Abstract
Research has documented immobilization of rodents, rabbits, guinea pigs and dogs by mechanical means, typically using neck clips or inversion (‘animal hypnosis’). In contrast, only a few studies of mechanical immobilization of cats are available, although some success has been reported in the literature. Domestic cats may be effectively immobilized by clips placed along the animal's dorsum. We use the term ‘pinch-induced behavioral inhibition’ (PIBI) for this behavior because it describes both the method and the response, while avoiding the more anthropomorphic term ‘hypnosis’. We investigated the effectiveness of PIBI and its neurological and habituation effects in healthy cats and cats with idiopathic cystitis (IC). Although not all cats were susceptible to PIBI and effectiveness varied among individuals, PIBI was useful for gentle restraint in most cats.
Animal hypnosis is sometimes used to describe a spectrum of immobility behaviors induced by a variety of means (Gallup 1974). The effect has been demonstrated in animals representing a variety of taxa, including insects, reptiles, birds and mammals (Gallup 1974). According to Fleischmann and Urca (1988), animal hypnosis describes a behavioral state in which the animal is both immobilized and desensitized to external stimuli. The practise was known to the ancient Egyptians, and reported in the western literature as early as 1646 (Klemm 1971). Immobility is assumed to have survival value in nature because many instances can be found in the wild. For example, snakes transfix prey with their gaze, and baby mammals become limp when their mother picks them up by the neck with her jaws. Both of these behaviors describe induction of immobilization by external stimuli, although the mechanisms may be quite different. Because there are many ways to induce immobilization, a variety of terms, such as hypnosis, mesmerism, scruff immobility reflex, behavioral arrest, bewitchment and fascination have been used to describe the behaviors (Lefebvre and Sabourin 1977).
There has been much research interest in the mechanisms underlying immobilization through mechanical inhibition in mice, rats, rabbits, and guinea pigs. In these animals, immobility is typically induced using neck clips or inversion. Although there has been little research on immobilization of cats, there have been several reports of success in immobilizing cats by placing clips along their dorsal midline (Lefebvre and Sabourin 1977, Toutain 1978a,b, Tarttelin 1991, 1993, Gagnon 2006). We also have found that cats are often effectively immobilized when clips are placed along the animal's neck. For cats, we have chosen to use the term ‘pinch-induced behavioral inhibition’ (PIBI) because it describes both the method and the response, while avoiding the more anthropomorphic term ‘hypnosis’. Furthermore, cats typically are not fully immobilized by the clips. The cat may be immobilized, but often the clipped animal retains some mobility with a decreased response to external stimuli. Because the cat may retain some mobility, PIBI is an inhibition response rather than true ‘animal hypnosis’. Therefore, ‘behavioral inhibition’ describes the clips' effects more accurately than does ‘immobility’. PIBI is more casually known as ‘clipnosis’.
Although this phenomenon has been reported previously, it has not been widely recognized as a safe and useful form of restraint for cats. The purpose of this report is to describe our experiences with PIBI and discuss the clinical applications of this form of restraint for cats. To this end, we investigated susceptibility to and some effects of PIBI in healthy cats and cats with idiopathic cystitis (IC). We included cats diagnosed with IC because our laboratory studies this syndrome, and cats with IC are unusually sensitive to stressors, including restraint (Westropp et al 2006).
Materials and methods
Animals
All cats used in this study were individually housed in stainless steel cages in the vivarium of The Ohio State University College of Veterinary Medicine and fed a standard dry commercial diet. The Animal Care and Use Committee of The Ohio State University approved all of the experimental procedures. Five healthy male and eight healthy female neutered cats ranging in age from 1 to 5 years, and 11 male and seven female neutered cats with IC ranging in age from 1 to 10 years, were studied. The evaluation of health status and diagnosis of IC was performed as previously described (Westropp et al 2006).
Clips
One or two standard 2 inch binder clips (Staples) were used to induce PIBI. The pressure applied was estimated by applying a clip to a blood pressure cuff attached to a Propper pressure gauge (Speidel+Keller, Jungingen, Germany) inflated to approximately 10 mmHg and recording the pressure rise.
