Abstract
Antagonistic effects of atipamezole (ATI), flumazenil (FLU) and 4-aminopyridine (4AP) alone and in various combinations after administration of medetomidine–midazolam–ketamine (MED–MID–KET) were evaluated in cats. Animals were anaesthetised with MED (50 μg/kg), MID (0.5 mg/kg) and KET (10 mg/kg) given intramuscularly. Twenty minutes later, physiological saline, ATI (200 μg/kg), FLU (0.1 mg/kg), 4AP (0.5 mg/kg), ATI–FLU, FLU–4AP, ATI–4AP or ATI–FLU–4AP was administered intravenously. FLU, 4AP alone, or FLU–4AP did not effectively antagonise the anaesthesia, hypothermia, bradycardia, and bradypnoea induced by MED–MID–KET. ATI alone was effective. ATI–FLU, ATI–4AP and ATI–FLU–4AP combinations produced an immediate and effective recovery from anaesthesia. The combination of ATI–FLU–4AP was the most effective in antagonising the anaesthetic effects, but was associated with tachycardia, tachypnoea, excitement, and muscle tremors. Combinations with ATI are more effective for antagonising anaesthesia, but ATI–FLU–4AP is not suitable.
The selective α2-adrenoceptor agonist, medetomidine (MED), is mainly used as a sedative, analgesic and muscle relaxant. However, it induces adverse cardiovascular effects such as hypertension and bradycardia in cats (Golden et al 1998, Lamont et al 2001, Selmi et al 2005). Midazolam (MID) is a water-soluble benzodiazepine that is used as an anxiolytic in human medicine (Brown et al 1979, Court and Greenbratt 1992). MID alone has not been used as a sedative agent for cats, because it induces ataxia, restlessness and abnormal behaviours that make cats more difficult to approach and restrain, and does not induce profound sedation in cats (Ilkiw et al 1996a). A combination of MED with MID has been reported to enhance the sedative and analgesic actions of the individual drug in rats (Salonen et al 1992) and pigs (Nishimura et al 1993), and to produce deep sedation in dogs (Itamoto et al 2000). This combination has also been reported to greatly reduce the anaesthetic induction dose of sodium thiopental and propofol in dogs (Kojima et al 2002). On the other hand, ketamine (KET) is widely used in feline practise as a dissociative anaesthetic agent. KET induces anaesthesia rapidly and causes minimal depression of the respiratory and cardiovascular systems, and has a wide margin of safety (Flecknell 1994). A wide range of KET doses are used for different purposes in feline practise. A MED–MID combination may be also used as a premedication before KET anaesthesia in cats. The combination of MID or MED with KET has been successfully used in cats (Verstegen et al 1991, Ilkiw et al 1996b, Sparkes et al 1996, Akkerdaas et al 2001). However, to the best of our knowledge, there are no reports on MED–MID or MED–MID–KET combinations in cats. Our preliminary studies indicated that a combination of MED–MID–KET produced good anaesthesia with excellent muscle relaxation and analgesia in cats.
Antagonism may be required when the anaesthetised animals show profound depression of vital signs, adverse effects of the given agents, and/or delayed recovery from anaesthesia. A selective α2-adrenoceptor antagonist, atipamezole (ATI), is used as an antagonist for sedation or bradycardia induced by MED in cats (Savola 1989, Vähä-Vahe 1990, Granholm et al 2006). Flumazenil (FLU), a potent and specific benzodiazepine antagonist, antagonises behavioural and neurological effects of benzodiazepines such as muscle relaxation and sedation (Brogden and Goa 1988, Sumida et al 1995). The effect of intravenous administration of variable-dose FLU following a fixed dose of KET and MID has been studied in healthy cats (Ilkiw et al 2002). 4-Aminopyridine (4AP) reverses non-depolarising neuromuscular and sympathetic ganglion blockade mainly because of the enhanced release of acetylcholine in cats (Durant et al 1980) and dogs (Rupp et al 1983), and partially antagonises the anaesthesia produced by KET or barbiturates in cats (Hatch et al 1983, 1984). In cats, however, there is little information available on the antagonistic effects of ATI, FLU and 4AP alone or in various combinations against the anaesthesia induced by a combination of MED, MID and KET.
The purpose of this study was to evaluate the antagonistic effects of intravenously administered ATI, FLU and 4AP alone and their combinations after anaesthesia produced by a fixed dose of MED, MID and KET injected intramuscularly in cats.
