Abstract
This study reports the prevalence of Cryptosporidium, Giardia and Isospora species in cats showing signs of gastrointestinal disease. Records from a United Kingdom commercial diagnostic laboratory between December 2003 and December 2005 were reviewed. Of 1355 cats, Cryptosporidium species oocysts were found in 13 cats (1%), Giardia species trophozoites in 74 (6%), and Isospora felis oocysts in 46 (3%). In a second group of 48 cats, prevalence of Giardia species was 15% using an immunoassay for detection of antigen compared to 4% detected with microscopy. Prevalence of Giardia (9%) and Isospora (9%) species was higher in cats less than 6 months old. Gender and breed did not affect prevalence. There was a trend for Cryptosporidium and Isospora species infections to be detected in late autumn and early winter. Regional differences in prevalence were not detected. None of these organisms show a characteristic pattern of clinical signs. This study demonstrates that enteric protozoal infection is common in domestic cats showing signs of alimentary disease.
The enteric protozoal parasites Cryptosporidium species, Isospora species and Giardia duodenalis are pathogenic to a wide range of mammalian hosts. In humans, cryptosporidiosis and giardiasis are both important ‘re-emerging diseases’ (Robertson et al 2000, Thompson 2000). The parasites are considered as a significant risk to immunocompromised people and are commonly recognised causes of diarrhoea in human infant day care centres (WHO 1996, Robertson et al 2000). Significant clinical infection has also been reported in immunocompetent adults (Martins and Guerrant 1995, Ramirez et al 2004).
In cats, infection with Cryptosporidium species and Giardia duodenalis is frequently subclinical. Clinical signs can occur, however, and generally include diarrhoea (Brightman and Slonka 1976, Wilkinson 1977, Bennett et al 1985, Barr and Bowman 1994, Robertson 2000). The reported prevalence of cryptosporidiosis and giardiasis in the cat population is variable, yet frequently high; worldwide studies show prevalences of up to 12% and 80%, respectively (Nash et al 1993, McGlade et al 2002). Variability is partly derived from the diversity in the techniques used for detection: notably, McGlade employed the more sensitive technique of polymerase chain reaction (PCR) to detect Giardia duodenalis. The role of the domestic cat in zoonotic transmission is unclear (Glaser et al 1998, Robertson et al 2000, Thompson 2004, Vasilopulos et al 2007).
Despite the potential risk for human spread, current prevalence data from the United Kingdom (UK) cat population are limited. Only one group has studied cats in the UK (Mtambo et al 1991, Nash et al 1993). This was limited geographically, looked at Cryptosporidium species exclusively and involved only small number of cats. The majority of overseas studies have investigated stray cats or cats obtained from a shelter, where the incidence of parasitism generally is considered higher than in pet cats (Swan and Thompson 1986, Hill et al 2000, Huber et al 2002, McGlade et al 2003a,b, Serra et al 2003). Most studies examining pet populations do not discriminate between cats showing signs of alimentary tract disease and those free of signs.
Isospora species commonly infect cats, yet clinical disease is mostly seen in young, immunocompetent or stressed hosts (Dubey 1993, Lindsay et al 1997). In kittens, Isospora species infection may result in diarrhoea that may be mucoid or bloody, with rapid improvement of clinical signs occurring following treatment with toltrazuril (Lloyd and Smith 2001, O'Brien et al 2002). The infection is common around the world, with a reported prevalence as high as 44% (Serra et al 2003). The prevalence of Isospora species infection in the UK pet cat population is unknown.
This study aimed to evaluate prevalence of Cryptosporidium species, Giardia duodenalis and Isospora species infection in a cohort of cats displaying signs of alimentary disease. Our purpose was to identify if demographic factors, such as signalment, region or season, were correlated with prevalence.
Materials and methods
Study population
Three populations were examined. The first (group 1) involved domestic cats, which had a faecal sample submitted to a commercial diagnostic laboratory (NationWide Laboratories, Lancashire, UK) between December 8 2003 and December 7 2005 for bacteriology and parasitology using standard UK laboratory techniques. The second (group 2) involved domestic cats, which had faecal samples submitted between March and May 2007 for bacteriology and parasitology. Inclusion of this additional cohort of cats aimed to assess whether the prevalence of Giardia species had been underestimated with conventional laboratory techniques, thus enzyme-linked immunosorbent assay (ELISA) was used for detection of Giardia duodenalis in addition to the standard technique. A third population (control) consisted of pet cats displaying no gastrointestinal signs.
Samples from groups 1 and 2 were submitted from mainland Great Britain, Northern Ireland, the Isle of Man and the Channel Islands. Typically, the reason for submission was investigation of gastrointestinal signs. Veterinarians were requested to send three faecal samples collected over a 48-h period. It is unclear whether all submissions contained multiple faecal samples. Computerised laboratory records were reviewed for age, breed, gender, date of sampling, and location of the submitting veterinary practise (by postcode). Seasonal prevalence trends were assessed in group 1 cats only as the duration for data collection from cats in group 2 did not span 12 months. Frequency and type of clinical signs in cats with documented enteric protozoal infections were recorded.
