data on pediatric malaria epidemiology that includes prevalence, risk factors, immune factors, socioeconomic factors, control methods, etc. were extracted from published literature using PubMed and Google Scholar. This data was further correlated with malaria incidence data from the World Health Organization (WHO) and the National Center for Vector Borne Diseases Control (NCVBDC)
Results
The younger age group is vulnerable to severe malaria due to an immature immune system. The risk of infection and clinical disease increases after the waning of maternal immunity. In the initial years of life, the developing brain is more susceptible to malaria infection and its after-effects. The pediatric population may act as a malaria transmission reservoir due to parasite density and asymptomatic infections. WHO recommended RTS,S/AS01 has limitations and may not be applicable in all settings to propel malaria elimination.
Conclusion
The diagnosis of malaria is based on clinical suspicion and confirmed with microscopy and/or rapid diagnostic testing. The school-age pediatric population serves as a transmission reservoir in the form of asymptomatic malaria since they have acquired some immunity due to exposure in early childhood. Targeting the hidden reservoir in the pediatric population and protecting this vulnerable group will be essential for malaria elimination from the countries targeting elimination.
Keywords: Pediatric malaria, Malaria transmission reservoir, Malaria elimination, Asymptomatic malaria, Malaria pathogenesis
Introduction
The World Health Organization (WHO) estimated 247 million malaria cases and 61,900 deaths globally in 2021. The Global burden of Malaria increased in 2021 compared to 2020 and most of the increase is coming from the countries in WHO African Region.1 This increase is likely attributed to disruptions to services during the COVID-19 pandemic. India accounted for the majority of malaria cases in the Southeast Asian region.2 Although it is a preventable disease but the infection is life-threatning in children under 5 years of age.3 Out of total deaths due to Malaria, 76 % of the deaths are estimated to be in children under 5 years of age.1 Pediatric plasmodium infections have varying clinical presentations ranging from asymptomatic to uncomplicated, severe, and cerebral malaria. It also affects children's quality of life.4 Symptomatic malaria in neonates and young infants leads to non-specific clinical manifestations that are difficult to discern from other conditions, such as bacterial sepsis. Malaria affects a child's physical and mental growth and also affects post-infection life of the child.3 Due to multiple exposures to malaria and the development of naturally acquired immunity, older children may harbor parasites without clinical symptoms and act as a reservoir for malaria transmission.5 This article highlights the various factors, including host and environmental, that play important role in pediatric malaria. It provides avenues of basic and applied research for a better understanding of malaria vulnerability in the pediatric population. The significance of pediatric population for malaria control and elimination through targeted strategies is emphasized.
Methodology
A comprehensive search strategy was devised, incorporating a variety of keywords and MeSH terms such as 'malaria,' 'Pv,' 'Pf,' 'prevalence,' 'risk factors,' 'predictors,' 'immune factors,' and 'control measures.' Boolean operators were skillfully applied in searches conducted on both PubMed and Google Scholar. The search results from both platforms were collated into a single dataset after eliminating duplicates. Subsequently, articles were meticulously screened based on their titles, abstracts, and full texts, and the relevant data were recorded in a Microsoft Excel spreadsheet. This data was then combined with malaria incidence data obtained from the National Central of Vector Borne Diseases Control (NCVBDC), New Delhi, under the Government of India, and the data from the World Malaria Report by WHO. The merged dataset was subjected to thorough analysis using Microsoft Excel, and the findings were visually presented through figures created with MS PowerPoint software.
Global burden of malaria in the pediatric age group
Of the different age groups defined by WHO, children up to 14 years of age are considered as the pediatric group in this manuscript.6 Children under five years old are the major contributors to malaria deaths. There is a reduction in the proportion of total deaths in the pediatric malaria group from 87 % in 2000 to 77 % in 2020.2
Severe malaria is concentrated in under 5 pediatric groups, and the number of severe cases increases with greater transmission intensity.2 Commonly, severe malaria is caused by P. falciparum infection but P. vivax also presents with severe disease in children.7 P. falciparum is the leading cause of child mortality due to infectious diseases.8 Most of the studies on pediatric malaria are from the African region. In Burkina Faso, a high burden of pediatric malaria was reported with two-thirds out of 6102 children included in the study being found positive for malaria.9 The malaria parasite prevalence increases with age and is found to be the highest at nine years.10 However the hospitalization requirement, severe malaria, and malaria mortality rate increase in children from 6 months to 4 years of age.10 In a study in Rwanda, the prevalence of malaria in children from 6 months to 14 years was found to be 14 % and was higher among children 5–9 years of age.11 74 % of malaria hospitalization was in under 5-year children, and severe anemia was the most common presentation in Uganda.12
The burden of pediatric malaria in India