Materials and methods
Animals and designs
Our experimental protocols were approved by the Animal Research Committee of Tottori University. Eight healthy intact, adult mixed-breed cats (five females and three males) ranging in body weight from 3.0 to 3.9 kg were used. The cats were housed individually and fed dry food and water ad libitum. Routine haematological and plasma biochemical tests were performed before the study commenced. All values were within the normal physiological range. Food was withheld for 12 h before the experiment. One hour before the experiment, the animals were placed in the experimental room controlled at 25°C by air conditioning. Eight cats received eight different treatments at the rate of one treatment per week in a randomised crossover study design.
Each cat was given the mixture of 0.05 mg/kg MED (MED HCl, 1 mg/ml, Domitor; Meiji Seika Kaisha, Japan) and 0.5 mg/kg MID (MID, 5 mg/ml, Dormicum; Yamanouchi Pharmaceutical, Japan) followed 10 min later by 10 mg/kg KET (KET HCl, 50 mg/ml, Ketalar; Japan) intramuscularly. MED and MID were mixed in the same syringe immediately before injection. MED–MID and KET were injected into the semi-membranosus muscle. The MED–MID administration caused rapid sedation and no painful response to the injection; lateral recumbency with excellent muscle relaxation was achieved within 10 min in all of cats. KET induced anaesthesia smoothly within 5–10 min, without signs of pain in response to intramuscular injection or a hypertonic or cataleptic state. In this study, general anaesthesia was defined as when the cat showed no behavioural responses to analgesic and other stimuli under complete lateral recumbency and was without the movements described below.
Twenty minutes after KET injection, the cats were given either physiological saline solution (PSS) at a dose of 0.1 ml/kg (control), 0.2 mg/kg ATI (ATI HCl, 5 mg/ml, Antisedan; Meiji Seika Kaisha, Japan), 0.1 mg/kg FLU (FLU, 0.1 mg/ml, Anexate; Yamanouchi Pharmaceutical, Japan), 0.5 mg/kg 4AP (4AP, Wako Pure Chemical Industries; Japan), ATI–FLU, FLU–4AP, ATI–4AP or ATI–FLU–4AP intravenously. 4AP was dissolved in PSS at a concentration of 2.5 mg/ml. The potential antagonists were mixed in the same syringe immediately before injection, and injected into the cephalic vein.
Measurements
Elapsed times to recovery of the palpebral reflex, pedal reflex and tail clamp reflex were recorded after the injection of antagonists. Pedal and tail clamp reflexes were defined as the reflex withdrawal to clamping of interdigital web of the paw of a limb and the tail during 3 s using Kocher's forceps. Recovery times to head movement, sternal recumbency, standing and walking were also recorded. The degree of antagonism for anaesthesia was assessed using a slight modification of previously published scoring methods (Itamoto et al 2000) as follows. (1) Posture score: 0=normal; 1=ataxia, but able to walk; 2=completely prone, unable to walk; 3=lateral recumbency, but able to move the tail or paw; and 4=complete lateral recumbency without movement. (2) Analgesic scores: a reflex withdrawal to clamping of the tail, the skin of body surface at the paramedian abdomen and the interdigital web of paw of all four limbs during 3 s using Kocher's forceps. 0=Normal response; 1=reduced response; 2=faint response; and 3=no reflex. (3) Jaw tone score: 0=normal resistance to open the mouth; 1=the jaw can be opened, but there is still some resistance; 2=little resistance to open the mouth and obvious muscle relaxation; and 3=no resistance. (4) Auricular score: in response to a clapping sound behind the auricula. 0=Normal response; 1=dull response, but able to move body or head; 2=no body movement; and 3=completely no reflex at all. Total score was calculated as the sum of four scores, including (1) posture, (2) analgesia, (3) jaw tone, and (4) auricular scores. Rectal temperature was measured prior to injection of MED–MID (pre-value), immediately before injection of potential antagonists (0 time), and 30, 60, 90, 120, 150, 180, 240, and 300 min after injection of antagonists. Heart rate, respiration rate and each of the above four scores were recorded before injection of MED–MID (pre-value), immediately before administration of antagonists (0 time), and 1, 5, 15, 30, 45, 60, 75, 90, 120, 150, 180, 240, and 300 min after injection of antagonists. At each time point a cat was placed on an observation table for scoring. Posture, analgesia, jaw tone, and auricular scores were recorded in that order. Heart rate was measured using a stethoscope. Respiration rate was measured by observations of movements of the thorax. Cats were observed for behavioural and visible side effects such as excitation, congestion of conjunctiva, rigidity of limbs, muscle tremors, piloerection and emesis for 300 min after injection of potential antagonists.
