Abstract
Interleukin(IL)-2 was originally characterized as an important T-cellular growth factor but later on, turned out to be a pivotal homeostatic factor for the establishment and maintenance of both natural(n)Treg and peripheral(p)Treg. In this review, it was aimed to connect the peculiar structural and functional aspects of IL-2 to the innovative advancements in tailoring its multifaceted functional behavior for targeting various IL-2 receptor types. The article includes detailed descriptions of modified versions of IL-2, obtained by either mutating or fusing IL-2 to heterologous molecules or by forming IL-2/(monoclonal) antibody complexes (IL-2C), and discusses their functional implications for addressing such heterologous pathological conditions in cancer, autoimmunity, and allergy. Additionally, this review sheds light on the underexplored contribution of autoantibodies to the endogenous regulation of IL-2 within the realms of both health and disease. The ongoing efforts to fine-tune IL-2 responses through antibody-dependent targeting or molecular engineering offer considerable translational potential for the future utility of this important cytokine.
Keywords: T cell growth factor, Interleukin-2, IL-2R, IL-2C, T regulatory cells, Treg, Muteins, Autoimmunity, Allergy, Cancer
Introduction: The T cell Growth Factor IL-2 and Its Cellular Receptors
In the mid-1970s, scientists were already able to grow malignant human T-cell lines in cell culture; however, the long-term expansion of primary human and murine T lymphocytes was still an enigma. This changed with the observation that T cells from unfractionated human bone marrow could be expanded for months if cultured with repeatedly refreshed conditioned medium obtained from phytohemagglutinin-stimulated, normal human lymphocytes [1]. The term “T-cell growth factor” (TCGF) was quickly coined for this activity in conditioned medium, which was soon joined by alternative names such as “thymocyte differentiation factor,” “costimulatory activity,” or “blastogenic factor,” all of which were eventually subsumed under the name interleukin-2 (IL-2). Only 7 years after the first description of TCGF, the structure of the IL-2 gene was deciphered [2] and its location on chromosome 4q26-28 was determined. The IL-2 gene spans approximately 5.7 kbp and consists of four exons.
The translated IL-2 protein consists of 153 amino acids, and during secretion the removal of a 20-amino acid leader leads to the mature 133 amino acid protein [3, 4]. With threonine at position 3, IL-2 contains a single O-linked glycosylation site, the posttranslational modification of which changes the IL-2’s molecular mass, while it does not seem to impact on its function [5]. The mature IL-2 protein has an Mr ranging from 13 to 17 kilodalton (kDa), which is explained by the variable degree of glycosylation. IL-2 contains three cysteine residues. Formation of a cysteine bridge between p.C58 and p.C105 [6], but not p.C125 [7], is essential for IL-2’s biological function. While, in wildtype human IL-2, p.C125 is not utilized for disulfide bridge formation, recombinant forms of IL-2 may differ in that respect and may, more or less frequently, involve also C125 into disulfide bridge formation. However, such versions almost entirely lose their biological activity. Consequently, recombinant forms of IL-2 may be expressed as “muteins,” introducing p.C125S or p.C125A modifications into the wildtype sequence [8]. Structure-wise, IL-2 represents a four α-helix bundle glycoprotein, consisting of domains A–D [9]. The region near the N-terminus of the A-helix has been suggested to bind to the IL-2R β-chain (CD122) [10]. Parts of the loop at which B and D helices are in close proximity are supposed to be involved in binding to IL-2Rα (CD25) [11]. The C-terminal helix of domain D may bind to CD132, the common IL-2R γ-chain [12].
The composition of the interleukin-2 receptor (IL-2R) mirrors the different biological activities of IL-2. In fact, the IL-2R can be configured in several different ways, it may be composed of up to three different molecules, referred to as IL-2 α- (CD25), β- (CD122), and γ-chain (CD132) [13]. The IL-2R α-chain is a 55-kDa type 1 membrane protein of 251 (272 long precursor) amino acids, with a short (13 amino acid long) intracellular tail. In addition, the IL-2R α-chain (CD25) has a substantial quaternary structure due to 5 disulfide bridges. The IL-2R α-chain gene is located on chromosome 10p14-15 [14]. Notably, a soluble form of IL-2R α-chain is constitutively formed, which serves as a biomarker for cellular activation [15] due to infection or alloreactivity [16, 17], exceedingly high serum sCD25 levels are diagnostic for hemophagocytic lymphohistiocytosis [18, 19]. The IL-2R β-chain is formed by a 75-kDa molecule also named CD122. It comprises a 525 amino acid long protein of which the smaller portion is located extracellularly (214 aa) while the larger portion is found cytoplasmically (286 aa) [20]. The gene for CD122 is located on chromosome 22q12. The IL-2R (common) γ-chain is a 64-kDa protein (CD132) with a 232 aa extracellular and a 64 aa intracellular domain [21]. It is encoded on the long arm of chromosome Xq13.1. Both, β- and γ-chains belong to the cytokine receptor superfamily and contain WSXWS motifs, which are commonly found in growth hormone receptors [22]. The IL-2R γ-chain, which forms part of other interleukin receptors also, such as the IL-4R, IL-7R, IL-9R, IL-15R, and IL-21R, is essential for IL-2R signaling [21].