Out of the total 5 million malaria cases and 35,000 deaths estimated in the Southeast Asia region, 83 % of cases and 82 % of deaths were estimated to be from India.2 Age-wise incidence data of malaria collated by the NCVBDCP is not available in the public domain. However, studies done in malaria-endemic regions of India have indicated a high burden in this age group. In one of the highest malaria-endemic states, Chhattisgarh, most deaths in children 1–14 years of age were due to malaria in 2013–14.13 61.5 % of malaria cases were in the pediatric population in 2017, in the district Bastar, Chhattisgarh.14 In Dhindori district, Madhya Pradesh, during the outbreak investigation about 67 % of malaria positive were from the pediatric age group of <15 years with the positivity rate highest in 1–4 years followed by 4–8 years.15 In a therapeutic efficacy study by Singh et al. 2012–2014 in Madhya Pradesh, about 90 % of enroled participants were under the age of 15, with the highest 46 % from 5 to 8 years, followed by 32.5 % from 9 to 14 years.16 In a study in the forested area of central India in 2013, about 82 % of the malaria positives were found from the age group below 15 years.17 Four decades of data analysis in the district Kheda, Gujrat revealed that about 48 % of P. falciparum and 52 % of P. vivax cases were reported in the 0–5 years age group.18 Kumar et al. estimated 3.8 million cases and 0.2 million deaths in India during 2015–16. About 27 % and 15 % of the deaths were estimated in < 5 and 6–14 age groups, respectively.18
Malaria pathophysiology and clinical manifestations in children
Malaria symptoms in children are non-specific and often mimic other childhood illnesses including pneumonia, gastroenteritis, and meningitis/encephalitis.8 There are no characteristic features of severe malaria in children. The clinical manifestations of malaria depend on the parasite, as well as host factors.8 Pediatric malaria cases were reported to have more gastrointestinal symptoms than adults, which may lead to clinical misdiagnosis.19 Fever is the key symptom in pediatric malaria with more likeliness of high fever (>40 °C) leading to convulsions. Malaria pathogenesis involves inflammation, anemia, and organ damage.20 Children below 5 years are more susceptible to severe malaria leading to organ damage.21 Malaria and anemia correlate with the adverse development index of children.22 Severe anemia correlates with significant morbidities and mortalities in pediatric malaria.23 The Hemoglobin (Hb) levels, Hematocrit levels, and RBC counts varied in asymptomatic as compared to symptomatic children in a field study in Burkina Faso, and the difference was more pronounced in young children.22 The asymptomatic children also have lower Hb levels, lymphocyte count, platelet count, and higher monocyte count compared to uninfected children.22 RBC sequestration is central to severe malaria pathogenesis. Acute kidney injury is one of the severe complications of P. falciparum infection in children.24 It may lead to mortality with disseminated intravascular coagulation (DIC), jaundice and parasite density as important factors for the same.24
Malnutrition and malaria
Malnutrition is one of the crucial factors associated with malaria severity in children.25 Blood transfusions were found to be more frequent in underweight children in a cross-sectional study at Ogun State, Nigeria.23 WHO has recommended artemisinin-based combination therapy as the first-line treatment for uncomplicated malaria globally. But, studies on the pharmacokinetics of artemisinin in the pediatric population are scarce. Understanding the pharmacodynamics and pharmacokinetics of Artemisinin Combination therapies (ACTs) in malnourished children needs to be improved for optimal antimalarial treatment for the vulnerable undernourished children living in hard-to-reach malaria-endemic areas.25 This should be one of the priority areas for the effective and safe use of artemisinin-based combination therapy in the pediatric population.
Pediatric immune system and malaria
Both congenital and neonatal malaria infections are rare. Newborns up to six months are thought to be protected from clinical malaria due to the inhibitory effect of maternal IgG, breastfeeding, and fetal hemoglobin on parasite growth.26 The risk of malaria infection and clinical disease rises after 6–9 months of age when the waning of maternal antibodies happens. Young children are most susceptible to malaria due to a lack of acquired functional immunity, which older children and adults develop due to multiple exposures.2 Clinical immunity to P. vivax malaria develops rapidly compared to P. falciparum.26 By the age of nine years, children acquire clinical immunity to P. vivax, but the acquisition of immunity to P. falciparum symptomatic infection remains incomplete.26
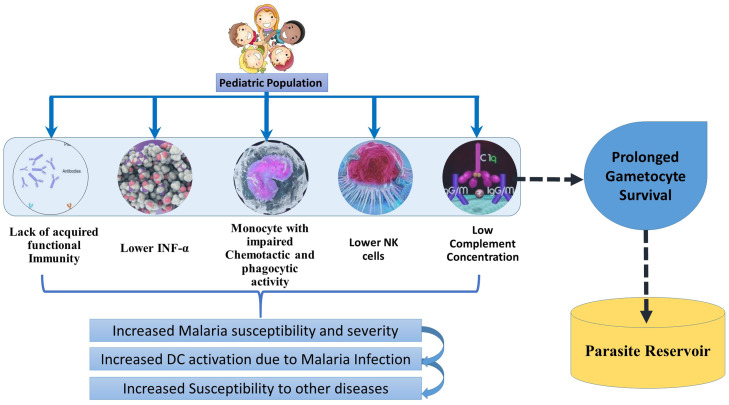
Neonate's immune system is immature due to impaired adaptive immune response, including B cell function, antibody production, and T cell response bias towards Th2 type.27 In high transmission settings Malaria negatively affects Dendritic Cell (DC) activation in children. The DC dysfunction persists even after malaria resolution, increasing the susceptibility to other diseases.28 Monocytes play an important role in plasmodium infection with increased count and pro-inflammatory cytokine production.29 Monocytes in neonates have impaired chemotactic and phagocytic activity compared to adults. It remains significantly low up to the age of 5–6 years.27 Natural Killer T cells play an important role in the immune response to pre-erythrocyte stages. The highest NK cells are found in cord blood, decline significantly in the first postnatal days, and progressive decline up to the 5th year of age. NK cells kill the pre-erythrocyte parasite by antibody-dependent cell-mediated cytotoxicity. It kills intrahepatic parasites by secreting type 1 interferon and INF-gamma.27
Antibodies are involved in killing plasmodium gametocytes through complement-mediated lysis.30 The complement component's serum concentration positively correlates with the individual's age.31 Lower complement concentration may lead to prolonged gametocyte survival in the lower age group.32 Reports from different continents showed contrasting cytokine production in the pediatric malaria population.32 Pro-inflammatory cytokines play a role in parasite clearance, but their high levels are also implicated in severe malaria.32 The pro-inflammatory cytokine level was found to be much higher in young children and it may be one of the reasons for malaria severity at a younger age.33 Type 1 interferons inhibit liver and blood stages of parasite infection. INFα production from PBMC from under five years children was significantly lower than in adults.27 The various immune components that increase the susceptibility and severity in the pediatric population are depicted in Fig. 1.