Statistics
Data of rectal temperature, heart and respiration rates: one-way analysis of variance (ANOVA) for repeated measures was used to examine the effect of time within each treatment group and one-way ANOVA for treatment effect at each time point. When ANOVA was significant, the Tukey test was used for multiple comparisons of the means. Data of elapsed times to recovery from anaesthesia were also analysed by one-way ANOVA for treatment effect, and Tukey's multiple comparison test was used to identify differences between means. For comparisons between treatment groups in the score data, the non-parametric, Mann–Whitney test was used. The significance level of all tests was set at P<0.05.
Results
Recovery time from anaesthesia
Mean elapsed times to recovering the eyelid, pedal and tail clamp reflex after injection of potential antagonists were significantly shortened in the antagonists-injected groups when compared with the PSS-injected control (Table 1). Recovery times to head-up motion, prone position, standing and walking were not significantly different among the control, FLU and 4AP groups. These times in the groups given ATI were significantly shortened in comparison with the control or non-ATI groups. Mean elapsed times to head-up motion or prone position in ATI–FLU, ATI–4AP and ATI–FLU–4AP groups were significantly shortened compared to those in the ATI group. Mean elapsed times to either head-up motion or prone position in ATI–FLU–4AP group were significantly shorter than those in the ATI–FLU and ATI–4AP groups. Recovery to prone position was most rapid in the ATI–FLU–4AP group. However, there were no significant differences in recovery times to standing and walking among the ATI–FLU, ATI–4AP and ATI–FLU–4AP groups (Table 2).
Table 1.
Recovery times to eyelid, pedal and tail clamp reflexes after administrations of ATI, FLU, and 4AP alone or their combinations in cats anaesthetised with MED–MID–KET
| Groups | Elapsed time (min) | ||
|---|---|---|---|
| Eyelid reflex | Pedal reflex | Tail clamp reflex | |
| Control | 58±34 | 83±28 | 98±31 |
| ATI | 1±0 a | 2±1 a | 5±10 a |
| FLU | 6±10 a | 62±15a,b | 64±22a,b |
| 4AP | 12±17 a | 32±24a,b,c | 51±29a,b |
| ATI–FLU | 1±0 a | 1±0a,c,d | 1±0a,c,d |
| FLU–4AP | 3±5 a | 31±24a,b,c,e | 49±22a,b,e |
| ATI–4AP | 1±0 a | 1±0a,c,d,f | 2±1a,c,d,f |
| ATI–FLU–4AP | 1±0 a | 1±0a,c,d,f | 1±0a,c,d,f |
Each value represents mean±SD of eight cats;
=significantly different from control (P<0.05);
=significantly different from ATI (P<0.05);
=significantly different from FLU (P<0.05);
=significantly different from 4AP (P<0.05);
=significantly different from ATI–FLU (P<0.05);
=significantly different from FLU-4AP (P<0.05).
Table 2.
Recovery times to head-up motion, prone position, standing and walking after administrations of ATI, FLU, and 4AP alone or their combinations in cats anaesthetised with MED–MID–KET
| Groups | Elapsed time (min) | ||
|---|---|---|---|
| Head-up | Prone position | Standing and walking | |
| Control | 189±47 | 197±50 | 225±48 |
| ATI | 33±21 a | 44±21 a | 76.3±14 a |
| FLU | 194±52 b | 209±52 b | 236±60 b |
| 4AP | 143±35b,c | 155±38b,c | 186±33b,c |
| ATI–FLU | 8±17a,b,c,d | 31±24a,c,d | 76±26a,c,d |
| FLU–4AP | 139±31a,b,c,e | 145±32a,b,e | 183±44b,e |
| ATI–4AP | 15±16a,c,d,f | 21±21a,b,c,d,f | 68±20a,c,d,f |
| ATI–FLU–4AP | 2±1a,b,c,d,f,g | 8±6a,b,c,d,e,f | 58±17a,b,c,d,f |
Each value represents mean±SD of eight cats;
=significantly different from control (P<0.05);
=significantly different from ATI (P<0.05);
=significantly different from FLU (P<0.05);
=significantly different from 4AP (P<0.05);
=significantly different from ATI–FLU (P<0.05);
=significantly different from FLU–4AP (P<0.05);
=significantly different from ATI–4AP (P<0.05).