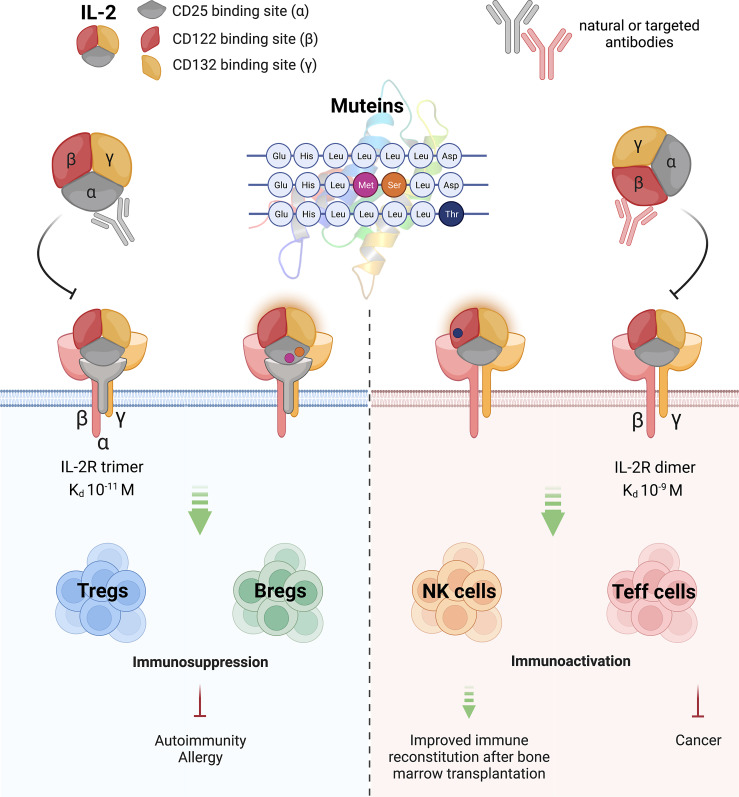
Notably, the different forms of the IL-2R afford highly different binding affinities for IL-2 (Table 1; Fig. 1). The IL-2R α-chain alone binds IL-2 with low affinity (Kd 10−8 M; low-affinity receptor) while the isolated β-chain has even inferior binding affinity (Kd 10−7 M). In contrast, the isolated γ-chain does not show any IL-2 binding activity. However, the situation changes when heterodimers (αγ; βγ; Kd 10−9 M) or the trimer (αβγ; [Kd 10−11 M]) become expressed and form a cell surface receptor, which displays up to 103–104-fold higher affinities (i.e., up to Kd 10−11) than the low-affinity receptors [23]. In addition, the α-chain has been found to be stably present on the cell surface for about 48 h, while β- and γ-chains have significantly shorter half-lives, lasting for no more than 1 h [24, 25].
Table 1.
Forms of human interleukin-2 receptors and their affinities for IL-2
Fig. 1.
Interleukin-2 takes center stage. The different functional aspects of IL-2 and possibilities for its modulation are shown, and the major epitopes of IL-2, their receptor binding preferences, along with the major cell surface receptors, and their binding affinities are indicated. Moreover, cellular IL-2 targets and their effects on disease processes and bone marrow regeneration are indicated. Graphics is inspired by Amit et al. [28].
From the differential affinity of IL-2 for the different forms of the IL-2 receptor, it is easy to deduce that the local or systemic availability of IL-2 is highly dependent on the surrounding cellular environment and their IL-2R decoration, which are competing for the available IL-2 present at a given time and a given place. This suggests that IL-2 is not only controlled by its neo-expression but also, if not primarily, by its differential consumption. Especially under steady-state conditions, low levels of IL-2 can be exclusively utilized by those cells, which express high levels of the CD25-containing tripartite high-affinity IL-2R. Consequently, such CD25high cells may act as an “IL-2 sink” by removing the available IL-2 at a given site while using it for their own propagation/differentiation as also demonstrated by Kueng et al. [29].
Distinct Monoclonal Antibodies Shape the Function and Increase the Bioavailability of IL-2 in vivo
A number of cell types express IL-2 receptors. Among them, resting T and B lymphocytes, NK cells, and also myeloid cell types belonging to the monocytic lineage. In the resting state, most of these cells express low levels of the medium-affinity βγ IL-2R, which requires higher concentrations of IL-2 to transduce significant signals to them. Some cell types, however, such as memory CD8+ T cells express high levels of CD122, which tend to proliferate in response to IL-2 or IL-15 in vitro. Injections of high doses of IL-2 but also of distinct IL-2 mAbs induce the proliferation of such cells with memory phenotype. Upon cellular activation but also upon treatment with IL-2 itself, T cells, B cells, and ILC2 upregulate CD25, the IL-2R α-chain, which leads to the expression of the tripartite high-affinity IL-2R, consisting of αβγ-chains [30]. These features are used as therapeutic principles during the treatment with, e.g., high-dose recombinant human IL-2, which in the form of aldesleukin (Proleukin) is successfully applied clinically as an “immune booster” to fight metastatic renal cell carcinoma [31]. This form of treatment is still considered to be the only curative option for this otherwise deadly disease [32].
Notably, the high-affinity IL-2R is constitutively expressed on (resting) natural T regulatory cells (nTreg) and is also a salient feature of peripherally induced Tregs (pTreg) [33, 34]. The presence of the tripartite IL-2R enables the respective cells to respond to minute amounts of IL-2 with their cellular activation and allows them to execute cell type and IL-2-dependent effector functions. For instance, the expression of the high-affinity IL-2R enables CD25+Foxp3+ T regulatory cells to “steal” the often minute amounts of available IL-2, which is considered to be one of the major effector functions of Tregs [35].