Fig. 1.
Immune factors in Pediatric Malaria: Due to lack of acquired functional immunity, Lower INF-α, impaired monocytes, Low NK cells and complement concentration, malaria susceptibility is increased in children below 5 years of age. Because of low complement concentration, the antibody-based killing of gametocytes gets hampered in children, including in older children. They can act as parasite reservoirs due to gametocyte survival for extended periods.
Neurological development and malaria infection
Under-five age is the period when rapid brain growth and physiologic changes in the blood-brain barrier occur. The developing brain in children is very sensitive to traumatic, ischemic, noxious, and inflammatory snubs. Plasmodium infection at the time of rapid brain development may lead to various aftereffects. The number of synaptic connections increases until pre-school years up to a level twice the adult brain.34 Due to elevated complement activation, synaptic pruning dysregulation in malaria infection may lead to neurocognitive impairment.34 Plasmodium infection is an independent risk factor for low cognitive development in children.35 Plasmodium exposure in early childhood may cause deficits in attention, memory, visuospatial skills, language, and executive functions.35 Brain swelling is more severe in cerebral malaria in children than in adults.36 Pathological patterns of cerebral malaria are different in children and adults. The risk of epilepsy and other neurobehavioral sequelae increases in children with cerebral malaria.37 In pediatric fatal cases, brain volume increases, leading to augmented cerebral pressure and brainstem herniation.38 P. vivax infection also affects the neurological system.39 P. vivax infection was reported to cause multifocal hemorrhagic infarcts in the brain of a 10-year-old boy in New Delhi, India.39
Biomarkers for severe pediatric malaria
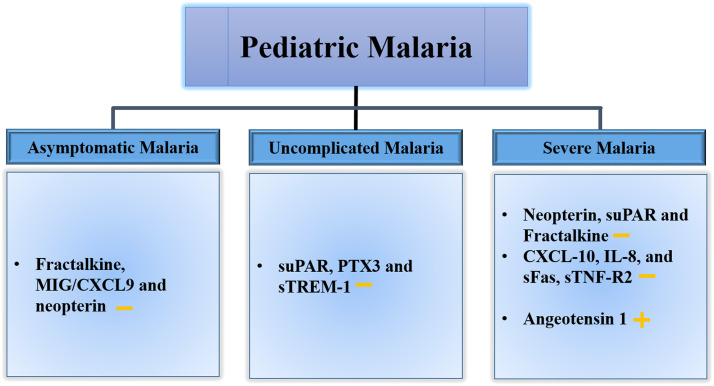
The levels of biomarkers of immune responses change with the clinical manifestation of malaria and these may help understand the clinical syndrome in children.40 Currently, the diagnosis of cerebral malaria in children depends on clinical indicators. However distinct profiles of cytokines and chemokines, including CXCL-10, IL-8, and soluble fas (sFas), sTNF-R2 have been reported in cerebral malaria.41 Endothelial-based biomarker Angiotensin 1 was found to best distinguish between uncomplicated malaria and cerebral malaria.42 Plasma Pf histidine-rich protein (hrp) 2 concentrations are higher in cerebral malaria and are associated with coma depth and mortality in children.43 Cell-free DNA in the plasma of infected children increases with disease severity and may act as an important biomarker for disease severity and fatal outcomes.44 Fractalkine, Monokine induced by gamma (MIG)/ (CXCL9), and Neopterin were found to be good indicators for asymptomatic malaria, while soluble urokinase-type Plasminogen Activator (suPAR), Pentraxin 3 (PTX3) and soluble triggering receptor expressed on myeloid cells 1 (sTREM-1) combination are indicative of uncomplicated malaria. Neopterin, soluble urokinase-type Plasminogen Activator (suPAR) and Fractalkine were found to be associated with severe malaria.40 The reported biomarkers for different clinical presentations of pediatric malaria are shown in Fig. 2.