Anaesthetic and analgesic scores
In the PSS-injected control group, a profound anaesthesia was observed for approximately 90 min after MED–MID–KET injection. Thereafter, the cats recovered gradually, but ataxia continued until 300 min after PSS administration. In postural score, there were no significant differences between the control and non-ATI injected groups. Cats that received ATI alone had significantly lower postural scores at 45–240 min after injection when compared with PSS or non-ATI injected groups. Similarly, the cats receiving ATI–FLU, ATI–4AP and ATI–FLU–4AP had significantly lower postural scores at 1–240 min after injection of antagonists when compared with the control or non-ATI injected groups. There were no significant differences in postural score between the groups combined with ATI.
The differences among the groups in analgesic, jaw tone, and auricular scores were similar to those in postural score. In each component score, there was no significant difference between the control and FLU group. Analgesic and jaw tone scores in the 4AP group were significantly lower than those in the control group at 60–90 min after injection of 4AP. The cats in the FLU–4AP group had significantly lower analgesic, jaw tone, and auricular scores at 30–150 min when compared with controls. Each component score in the groups combined with ATI was significantly lower than that in the control cats at 1–180 min. The cats in ATI–FLU–4AP group had the lowest component score after injection of antagonists.
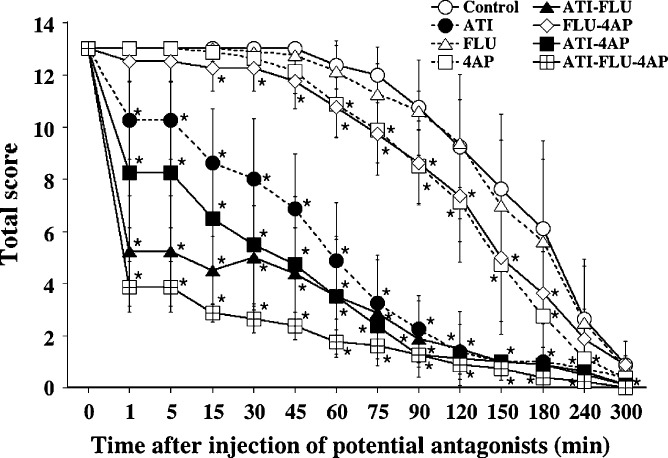
The results for total score are shown in Fig 1. In total score, there was no significant difference between the control and FLU groups. Total score in both 4AP and FLU–4AP groups was slightly and significantly lower than that in the control. The cats in the ATI group had a significantly lower total score at 15–240 min when compared with PSS or non-ATI injected groups. The cats in both ATI–FLU and ATI–4AP groups had significantly lower total scores at 1–180 min when compared with PSS or non-ATI injected groups. The cats in the ATI–FLU–4AP group had the lowest total score at 1–60 min after antagonist injection when compared with the other groups. There were no significant differences in total score between the groups combined with ATI from 75 to 300 min after injection of potential antagonists.
Fig 1.
Antagonistic effect of ATI, FLU and 4AP, as assessed by total score calculated as the sum of four component scores, in cats anaesthetised with MED–MID–KET. Each point and vertical bars indicate the mean value±SD of eight cats. *Significantly different from the control group (P<0.05).
Rectal temperature, heart and respiratory rates
Rectal temperature decreased significantly or tended to decrease from pre-values until 240 min in the control and non-ATI injected groups. Rectal temperature in ATI, ATI–FLU and ATI–4AP groups tended to decrease until 60 min, but recovered to pre-values at 180 min after administration of antagonists. Recovery time from the decreased rectal temperature was the fastest in the ATI–FLU–4AP group than the other groups.
Heart rates in the control and non-ATI injected groups were significantly reduced from pre-values. Heart rates in the ATI combined groups were significantly higher than those in control and non-ATI injected groups at 1–300 min after injection of antagonists. However, the cats in the ATI–FLU–4AP group showed tachycardia over 180 beats/min in the mean value at 1–120 min after injection. For example, heart rates [beats/min; mean±standard deviation (SD)] increased significantly from 142±15 of pre-value to 197±37 at 1 min, 218±28 at 5 min, 213±17 at 90 min, and 187±34 at 120 min after ATI–FLU–4AP injection.
Respiratory rates in the control, FLU and FLU–4AP groups decreased significantly or tended to decrease from pre-values until 300 min after injection. Respiratory rates in the ATI, ATI–FLU and ATI–4AP groups were significantly higher than those in the control and non-ATI injected groups at 1–150 min after injection of potential antagonists. There were no significant differences in respiratory rates among the ATI, ATI–FLU and ATI–4AP groups. Respiration rates in the ATI–FLU–4AP group tended to be higher than those in the other groups at 5–150 min after antagonist injection, and showed tachypnoea at 90 min after injection.