Procedure
The cats were removed from their home cages, placed on an examination table in the hallway of their housing room, and the clip(s) placed along the dorsal midline between C1 and C7 (Fig 1). The first clip was placed directly behind the ears (this is the scruff area, commonly used by queens to pick up and carry kittens). The second clip, if used, was placed immediately behind the first. After placing the clip(s) on the cat, PIBI was immediately ranked on a Likert scale ranging from −3 to +3. A rank of −3 indicated a marked negative response to the clip(s) (arousal, vocalization, attempts to remove clip), 0 indicated no response, and +3 indicated a marked positive response (behavioral inhibition). After ranking the response the clips were removed.
Fig 1.

Placement of clips along the dorsal cervical region of a cat.
Habituation to PIBI
All cats were clipped 1, 2, and 3 months after initial clipping. PIBI response was ranked and compared to the previous month's PIBI ranking.
Scruffing and PIBI
In month 4 (3 months after initial clipping), 12 of 13 healthy cats and 16 of 18 cats with IC were gently lifted by the scruff of the neck to determine whether the cat's response to scruffing would predict the response to PIBI using the same scale used for ranking PIBI.
Neurological examinations and PIBI
Four of 13 healthy cats (two males and two females), and six (three males and three females) of 18 cats with IC were examined both before and during clipping by a board certified veterinary neurologist (SW). Mentation, menace response, facial sensation, and front and back leg support capabilities were examined. The responses were scored before and after clipping as: 0=normal, 1=mildly decreased, 2=moderately decreased, 3=markedly decreased.
Statistical analysis
Response to PIBI over time was evaluated using Kruskal–Wallis test. Scruffing and clipping were evaluated using linear regression analysis, and the effect of clipping on all neurological parameters was evaluated using Wilcoxon signed rank test.
Results
Measurement of pressure applied by the clips ranged from 140 to 160 mmHg. On initial exposure, 12 of 13 healthy cats (92%) had a positive PIBI score, and one had a moderate negative PIBI score (−2). All 18 of the cats with IC (100%) had a positive PIBI score. No effects of age or gender were identified in either group.
At month 2, PIBI scores increased in seven of 13 healthy cats, stayed the same in four, and decreased in two. In month 3, PIBI scores increased in two cats, stayed the same in six, and decreased in five. The PIBI scores increased in three of 18 cats with IC, stayed the same in five, and decreased in 10 in month 2. In month 3, scores increased in five cats, stayed the same in 10, and decreased in three (Fig 2).
Fig 2.
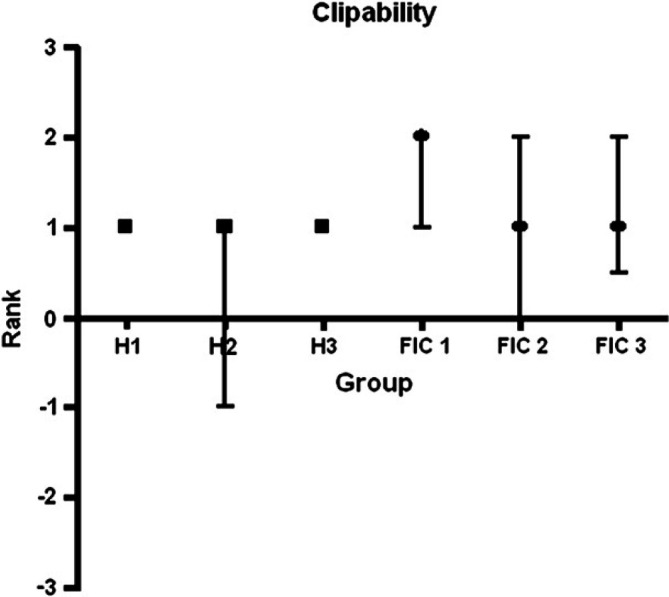
Effect of repeated clipping on response in healthy (H) cats and cats with IC. Only H2 and IC 1 were statistically different using Kruskal–Wallis test.
Scruffing and PIBI
A positive scruffing score almost always indicated a positive PIBI score. Likewise, a negative scruffing score almost always indicated a negative PIBI score. A closer relationship between responses to scruffing and clipping was observed in cats with IC than in healthy cats (r2=0.72, P<0.0001) (Fig 3).
Fig 3.