The biological importance of IL-2 is reflected by studies in mice lacking IL-2, which succumb to ulcerative colitis-like autoimmunity [36]. Moreover, disrupting the receptors for IL-2 resulted in dysregulated lymphocyte proliferation and autoinflammatory syndromes [37]. One of the mechanisms for overt autoimmunity in IL-2/IL-2R-deficient T cells is their lack of Treg development. In fact, it has been shown that IL-2 regulates the expression of Foxp3 in human Treg cells via STAT3 and STAT5 [38]. More recently, also distinct B-cell subsets have been shown to express CD25, which utilize low-dose IL-2 to differentiate into B regulatory cells (Breg) [39]. Conversely, deficiency of IL-2Rα as observed in patients, similar to Il2ra−/− mice, was found to be associated with overshooting lymphoproliferation and lymphocytic infiltration of multiple organs (lung, liver, and skin) with oligoclonal T cells. This phenotype is likely due to IL-2Rα’s role in both intrathymic negative selection and peripheral promotion of tolerance via the induction of pTregs [40].
Due to the complex composition of the different IL-2R structures, it was soon hypothesized that IL-2 itself may bind with distinct epitopes to the different parts of the high- and medium-affinity receptors [10, 11] (Fig. 1). These assumptions opened the door for the introduction of targeted modifications to IL-2 and its function based either on specific binding reagents, i.e., monoclonal antibodies, masking distinct IL-2R-binding epitopes on IL-2 while leaving others fully available for receptor interaction. For instance, if a monoclonal antibody covering the binding epitope responsible for interaction with CD25 was admixed with IL-2 or injected systemically, binding of the resulting interleukin-2 complex (IL-2C) should become restricted to the low-/medium-affinity versions of the receptor, i.e., IL-2Rβ and IL-2Rβγ. If, however, a monoclonal antibody covered the epitope responsible for binding to the medium-/low-affinity IL-2R, the resulting IL-2C would exclusively bind to the high-affinity IL-2R, thus stimulating exclusively those cells which (already under resting conditions) express that receptor [41].
Of note, such mAbs would not only change the receptor specificity of endogenously or exogenously applied IL-2 but may also significantly prolong the half-life of the otherwise short-lived IL-2 (T1/2 only 6–7 min) [42, 43] with renal elimination accounting for 75% of IL-2 clearance [44]. The first experimental studies to show such immunomodulatory capacities of injected anti-IL-2 mAbs were conducted by Jonathan Sprent and coworkers [41], and later on by Fred Finkelman and coworkers [43] who demonstrated that murine Treg cells can be selectively expanded by IL-2 complexes (IL-2C) formed upon injection of the rat anti-mouse IL-2 monoclonal antibody JES-6.1A12 (Table 2; Fig. 1). In preclinical models, IL-2C-expanded Tregs not only protected from experimental autoimmune encephalitis and prolonged survival of allogeneic islet grafts [45] but also ameliorated allergen-induced airway hyperreactivity in prophylactic and therapeutic settings, where they reduced allergen-specific IgE serum levels [46, 47] and expanded Treg during sublingual immunotherapy [48] and food allergy, respectively [49]. Moreover, it was shown that IL-2C-expanded Treg cells could be recalled upon allergen challenge, which was associated with suppression of lung-specific Th2 responses long after initial treatment [50]. These studies also revealed GARP expression, apart from CD25 expression as one of the most reliable markers for IL-2C-expanded Treg [50]. Alternatively, it was revealed that mAbs such as S4B6.1 or JES6-5H4, which block the CD25 binding site on IL-2 and thus enhance IL-2C binding to the IL-2R β-chain (CD122), can be used after bone marrow transplantation or in the case of cancer to foster fast T-cell regeneration but also strong effector functions [41].
Table 2.
Anti-IL-2 monoclonal antibodies influencing IL-2 function in vivo
| Clone name | Specificity on IL-2 | Function | Isotype | Species | Ref. |
|---|---|---|---|---|---|
| Mouse | |||||
| JES6.1A12 | CD122 binding site | Expands Treg | IgG2a | Rat | [41] |
| JES6-5H4 | CD25 binding site | Expands CD8+ memory cells | IgG2b | Rat | [41] |
| S4B6.1 | CD25 binding site | Expands CD8+ memory cells | IgG2a | Rat | [44, 46] |
| Human | |||||
| 1C6 | CD122 binding site | Expands Treg | – | Mouse | [49] |
| 5344 | CD122/132 binding site | Expands Treg | IgG1 | Mouse | [48] |
| F5111.2 | CD122 binding site | Expands Treg | scFv | Human | [51] |
| UFKA-20* | CD122 binding site | Expands Treg | IgG2b | Mouse | [52] |
| AU-007 | CD25 binding site | Expands CD8+ memory cells, NK cells and NKT cells | IgG1 | Human | [28] |
| MAB602 (5355) | CD25 binding site | Expands CD8+ memory cells | IgG2a | Mouse | [41] |
| TCB2 (hIL-2/TCB2:SLC-3010) | CD25 binding site | Expands CD8+ memory cells and NK cells | – | Mouse | [53] |
*Also works on human T cells ex vivo, on rhesus macaques, and in mice also in vivo.