Behavioural side effects
Excitement, vocalising and aversion to body touch were observed in some of the cats that received ATI, 4AP, ATI–FLU or ATI–4AP, and in most of the cats that received ATI–FLU–4AP. Congestion of the conjunctiva was observed in some of the cats receiving ATI, ATI–4AP or ATI–FLU–4AP. Rigidity of limbs was observed in some cats that received FLU and 4AP, and in most after ATI, ATI–FLU, ATI–4AP or ATI–FLU–4AP. Emesis was observed in the control and non-ATI injected groups during recovery. Muscle tremors were observed in many cats that received ATI–4AP and ATI–FLU–4AP (Table 3).
Table 3.
Behavioural side effects after administrations of ATI, FLU, and 4AP alone or their combinations in cats anaesthetised with MED–MID–KET
| Groups | Excitement signs (initiation; duration) | Congestion of conjunctiva (initiation; duration) | Rigidity of four limbs (initiation; duration) | Emesis (initiation) | Muscle tremors (initiation; duration) |
| Control | 0/8* | 0/8 | 0/8 | 3/8 (148, 158, 189) | 0/8 |
| ATI | 2/8 (68±11; 83±32)† | 1/8 (90; 30) | 4/8 (53±19; 41±26) | 0/8 | 0/8 |
| FLU | 0/8 | 0/8 | 1/8 (75; 15) | 3/8 (187, 212, 214) | 0/8 |
| 4AP | 1/8 (120; 30) | 0/8 | 2/8 (135±21; 25±7) | 2/8 (144, 202) | 0/8 |
| ATI–FLU | 3/8 (110±17; 80±35) | 0/8 | 4/8 (34±14; 40±41) | 0/8 | 0/8 |
| FLU–4AP | 0/8 | 0/8 | 0/8 | 1/8 (140) | 0/8 |
| ATI–4AP | 3/8 (60±26; 62±15) | 2/8 (68±32; 23±11) | 3/8 (37±28; 33±24) | 0/8 | 4/8 (71±38; 49±43) |
| ATI–FLU–4AP | 7/8 (74±36; 35±27) | 3/8 (70±9; 25±17) | 6/8 (7±4; 62±69) | 0/8 | 5/8 (52±34; 27±20) |
Positive/examined cats.
Time (mean±SD, min) after injection of potential antagonists in positive cats.
Discussion
The present study showed that MED–MID–KET at the doses used produced good anaesthesia with excellent muscle relaxation and analgesia in cats. The clinically recommended dose of MED as a sedative-analgesic in cats is reported to be 0.01–0.04 mg/kg intravenously and 0.04–0.08 mg/kg intramuscularly (Sinclair 2003). Also, it is well known that the wide ranges in KET doses (2–4 mg/kg intravenously and 10–30 mg/kg intramuscularly) are used for different purposes in feline practise (Flecknell 1994). A previous study reported that intravenous administration of 0.05 and 0.5 mg/kg MID after 3 mg/kg KET had beneficial effects on behavioural responses in cats (Ilkiw et al 1996b). It caused a greater proportion of cats to assume a laterally recumbent position with head down compared with KET alone. In addition, doses of MID of 0.5 mg/kg or above reduced muscle rigidity observed in cats which received KET alone, and greatly diminished a nociceptive response to the tail or paw clamp in cats (Ilkiw et al 1996b). Based on the previous findings described above, we determined intramuscular doses of 0.05 mg/kg MED, 0.5 mg/kg MID and 10 mg/kg KET for this study. Therefore, this fixed dose of MED–MID–KET produced general anaesthesia with excellent muscle relaxation and analgesia for approximately 90 min in cats.
In this study, the ATI dose of 0.2 mg/kg was selected for the reversal of 0.05 mg/kg MED, because the effective reversal dose of ATI in cats has been found to be 2–4 times that of MED (Vähä-Vahe 1990, Cullen 1996, Granholm et al 2006). An FLU dose of 0.1 mg/kg was determined based on a previous report that, assuming a agonist–antagonist ratio of 13:1, an FLU dose of 0.04 mg/kg or above would be enough for complete reversal of 0.5 mg/kg MID, and that an intravenous administration of 0.1 mg/kg FLU shortened the period of initial recovery stages following 0.5 mg/kg MID and 3 mg/kg KET in cats (Ilkiw et al 2002). 4AP dose of 0.5 mg/kg was selected based on reports it partially antagonised the effects of KET or pentobarbital anaesthesia in cats (Hatch et al 1983, 1984).
