Abstract
Background:
Although opioids are initiated on hospital discharge in millions of older adults each year, there are no studies examining patient- and prescribing-related risk factors for opioid-related adverse drug events (ADEs) after hospital discharge among medical patients.
Methods:
Retrospective cohort study of a national sample of Medicare beneficiaries age 65 and older, hospitalized for a medical reason, with at least 1 claim for an opioid within 2 days of hospital discharge. We excluded patients receiving hospice care and patients admitted from or discharged to a facility. We used administrative billing codes and medication claims to define potential opioid-related ADEs within 30 days of hospital discharge, and competing risks regression to identify risk factors for these events.
Results:
Among 22,879 medical hospitalizations (median age 74, 36.9% female) with an opioid claim within 2 days of hospital discharge, a potential opioid-related ADE occurred in 1,604 (7.0%). Independent risk factors included age 80 years and older (HR 1.18, 95% CI 1.05-1.33); clinical conditions, including kidney disease (HR 1.22, 95% CI 1.08-1.37), dementia/delirium (HR 1.38, 95% CI 1.22-1.56), anxiety disorder (HR 1.20, 95% CI 1.06-1.36), opioid use disorder (HR 1.20, 95% CI 1.03-1.39), intestinal disorders (HR 1.31, 95% CI 1.15-1.49), pancreaticobiliary disorders (HR 1.32, 95% CI 1.09-1.61), and musculoskeletal and nervous system injuries (HR 1.35, 95% CI 1.17-1.54); red flags for opioid misuse (HR 1.37, 95% CI 1.04-1.80); opioid use in the 30 days prior to hospitalization (HR 1.20, 95% CI 1.08-1.34; and prescription of long-acting opioids (HR 1.34, 95% CI 1.06-1.70).
Conclusions:
Potential opioid-related ADEs occurred within 30 days of hospital discharge in 7.0% of older adults discharged from a medical hospitalization with an opioid prescription. Identified risk factors can be used to inform physician decision-making, conversations with older adults about risk, and development and targeting of harm reduction strategies.
Keywords: Opioids, adverse drug events, hospitalization
INTRODUCTION
Opioid use is common in the inpatient setting1, 2 and often continued after hospital discharge, with 15% of previously opioid naïve older adults filling a new opioid prescription within 7 days of hospital discharge.3 With more than 12 million discharges age 65 and older from United States (U.S.) hospitals each year, this suggests that almost 2 million older adults are newly initiated on opioids after a hospitalization annually. Prior studies have demonstrated that opioids are consistently among the top medication classes responsible for adverse drug events (ADEs) both during hospitalization and in the post-discharge period.4-7 However, the majority of these studies have not specifically examined older adults, who are at heightened risk for ADEs due to greater degrees of comorbidity and polypharmacy, as well as altered pharmacokinetics and pharmacodynamics. Using a national cohort of older adults hospitalized for medical reasons, we sought to determine the incidence and independent risk factors for potential opioid-related ADEs within 30 days of hospital discharge.
METHODS
This study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board with a waiver of informed consent.
Study Population
We conducted a cohort study of the 20% sample of U.S. Medicare beneficiaries with a short-stay hospitalization in 2016. We focused on hospitalizations with a medical reason for hospitalization (based on diagnosis-related group), and excluded hospitalizations with discharge to a facility (since prescription claims would be unavailable), and hospitalizations with discharge to hospice or with cancer as a discharge diagnosis (since opioids are used differently in these patient populations). See Supplementary Figure S1 for full inclusion and exclusion criteria. We used the Centers for Disease Control (CDC) National Center for Injury Prevention and Control compilation of opioid analgesics to identify beneficiaries with opioid claims occurring within 2 days after hospital discharge, excluding claims for buprenorphine formulations intended for treatment of opioid use disorder, as defined by the CDC algorithm.8
Outcomes
We measured the occurrence of any potential opioid-related ADE in the time period between the date of the first claim for an opioid and 30 days after hospital discharge. We defined potential opioid-related ADEs as any unplanned9 emergency department (ED) visit, observation stay, or acute hospitalization with a diagnosis code for any of the following known opioid-related adverse effects, defined using International Classification of Disease (ICD) 9 or 10 codes occurring in any position (see Supplementary Table S1): delirium, nausea/vomiting, slowed colonic motility (including constipation, ileus, obstruction, fecal impaction), fall/fracture, urinary retention, and poisoning/adverse effects from opioids.