The above-described mouse studies performed with epitope-specific anti-IL-2 antibodies were soon translated into the human system by testing whether anti-human IL-2 mAbs targeting the different binding epitopes of IL-2 would afford similar functions. Along those lines, the seminal studies by Jeffrey Bluestone and colleagues have shown that human(h)IL-2 and the anti-hIL-2 antibody F5111.2 fulfill similar functions as murine IL-2 and the JES6.1A12 mAb [51]. Similar to JES6.1A12, F5111.2 sterically blocks the binding of hIL-2 to IL-2Rβ while it also allosterically reduces hIL-2 affinity for IL-2Rα. Receptor activation is regulated by IL-2C dissociation, which is induced by IL-2Rα, leading to selective stimulation of cells expressing high levels of IL-2Rα, such as Treg cells in the form of an exchange release mechanism [51]. Further studies by the Boyman laboratory led to the discovery of UFKA-20, an anti-human IL-2 mAb that proved effective in vivo in mice and rhesus macaques and ex vivo with human T cells by increasing Treg cells [52]. Several related approaches were followed in the past to generate immunoactivating IL-2C. The hIL-2C referred to as SLC-3010 (Selecxine, Seoul, Republic of Korea) consists of hIL-2 complexed to the humanized anti-IL-2 antibody TCB2, which obstructs the CD25 binding site on IL-2, thus fostering the stimulation of memory/effector cells [53]. A similar approach was taken with hIL-2 complexed to the AU-007 (Aulos Biosciences, Larkspur, CA) anti-IL-2 mAb, which blocks the CD25 binding site of IL-2 and thus interrupts the IL-2 auto-inhibitory loop by preventing Treg expansion [28, 54].
However, a constant aspect of concern was the fact that IL-2Cs critically depend on the correct stoichiometry of two components mixed together. The pharmacokinetic control of adjusting the two components in optimal proportions proved to be difficult. For that very reason, structural information was obtained from anti-IL-2 mAb and used for the construction of IL-2/mAb complexes consisting of a single translational product (please refer to chapter modication of IL-2 function by mutation or gene fusion). Consequently, the concept of mAbs blocking one biological function of IL-2 while activating another one with an IL-2/scFv-fusion protein clearly reduces the complexity of a previously mentioned two-component drug (immune complex), which improves dosing and control of off-target effects.
Apart from modifying IL-2 function with mAbs, levels of secreted IL-2 can also be modulated with small molecule inhibitors eventually leading to Treg expansion. Along those lines, Tauber et al. [55] have shown recently that the small molecule inhibitor BX-795 increased IL-2 levels produced by antigenically activated T cells which led to the generation of Tregs, which at large resembled iTregs with the exception that they lacked Foxp3 expression as revealed by RNASeq and qPCR [55].
In summary, studies with mAbs targeting distinct epitopes of IL-2 were instrumental to understand IL-2R biology and paved the way for a number of clinical trials intended to shape the biological function of this important cytokine to expand either regulatory or effector T and B cells.
Natural and Disease-Associated Anti-Human Interleukin-2 Antibodies
While the effects of IL-2 on target cells can be modulated by the introduction of designer epitope-specific monoclonal antibodies, we here also want to discuss (naturally) existing mechanisms for the formation of autoantibodies against IL-2. Furthermore, the extent to which this “discrete” branch of the cytokine regulatory network is useful for the homeostasis of the IL-2 amplitude and control of its function independent of regulation at the level of protein synthesis is being scrutinized.
Molecular mimicry [56, 57], the release of self-antigens from dying cells (immunogenic cell death) [58], altered posttranslational modifications of self-antigens (neoantigens) [59], inborn errors of immunity [60, 61], or unknown escape from tolerance are some of the reported mechanisms whereby the immune system can develop autoreactive antibodies despite the elimination of high-affinity self-reactive T and B cells through negative selection and clonal anergy [62]. For instance, up to 20% of antibodies produced by mature human B cells have an autoreactive character [63], likely as a result of persisting low-affinity self-reactive B cells. However, such autoantibodies are “restricted” to manifest a pathological state, a phenomenon called horror autotoxicus by Paul Ehrlich in the late 1890s [64].
Accordingly, in various disease groups, a number of studies have reported the presence of autoantibodies directed against a whole collection of cytokines, the most prominent targets being IL-1, IFNs, and GM-CSF, among others (reviewed in [65, 66]). While such autoantibodies predispose (a phenomenon recognized as secondary immunodeficiency) or exacerbate disease prognosis in most cases [67], the presence of, f.i., anti-IL-1α autoantibodies has been shown to attenuate disease, e.g., joint destruction in rheumatoid arthritis patients [68] or overt inflammation in APS1/APECED patients supporting their survival [60]. Of note in that respect, T2D patients were shown to have a deficiency in natural serum anti-IL-6, -IL-8 and -TNF-α IgG autoantibodies, which improved after 6-months of glucose-lowering medication. After therapy, HbA1c levels inversely correlated with anti-IL-1α and anti-IL-6 autoantibodies [69].