Group 3 comprised a total of 45 cats without signs of alimentary disease. Sixteen were owned by staff from Liverpool University, while clinic-visiting cats at first-opinion practises in Liverpool or rural Scotland (nine and 20 cats, respectively) made up the remainder of cats.
Laboratory techniques
To detect Cryptosporidium species oocysts, air-dried, auramine phenol-stained faecal smears were examined microscopically under ultraviolet illumination. Positive results were confirmed by modified Ziehl–Nielsen staining and microscopic examination under 1000×.
Wet faecal preparations were prepared for the identification of Giardia species trophozoites. An ELISA (ProSpecT Giardia Microplate Assay, Remel, Dartford, Kent) detecting Giardia specific antigen 65 was also employed for cats from groups 2 and 3 for Giardia species detection, following manufacturer's directions. Faeces were frozen at −20 to −70°C for up to 2 months prior to ELISA testing, as authorised by the assay instructions.
Isospora felis oocysts were concentrated by the McMaster flotation technique using a saturated zinc sulphate salt solution and examined under 100× magnification. The same technique was used to identify helminth ova. For all organisms, known positive and negative samples served as controls.
Microbiology techniques performed on the faeces included inoculation of blood agar and MacConkey agar plates for aerobic incubation, of blood agar for anaerobic incubation, of Selenite broth for selective culture of Salmonella species and campylobacter agar for selective culture of Campylobacter species in a 10% carbon dioxide environment. Agar plates were examined at 24 h, and re-incubated for a further 24 h if negative.
Statistical analysis
Data were entered into a statistical software programme (Minitab 14.0, Minitab Ltd, State College, PA, USA). Simple descriptive statistics were produced. Groups were analysed separately for calculation of prevalence data unless specified. Comparison of categorical variables was by χ2 analysis or the Fisher's exact test, as appropriate. Significance was set at P<0.05.
Results
Cat population sampled
Group 1 of the study consisted of 1355 cats. Age was known for 1152 cats for which the median age of the population was 24 months (range 1–240). Eighteen percent of cats were less than 6 months of age and 31% were less than 1 year old. Males comprised 54% of the population. Breed was recorded for 1260 cats; of the 27 different breeds represented, Siamese (63) and Persian (66) cats were most common. In total, there were 379 pedigree and 881 mixed breed cats.
Forty-eight cats were included in group 2. For 38 cats where age was recorded, the median age was 84 months (range 2–204). Gender was known for 46 cats, with 25 (54%) males and 21 females (46%). Four cats (10%) were less than 6 months and 10 (25%) were less than 1 year old. Breed was recorded for 45 cats, with 32 mixed breed and 13 pedigree cats.
Forty-five cats were included in group 3. The median age for this population was 96 months (range 1–240). Three cats (6%) were less than 6 months and seven (16%) were less than 1 year old. There were 26 females (60%) and 19 males (40%), and four (8%) pedigree and 41 (92%) mixed breed cats.
Samples from groups 1 and 2 were submitted from mainland Great Britain, Northern Ireland, the Isle of Man and the Channel Islands. Five-hundred and sixty-four (43%) samples came from north-west England, 181 (14%) from the Midlands, 221 (17%) from south-east England, 129 (10%) from north-east England, 65 (5%) from East Anglia, 65 (5%) from south-west England, 31 (2%) from Wales, 26 (2%) from Scotland, and the remainder from Northern Ireland, the Isle of Man and the Channel Islands. Group 3 cats were owned by staff from Liverpool University (16), or were clinic-visiting cats at first-opinion practises in Liverpool (nine) or rural Scotland (20).
Overall prevalence of enteric protozoal infections
Cryptosporidium, Giardia and Isospora species were identified by microscopy in 129 of 1355 cats in group 1 (10%). Thirty-two cats in total were infected with more than one organism (25% of total infected). Of these, four cats were infected with more than one protozoal organism (3%). The remainder had concurrent infection with either Salmonella species, Campylobacter species or Toxocara cati.
Cryptosporidium species infection
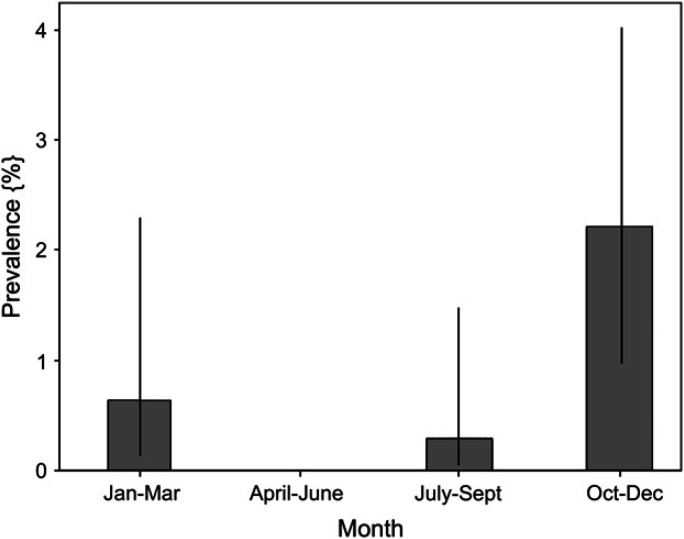
Cryptosporidium species oocysts were identified in 13 of 1355 group 1 cats (1%) only. In 12 cats, Cryptosporidium species was the only isolate. One cat was co-infected with Giardia species. There was no significant difference in prevalence between genders or age, and the prevalence in pedigree and mixed breed cats was similar. Median age of infected cats was 36 months (range 4–171). A higher prevalence of oocyst shedding was found in the period from October to December (Fig 1). No significant differences in prevalence were found between regions.