Candidate Risk Factors
We included candidate risk factors hypothesized to be associated with opioid-related ADEs based on clinical rationale, and prior studies in other populations and settings (see Table 1). They included patient characteristics, as well as characteristics of the discharge opioid prescriptions themselves. Red flags for opioid misuse was defined as having an average daily morphine-equivalent dose greater than 120 mg for any 90-day period, and at least 90 days of opioid use and opioid prescriptions from 4 or more prescribers during the year prior to hospital admission.10
Table 1.
Patient and opioid prescription characteristics and associations with opioid-related adverse drug events (ADEs) within 30 days of hospital discharge.
| Characteristics, n(%) | Overall n = 22,879 |
Without ADE n = 21,275 |
With ADE n= 1,604 |
Adjusted Hazard Ratio [95% CI] |
|---|---|---|---|---|
| Demographics | ||||
| Age ≥ 80 | 6555 (28.7) | 6038 (28.4) | 517 (32.2) | 1.18 [1.05-1.33] |
| Female | 8445 (36.9) | 7851 (36.9) | 594 (37.0) | 1.09 [0.98-1.22] |
| Clinical conditionsa | ||||
| Heart failure | 12303 (53.8) | 11321 (53.2) | 982 (61.2) | 1.11 [0.98-1.25] |
| Cerebrovascular disease | 6274 (27.4) | 5746 (27.0) | 528 (32.9) | 1.08 [0.96-1.21] |
| Respiratory illness | 15055 (65.8) | 13929 (65.5) | 1126 (70.2) | 1.00 [0.89-1.13] |
| Kidney disease | 13865 (60.6) | 12768 (60.0) | 1097 (68.4) | 1.22 [1.08-1.37] |
| Dementia/delirium | 5170 (22.6) | 4647 (21.8) | 523 (32.6) | 1.38 [1.22-1.56] |
| Mood disorder | 13063 (57.1) | 12034 (56.6) | 1029 (64.2) | 0.99 [0.88-1.13] |
| Anxiety disorder | 10982 (48.0) | 10065 (47.3) | 917 (57.2) | 1.20 [1.06-1.36] |
| Alcohol use disorder | 2283 (10.0) | 2091 (9.8) | 192 (12.0) | 1.04 [0.88-1.23] |
| Opioid use disorder | 2891 (12.6) | 2589 (12.2) | 302 (18.8) | 1.20 [1.03-1.39] |
| Other substance use disorders | 2776 (12.1) | 2576 (12.1) | 200 (12.5) | 0.97 [0.83-1.14] |
| Liver disease | 4954 (21.7) | 4543 (21.4) | 411 (25.6) | 1.09 [0.97-1.23] |
| Intestinal disorders | 3488 (15.2) | 3195 (15.0) | 293 (18.3) | 1.31 [1.15-1.49] |
| Pancreaticobiliary disorders | 1332 (5.8) | 1219 (5.7) | 113 (7.0) | 1.32 [1.09-1.61] |
| Musculoskeletal/connective tissue diseases | 12498 (54.6) | 11618 (54.6) | 880 (54.9) | 0.93 [0.84-1.04] |
| Injuries (musculoskeletal and nervous system) | 3235 (14.1) | 2973 (14.0) | 262 (16.3) | 1.35 [1.17-1.54] |
| Multi-morbidityb | 7287 (31.9) | 6578 (30.9) | 709 (44.2) | 1.06 [0.89-1.25] |
| Red Flags for Opioid Misusec | 468 (2.0) | 404 (1.9) | 64 (4.0) | 1.37 [1.04-1.80] |
| Medication exposures | ||||
| Opioidsd | 9994 (43.7) | 9168 (43.1) | 826 (51.5) | 1.20 [1.08-1.34] |
| Benzodiazepinese | 4909 (21.5) | 4500 (21.2) | 409 (25.5) | 1.02 [0.90-1.16] |
| Muscle relaxantse | 1548 (6.8) | 1414 (6.6) | 134 (8.4) | 1.14 [0.95-1.37] |
| Gabapentin/pregabaline | 4230 (18.5) | 3879 (18.2) | 351 (21.9) | 1.07 [0.95-1.21] |
| Polypharmacyf | 9409 (41.1) | 8641 (40.6) | 768 (47.9) | 1.06 [0.94-1.18] |
| Opioid Prescription Characteristics | ||||
| Short-acting opioids only | 21225 (92.8) | 19803 (93.1) | 1422 (88.7) | Reference |
| Long-acting opioids only | 774 (3.4) | 691 (3.2) | 83 (5.2) | 1.34 [1.06-1.70] |
| Short- and long-acting opioids | 880 (3.8) | 781 (3.7) | 99 (6.2) | 1.38 [1.08-1.75] |
| MME per day – mean (median, Q1-Q3) | 42 (30, 20-45) | 41 (30, 20-45) | 47 (30, 20-50) | 1.00 [1.00-1.00] |
| Days supply – mean (median, Q1-Q3) | 13 (7, 5 – 20) | 13 (7, 5 – 20) | 13 (8, 5 – 23) | 1.00 [0.99-1.00] |
Abbreviations: MME = milligram morphine equivalent