How is the situation regarding IL-2? Are autoantibodies also formed against IL-2? Of note, early research in lupus mice (NZBWF1 and BXSB) revealed equal or even higher levels of IL-2-producing lymphocytes which were, however, paralleled by reduced IL-2 serum levels. These observations have prompted studies to determine whether anti-IL-2 antibodies might be responsible for these paradoxical findings in “autoimmune” mice. Autoimmune mice that have a strong tendency to produce (anti-nuclear) autoantibodies were well-known before [70]. Subsequent studies indeed showed that autoantibodies against IL-2 could be detected in lupus mice [70] (Table 3). Notably, the number of anti-IL-2-producing B cells increased with age (at 20 weeks higher than 6 weeks) and their in vitro expansion could be further driven by inflammatory stimuli such as lipopolysaccharide. Apart from connective tissue disease models, immune tolerance to IL-2 is also lost at large in non-obese diabetic (NOD) mice as revealed by Pérol et al. [71] and colleagues [71]. Similar to lupus mice, anti-IL-2 antibody titers in NOD mice seem to increase with age. Their titers could be further increased by injecting toxically high amounts of rIL-2. The mere induction and the IL-2 neutralization capacity of the antibodies significantly correlated with the survival of such challenged mice. Apart from B-cell responses, also IL-2-specific T-cell responses directed against immunodominant peptide sequences of IL-2 were detectable in NOD mice. Notably, Pérol et al. [71] and colleagues also found significant anti-IL-2 autoantibody levels in human T1D, SLE, and RA patients when compared to healthy controls [71]. Although convincingly argued and experimentally described, the significantly greater frequency of anti-IL-2 antibodies in patients with T1D compared with healthy controls, and thus the relevance of these antibodies with regards to the pathogenesis of T1D, could not be confirmed by other research groups [72]. The significant differences in these results were attributed to the use of different experimental procedures to detect anti-IL-2 autoantibodies, i.e., plate-bound ELISA versus fluid phase IL-2 detection [72, 73]. While another study performed by Churlaud et al. [73] confirmed that T1D patients present with significantly higher serum anti-IL-2 reactivity compared to healthy control individuals in plate-bound IL-2 ELISA assays, this reactivity was not comparable in magnitude to the high autoantibody levels detectable after high-dose IL-2 therapy performed for the treatment of neuroblastoma or renal adenocarcinoma [73, 74]. From these results, the authors concluded that patients with T1D have at best something like an “anti-IL-2 reactivity” but not full-fledged anti-IL-2 antibodies.
Table 3.
Natural and disease-associated (autoimmune) anti-IL-2 antibodies
| Disease-associated antibodies | Frequency | Detection method | Ref. |
|---|---|---|---|
| Mouse | |||
| Lupus | Not specified | PFC assay | [70] |
| T1D-NOD | 100% | ELISA | [71] |
| Human | |||
| T1D, RA, Sjögren, SLE | 25%, 15%, 18%, 20% | ELISA | [71] |
| T1D | 0% | Liquid phase assay | [72] |
| T1D | Significantly more than HC | ELISA | [73] |
| High-dose rIL-2 therapy | 65% | IB, ELISA | [75] |
| rIL-2-treated renal cancer patients | 60% | ELISA | [74] |
| HIV | 96% | ELISA | [76] |
| HIV; rIL-2-treated cancer patients | Approx. 50%; 100% | ELISA, IB | [77, 78] |
| HIV | 100% | ELISA | [66] |
| Natural antibodies | |||
| Healthy individuals (pool) | n.a. | IB | [79] |
| Healthy individuals | Approx. 30% | ELISA | [77, 78] |
| Healthy individuals | 100%, no autoantibodies found in IgG-free serum/plasma | ELISA | [80] |
| Healthy individuals | 100% (IL-2/anti-IL-2 autoantibodycomplexes), free IL-2 (less frequent) | ELISA | [66] |
| Healthy individuals | 3.6% | ELISA | [71] |
PFC, plaque forming cell assay; n.a., not applicable; ELISA, enzyme-linked immunosorbent assay; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; IB, immunoblot; HC, healthy controls.
In addition to high-dose rIL-2 therapy, high anti-IL-2 antibody levels can also be detected in HIV patients. In a study enrolling 25 HIV patients, 14 patients presented with clearly detectable anti-IL-2 antibodies [76]. However, the herein detectable anti-IL-2 antibody reactivity was attributed to molecular mimicry between IL-2 and gp41 of HIV, which share substantial sequence homology in a specific segment [81, 82]. It was speculated that such cross-reactive autoantibodies directed against residues 14–19 of mature IL-2 may alter the function of IL-2 and thus contribute to the immunodeficiency observed in AIDS patients [76] because mutation studies have shown that the IL-2 epitope targeted by the antibodies is involved in IL-2R binding [6]. This assumption was spurred by the facts that the described cross-reactive antibodies did not only bind to the homologous peptide sequence of IL-2 but also to full-length IL-2 coated onto ELISA plates, the binding of which could not only be inhibited by the gp41-derived LERILL peptide but also with full-length IL-2 [76]. Besides, it was shown that sera fully absorbed for their gp41 reactivity retained IL-2 reactivity, suggesting that additional (broader) anti-IL-2 antibody specificities are present in HIV sera (Table 3) [76, 77].