Fig. 2.
The biomarkers for different clinical presentations of pediatric malaria: Potential biomarkers have been identified for different clinical presentations. Fractalkine, Monokine induced by gamma (MIG)/ (CXCL9), and Neopterin were found to be good indicators of asymptomatic infections. A combination of three biomarkers, soluble urokinase-type Plasminogen Activator (suPAR), Pentraxin 3 (PTX3), and soluble triggering receptor expressed on myeloid cells 1 (sTREM-1), is a good indicator of uncomplicated plasmodium infection. Along with other biomarkers Neopterin, suPAR, and Fractalkine are found to be good predictors of severe malaria. + represents increase and – represents decrease.
Children as plasmodium reservoir
Parasite densities and the likelihood of passing the infection to mosquitoes were found to be highest in children.45 The hospitalization risk and the risk of severe malaria decrease after four years of age, but the parasite prevalence remains high.11 The pediatric population acts as an infection reservoir at the beginning of the dry season due to the slow clearance of infection. Children contribute more to the parasite reservoir in high transmission settings than in low transmission.5,46
According to studies, School-age children have a higher risk of infection, including asymptomatic infection. Active surveillance is more successful in detecting pediatric malaria cases compared to facility-based passive surveillance.47 The use of personal protection measures like bed nets is less often.45 They are brought less frequently to hospitals for treatment and more often to unreliable treatment sources.45 In a study in central India, 77 % of asymptomatic malaria infections were found in school-age children (6–14 years). A good understanding of the transmission source and infection risk is required to adequately progress toward malaria elimination.
Socio-demographic factors
The malaria prevalence is higher in rural households than in urban, ones and houses constructed from poorly suited materials in Rawanda.11 It was found to be associated with the gender of the child, the number of household members, mosquito bed nets for sleeping, Insecticide Residual Spray (IRS), the location source of drinking water, and the age of the head of the household.11 Older children, poor households, distance from health facilities <5 km and localities with an inadequate number of nurses are major risk factors for asymptomatic malaria in Burkina Faso.48 Practicing safe living habits like decreasing outdoor activity during peak mosquito-biting hours can help reduce the malaria burden.49 LLIN usage significantly affects malaria prevalence among school-age children. It is affected by socioeconomic factors (access to electricity, the mother's occupation, and household size).50 Promoting and improving access to primary education for women can be crucial aspects of the future of malaria and other pediatric disease control. One extra year of maternal education in Uganda was responsible for a 7.5 % reduced risk of malaria infection in children. It also showed a positive association with LLIN usage.51 The 6–14 age group is least likely to benefit from malaria control interventions, like bed nets and prompt diagnosis and treatment, due to low LLIN usage and seeking treatment from non-standard sources, more so in resource-poor settings.52 They are at increased risk of malaria vector bites due to their sleep and wake-up time. They tend to sleep after 9 p.m. and get up before 6 a.m.53,54
Preventive malaria treatment in school-aged children effectively reduces P. falciparum prevalence, anemia, and subsequent clinical malaria risk.55 Schools provide easy access to this age group to deliver strategies targeting this age group. School-based health packages may be deployed that can be more cost-effective.56 Improving disease knowledge, intervention coverage, and socioeconomic conditions will be necessary for malaria control.9
Significance and strategies for targeting pediatric malaria
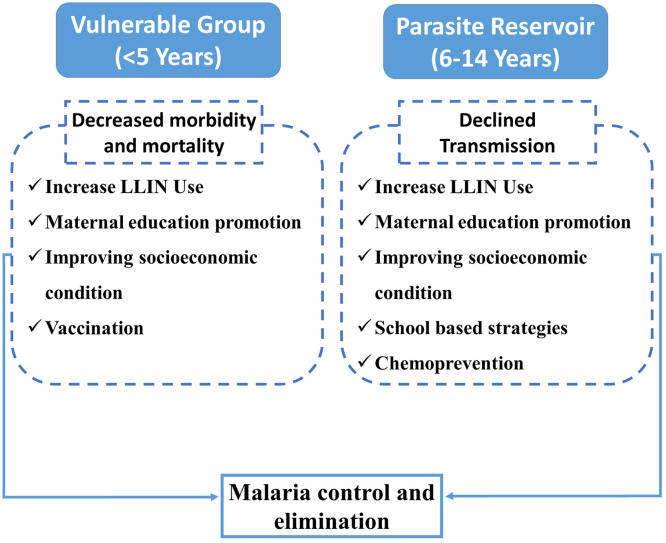
Targeting the pediatric malaria reservoir and protecting the vulnerable group is required for achieving the goal of malaria elimination (Fig. 3). In highly endemic countries, malaria may contribute up to 10 % of all mortality in the under-five age group.57 Malaria interventions targeting malaria mortality should focus on the specific age group that is highly sensitive to malaria mortality (Fig. 3). LLIN usage has been reported to decrease malaria morbidity and mortality. It decreases malaria deaths by about 50 % in children below 5 years of age.58 In one study focused on the characteristics of sub-patent malaria in a pre-elimination setting, it was found that about 27 % of sub-patent malaria is reported to have detectable gametocytaemia, suggesting the importance of active and reactive case detection using molecular methods.59 Focal screening using polymerase chain reaction to find the hidden parasite reservoir in the asymptomatic individuals, especially the school-age group, may effectively interrupt transmission and accelerate the progress toward malaria elimination. Older children need more attention in previously malaria-endemic areas due to loss of naturally acquired immunity and non-regular use of LLINs by the age group.60 With decreased transmission, the mean age of malaria-related hospital admission increases towards older children.2 Strategies are required to target older children without declining attention to under-five children. Strategies focused on school-age children and adults may be useful in achieving the target of pre-elimination.61 Fig. 3 summarizes the interventions required for different pediatric age groups from a malaria elimination perspective.