The present study demonstrated that either FLU or 4AP alone did not markedly antagonise MED–MID–KET anaesthesia. On the other hand, ATI alone, ATI–FLU, ATI–4AP and ATI–FLU–4AP significantly hastened the recovery from anaesthesia induced by MED–MID–KET and a combination of ATI–FLU–4AP was most effective. It is, therefore, concluded that ATI alone and combinations with ATI are much more useful for antagonising MED–MID–KET anaesthesia in cats. However, the present study indicated that the quality of recovery was smoother in ATI alone or ATI–FLU than after both ATI–4AP and ATI–FLU–4AP, because the rigidity of limbs, muscle tremors and excitement that were observed in most cats received ATI–FLU–4AP, and muscle tremors were observed in many cats after ATI–4AP during recovery process.
The present study revealed that combinations with ATI were effective in antagonising the hypothermia, bradycardia and hypopnoea induced by MED–MID–KET anaesthesia. Reversal of bradycardia is mainly due to ATI (Granholm et al 2006). On the other hand, Savola (1989) reported that the anti-cholinergic drug, atropine, was not effective in antagonising MED-induced bradycardia in anaesthetised rats. However, Short et al (1991) and Ko et al (2001) have reported in dogs that atropine and/or glycopyrrolate were more effective in preventing MED-induced bradycardia, but induced hypertension and pulsus alternans. In addition, Bergstom (1988) and Alibhai et al (1996) have reported that an anti-cholinergic drug enhances hypertension produced by MED in dogs, although there are no published data showing such findings in cats. Therefore, if undesirable events occurred on cats when an anti-cholinergic drug was given as a premedicant to MED administration, the administration of ATI would be recommended for the reversal of these effects.
MED induces second-degree atrioventricular block and vomiting (Short 1991). In our study, both cardiac arrhythmia assessed by auscultation and vomiting occurred more frequently in the control and non-ATI injected groups than the other groups. Therefore, combinations with ATI can prevent these side effects. In the present study, excitement was not observed in the FLU group, but occurred frequently in the groups combined with ATI and most frequently in the ATI–FLU–4AP group. Ilkiw et al (1996a) reported that MID alone induced abnormal behaviours or excitement-like signs such as ataxia and restlessness in cats. In our study, MID might play a minor role in the observed excitement because these signs occurred frequently in the ATI–FLU and ATI–FLU–4AP groups in which the effects of MID were antagonised by FLU. Although a combination of ATI, FLU and 4AP was most effective in antagonising the anaesthesia induced by MED–MID–KET in our study, it induced limb rigidity and muscle tremors during the recovery phase. These results indicate that the use of a mixture of ATI, FLU and 4AP as antagonists for MED–MID–KET anaesthesia is not always suitable for a smooth recovery.
In the present study, ATI, ATI–FLU, ATI–4AP and ATI–FLU–4AP combinations were effective in antagonising the anaesthesia and adverse effects induced by MED–MID–KET in cats. ATI alone effectively reversed the anaesthesia, hypothermia, bradycardia and hypopnoea produced by MED–MID–KET, with minimal adverse effects. However, the ATI–FLU combination may have some disadvantages because FLU is required at high dose in cats and is expensive. The ATI–4AP combination may be practical because 4AP is cheaper than FLU and this combination effectively hastens the recovery from anaesthesia. However, the dose of 4AP should be carefully chosen because of its toxicity (Verstegen et al 1991). A combination of ATI–FLU–4AP is most effective in antagonising the MED–MID–KET anaesthesia, but tachypnoea, excitement signs and muscle tremors occur frequently. Therefore, ATI alone can be used to shorten the effects of MED–MID–KET in cats. The combination of ATI, FLU and 4AP may be used after overdosage of MED–MID–KET.
In conclusion, ATI alone and combinations with ATI are much more effective for antagonising the anaesthesia and side effects induced by MED–MID–KET. ATI alone can be used as a safe and effective agent for antagonising the MED–MID–KET anaesthesia in cats. The use of ATI–FLU–4AP maybe not always produce smooth recovery from anaesthesia.
Acknowledgements
This work was supported by the fund from the Meiji Seika Co, and in part by Grant-in-Aid for Scientific Research (C) (14560266 and 18580316) from the Japanese Ministry of Education, Science, Sports and Culture, Japan. The authors thank Dr T. Sato for technical assistance.