Fig 1.
Bar chart showing seasonal prevalence for shedding of Cryptosporidium species oocysts. Bars indicate observed prevalence, and dark vertical bars show the 95% confidence interval. Increased shedding occurred during the months of October to December (P=0.005; Fisher's exact test).
Giardia duodenalis infection
Giardia species infection was found by microscopy in 74 of 1355 (6%) group 1 cats and 2 of 48 (4%) group 2 cats and 0 of 45 (0%) group 3 cats. In the cats from group 2, ELISA detected a higher prevalence of Giardia duodenalis (seven cats, 15%) compared to the number detected using microscopy (2/48, 4%), although the difference did not reach significance (P=0.09). Two cats in group 3 (4%) were positive for Giardia duodenalis using ELISA.
Of the cats known for certain to have Giardia species infections based either on microscopy or ELISA, 70 were infected by giardia alone and 13 were co-infected by another enteropathogen (Campylobacter species, n=8; Isospora felis, n=2; Toxocara cati, n=1; Cryptosporidium species, n=1; both Campylobacter species and Isospora felis, n=1). There was no significant difference in prevalence between genders or amongst breeds for any group. Median age of infected animals showing signs was 12 months (range 1–182 months). In cats for which age was known, the prevalence of giardia in cats less than 6 months old (22/251; 9%) was significantly higher than in cats aged 6 months or older (47/939; 5%, P=0.03) for cats showing clinical signs. No significant seasonal or regional trends were evident.
Isospora felis infection
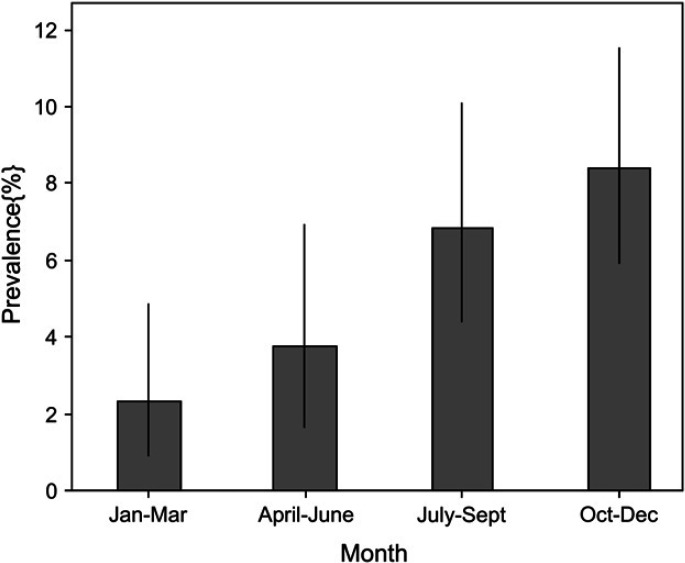
Isospora felis infection was found in 46 of 1355 (4%) cats, all in group 1. Thirty-five cats were infected by I felis alone, whereas 11 were co-infected by another potential pathogen (Campylobacter species, n=6; Giardia species, n=2; Salmonella species, n=2; both Campylobacter species and Giardia species, n=1). There was no significant difference in prevalence between genders or amongst breeds. Median age of infected animals was 4 months (range 1–156 months). In cats for which age was known, prevalence in cats <6 months of age (22/251 positive, 9%) was significantly higher than in cats ≥6 months (17/939, 2%) (P<0.001). A higher prevalence of infection was detected during October to December (P<0.001, Fig 2).
Fig 2.
Bar chart showing the seasonal prevalence for shedding Isospora felis cysts. Bars indicate observed prevalence, and dark vertical bars show the 95% confidence interval. Increased shedding occurred during the months of October to December (P<0.001).
Clinical signs in infected cats
Signalment and clinical signs are summarised in Table 1. Whilst diarrhoea was the most frequently reported sign, others included haematochezia, weight loss, vomiting and systemic malaise. The identity of the protozoal parasite did not produce significantly different clinical signs in infected cats. The presence or absence of protozoal parasites was not correlated with the duration of diarrhoea (ie, chronic versus acute).
Table 1.