Fig. 3.
Age-group specific interventions for sustained Malaria control and elimination: Protecting the under-five vulnerable group and targeting the parasite reservoir in older children with particular interventions will help achieve the target of malaria control and elimination.
Recent advances in pediatric malaria prevention
WHO recommended RTS,S/AS01 malaria vaccine which induces antibodies against the circumsporozoite protein (CSP) protein of the parasite. It induces protection from infection and severe disease but does not prevent disease transmission. It is recommended for children living in moderate to high transmission settings, mostly in African countries, and is an intervention to prevent clinical and severe malaria. The phase III trial conducted in seven sub-Saharan countries showed modest clinical efficacy of 51 % in the 5–17 months’ age group after 12 months follow up after 3rd dose which reduced to 26 % after 48 months and 39 % among those who got 4 doses. WHO did not recommend it for 6–12 weeks infants as the efficacy was low. The pilot implementation trial conducted in three sub-Saharan countries in real-life settings, 40 % reduction in malaria episodes and one death prevented per 200 vaccines prompted WHO to recommend the vaccine in moderate to high transmission settings.62 RTS,S/AS01 is recommended by WHO to be used in sub-Saharan Africa with moderate to high transmission settings. It may not be useful for implementation in India, as India is a low-transmission setting.63 Seasonal vaccination with the RTS,S/AS01 was found to be almost equally protective to four annual courses of chemoprevention. But the combination of both was superior to chemoprevention and vaccination alone. The protective efficacy of combination against clinical malaria is 62.8 % and 59.6 % higher compared to chemoprevention and vaccination alone respectively.64 R21/Matrix-M is another novel pre-erythrocytic malaria vaccine that has shown an efficacy of more than 75 % in children and has been approved in Ghana for use in children 5–36 months of age.65,66 Recently radiation-attenuated Plasmodium falciparum (Pf) sporozoites (SPZ) in PfSPZ Vaccine has been found to have an efficacy of more than 75 % against the controlled human malaria infection.67
Future aspects for malaria control and elimination
Specific tailored interventions are required for the different transmission intensities. Implementation research is required to understand the effectiveness of interventions in different transmission settings and targeted interventions for specific age groups. Research studies focused on the pediatric population are needed on the strategies to tackle the malaria reservoir in school-age children. Focusing on malaria in children allows for controlling malaria transmission by strategies focused on older children, and malaria deaths by focusing on children under 5 years of age. A highly efficacious vaccine is required that can be administered to children under 5 years as well as older children for applicability in low transmission as well as high transmission settings. More research is required to understand the effectiveness of vaccines in different transmission settings. LLINs with more than one insecticide to control the malaria vector with developing insecticide resistance. Highly sensitive tools that can be deployed in the field settings are required to detect and treat asymptomatic malaria. Research is required to understand the pharmacodynamics and pharmacokinetics of Artemisinin Combination therapies (ACTs) in malnourished children.
Conclusion
Pediatric malaria includes different age groups with different susceptibilities and clinical profiles after Plasmodium infection. The under-five age group is most susceptible to malaria morbidity and mortality, due to an immature immune system. Child development in the context of malaria exposure needs to update the national programmes about this under-recognized malaria burden. Prognostic biomarkers for severe malaria may be very effective for saving children under 5 years of age from malaria mortality. Education and socioeconomic conditions are the two most important factors for malaria in children. The age group 6–14 years is less susceptible but harbours a high asymptomatic malaria parasite load. Targeting the asymptomatic reservoir in children may prove to be helpful in accelerating the malaria elimination drive. Increasing LLIN usage, promoting girls/women education, and improving the family's socioeconomic situation will help decrease pediatric malaria morbidity and mortality. An efficacious vaccine, highly sensitive tools and LLINs with more than one insecticide are required for elimination of Malaria.
Declaration of Competing Interest
All the authors agree with the content of the manuscript and give their consent to submit and consent has also been obtained from authorities in the institutes. Authors declare no conflict of Interest.
Acknowledgment
The authors are thankful to Dr. CP Yadav, Scientist C, ICMR-National Institute of Malaria Research for helping in the figures for the manuscript. We are thankful to ICMR- national Institute of Malaria Research for the logistic support.
Contributor Information
Ritesh Ranjha, Email: dr.ranjha01@gmail.com.
Praveen K. Bharti, Email: saprapbs@yahoo.co.in.