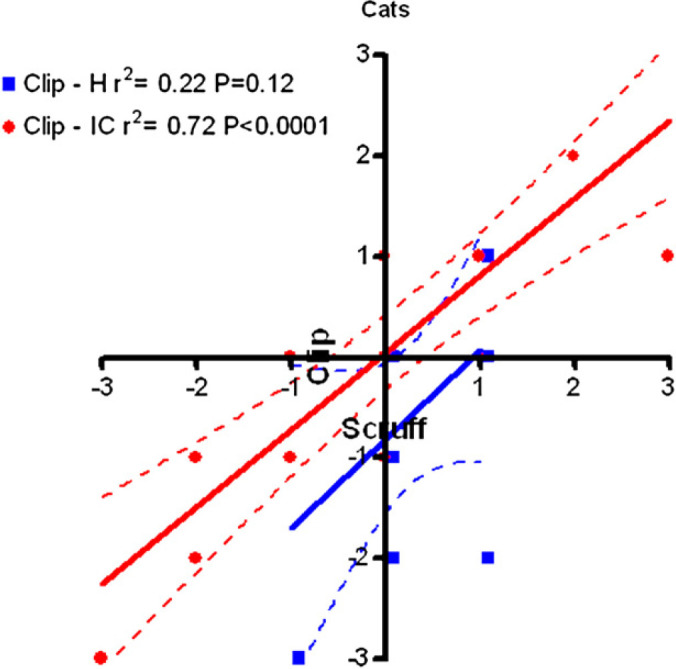
Regression (best fit line and 95% confidence limits) of response to scruffing onto response to clipping in healthy cats and cats with IC.
Neurological examinations and PIBI
No differences were observed between responses of healthy cats and cats with IC or gender, so the data were pooled for analysis. Only mentation (25th percentile=2, median=2, 75th percentile=1; P=0.004) was significantly decreased by PIBI. During clip application, miosis was observed in all cats.
Discussion
The scruff response in cats is ‘almost identical to the immobility produced in small kittens when they are picked up by the skin of the neck by their mother and transported from one nest site to another’ (Hart 1978). The kitten's tail curls up under the body, the back ventroflexes, and the animal becomes passive. Veterinarians and cat owners alike have long recognized that ‘scruffing’ a cat can provide restraint for minor procedures such as administering injections or trimming claws. PIBI induced a comparably passive behavior when the clips were applied to the scruff of the neck.
We became interested in using PIBI because of its effectiveness in inhibiting mobility during phlebotomy and other minor procedures, and because it seemed to have a ‘relaxing’ effect on many cats even after removal of the clips. Cats usually seemed more content, even purring, and less fearful during veterinary procedures when clips were used instead of restraint by other means. Some veterinarians have even called clipping their ‘third hand’ because of its usefulness in facilitating veterinary procedures in a clinical setting (Gagnon 2006). PIBI has provided a generally effective alternative to more traditional means of restraining cats.
We found that, as with scruffing, not all cats were susceptible to PIBI, and the effectiveness varied among individuals. In month 1 (initial clipping), 92% of 13 healthy cats and 100% of 18 cats with IC responded positively to clipping. Tarttelin (1991) reported that 67% of 60 cats responded to clipping, while 33% showed no response. Gagnon (2006) has found that 69% of 100 cats responded to PIBI (using two fingers instead of clips). In our study, one healthy cat (PIBI score −2) responded negatively to the clips with vocalization and biting, and one cat with IC (PIBI score +3) immediately fell to his side and appeared almost catatonic. However, the healthy cat's PIBI score increased the following month. Although we did not identify an effect of time on response to PIBI, our subsequent experience has suggested that most cats become more rather than less tolerant to PIBI after repeated experiences with the procedure. Individual IC cats' responses were more variable than those of the healthy cats. We were not able to identify a particular source of this variation, although it may be related to environmental circumstances in the vivarium. For example, the absence of the cats' regular caretaker may have affected the response to PIBI among the cats with IC in month 2. We have observed that calm, quiet cats respond much better to clipping than do agitated cats. In clinical settings, we recommend clipping before attempting any procedures, and not as a last resort. Cats that are already aroused are less likely to respond positively to application of clips.
The physiological response to PIBI was somewhat similar to that of scruffing. For example, miosis and ventroflexion of the back with the tail (Fig 4) curled up under the body occur during both procedures (Reiner 1986). Although we believe that PIBI is preferable to scruffing, we investigated scruffing as a possible predictor of positive PIBI response because of their similarities. We found that scruffing provided a reasonable prediction of whether or not cats, particularly cats with IC, would respond positively to the clips. This assessment may be useful in some clinical settings to save time and energy that might otherwise be wasted trying to clip a cat that would not be likely to respond to PIBI.
Fig 4.

Ventroflexion of the back and tail placement during positive PIBI.