Clinical signs in animals from groups 1 and 2 infected with Cryptosporidium, Giardia, and Isospora species
| Clinical signs | Cryptosporidium infection | Giardia infection | Isospora infection |
|---|---|---|---|
| Diarrhoea | 5/5 (100%) | 37/37 (100%) | 17/17 (100%) |
| Chronicity | |||
| Acute | 0/5 (0%) | 3/37 (8%) | 2/17 (12%) |
| Chronic | 4/5 (80%) | 21/37 (57%) | 7/17 (41%) |
| Unknown | 1/5 (20%) | 13/37 (35%) | 8/17 (48%) |
| Fresh blood present | 1/5 (20%) | 8/37 (22%) | 2/17 (12%) |
| Melaena | 0/5 (0%) | 0/37 (0%) | 0/17 (0%) |
| Weight loss | 1/5 (20%) | 2/37 (5%) | 2/17 (12%) |
| Vomiting | 0/5 (0%) | 0/37 (0%) | 1/17 (6%) |
| Systemic malaise | 0/5 (0%) | 1/37 (3%) | 1/17 (6%) |
| In-contact cats with signs | 0/5 (6%) | 2/37 (5%) | 4/17 (2%) |
Numbers are expressed as the proportion of cats for which accurate information was available (percentage in brackets).
Discussion
Cryptosporidium, Giardia and Isospora species are commonly detected in the faeces of domestic cats showing signs of alimentary disease across the UK. The extent to which they actually contribute to disease is currently unclear, because in order to determine disease causation it is necessary to not only show the presence of an enteropathogen, but to show that its elimination results in resolution of the observed clinical signs. The present findings generally agree with similar work examining their prevalence in various populations worldwide. Such studies show the prevalence of Cryptosporidium species in cats to be as high as 12.3% (Arai et al 1990, Mtambo et al 1991, Nash et al 1993, Sargent et al 1998, McReynolds et al 1999, Hill et al 2000, Spain et al 2001, Huber et al 2002, Nutter et al 2004, Lopez et al 2006). Prevalence of Cryptosporidium species varies, however, with some studies failing to detect the organism (Kirkpatrick 1988, Vanparijs et al 1991, Nolan and Smith 1995, Barutzki and Schaper 2003, Epe et al 2004). Data on Giardia species infection in cats show a similarly wide range of prevalence (1–52%) (Swan and Thompson 1986, Kirkpatrick 1988, Winsland et al 1989, Tonks et al 1991, Meloni et al 1993, Hill et al 2000, Spain et al 2001, Huber et al 2002, Barutzki and Schaper 2003, McGlade et al 2003a,b, Serra et al 2003, Bianciardi et al 2004, Cirak and Bauer 2004, Epe et al 2004, Gookin et al 2004, Nutter et al 2004, Robben et al 2004, De Santis-Kerr et al 2006). Studies reporting the prevalence of I felis infection are limited; studies state the prevalence for coccidia to be 1–44%, assuming the majority to be I felis (Kirkpatrick 1988, Vanparijs et al 1991, Nolan and Smith 1995, Barutzki and Schaper 2003, Serra et al 2003, Epe et al 2004, Robben et al 2004, De Santis-Kerr et al 2006).
Across previous studies, population source (domestic pet versus non-pet) has been shown to influence the observed prevalence (Swan and Thompson 1986, Hill et al 2000, Huber et al 2002, McGlade et al 2003a,b, Serra et al 2003). Whilst this may reflect differences in general health amongst populations, higher prevalence in dense populations may be expected because of increased ease of transmission. The high prevalence of Cryptosporidium species found in the Glasgow study (12.3%) was derived from a small population from just eight farms (Nash et al 1993). Similarly, a high prevalence of Giardia species (50%) was detected in a Persian cattery by Kirkpatrick (1988). A domestic population consisting of owned cats, visiting a veterinary surgeon, was included in the current study, which may partially explain the low levels of infection detected compared to some previous studies. In contrast, the prevalence of Giardia species and I felis in the current study is higher than that reported in a similar vet-visiting population in the US, which documented the lowest recorded prevalence for Giardia species (0.6%) and coccidia (1.4%) worldwide (De Santis-Kerr et al 2006).
The methods used for detection of parasites are likely to play a great role in the variable prevalence detected worldwide. In the current study, the true prevalence of protozoans might be higher due to intermittent excretion of trophozoites and oocysts or low sensitivities of commercial laboratory techniques. Microscopy was used for detection of Cryptosporidium species oocysts. Whilst microscopic examination of two consecutive feline faecal samples for Cryptosporidium species oocysts has similar or superior sensitivities to commercial enzyme immunoassays (EIAs) tested, it is unclear whether all submissions contained multiple faecal samples as requested by the laboratory (Marks et al 2004). PCR assay may be a more sensitive technique (Scorza et al 2003).