References
- 1.World Health Organization; Geneva: 2022. World Malaria Report 2022. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 2.World Health Organization; Geneva: 2021. World Malaria Report 2021. Licence:CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 3.Ashley E.A., Poespoprodjo J.R. Treatment and prevention of malaria in children. Lancet Child Adolesc Health. 2020;4(10):775–789. doi: 10.1016/S2352-4642(20)30127-9. [DOI] [PubMed] [Google Scholar]
- 4.Lufungulo Bahati Y., Delanghe J., Bisimwa Balaluka G., Sadiki Kishabongo A., Philippe J. Asymptomatic submicroscopic plasmodium infection is highly prevalent and is associated with anemia in children younger than 5 Years in South Kivu/Democratic Republic of Congo. Am J Trop Med Hyg. 2020;102(5):1048–1055. doi: 10.4269/ajtmh.19-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranjha R., Dutta G.D.P., Gitte S.V. School-age children as asymptomatic malaria reservoir in tribal villages of Bastar Region, Chhattisgarh. Indian Pediatr. 2019;56(10):873–875. [PubMed] [Google Scholar]
- 6.World Health Organization; 2023. Regional Health Observatory - South East Asia. [Google Scholar]
- 7.Nema S., Ghanghoria P., Bharti P.K. Malaria elimination in India: bridging the gap between control and elimination. Indian Pediatr. 2020;57(7):613–617. doi: 10.1007/s13312-020-1888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher R.F., Spinelli E. Malaria in children. Mediterr J Hematol Infect Dis. 2012;4(1) doi: 10.4084/MJHID.2012.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samadoulougou S., Maheu-Giroux M., Kirakoya-Samadoulougou F., De Keukeleire M., Castro M.C., Robert A. Multilevel and geo-statistical modeling of malaria risk in children of Burkina Faso. Parasit Vectors. 2014;7:350. doi: 10.1186/1756-3305-7-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamau A., Mtanje G., Mataza C., et al. Malaria infection, disease and mortality among children and adults on the coast of Kenya. Malar J. 2020;19(1):210. doi: 10.1186/s12936-020-03286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habyarimana F., Ramroop S. Prevalence and risk factors associated with malaria among children aged six months to 14 years old in Rwanda: evidence from 2017 Rwanda malaria indicator survey. Int J Environ Res Public Health. 2020;17(21):7595. doi: 10.3390/ijerph17217975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mpimbaza A., Walemwa R., Kapisi J., et al. The age-specific incidence of hospitalized paediatric malaria in Uganda. BMC Infect Dis. 2020;20(1):503. doi: 10.1186/s12879-020-05215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Community based monitoring of health in Chhattisgarh 2013-14: State Health Resource Center, Raipur, Chhattisgarh. https://shsrc.org/index.php/studies-2/.
- 14.Krishna S., Yadav A., Bhandari S., et al. Prevalence of malaria in two highly endemic Community Health Centers in the Bastar district, Chhattisgarh showing mixed infections with Plasmodium species. Sci Rep. 2017;7(1):16860. doi: 10.1038/s41598-017-16974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh N., Shukla M.M., Chand G., et al. Epidemic of Plasmodium falciparum malaria in Central India, an area where chloroquine has been replaced by artemisinin-based combination therapy. Trans R Soc Trop Med Hyg. 2011;105(3):133–139. doi: 10.1016/j.trstmh.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Mishra S., Bharti P.K., Shukla M.M., et al. Clinical and molecular monitoring of Plasmodium falciparum resistance to antimalarial drug (artesunate+sulphadoxine-pyrimethamine) in two highly malarious district of Madhya Pradesh, Central India from 2012-2014. Pathog Glob Health. 2017;111(4):186–194. doi: 10.1080/20477724.2017.1331875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N., Chand S.K., Bharti P.K., et al. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One. 2013;8(9):e73730. doi: 10.1371/journal.pone.0073730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baharia R.K., Yadav C.P., Sharma A. Four decades of epidemiological data reveal trajectories towards malaria elimination in Kheda district (Gujarat), western part of India. BMJ Glob Health. 2021;6(12) doi: 10.1136/bmjgh-2021-005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman-Yassen A.E., Mony V.K., Arguin P.M., Daily J.P. Higher rates of misdiagnosis in pediatric patients versus adults hospitalized with imported malaria. Pediatr Emerg Care. 2016;32(4):227–231. doi: 10.1097/PEC.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohee L.M., Laufer M.K. Malaria in children. Pediatr Clin N Am. 2017;64(4):851–866. doi: 10.1016/j.pcl.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyburn H., Mbatia R., Drakeley C., et al. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293(12):1461–1470. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- 22.Gansane A., Ouedraogo I.N., Henry N.B., et al. Variation in haematological parameters in children less than five years of age with asymptomatic Plasmodium infection: implication for malaria field studies. Mem Inst Oswaldo Cruz. 2013;108(5):644–650. doi: 10.1590/0074-0276108052013017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogunlesi T., Fetuga B., Olowonyo M., Adekoya A., Adetola O., Ajetunmobi A. Severe childhood anaemia and blood transfusion in a Nigerian secondary level facility. J Trop Pediatr. 