It is interesting to note that the scruff is not the only location on the cat that stimulates PIBI (Beyaert et al 2003). Clips placed anywhere along the dorsal midline may produce some response. Although it is unknown whether the PIBI mechanism is related to acupuncture point stimulation, the dorsal midline has been called the ‘governing vessel meridian’ by acupuncturists, and stimulation of points along this line is reported to decrease activity (Gagnon 2006). In our experience, however, application of clips to the neck seems to maximize clip effectiveness. Clip pressures also affected PIBI induction. We experimented with a variety of clips, including clothes' pins (Fig 5), kitchen clips, and binder clips. We found that the most effective clips were those that produced pressures in the range of systolic blood pressure. This same relationship between pressure and PIBI was found in rats (Meyer 1990) and cats (Toutain 1978a). Although we used clip pressures that did not exceed physiological blood pressures to ensure that clip application did not result in ischemic damage to the skin of the animal, pressures greater than 300 mmHg for prolonged periods of time (>3 h) have been reported to be necessary to induce ischemia-related injury (discussed in Tsuji et al 2005).
Fig 5.
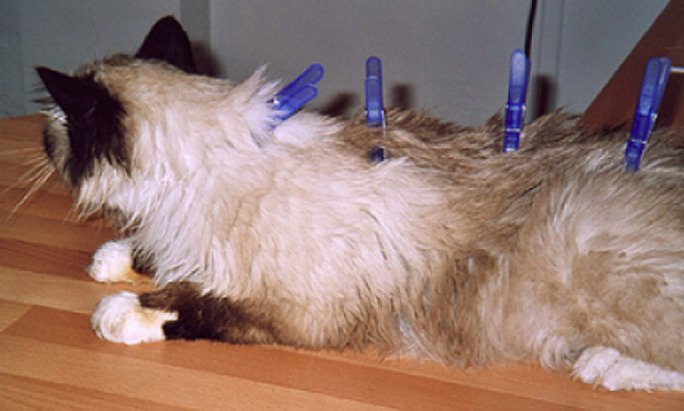
Clipnosis using clothes' pins.
The only difference in neurological function observed in the cats after application of the clips was a decrease in mentation, suggesting a forebrain site of action. The altered mentation was not characteristic of decreased arousability, as would be observed in obtunded or stuporous animals. Instead, the difference was in content; the changes were not appropriate in the given environment, in which more active behavior was observed without the clips. Interestingly, the mentation change appeared to have positive characteristics associated with it. The cats appeared calmer, and most purred and kneaded with their paws while the clips were attached. In the cats that had the most pronounced responses, the menace response also was decreased or absent, further supporting a forebrain localization of the effect.
Reiner (1986) reported significant pupillary miosis and decreased central and peripheral sympathetic neuron activities during the induction of the scruff immobility reflex, suggesting that the reflex is not noxious (Boscan et al 2005). In contrast to PIBI, tonic (or unresponsive) immobility is ‘the sudden onset of prolonged stillness and decreased responsivity in a previously active animal in the face of threatening stimulation’ (Marks 1987, reviewed in Moskowitz 2004). Tonic immobility represents a fear response, and can be elicited in a wide range of animals, ranging from arthropods to fish, amphibians, reptiles, birds, and mammals. Although tonic immobility also is characterized by lack of movement, animals reportedly maintain unusual postures for prolonged periods, with muscular rigidity and waxy flexibility of limbs. Vocal behavior is typically suppressed, and the animal is unresponsive to even intense or painful stimulation. This strategy is presumed to have developed to increase the probability of survival, as many predators lose interest in prey that remains motionless for a period of time.
PIBI also should be distinguished from attentive immobility, which is more appropriately described as freezing (Moskowitz 2004). Attentive immobility is primarily a transient defense strategy while the animal stops moving to avoid detection and to better locate a predator and plan escape. In contrast to tonic immobility, the freezing animal is highly responsive to stimuli, such as touch, and remains in the alert posture typical for that species (Marks 1987).
We conclude that PIBI is not a fear or pain response, and instead may have evolved to facilitate transportation of the kitten by the mother cat. We have observed that young cats and kittens are more responsive to PIBI than adults, suggesting that this may be a procedure to be introduced to owners as early in the cat's life as possible to enhance effects. The profound PIBI response in young cats and kittens also suggests that this may be the residue of a conserved juvenile mechanism in adult cats. The mechanisms underlying immobility remain uncertain (Klemm 1971, Fleischmann and Urca 1988, Klemm 2001, Beyaert et al 2003).