Diagnoses over the year prior to hospital discharge, defined by the Centers for Medicare and Medicaid Services (CMS) Chronic Conditions Warehouse (CCW), or on hospital discharge, defined by the Healthcare Cost and Utilization Project multi-level diagnosis and procedure Clinical Classification System25, 26
Defined as presence of six or more of the specified clinical conditions
Defined as average daily morphine-equivalent dose greater than 120 mg for any 90-day period, and at least 90 days of opioid use, and opioid prescriptions from 4 or more prescribers during the year prior to hospital admission10
Presence of any opioid claims in the 30 days prior to hospital admission
Presence of any claims in the 30 days prior to hospital admission or within 7 days after hospital discharge
Defined as 10 or more preadmission medications; we defined preadmission medications as medications in which the day supply of the most recent fill prior to hospitalization was sufficient to last until the admission date plus a grace period equal to the day supply, consistent with prior literature27
Statistical analysis
We performed a multivariable competing risks regression analysis using the Fine-Gray subdistribution hazards function to estimate adjusted hazard ratios for the hazard of a potential opioid-related ADE.11 We used this approach to account for the occurrence of death or hospitalization for a non-opioid-related reason, both of which would impact the patient’s eligibility for the outcome of interest. For continuous predictors, we examined the incidence of our outcome by quartiles of each predictor, to identify if there were any natural cutpoints of risk at which to dichotomize; if not, the variable was kept as continuous. We assessed for collinearity between candidate predictors by examining correlation between each predictor variable and each of the others. All analyses were carried out using version 9.4 of SAS software (SAS Institute Inc, Cary, North Carolina).
Sensitivity Analyses
Since most opioid prescriptions were for a 7-14 day supply, we ran a sensitivity analysis in which we re-ran our analysis using a shortened outcome window of 14 days after hospital discharge. Additionally, because beneficiaries could have been hospitalized multiple times during the study period, resulting in clustering, we ran another sensitivity analysis in which we included only one randomly chosen hospitalization for each beneficiary, to ensure similar effect estimates.
RESULTS
After applying our exclusions (Supplementary Figure S1), there were 299,239 medical hospitalizations remaining, of whom 22,879 (7.6%) had a claim for an opioid within 2 days of hospital discharge and were included in our analytic cohort. See Table 1 for cohort characteristics. The median age was 74, and females represented 36.9% of the cohort.
Incidence of Potential Opioid-Related ADEs
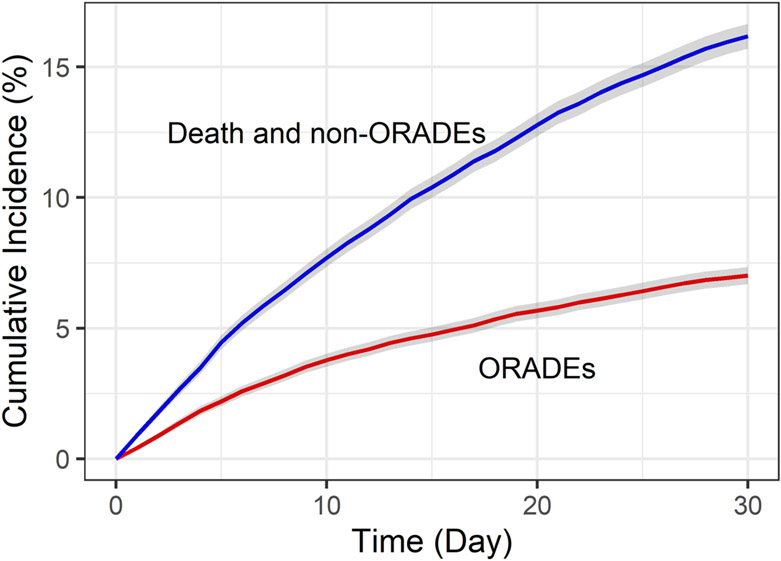
At least one potential opioid-related ADE occurred within 30 days of hospital discharge in 1,604 (7.0%) hospitalizations (Table 2), and within 14 days of hospital discharge in 1,091 (4.8%) hospitalizations (Supplementary Table S2). Figure 1 shows the cumulative incidence of potential opioid-related ADEs and the competing risks of death or hospitalization for a non-opioid-related reason. The majority of potential opioid-related ADEs were identified during a rehospitalization (1,134; 70.7%), with the remainder identified during an ED visit without hospitalization (470; 29.3%). The most common potential opioid-related ADEs were slowed colonic motility (2.7%), delirium (1.8%) and nausea/vomiting (1.5%).