2016;62(2):107–115. doi: 10.1093/tropej/fmv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad R., Mishra O.P. Acute kidney injury in children with plasmodium falciparum malaria: determinants for mortality. Perit Dial Int. 2016;36(2):213–217. doi: 10.3747/pdi.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das D., Grais R.F., Okiro E.A., et al. Complex interactions between malaria and malnutrition: a systematic literature review. BMC Med. 2018;16(1):186. doi: 10.1186/s12916-018-1177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbs K.R., Dent A.E. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology. 2016;143(2):129–138. doi: 10.1017/S0031182015001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgountzou A., Papadopoulos N.G. Postnatal Innate immune development: from birth to adulthood. Front Immunol. 2017;8:957. doi: 10.3389/fimmu.2017.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yap X.Z., Lundie R.J., Beeson J.G., O'Keeffe M. Dendritic cell responses and function in malaria. Front Immunol. 2019;10:357. doi: 10.3389/fimmu.2019.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega-Pajares A., Rogerson S.J. The Rough Guide to Monocytes in Malaria Infection. Front Immunol. 2018;9:2888. doi: 10.3389/fimmu.2018.02888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belachew E.B. Immune response and evasion mechanisms of plasmodium falciparum parasites. J Immunol Res. 2018;2018 doi: 10.1155/2018/6529681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roach B., Kim Y., Jerome E., Michael A.F. Influence of age and sex on serum complement components in children. Am J Dis Child. 1981;135(10):918–920. doi: 10.1001/archpedi.1981.02130340030011. [DOI] [PubMed] [Google Scholar]
- 32.Mandala W.L., Msefula C.L., Gondwe E.N., Drayson M.T., Molyneux M.E., MacLennan C.A. Cytokine profiles in malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin Vaccine Immunol. 2017;24(4) doi: 10.1128/CVI.00533-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrington L., Vance H., Rek J., et al. Both inflammatory and regulatory cytokine responses to malaria are blunted with increasing age in highly exposed children. Malar J. 2017;16(1):499. doi: 10.1186/s12936-017-2148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkes M., Elphinstone R.E., Conroy A.L., Kain K.C. Contrasting pediatric and adult cerebral malaria: the role of the endothelial barrier. Virulence. 2013;4(6):543–555. doi: 10.4161/viru.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kihara M., Carter J.A., Newton C.R. The effect of Plasmodium falciparum on cognition: a systematic review. Trop Med Int Health. 2006;11(4):386–397. doi: 10.1111/j.1365-3156.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 36.Sahu P.K., Duffy F.J., Dankwa S., et al. Determinants of brain swelling in pediatric and adult cerebral malaria. JCI Insight. 2021;6(18) doi: 10.1172/jci.insight.145823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birbeck G.L., Molyneux M.E., Kaplan P.W., et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9(12):1173–1181. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahu P.K., Hoffmann A., Majhi M., et al. Brain magnetic resonance imaging reveals different courses of disease in pediatric and adult cerebral malaria. Clin Infect Dis. 2021;73(7):e2387–e2e96. doi: 10.1093/cid/ciaa1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathia S.K., Sankar J., Kandasamy D., Lodha R. Plasmodium vivax malaria presenting with multifocal hemorrhagic brain infarcts in a school-going child. J Trop Pediatr. 2016;62(4):341–344. doi: 10.1093/tropej/fmw007. [DOI] [PubMed] [Google Scholar]
- 40.Tahar R., Albergaria C., Zeghidour N., Ngane V.F., Basco L.K., Roussilhon C. Plasma levels of eight different mediators and their potential as biomarkers of various clinical malaria conditions in African children. Malar J. 2016;15:337. doi: 10.1186/s12936-016-1378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucchi N.W., Jain V., Wilson N.O., Singh N., Udhayakumar V., Stiles J.K. Potential serological biomarkers of cerebral malaria. Dis Markers. 2011;31(6):327–335. doi: 10.3233/DMA-2011-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conroy A.L., Phiri H., Hawkes M., et al. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case-control study. PLoS One. 2010;5(12):e15291. doi: 10.1371/journal.pone.0015291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubach M.P., Mukemba J., Florence S., et al. Plasma Plasmodium falciparum histidine-rich protein-2 concentrations are associated with malaria severity and mortality in Tanzanian children. PLoS One. 2012;7(5):e35985. doi: 10.1371/journal.pone.0035985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vera I.M., Kessler A., Ting L.M., et al. Plasma cell-free DNA predicts pediatric cerebral malaria severity. JCI Insight. 2020;5(12) doi: 10.1172/jci.insight.136279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bousema T., Drakeley C. Determinants of malaria transmission at the population level. Cold Spring Harb Perspect Med. 2017;7(12) doi: 10.1101/cshperspect.a025510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerardin J., Ouedraogo A.L., McCarthy K.A., Eckhoff P.A., Wenger E.A. Characterization of the infectious reservoir of malaria with an agent-based model calibrated to age-stratified parasite densities and infectiousness. Malar J. 2015;14:231. doi: 10.1186/s12936-015-0751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh N., Bharti P.K., Kumre N.S. Active v. passive surveillance for malaria in remote tribal belt of Central India: implications for malaria elimination. Pathog Glob Health. 