References
- Akkerdaas L.C., Mioch P., Sap R., Hellebrekers L.J. Cardiopulmonary effects of three different anaesthesia protocols in cats, Veterinary Quarterly 23, 2001, 182–186. [DOI] [PubMed] [Google Scholar]
- Alibhai H.I., Clarke K.W., Lee Y.H., Thompson J. Cardiopulmonary effects of combinations of medetomidine hydrochloride and atropine sulphate in dogs, Veterinary Record 138, 1996, 11–13. [DOI] [PubMed] [Google Scholar]
- Bergstom K. Cardiovascular and pulmonary effects of a new sedative/analgesic (medetomidine) as a preanaesthetic drug in the dog, Acta Veterinaria Scandinavica 29, 1988, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R.N., Goa K.L. Flumazenil. A preliminary review of its benzodiazepine antagonist properties, intrinsic activity and therapeutic use, Drugs 35, 1988, 448–467. [DOI] [PubMed] [Google Scholar]
- Brown C.R., Sanquist F.H., Canup C.A., Pedley T.A. Clinical, electroencephalographic and pharmacokinetic studies of a water-soluble benzodiazepine, midazolam maleate, Anesthesiology 50, 1979, 467–470. [DOI] [PubMed] [Google Scholar]
- Court M.H., Greenbratt D.J. Pharmacokinetics and preliminary observations of behavioral changes following administration of midazolam to dogs, Journal of Veterinary Pharmacology and Therapeutics 15, 1992, 343–350. [DOI] [PubMed] [Google Scholar]
- Cullen L.K. Medetomidine sedation in dogs and cats: a review of its pharmacology, antagonism and dose, British Veterinary Journal 152, 1996, 519–535. [DOI] [PubMed] [Google Scholar]
- Durant N.N., Lee C., Katz R.L. 4-Aminopyridine reversal of sympathetic ganglionic blockade in the anesthetized cat, Anesthesiology 52, 1980, 381–384. [DOI] [PubMed] [Google Scholar]
- Flecknell P.A. Injectable anaesthetics. Taylor Hall. Anaesthesia of the Cat, 1994, Bailliere Tindall, WB Saunders: Philadelphia, 129–156. [Google Scholar]
- Granholm M., McKusick B.C., Westerholm F.C., Aspegren J.C. Evaluation of the clinical efficacy and safety of dexmedetomidine or medetomidine in cats and their reversal with atipamezole, Veterinary Anaesthesia and Analgesia 33, 2006, 214–223. [DOI] [PubMed] [Google Scholar]
- Golden A.L., Bright J.M., Daniel G.B., Fefee D., Schmidt D., Harvey R.C. Cardiovascular effects of the alpha2-adrenergic receptor agonist medetomidine in clinically normal cats anesthetized with isoflurane, American Journal of Veterinary Research 59, 1998, 509–513. [PubMed] [Google Scholar]
- Hatch R.C., Booth M.H., Kitzman J.V., Wallner B.M., Clark J.D. Antagonism of ketamine anesthesia in cats by 4-aminopyridine and yohimbine, American Journal of Veterinary Research 44, 1983, 417–423. [PubMed] [Google Scholar]
- Hatch R.C., Kitzman J.V., Clark J.D., Zahner J.M., Booth N.H. Reversal of pentobarbital anesthesia with 4-aminopyridine and yohimbine in cats pretreated with acepromazine and xylazine, American Journal of Veterinary Research 45, 1984, 2586–2590. [PubMed] [Google Scholar]
- Ilkiw J.E., Farver T.B., Suter C.M., McNeal D., Steffey E.P. The effect of intravenous administration of variable-dose flumazenil after fixed-dose ketamine and midazolam in healthy cats, Journal of Veterinary Pharmacology and Therapeutics 25, 2002, 181–188. [DOI] [PubMed] [Google Scholar]
- Ilkiw J.E., Suter C.M., Farver T.B., McNeal D., Steffey E.P. The behaviour of healthy awake cats following intravenous and intramuscular administration of midazolam, Journal of Veterinary Pharmacology and Therapeutics 19, 1996a, 205–216. [DOI] [PubMed] [Google Scholar]
- Ilkiw J.E., Suter C.M., McNeal D., Farver T.B., Steffey E.P. The effect of intravenous administration of variable-dose midazolam after fixed-dose ketamine in healthy awake cats, Journal of Veterinary Pharmacology and Therapeutics 19, 1996b, 217–224. [DOI] [PubMed] [Google Scholar]