Table 2.
Incidence of potential opioid-related adverse drug events (ADEs) within 30 days of hospital discharge (n = 22,879).
| Outcomes | n | % | |
|---|---|---|---|
| Any potential opioid-related ADE | 1604 | 7.0 | |
| Individual potential opioid-related ADEsa | |||
| Slowed colonic motility | 613 | 2.7 | |
| Delirium | 417 | 1.8 | |
| Nausea/vomiting | 353 | 1.5 | |
| Fall/Fracture | 200 | 0.9 | |
| Urinary retention | 203 | 0.9 | |
| Opioid-Related Adverse Effects | 138 | 0.6 | |
Number of individual potential opioid-related ADEs sum to greater than the number with any potential opioid-related ADE since beneficiaries may have had more than one type
Figure 1:
Cumulative incidence of potential opioid-related ADEs and the competing risks of death or hospitalization for a non-opioid-related reason within 30 days of hospital discharge.
Abbreviation: ORADE = opioid-related adverse drug event
Associations Between Candidate Risk Factors and Potential Opioid-Related ADEs
Table 1 shows patient and prescription characteristics overall, and stratified by presence or absence of a potential opioid-related ADE. After examining outcome incidence by quartiles of each continuous predictor variable, we dichotomized age at 80 and multi-morbidity at 6 based on observed thresholds above which risk increased non-linearly. In multivariable competing risks regression, we identified several characteristics associated with a potential opioid-related ADE (Table 1), including age 80 years and older (HR 1.18, 95% CI 1.05-1.33); clinical conditions, including kidney disease (HR 1.22, 95% CI 1.08-1.37), dementia/delirium (HR 1.38, 95% CI 1.22-1.56), anxiety disorder (HR 1.20, 95% CI 1.06-1.36), opioid use disorder (HR 1.20, 95% CI 1.03-1.39), intestinal disorders (HR 1.31, 95% CI 1.15-1.49), pancreaticobiliary disorders (HR 1.32, 95% CI 1.09-1.61), and musculoskeletal and nervous system injuries (HR 1.35, 95% CI 1.17-1.54); red flags for opioid misuse (HR 1.37, 95% CI 1.04-1.80); opioid use in the 30 days prior to hospitalization (HR 1.20, 95% CI 1.08-1.34; and prescription of long-acting opioids (HR 1.34, 95% CI 1.06-1.70 for long-acting only, and HR 1.38, 95% CI 1.08-1.75 for short- and long-acting opioids). After restricting the outcome window to 14 days after hospital discharge, results were similar, with the addition of female gender (HR 1.18, 95% CI 1.03-1.34) as a significant predictor (Supplementary Table S3). After randomly selecting one hospitalization per beneficiary (n=21,436), characteristics associated with opioid-related ADEs were unchanged (Supplementary Table S4).
DISCUSSION
In this large national cohort of older adults hospitalized for medical reasons, we found that a potential opioid-related ADE occurred in 7% of those discharged with an opioid prescription. Gastrointestinal issues (slowed colonic motility and nausea/vomiting) and delirium were the most common potential opioid-related ADEs. We identified several patient and opioid prescription characteristics associated with potential opioid-related ADEs, the strongest of which were presence of a dementia or delirium diagnosis, presence of red flags for opioid misuse, and prescription of long-acting opioid formulations. Awareness of these risk factors may help physicians, hospital-systems, and researchers identify patients at higher risk for an opioid-related ADE, to inform opioid prescribing decisions, conversations with older adults, and development or deployment of risk-mitigation strategies, such as pharmacist follow up or transitional care management visits.