The chapter regarding the presence and significance of the levels of anti-IL-2 autoantibodies has not been closed as of yet. Of note, anti-IL-2 antibody reactivity was also found in healthy individuals, who possess weaker yet considerable titers of anti-IL-2 autoantibodies (Table 3). Such antibodies were first isolated from serum pools of healthy donors by immunoaffinity purification using rIL-2 coupled to Sepharose. The eluted anti-IL-2 F(ab)2-fraction of IgG antibodies was able to block IL-2-induced lymphocyte proliferation in vitro by more than 70% [79]. Later on, such purified antibodies have been shown to also block the mixed lymphocyte reaction and lymphokine-activated killer cell expansion [77, 78]. Another study detected anti-IL-2 antibodies in 3.8% of healthy individuals [71]. In contrast, all 15 healthy participants enrolled in a study were reported to have autoantibodies against IL-2 [80]. However, these antibodies became only detectable upon isolation and subsequent acidification of IgG fractions followed by removal through size exclusion ultrafiltration of the small (13–17 kDa) IL-2. Such “un-complexed” anti-IL-2 antibodies clearly reacted with IL-2 resolved by SDS-PAGE under reducing conditions in immunoblotting experiments [80].
Accordingly, even low-affinity antibodies directed against a localized target, such as IL-2, may contribute to altered biological responses in vivo since they may (i) contribute to the cytokine’s transportation in inactive form, (ii) enhance its serum half-life, preventing its proteolytic degradation and/or renal secretion, regulating its availability to target specific receptors/cells, and (iii) act as reservoirs guaranteeing its sustained release, apart from merely neutralizing the cytokine. The basic fact that the autoantigenic ligand (IL-2) and the autoantibody (anti-IL-2) are present in variant and sometimes rapidly changing amounts calls for a differentiated view on the matter. In fact, one must take into consideration that such autoantibodies are difficult to detect because they may form inactive immune complexes with endogenous IL-2 (IL-2C) [80], interfere with the release of low quantities of matrix-bound rIL-2 protein during affinity purification of IL-2-specific autoantibodies [79] or, alternatively, may not be clearly detectable in complex assay formats [83] which can obscure their “true” measurement. Future studies will have to clarify the epitope specificity of human anti-IL-2 autoantibodies and their putative relevance for health and disease.
Modification of IL-2 Function by Mutation or Gene Fusion
IL-2 is a versatile cytokine whose function can be modulated with the help of endogenous or adoptively transferred antibodies. Since the determination of the IL-2 sequence, structure, and the early experimental discoveries as a lymphocyte stimulatory agent (T and NK cells) [5, 84–86] scientists have been keen to develop a multitude of targeted alterations within the IL-2 protein sequence, known as muteins [87], to enhance IL-2-based therapeutics. After the discovery of the key disulfide bridge in the IL-2 molecule between the positions p.C58 and p.C105 [7], it became obvious that any modifications around this region may render IL-2 as biologically inactive. In therapeutic proteins, chemical oxidation of methionine residues to methionine sulfoxide during the upstream and downstream processing has been a challenge for pharmaceutical industries. An IL-2 mutein containing p.M194A substitution was, thus, developed by Cetus Corporation which, while retaining the optimal biological activity, showed enhanced stability against the chemical oxidation [88]. As highlighted before that IL-2 imparts different effector functions depending on the expression of IL-2R composition on target cells, for the simplicity of the readers, we now individually discuss the novel, functionally relevant mutations in the IL-2 sequence targeting one or the other receptor configuration (Table 4; Fig. 1).
Table 4.
Structure and function of interleukin-2 muteins
| Name | Site mutated in IL-2 | Function | Ref. |
|---|---|---|---|
| Medium (or low)-affinity IL-2R-specific | |||
| – | p.D20N | 80–90% reduced proliferation with HUT-102 cells with similar reduction in binding affinity to IL-2Rαβγ | [89] |
| FSD13 | p.P65L | Enhanced stimulation of effector T cells and NK cells over Tregs | [90] |
| Super-2 | p.L80F/R81D/L85V/I86V/I92F | Increased binding affinity for IL-2Rβ compensating for the absence of IL-2Rα expression on cytotoxic T cells and NK cells leading to improved anti-tumor response | [89] |
| IL-2v | p.F42A/Y45A/L72G | Abolished IL-2Rα interaction. CEA-IL2v fusion improved anti-tumor targeting in MC38-CEA mouse model. Completed phase 1 clinical trial on CEA-positive solid tumor patients | [90, 91] |
| High-affinity IL-2R-specific | |||
| M1–M6 | p.V69A/Q74Pp.V69A/Q74P/I128T | 15-30-fold higher affinity for IL-2Rα and reduced binding with IL-2Rβγ alongside increased stimulation of Kit 225 cells. M6 mediates its elevated potency through IL-2Rα-dependent ligand-reservoir effect | [92, 93] |
| 2–4 | p.N29S/Y31H/K35R/T37A/K48E/V69A/N71R/Q74P/N88D/I89V | Affinity for IL-2Rα is greater than 160-fold compared to wildtype IL-2. May reduce toxicity as low-dose IL-2 can be administered in vivo | [94] |
| BAY 50-4798 | p.N88R | 1000-fold increased selectivity for IL-2Rαβγ to IL-2Rβγ compared to wildtype IL-2. Finished phase 1 clinical study on melanoma and renal cancer patients | [95, 96] |
| – | p.N88D | Mutein with reduced affinity for the IL-2Rβγ and fused with an engineered IgG1 antibody to enhance serum half-life | [97] |