The methods used for detection of Giardia species may also markedly affect prevalence (McGlade et al 2003a,b), and this has been further demonstrated in the current study with a threefold difference in prevalence detected with ELISA compared to direct-smear microscopy for trophozoites. Trophozoites rapidly disintegrate in faeces over time, thus reducing the likelihood of microscopic identification (Bianciardi et al 2004), while oocysts are excreted intermittently, making submission of multiple specimens mandatory if relying on this older methodology. In dogs, there is marked variation in the sensitivity for detecting Giardia species using zinc sulphate concentration techniques (ZSCT) depending on the number of faecal samples tested (Barr 2006). This may also be true in cats, and when using a direct-smear technique. A further limitation of the direct-smear technique employed is its lower sensitivity compared to the ZSCT and antigen detection (Barr 2006), however, both the antigen detection assay used in the current study and a similar commercial ELISA test are more sensitive than ZSCT in detecting Giardia species in cats (McGlade et al 2003b, Cirak and Bauer 2004). Thus, while the microplate assay used in the current study has not been validated for cats, it is clear from previous data that its sensitivity should exceed that of the previously established ZSCT technique (Cirak and Bauer 2004). There is no established gold standard detection method for Giardia species, and there is very limited data on specificity and sensitivity of different ELISA tests (Groat 2003), but it seems likely that underestimation of infection is the most likely error.
Numbers in group 2 were small, and sampling was restricted to a 3-month period, but 15% of cats were Giardia species antigen positive. Ideally, samples obtained from a larger cohort of cats over a 12-month period would provide confirmation of this, and would allow further assessment of any effects of season. Finally, using PCR for detection of Giardia species may be superior still, although disadvantages of the technique include the current lack of commercial availability and the potential for contamination causing false positive results (McGlade et al 2003a,b). Given that the prevalence results are likely to be an underestimate, this further highlights the presence of significant number of potentially pathogenic and zoonotic enteric pathogens in our pet population. Additionally, the data reinforce to practitioners the importance of requesting use of the more sensitive techniques in commercial UK laboratories, such as an ELISA for detection of Giardia species antigen, in particular when multiple faecal samples are not obtained. In house ‘cage side’ kits using ELISA technology, therefore, have much to recommend them.
The current study demonstrated G duodenalis and I felis infection to be more common in cats under 6 months of age. Data examining age as a risk factor for individual pathogens in cats are limited; however, the age distribution of I felis in this study supports findings from recent work in cats in Chile and North America (De Santis-Kerr et al 2006, Lopez et al 2006). The latter study also identified a higher prevalence of Giardia species infection in cats less than 4 years old. Immaturity is considered to be a significant risk factor for giardiasis in humans and dogs, suggesting that immunity develops with age (Bianciardi et al 2004). Similarly, cats may develop immunity to I felis and have no or decreased oocyst production after oocyst challenge (Lindsay et al 1997). Development of humoural immunity with age may have contributed to the lower prevalence seen in mature cats in this study.
Seasonal trends were detected for Cryptosporidium species and I felis infection, with a higher prevalence found in October to December. Whilst a similar seasonal trend has been documented for C hominis infection in humans and a seasonal trend for coccidial infection in general was reported for summer, spring and autumn (McLaughlin et al 2000, De Santis-Kerr et al 2006), no previous studies have shown seasonality for Cryptosporidium species or I felis in dogs or cats. The reason for our finding is unclear; seasonal variation may be caused by the effect of climate on the parasite or host physiology (Becker et al 1977, Kirkpatrick 1988). An expected confounding factor was age, considering the seasonal feline reproductive cycle in the UK and the impact of age on prevalence of I felis. However, cats sampled during October to December were not younger. This may suggest the trend was genuine, however, the possibility that the kittens act as a source of re-infection for adults in multi-cat households must be considered. A limitation of this work was the 2-year duration of the study for analysis of group 1; ideally, serial time studies over several years allow more robust conclusions over seasonality to be drawn. Nonetheless, the trend is interesting and should be explored further.
The clinical significance of feline protozoal enteric pathogens remains unclear. Diarrhoea has been reported as the major clinical disease for Cryptosporidium species, Giardia and Isospora species infections, with clinical infection more frequent in young immunologically naive or immunocompromised individuals (Brightman and Slonka 1976, Wilkinson 1977, Bennett et al 1985, Robertson 2000). However, subclinical infection is likely to be common also, and identifying pathogens does not necessarily indicate that they are the cause of disease (Arai et al 1990, Asahi et al 1991, Lindsay and Blagburn 1991, Mtambo et al 1991, Dubey 1993, Nash et al 1993, Thompson 2004). Interestingly, Giardia duodenalis was detected in small number in the control population (group 3), which by definition was devoid of clinical signs. Few studies have examined populations displaying signs typical of alimentary disease, the population most likely to be tested by veterinarians. Caution is needed in making conclusions about the frequency of specific clinical signs associated with parasitism in this study, as it cannot be certain that all signs were recorded by the submitting veterinary surgeon. Nevertheless, it is clear that while a substantial proportion of animals with diarrhoea may harbour enteric protozoa, the majority do not. Additionally, to accurately address the differences in parasite prevalence between a cat population with signs of alimentary disease and one free of clinical signs, a large, nationally derived control population accrued over a similar 2-year period would be desirable.
Finally, prevalence data are an essential component for evaluation of zoonotic risk, but knowledge of the specific genotype and species of Giardia and Cryptosporidium species, respectively, would be vital for this assessment. Infection of humans by C felis has been reported (Pedraza-Diaz et al 2001, Caccio et al 2002, Gatei et al 2002). However, the majority of studies examining zoonotic risk were performed prior to routine use of PCR, making assessment of risk difficult.