Prior studies have examined risk factors for opioid-related ADEs in medical and surgical patients in the inpatient and outpatient settings, but none have focused on the immediate post-discharge period among older adults. Our findings are consistent with prior studies, which have demonstrated age,2, 12-17 renal failure,17, 18 mental health comorbidities,17, 19 prior substance use disorders,17, 19, 20 and use of long-acting opioid formulations as risk factors for opioid-related ADEs.17, 21 Although guidelines recommend against initiation or modification of long-acting opioids as part of a hospitalization episode,22 we found that 7% of patients discharged with an opioid prescription received a long-acting opioid formulation. Our findings may help to further discourage this practice. In contrast to prior studies in other settings and patient populations, although patients with potential opioid-related ADEs received higher average daily doses of opioids, opioid dose was not a significant predictor of potential opioid-related ADEs after multivariable adjustment.23, 24 Although opioid dose is clearly a risk factor for adverse outcomes based on pharmacokinetic principles, this effect may have been overshadowed by other stronger predictors in our model that tend to be collinear with dose, such as long-acting opioid formulations. The heterogeneity of our patient population may also have resulted in confounding by indication, not fully captured by the clinical conditions in our model. Although studies in more narrow patient populations may provide additional insight, we chose to keep our focus broad given the wide range of clinical conditions managed by inpatient medical providers.
Our finding that opioid prescription in the 30 days prior to hospitalization was associated with increased risk of a potential opioid-related ADE warrants further investigation to understand the factors contributing to this heightened risk. It is possible that receipt of an opioid prescription after hospital discharge in the presence of a pre-existing opioid prescription contributes to confusion around which or how much to take. Alternatively, it may be that prior opioid use places a patient at higher risk of an opioid-related ADE for other reasons, such as tendency towards higher doses. We did not, however, find collinearity between prior use and discharge prescription dose. Further research is necessary to better understand this finding.
There are limitations to this analysis. First, reliance on billing codes could have resulted in undercapture of potential opioid-related ADEs. Second, since we cannot know for certain if the adverse event was due to the opioid itself or another factor, we can only comment on potential opioid-related ADEs.
In conclusion, in this large, national cohort of medically hospitalized older adults, a potential opioid-related ADE occurred in 7% of those discharged with an opioid prescription. We identified several risk factors for severe opioid-related ADEs, which can be used to inform physician decision-making around pain management, conversations with older adults, or to target at-risk populations for design and testing of interventions to mitigate opioid-associated risk.
Supplementary Material
Supplementary Figure S1: Consort Diagram
Supplementary Table S1: Outcome Operationalization
Supplementary Table S2: Incidence of potential opioid-related adverse drug events (ADEs) within 14 days of hospital discharge
Supplementary Table S3: Patient and opioid prescription characteristics and associations with opioid-related adverse drug events (ADEs) within 14 days of hospital discharge
Supplementary Table S4: Association between patient and opioid prescription characteristics and opioid-related adverse drug events (ADEs) after restricting to a single hospitalization per beneficiary
Key Points:
In this large national cohort of older adults hospitalized for medical reasons, we found that a potential opioid-related adverse drug event (ADE) occurred in 7% of those discharged with an opioid prescription.
Gastrointestinal issues (slowed colonic motility and nausea/vomiting) and delirium were the most common potential opioid-related ADEs.
The strongest factors associated with potential opioid-related ADEs were presence of a dementia or delirium diagnosis, presence of red flags for opioid misuse, and prescription of long-acting opioid formulations.
Why does this matter?
Awareness of these risk factors may help physicians, hospital-systems, and researchers identify older adults at higher risk for an opioid-related ADE, to inform opioid prescribing decisions, conversations, and development or deployment of risk-mitigation strategies, such as pharmacist follow up or transitional care management visits.
ACKNOWLEDGMENTS
Funding: The study was funded by grant number R01HS026215 from the Agency for Healthcare Research and Quality.
Sponsor’s Role:
The funding agency had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agency.
Footnotes
Conflict of Interest: The authors have no conflicts.