| NHS-IL2, Selectikine | p.D20T | Improved selectivity for activated T cells versus NK cells. Increased safety profile due to reduced binding to endothelial cells | [98, 99] |
| hIL-2/F5111.2 | hIL-2 fused with F5111.2 antibody | Improved Treg specificity in preclinical models of diabetes mellitus and colitis | [100] |
| NARA1leukin (ANV419) | Split hIL-2 fused with NARA1 antibody | Hinders IL-2Rα binding, exhibiting enhanced anti-tumor response by effector T cells and NK cells | [101] |
| Affecting function without modulating receptor binding | |||
| – | p.T51P | Retards ligand-induced receptor internalization on T cells expressing IL-2Rα | [102, 103] |
| 2D1 | p.L18M/L19S | Increased ligand recycling causing 10-fold greater potency for cells expressing heterotrimeric receptors | [104] |
Muteins with Increased Binding to Medium-(or Low-)Affinity Receptor
As early as in 1988, Weigel and colleagues attempted to systematically evaluate a set of IL-2 muteins with regards to their biological activity on murine CTLL cell line and in human concanavalin A-activated T cells. For instance, p.D20N exchange reduced the relative proliferation inducing activity to only 10–20%. This decrease was accompanied by a similar reduction in the binding to the high-affinity IL-2 receptor on HUT-102 cells, a human T-cell line, while affinity towards the low-affinity receptor was unchanged. The authors, thus, speculated p.D20 may be involved in binding to the IL-2R α-chain [89]. Another study documented an IL-2 mutein, FSD13, with a single amino acid mutation at p.P65L, which results in reduced Treg-inducing potential and enhanced stimulatory capacity of CD4+ and CD8+ effector T cells as well as NK cells and reduced organ cytotoxicity compared to wildtype IL-2 [90]. In a parallel fashion, Super-2, an IL-2 “superkine” has been engineered to possess an increased affinity to IL-2Rβ without the need for expression of IL-2Rα by naïve T cells to capture even trace amounts of IL-2 with high-affinity trimeric IL-2R. Super-2 induced robust proliferation of cytotoxic T cells and improved anti-tumor response, with the advantage of proportionally reduced expansion of Tregs and lesser induction of pulmonary edema [91]. Likewise, Klein et al. [105] designed IL-2v mutein (p.F42A/Y45A/L72G) which prevented binding to the IL-2Rα, while the interaction with IL-2Rβγ remained unaffected as revealed by X-ray co-crystallization studies. It was also shown that CEA-IL2v, IL-2v fused with a humanized carcinoembryonic antigen (CEA)-specific antibody CH1A1A-2F1, indiscriminately activated cytotoxic T cells, NK cells, and Tregs at similar concentrations, suggesting that Tregs bearing IL-2R α-chain have no significant advantage upon administration of IL-2v. As a consequence, CEA-IL2v showed enhanced tumor targeting compared with CEA-IL2wt in the MC38-CEA mouse model. A phase 1 clinical trial using CEA-IL2v in patients CEA-positive solid tumors was completed in 2018 [105, 106].
Muteins with Increased Binding to High-Affinity IL-2 Receptor
In contrast to the attempt to develop IL-2 muteins with increased potential to expand cytotoxic T cells in vivo is the generation of IL-2 variants with reduced effector T cell- but increased Treg-activating function. Using yeast surface display, one study attempted to screen IL-2 mutants with increased potency for soluble IL-2R α-chain [92]. It was found that M1 (p.V69A/Q74P) and M6 (p.V69A/Q74P/I128T) IL-2 muteins exhibited improved IL-2R α-chain binding (15–30-fold) which is expressed at roughly 30–70-fold higher levels on the surface of a human T-cell line Kit 225 compared to the trimeric IL-2Rαβγ (3–7 × 103 molecules) [27, 107]. However, binding to the medium-affinity IL-2Rβγ, expressed on the human NK cell line YT-2C2 [108], was significantly decreased by M1 and slightly by M6 compared to the wildtype-like IL-2 (p.C125S). It remained interesting that, of the two, only M6 caused increased proliferation of Kit 225 cells in the picomolar range. The authors argued that an increased affinity to IL-2Rα alone may not necessarily translate into augmented stimulatory potency. To resolve this conundrum, they mimicked the physiologically rapid systemic clearance of IL-2 in a pulse bioassay where M6 and M1 were briefly (30 min) exposed to the cytokine-starved Kit 225 cells [93]. Here, both M6 and M1 enabled improved proliferation of Kit 225 cells, strongly implying the use of appropriate assays to decipher the physiological importance of IL-2 muteins. In the pulse assay, it was also discovered that IL-2 mutein, M6, continued to persist on the surface of Kit 225 cells despite washing, which gradually declined upon the introduction of the soluble IL-2R α-chain in the medium. Hence, they attributed the increased potency of M6 to the IL-2Rα-mediated ligand-reservoir effect which increased the half-life of the M6 on cells overexpressing IL-2Rα without altering serum levels of IL-2. The very research group generated an IL-2 mutant, 2–4, with significantly higher IL-2R α-chain affinity, i.e., Kd 10−10 M for IL-2Rα (∼5-fold > M6) compared to the nanomolar affinity range of the wildtype IL-2. The 2–4 mutein showcased ten mutations (p.N29S/Y31H/K35R/T37A/K48E/V69A/N71R/Q74P/N88D/I89V) and thus low-dose IL-2 can be administered to induce proliferation of activated T cells transiently expressing the trimeric IL-2Rαβγ with consequently lower toxicity in clinical settings [94]. Since IL-2Rα is also expressed on Tregs and has a surface reservoir effect, in