No cat exhibited behavior that was interpreted as evidence that they were in pain, eg, tachypnea, tachycardia, mydriasis. Furthermore, we observed the effects of clip application on 15 cats (eight cats with IC and seven healthy cats) with implanted telemetry devices and detected no significant changes in heart rate, blood pressure, or body temperature. These observations, particularly the absence of stress-induced hyperthermia, lead us to conclude that the clips were not painful for the cats (unpublished data). Moreover, in clinical use we regularly observe miosis, bradypnea, bradycardia, and less resistance to venepuncture, which further suggests the absence of pain. Additional research will be necessary to determine potential analgesic effects of PIBI in cats. However, we conclude that PIBI can be a safe, convenient, benign method of restraint for a variety of routine veterinary procedures such as physical examination, minor wound care, venepuncture, vaccinations, blood glucose monitoring, and nail trimming.
References
- Beyaert C.A., Haouzi P., Marchal F. Inhibition of midbrain-evoked tonic and rhythmic motor activity by cutaneous stimulation in decerebrate cats, Experimental Brain Research 149, 2003, 159–166. [DOI] [PubMed] [Google Scholar]
- Boscan P., Dutschmann M., Herbert H., Paton J.F. Neurokininergic mechanism within the lateral crescent nucleus of the parabrachial complex participates in the heart-rate response to nociception, Journal of Neuroscience 25, 2005, 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A., Urca G. Clip-induced analgesia and immobility in the mouse: pharmacological characterization, Neuropharmacology 27, 1988, 641–648. [DOI] [PubMed] [Google Scholar]
- Gagnon AC. (2006) Unpublished observations.
- Gallup G.G., Jr. Animal hypnosis: factual status of a fictional concept, Psychological Bulletin 81, 1974, 836–853. [DOI] [PubMed] [Google Scholar]
- Hart B.L. Handling and restraint of the cat, Feline Practice 5, 1978, 10–11. [Google Scholar]
- Klemm W.R. Neurophysiologic studies of the immobility reflex (‘animal hypnosis’), Neurosciences Research 4, 1971, 165–212. [PubMed] [Google Scholar]
- Klemm W.R. Behavioral arrest: in search of the neural control system, Progress in Neurobiology 65, 2001, 453–471. [DOI] [PubMed] [Google Scholar]
- Lefebvre L., Sabourin M. Response differences in animal hypnosis: a hypothesis, Psychological Record 1, 1977, 77–87. [Google Scholar]
- Marks I.M. Fears, Phobias, and Rituals: Panic, Anxiety, and their Disorders, 1987, Oxford University Press: New York. [Google Scholar]
- Meyer M.E. Dorsal pressure potentiates the duration of tonic immobility and catalepsy in rats, Physiology and Behaviour 47, 1990, 531–533. [DOI] [PubMed] [Google Scholar]
- Moskowitz A.K. ‘Scared stiff’: catatonia as an evolutionary-based fear response, Psychological Review 111, 2004, 984–1002. [DOI] [PubMed] [Google Scholar]
- Reiner P.B. Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats, Brain Research 378, 1986, 86–96. [DOI] [PubMed] [Google Scholar]
- Tarttelin M.F. Restraint in the cat induced by skin clips, International Journal of Neuroscience 57, 1991, 288. [Google Scholar]
- Tarttelin M.F. Restraint induced by non-noxious skin clips: modifications of this technique results in a greater success rate in the adult cat, International Journal of Neuroscience 71, 1993, 131. [Google Scholar]
- Toutain P.L. L'hypnose Animale, Revue Médecine Vétérinaire 129, 1978a, 1289–1304, (in French) [Google Scholar]
- Toutain P.L. L'hypnose, moyen de contention chez le chat, L'Animale de Compagnie 6, 1978b, 725–729, (in French) [Google Scholar]
- Tsuji S., Ichioka S., Sekiya N., Nakatsuka T. Analysis of ischemia–reperfusion injury in a microcirculatory model of pressure ulcers, Wound Repair and Regeneration 13, 2005, 209–215. [DOI] [PubMed] [Google Scholar]
- Westropp J.L., Kass P.H., Buffington C.A.T. Evaluation of the effects of stress in cats with idiopathic cystitis, American Journal of Veterinary Research 67, 2006, 731–736. [DOI] [PubMed] [Google Scholar]