2016;110(4–5):178–184. doi: 10.1080/20477724.2016.1223920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouedraogo M., Samadoulougou S., Rouamba T., et al. Spatial distribution and determinants of asymptomatic malaria risk among children under 5 years in 24 districts in Burkina Faso. Malar J. 2018;17(1):460. doi: 10.1186/s12936-018-2606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts D., Matthews G. Risk factors of malaria in children under the age of five years old in Uganda. Malar J. 2016;15:246. doi: 10.1186/s12936-016-1290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omonijo A.O., Omonijo A., Okoh H.I., Ibrahim A.O. Relationship between the usage of long-lasting insecticide-treated bed nets (LLITNs) and malaria prevalence among school-age children in Southwestern Nigeria. J Environ Public Health. 2021;2021 doi: 10.1155/2021/8821397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masuda K. Length of maternal schooling and children's risk of malaria infection: evidence from a natural experiment in Uganda. BMJ Glob Health. 2020;5(2) doi: 10.1136/bmjgh-2019-001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohee L.M., Nankabirwa J.I., Greenwood B., Djimde A., Mathanga D.P. Time for malaria control in school-age children. Lancet Child Adolesc Health. 2021;5(8):537–538. doi: 10.1016/S2352-4642(21)00158-9. [DOI] [PubMed] [Google Scholar]
- 53.van Eijk A.M., Choubey S., Barla P., et al. Malaria in Sundargarh district, Odisha, India: epidemiological and behavioral aspects from surveys. Acta Trop. 2020;211 doi: 10.1016/j.actatropica.2020.105647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subbarao S.K., Nanda N., Rahi M., Raghavendra K. Biology and bionomics of malaria vectors in India: existing information and what more needs to be known for strategizing elimination of malaria. Malar J. 2019;18(1):396. doi: 10.1186/s12936-019-3011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohee L.M., Opondo C., Clarke S.E., et al. Preventive malaria treatment among school-aged children in sub-Saharan Africa: a systematic review and meta-analyses. Lancet Glob Health. 2020;8(12):e1499–ee511. doi: 10.1016/S2214-109X(20)30325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chinkhumba J., Kadzinje V., Jenda G., Kayange M., Mathanga D.P. Impact of school-based malaria intervention on primary school teachers' time in Malawi: evidence from a time and motion study. Malar J. 2022;21(1):301. doi: 10.1186/s12936-022-04324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehta P.N. Medscape; 2020. Pediatric Malaria. [Google Scholar]
- 58.Odeyemi A., Olasinde Y., Ojewuyi A., Odeyemi A., Ala O., Agelebe E. Utilization of long lasting insecticidal net among children aged less than five years in a tertiary health facility in south-west Nigeria. Alex J Med. 2022;58(1):44–51. [Google Scholar]
- 59.Kobayashi T., Kanyangarara M., Laban N.M., et al. Characteristics of subpatent malaria in a pre-elimination setting in Southern Zambia. Am J Trop Med Hyg. 2019;100(2):280–286. doi: 10.4269/ajtmh.18-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wotodjo A.N., Doucoure S., Diagne N., et al. Another challenge in malaria elimination efforts: the increase of malaria among adults after the implementation of long-lasting insecticide-treated nets (LLINs) in Dielmo, Senegal. Malar J. 2018;17(1):384. doi: 10.1186/s12936-018-2536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bashir I.M., Nyakoe N., van der Sande M. Targeting remaining pockets of malaria transmission in Kenya to hasten progress towards national elimination goals: an assessment of prevalence and risk factors in children from the Lake endemic region. Malar J. 2019;18(1):233. doi: 10.1186/s12936-019-2876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laurens M.B. RTS,S/AS01 vaccine (Mosquirix): an overview. Hum Vaccin Immunother. 2020;16(3):480–489. doi: 10.1080/21645515.2019.1669415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahi M., Sharma A. Should India be considering deployment of the first malaria vaccine RTS,S/AS01? BMJ Glob Health. 2022;7(1) doi: 10.1136/bmjgh-2021-007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chandramohan D., Zongo I., Sagara I., et al. Seasonal malaria vaccination with or without seasonal malaria chemoprevention. N Engl J Med. 2021;385(11):1005–1017. doi: 10.1056/NEJMoa2026330. Sep 9. [DOI] [PubMed] [Google Scholar]
- 65.Datoo M.S., Natama H.M., Somé A., et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years' follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 2022;22(12):1728–1736. doi: 10.1016/S1473-3099(22)00442-X. [DOI] [PubMed] [Google Scholar]
- 66.University of Oxford; 2023. R21/Matrix-M™ Malaria Vaccine Developed By University of Oxford receives Regulatory Clearance for Use in Ghana. [Google Scholar]
- 67.Mordmuller B., Sulyok Z., Sulyok M., et al. A PfSPZ vaccine immunization regimen equally protective against homologous and heterologous controlled human malaria infection. NPJ Vaccines. 2022;7(1):100. doi: 10.1038/s41541-022-00510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]