- Itamoto K., Hikasa Y., Sakonjyu I., Itoh H., Kakuta T., Takase K. Anaesthetic and cardiopulmonary effects of balanced anaesthesia with medetomidine–midazolam and butorphanol in dogs, Journal of Veterinary Medicine Series A 47, 2000, 411–420. [DOI] [PubMed] [Google Scholar]
- Ko J.C., Fox S.M., Mandsager R.E. Effects of preemptive atropine administration on incidence of medetomidine-induced bradycardia in dogs, Journal of the American Veterinary Medical Association 218, 2001, 52–58. [DOI] [PubMed] [Google Scholar]
- Kojima K., Nishimura R., Mutoh T., Hong S.H., Mochizuki M., Sasaki N. Effects of medetomidine–midazolam, acepromazine–butorphanol, and midazolam–butorphanol on induction dose of thiopental and propofol and on cardiopulmonary changes in dogs, American Journal of Veterinary Research 63, 2002, 1671–1679. [DOI] [PubMed] [Google Scholar]
- Lamont L.A., Bulmer B.J., Grimm K.A., Tranquilli W.J., Sisson D.D. Cardiopulmonary evaluation of the use of medetomidine hydrochloride in cats, American Journal of Veterinary Research 62, 2001, 1745–1749. [DOI] [PubMed] [Google Scholar]
- Nishimura R., Kim H., Matsunaga S., Hayashi K., Tamura H., Sasaki N., Takeuchi A. Sedative effect induced by a combination of medetomidine and midazolam in pigs, Journal of Veterinary Medical Science 55, 1993, 717–722. [DOI] [PubMed] [Google Scholar]
- Rupp S.M., Shinohara Y., Fisher D.M., Miller R.D., Castagnoli N., Jr. Pharmacokinetics and pharmacodynamics of 4-aminopyridine in anesthetized dogs, Journal of Pharmacology and Experimental Therapeutics 225, 1983, 351–354. [PubMed] [Google Scholar]
- Salonen M., Reid K., Maze M. Synergistics interaction between α2-adrenergic agonists and benzodiazepines in rats, Anesthesiology 76, 1992, 1004–1011. [DOI] [PubMed] [Google Scholar]
- Savola J.M. Cardiovascular action of medetomidine and their reversal by atipamezole, Acta Veterinaria Scandinavica 85, 1989, 38–47. [PubMed] [Google Scholar]
- Selmi A.L., Mendes G.M., Lins B.T., Figueiredo J.P., Barbudo-Selmi G.R. Comparison of xylazine and medetomidine as premedicants for cats being anaesthetised with propofol–sevoflurane, Veterinary Record 157, 2005, 139–143. [DOI] [PubMed] [Google Scholar]
- Short C.E. Effect of anticholinergic treatment on the cardiac and respiratory systems in dogs sedated with medetomidine, Veterinary Record 129, 1991, 310–313. [DOI] [PubMed] [Google Scholar]
- Sinclair M.D. A review of the physiological effects of α2-agonists related to the clinical use of medetomidine in small animal practice, Canadian Veterinary Journal 44, 2003, 885–897. [PMC free article] [PubMed] [Google Scholar]
- Sparkes A.H., Papasouliotis K., Viner J., Cripps P.J., Gruffydd-Jones T.J. Assessment of orocaecal transit time in cats by the breath hydrogen method: the effects of sedation and a comparison of definitions, Research In Veterinary Science 60, 1996, 243–246. [DOI] [PubMed] [Google Scholar]
- Sumida T., Tagami M., Ide Y., Nagase M., Sekiyama H., Hanaoka K. Intravenous midazolam suppresses noxiously evoked activity of spinal wide dynamic range neurons in cats, Anesthesia and Analgesia 80, 1995, 58–63. [DOI] [PubMed] [Google Scholar]
- Vähä-Vahe T. Clinical effectiveness of atipamezole as a medetomidine antagonist in cats, Journal of Small Animal Practice 31, 1990, 193–197. [DOI] [PubMed] [Google Scholar]
- Verstegen J., Fargetton X., Zanker S., Donnay I., Ectors F. Antagonistic activities of atipamezole, 4-aminopyridine and yohimbine against medetomidine/ketamine-induced anaesthesia in cats, Veterinary Record 128, 1991, 57–60. [DOI] [PubMed] [Google Scholar]