In conclusion, this is the largest study of its kind investigating prevalence of Cryptosporidium, Giardia and Isospora species infection in domestic cats with alimentary signs. The results show that enteric protozoal infection is prevalent in the UK, but that none of these organisms show a characteristic pattern of clinical signs. Importantly, infection with Giardia duodenalis may be missed using conventional microscopy for diagnosis. Both Giardia species and I felis infection are more common in cats less than 6 months of age and Cryptosporidium species and I felis infection are more prevalent towards the end of the year. The results highlight the potential role of domestic cats for zoonotic transmission of cryptosporidiosis and giardiasis. Routine treatment of young cats with toltrazuril (for coccidia) and subsequently febantel/pyrantel for three consecutive days (for helminths and Giardia species) has much to recommend it, both for the benefit of the cat and also for the reduced zoonotic risk to its owners and other human contacts.
Acknowledgements
D.J.B. is funded by Hill's Pet Nutrition Ltd. A.J.G.'s lectureship is funded by Royal Canin. The authors are grateful to the staff of NationWide Laboratories for their co-operation in this research, and to the numerous veterinarians who submitted faecal samples for analysis. The ProSpecT Giardia microplate assays were kindly donated by Oxoid, Thermo Fisher Scientific.
References
- Arai H., Fukuda Y., Hara T., Funakoshi Y., Kaneko S., Yoshida T., Asahi H., Kumada M., Kato K., Koyama T. Prevalence of Cryptosporidium infection among domestic cats in the Tokyo Metropolitan District, Japan, Japanese Journal of Medical Science and Biology 43, 1990, 7–14. [DOI] [PubMed] [Google Scholar]
- Asahi H., Koyama T., Arai H., Funakoshi Y., Yamaura H., Shirasaka R., Okutomi K. Biological nature of Cryptosporidium species isolated from a cat, Parasitology Research 77, 1991, 237–240. [DOI] [PubMed] [Google Scholar]
- Barr S.C., Bowman D.D. Giardiasis of dogs and cats, Compendium of Continuing Education for the Practising Veterinarian 16, 1994, 603–614. [Google Scholar]
- Barr S.C. Enteric protozoal infections. Greene C.E. Infectious Disease of the Dog and Cat, 2006, Elsevier Saunders: St Louis, 736–750. [Google Scholar]
- Barutzki D., Schaper R. Endoparasites in dogs and cats in Germany 1999–2002, Parasitology Research 90 (Suppl. 3), 2003, S148–S150. [DOI] [PubMed] [Google Scholar]
- Becker S.V., Selby L.A., Hutcheson D.P., Hacker D.V. The association of selected climatic factors with natural alimentary parasites of the dog, Environmental Research 14, 1977, 141–151. [DOI] [PubMed] [Google Scholar]
- Bennett M., Baxby D., Blundell N., Gaskell C.J., Hart C.A., Kelly D.F. Cryptosporidiosis in the domestic cat, Veterinary Record 116, 1985, 73–74. [DOI] [PubMed] [Google Scholar]
- Bianciardi P., Papini R., Giuliani G., Cardini G. Prevalence of Giardia antigen in stool samples from dog and cats, Revue Medecine Veterinaire 155, 2004, 417–421. [Google Scholar]
- Brightman A.H., II, Slonka G.F. A review of five clinical cases of giardiasis in cats, Journal of the American Animal Hospital Association 12, 1976, 492–497. [Google Scholar]
- Caccio S.M., Pinter E., Fantini R., Mezzaroma I., Pozio E. Human infection with Cryptosporidium felis: case report and literature review, Emerging Infectious Diseases 8, 2002, 85–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirak V.Y., Bauer C. Comparison of conventional coproscopical methods and commercial coproantigen ELISA kits for the detection of Giardia and Cryptosporidium infections in dogs and cats, Berliner und Muenchener Tieraerztliche Wochenschrift 117, 2004, 410–413. [PubMed] [Google Scholar]
- Dubey J.P. Intestinal protozoa infections, Veterinary Clinics of North America Small Animal Practice 23, 1993, 37–55. [DOI] [PubMed] [Google Scholar]
- De Santis-Kerr A.C., Raghavan M., Glickman N.W., Caldanaro R.J., Moore G.E., Lewis H.B., Schantz P.M., Glickman L.T. Prevalence and risk factors for Giardia and coccidia species of pet cats in 2003–2004, Journal of Feline Medicine and Surgery 8, 2006, 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epe C., Coati N., Schnieder T. Results of parasitological examinations of faecal samples from horses, ruminants, pigs, dogs, cats, hedgehogs and rabbits between 1998 and 2002, Deutsche Tieraerztliche Wochenscherift 111, 2004, 243–247. [PubMed] [Google Scholar]
- Gatei W., Suputtamongkol Y., Waywa D., Ashford R.W., Bailey J.W., Greensill J., Beeching N.J., Hart C.A. Zoonotic species of Cryptosporidium are as prevalent as the anthroponotic in HIV-infected patients in Thailand, Annals of Tropical Medicine and Parasitology 96, 2002, 797–802. [DOI] [PubMed] [Google Scholar]
- Glaser C.A., Safrin S., Reingold A., Newman T.B. Association between Cryptosporidium infection and animal exposure in HIV-infected individuals, Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 17, 1998, 79–82. [DOI] [PubMed] [Google Scholar]
- Gookin J.L., Stebbins M.E., Hunt E., Burlone K., Fulton M., Hochel R., Talaat M., Poore M., Levy M.G. Prevalence of and risk factors for feline Tritrichomonas foetus and giardia infection, Journal of Clinical Microbiology 42, 2004, 2707–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat R. (2003) Survey of clinic practices and testing for diagnosis of Giardia infections in dogs and cats. 2003 ACVIM Forum, June 4–8, Charlotte, NC.