References:
- [1].Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kessler ER, Shah M, S KG, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33: 383–391. [DOI] [PubMed] [Google Scholar]
- [3].Jena AB, Goldman D, Karaca-Mandic P. Hospital Prescribing of Opioids to Medicare Beneficiaries. JAMA Intern Med. 2016;176: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138: 161–167. [DOI] [PubMed] [Google Scholar]
- [5].Tsilimingras D, Schnipper J, Duke A, et al. Post-Discharge Adverse Events Among Urban and Rural Patients of an Urban Community Hospital: A Prospective Cohort Study. J Gen Intern Med. 2015;30: 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274: 29–34. [PubMed] [Google Scholar]
- [7].Kanaan AO, Donovan JL, Duchin NP, et al. Adverse drug events after hospital discharge in older adults: types, severity, and involvement of Beers Criteria Medications. J Am Geriatr Soc. 2013;61: 1894–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].National Center for Injury Prevention and Control. CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2018 version. Atlanta, GA: Centers for Disease Control and Prevention; 2018. Available at https://www.cdc.gov/drugoverdose/resources/data.html. Accessed May 5, 2020. [Google Scholar]
- [9].Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation (YNHHSC/CORE). 2021 Measure updates and specifications report hospital-wide all-cause unplanned readmission. Version 10.0, April 2021. https://qualitynet.cms.gov/inpatient/measures/readmission/methodology. Accessed June 22, 2021.
- [10].U.S. Department of Health and Human Services Office of Inspector General. Toolkit: Using Data Analysis To Calculate Opioid Levels and Identify Patients At Risk of Misuse or Overdose. Available at: https://oig.hhs.gov/oei/reports/oei-02-17-00560.asp. Accessed May 17, 2021.
- [11].Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94: 496–509. [Google Scholar]
- [12].Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74: 102–112. [DOI] [PubMed] [Google Scholar]
- [13].Minkowitz HS, Gruschkus SK, Shah M, Raju A. Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm. 2014;71: 1556–1565. [DOI] [PubMed] [Google Scholar]
- [14].Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32: 6S–11S. [DOI] [PubMed] [Google Scholar]
- [15].Taylor S, Kirton OC, Staff I, Kozol RA. Postoperative day one: a high risk period for respiratory events. Am J Surg. 2005;190: 752–756. [DOI] [PubMed] [Google Scholar]
- [16].Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260: 76–87. [DOI] [PubMed] [Google Scholar]
- [17].Herzig SJ, Stefan MS, Pekow PS, et al. Risk Factors for Severe Opioid-Related Adverse Events in a National Cohort of Medical Hospitalizations. J Gen Intern Med. 2020;35: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brunton LL, ed. Goodman and Gilman’s the Pharmacologic Basis of Therapeutics. 9 ed. New York, NY:McGraw-Hill; 1996. [Google Scholar]
- [19].Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129: 355–362. [DOI] [PubMed] [Google Scholar]
- [20].Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O'Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175: 608–615. [DOI] [PubMed] [Google Scholar]
- [22].Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement From the Society of Hospital Medicine. J Hosp Med. 2018;13: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305: 1315–1321. [DOI] [PubMed] [Google Scholar]
- [24].Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort Study of the Impact of High-Dose Opioid Analgesics on Overdose Mortality. Pain Med. 2016;17: 85–98. [DOI] [PubMed] [Google Scholar]
- [25].HCUP CCS. Healthcare Cost and Utilization Project (HCUP). March 2017. Agency for Healthcare Research and Quality, Rockville, MD. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed July 19, 2021. [Google Scholar]
- [26].Tools Archive for Clinical Classifications Software Refined. Healthcare Cost and Utilization Project (HCUP). March 2021. Agency for Healthcare Research and Quality, Rockville, MD. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccsr_archive.jsp. Accessed July 19, 2021. [Google Scholar]
- [27].Anderson TS, Jing B, Wray CM, et al. Comparison of Pharmacy Database Methods for Determining Prevalent Chronic Medication Use. Med Care. 2019;57: 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Consort Diagram
Supplementary Table S1: Outcome Operationalization
Supplementary Table S2: Incidence of potential opioid-related adverse drug events (ADEs) within 14 days of hospital discharge
Supplementary Table S3: Patient and opioid prescription characteristics and associations with opioid-related adverse drug events (ADEs) within 14 days of hospital discharge
Supplementary Table S4: Association between patient and opioid prescription characteristics and opioid-related adverse drug events (ADEs) after restricting to a single hospitalization per beneficiary