- Hill S.L., Cheney J.M., Taton-Allen G.F., Reif J.S., Bruns C., Lappin M.R. Prevalence of enteric zoonotic organisms in cats, Journal of the American Veterinary Medical Association 216, 2000, 687–692. [DOI] [PubMed] [Google Scholar]
- Huber F., Bomfin T.C.B., Gomes R.S. Comparison among infection with Cryptosporidium sp. and Giardia sp. in cats under two breeding systems, Revista Brasileira de Parasitologia Veterinaria 11, 2002, 7–12. [Google Scholar]
- Kirkpatrick C.E. Epizootiology of endoparasitic infections in pet dogs and cats presented to a veterinary teaching hospital, Veterinary Parasitology 30, 1988, 113–124. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Blagburn B.L. Coccidial parasites of cats and dogs, Compendium on Continuing Education for the Practicing Veterinarian 13, 1991, 759–765. [Google Scholar]
- Lindsay D.S., Dubey J.P., Blagburn B.L. Biology of Isospora species from humans, non-human primates, and domestic animals, Clinical Microbiology Reviews 10, 1997, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S., Smith J. Activity of toltrazuril and diclazurial against Isospora species in kittens and puppies, Veterinary Record 148, 2001, 509–511. [DOI] [PubMed] [Google Scholar]
- Lopez J.D., Abarca K.V., Paredes M.P., Inzunza T.E. Intestinal parasites in dogs and cats with gastrointestinal symptoms in Santiago, Chile, Revista Médica de Chile 134, 2006, 193–200. [DOI] [PubMed] [Google Scholar]
- Marks S.L., Hanson T.E., Melli A.C. Comparison of direct immunofluorescence, modified acid-fast staining, and enzyme immunoassay techniques for detection of Cryptosporidium species in naturally exposed kittens, Journal of the American Veterinary Medical Association 225, 2004, 1549–1553. [DOI] [PubMed] [Google Scholar]
- Martins C.A., Guerrant R.L. Cryptosporidium and cryptosporidiosis, Parasitology Today 11, 1995, 434–436. [DOI] [PubMed] [Google Scholar]
- McGlade T.R., Robertson I.D., Elliot A.D., Read C., Thompson R.C. Gastrointestinal parasites of domestic cats in Perth, Western Australia, Veterinary Parasitology 117, 2003a, 251–262. [DOI] [PubMed] [Google Scholar]
- McGlade T.R., Robertson I.D., Elliot A.D., Thompson R.C. High prevalence of Giardia detected in cats by PCR, Veterinary Parasitology 110, 2003b, 197–205. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Amar C., Pedraza-Diaz S., Nichols G.L. Molecular epidemiological analysis of Cryptosporidium species in the United Kingdom: results of genotyping Cryptosporidium species in 1,705 fecal samples from humans and 105 fecal samples from livestock animals, Journal of Clinical Microbiology 38, 2000, 3984–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds C.A., Lappin M.R., Ungar B., McReynolds L.M., Bruns C., Spilker M.M., Thrall M.A., Reif J.S. Regional seroprevalence of Cryptosporidium parvum-specific IgG of cats in the United States, Veterinary Parasitology 80, 1999, 187–195. [DOI] [PubMed] [Google Scholar]
- Meloni B.P., Thompson R.C., Hopkins R.M., Reynoldson J.A., Gracey M. The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley, Medical Journal of Australia 158, 1993, 157–159. [DOI] [PubMed] [Google Scholar]
- Mtambo M.M., Nash A.S., Blewett D.A., Smith H.V., Wright S. Cryptosporidium infection in cats: prevalence of infection in domestic and feral cats in the Glasgow area, Veterinary Record 129, 1991, 502–504. [PubMed] [Google Scholar]
- Nash A.S., Mtambo M.M., Gibbs H.A. Cryptosporidium infection in farm cats in the Glasgow area, Veterinary Record 133, 1993, 576–577. [PubMed] [Google Scholar]
- Nolan T.J., Smith G. Time series analysis of the prevalence of endoparasitic infections in cats and dogs presented to a veterinary teaching hospital, Veterinary Parasitology 59, 1995, 87–96. [DOI] [PubMed] [Google Scholar]
- Nutter F.B., Dubey J.P., Levine J.F., Breitschwerdt E.B., Ford R.B., Stoskopf M.K. Seroprevalences of antibodies against Bartonella henselae and Toxoplasma gondii and fecal shedding of Cryptosporidium species, Giardia species, and Toxocara cati in feral and pet domestic cats, Journal of the American Veterinary Medical Association 225, 2004, 1394–1398. [DOI] [PubMed] [Google Scholar]
- O'Brien C.R., Pope S.E., Malik R. Vomiting, diarrhoea and inappetence in a young cat with hypoproteinaemia, Australian Veterinary Journal 80, 2002, 544–551. [DOI] [PubMed] [Google Scholar]
- Pedraza-Diaz S., Amar C., Iversen A.M., Stanley P.J., McLauchlin J. Unusual Cryptosporidium species recovered from human faeces: first description of Cryptosporidium felis and Cryptosporidium ‘dog type’ from patients in England, Journal of Clinical Microbiology 50, 2001, 293–296. [DOI] [PubMed] [Google Scholar]
- Ramirez N.E., Ward L.A., Sreevatsan S. A review of the biology and epidemiology of cryptosporidiosis in humans and animals, Microbes and Infection 6, 2004, 773–785. [DOI] [PubMed] [Google Scholar]
- Robben S.R., le Nobel W.E., Dopfer D., Hendrikx W.M., Boersema J.H., Fransen F., Eysker M.E. Infections with helminths and/or protozoa in cats in animal shelters in the Netherlands, Tijdschrift Diergeneeskd 129, 2004, 2–6. [PubMed] [Google Scholar]
- Robertson R.C.A. Giardiasis as a re-emerging infectious disease and its zoonotic potential, International Journal for Parasitology 30, 2000, 1259–1267. [DOI] [PubMed] [Google Scholar]
- Robertson I.D., Irwin P.J., Lymbery A.J., Thompson R.C. The role of companion animals in the emergence of parasitic zoonoses, International Journal of Parasitology 30, 2000, 1369–1377. [DOI] [PubMed] [Google Scholar]
- Sargent K.D., Morgan U.M., Elliot A., Thompson R.C. Morphological and genetic characterisation of Cryptosporidium oocysts from domestic cats, Veterinary Parasitology 77, 1998, 221–227. [DOI] [PubMed] [Google Scholar]
- Scorza A.V., Brewer M.M., Lappin M.R. Polymerase chain reaction for the detection of Cryptosporidium spp. in cat feces, Journal of Parasitology 89, 2003, 423–426. [DOI] [PubMed] [Google Scholar]
- Serra C.M., Uchoa C.M., Coimbra R.A. Parasitological study with faecal samples of stray and domiciliated cats (Felis catus domesticus) from the Metropolitan Area of Rio de Janeiro, Brazil, Revista da Sociedade Brasileira de Medicina Tropical 36, 2003, 331–334. [DOI] [PubMed] [Google Scholar]
- Spain C.V., Scarlett J.M., Wade S.E., McDonough P. Prevalence of enteric zoonotic agents in cats less than 1 year old in central New York State, Journal of Veterinary Internal Medicine 15, 2001, 33–38. [DOI] [PubMed] [Google Scholar]
- Swan J.M., Thompson R.C. The prevalence of Giardia in dogs and cats in Perth, Western Australia, Australian Veterinary Journal 63, 1986, 110–112. [DOI] [PubMed] [Google Scholar]
- Thompson R.C. Giardiasis as a re-emerging infectious disease and its zoonotic potential, International Journal of Parasitology 30, 2000, 1259–1267. [DOI] [PubMed] [Google Scholar]
- Thompson R.C. The zoonotic significance and molecular epidemiology of Giardia and giardiasis, Veterinary Parasitology 126, 2004, 15–35. [DOI] [PubMed] [Google Scholar]
- Tonks M.C., Brown T.J., Ionas G. Giardia infection of cats and dogs in New Zealand, New Zealand Veterinary Journal 39, 1991, 33–34. [DOI] [PubMed] [Google Scholar]
- Vanparijs O., Hermans L., van der Flaes L. Helminth and protozoan parasites in dogs and cats in Belgium, Veterinary Parasitology 38, 1991, 67–73. [DOI] [PubMed] [Google Scholar]
- Vasilopulos R.J., Rickard L.G., Mackin A.J., Pharr G.T., Huston C.L. Genotypic analysis of Giardia duodenalis in domestic cats, Journal of Veterinary Internal Medicine 21, 2007, 352–355. [DOI] [PubMed] [Google Scholar]
- WHO (1996) The world health report 1996. Geneva: World Health Organisation. [PubMed] [Google Scholar]
- Wilkinson G.T. Coccidial infection in a cat colony, Veterinary Record 100, 1977, 156–157. [DOI] [PubMed] [Google Scholar]
- Winsland J.K., Nimmo S., Butcher P.D., Farthing M.J. Prevalence of Giardia in dogs and cats in the United Kingdom: survey of an Essex veterinary clinic, Transactions of the Royal Society of Tropical Medicine and Hygiene 83, 1989, 791–792. [DOI] [PubMed] [Google Scholar]