what manner the pivot shifts with 2–4 mutein remains uncertain. Also, in the context of tumor immunotherapy and IL-2-mediated immunoproliferation, the role of antigen-independent NK cells expressing medium-affinity IL-2Rβγ is highly debated. Studies have attributed the toxicity of high-dose aldesleukin, a clinically approved human IL-2, to NK responses [109] while others do not rule out the therapeutic benefit of NK cells mediating essential anti-tumor effects [110]. The associated toxicities also result in vascular leak syndrome which is a major hurdle in dose-escalation in the treatment of carcinomas with IL-2 [111]. Besides, it was reported that a region around p.D20 in IL-2, which directly acts as disintegrin on endothelial cells [112], may cause vascular leak syndrome, while others linked capillary leak syndrome and vasopermeability to the linear fragments of IL-2 within amino acids 15–22 and 22–58, respectively, the latter was also shown to be involved in receptor binding, though is biologically inactive [99, 100]. Interestingly, another study reported low-to-moderate expression of the individual IL-2R αβγ-subunits on murine lung endothelial cells at both the mRNA and protein levels and considered this a singular cause of IL-2-dependent development of pulmonary edema [113]. Therefore, attempts to improve IL-2Rα affinity with IL-2 muteins to preferentially expand activated T cells, bearing the transient trimeric IL-2R, over NK cells in the context of cancer therapy have been continuously entertained. Using an analogous approach, BAY 50-4798, a p.N88R mutated version of IL-2, displayed 103-fold greater selectivity for IL-2Rαβγ compared to IL-2Rβγ. After a positive clinical phase 1 study evaluating this mutein in advanced melanoma and renal cancer patients it did not, however, show an improved anti-tumor activity with one-half of the dosage to wildtype IL-2 [95, 96].
By modification of the same amino acid position, a very interesting approach was presented by Peterson et al. [97] In their study, a mutant form of IL-2 (p.N88D), which has a lower affinity for IL-2Rβγ and thus a higher capacity to expand regulatory T cells, was fused with IgG1 to form a moiety consisting of two IL-2 and one IgG1 molecule [97]. The altered IgG1 has no binding affinity for Fcγ receptors and cannot activate complement [97]. Moreover, its Fab fragments do not bind antigen as non-binding germ line encoded variable domains are used [97]. However, its binding to the neonatal Fc receptor (Fcn) is still functional, resulting in a highly prolonged half-life of this construct [97]. Usually, antibodies are recycled in vivo, being taken up from cells via the Fcn, and then released again [114]. In a parallel effort, Selectikine (p.D20T), an IL-2 mutant, fused to NHS76 antibody demonstrated increased selectivity for activated effector T cells and reduced vascular toxicity which allowed administration of higher therapeutic doses compared to wildtype IL-2 leading to an at least 5-fold greater therapeutic index in a mouse tumor model. Selectikine also completed its dose-escalation phase 1 trial in patients with advanced solid tumors [98, 99].
Similarly, structural information obtained from the hIL-2/F5111.2 complexes was used to engineer single-chain variable fragments (scFv) of hIL-2 and F5111.2, thus combining both components in one translational product which displayed high stability and increased half-life and efficiency in preclinical models of checkpoint inhibitor-induced diabetes mellitus and colitis [100]. A related fusion approach between hIL-2 and an anti-IL-2 mAb was followed to obtain ANV419, NARA1leukin (Anaveon) [102]. This fusion protein mimics hIL-2/NARA1 complexes and was generated by permanently grafting two unmutated fragments (1–76aa; 77–133aa) to the antigen-binding grove of the humanized anti-IL-2 mAb NARA1, which obscures the IL-2Rα binding site on IL-2 [101], generating an IL-2Rβγ (medium-affinity)-biased version of IL-2 [102], with enhanced efficacy against localized and metastatic tumors as shown in preclinical models.
Muteins Affecting Target Function without Altering Receptor Binding
Besides affinity modulation for IL-2R heteromers, IL-2 muteins have been reported to impart targeted outcomes by leveraging additional mechanisms. For instance, David Z. Chang and colleagues demonstrated that by substituting p.T51P, the bioactivity of IL-2 can be maintained despite a 10-fold reduced affinity for IL-2Rαβ as the analog retards ligand-induced receptor internalization in T cells compared to wildtype IL-2 [103, 104]. Receptor internalization regulates lymphocyte responsiveness to IL-2 by degrading the agonist as well as IL-2Rβ and IL-2Rγ chains however, IL-2Rα recycles back to the surface [25]. By manipulating the endosomal trafficking of ligand-bound IL-2 receptors, a 10-fold increased potency of IL-2, but with similar binding affinity, was noted. In fact, the double mutant version p.L18M/L19S, named 2D1, was found to be resistant to endosomal degradation and thus reappeared on the cell surface along with the recycled IL-2R α-chain as affinity for the IL-2Rα increases at the typically low pH in endosomal compartments [115, 116]. This mutein-based superagonism of IL-2 is dependent on the expression of the heterotrimeric high-affinity IL-2 receptor which is abundantly expressed on Tregs, Bregs and recently activated T cells. Of note, the inventors also showed reduced IL-2-induced production of IFN-γ by NK cells expressing the intermediate-affinity receptor, IL-2Rβγ, which is also found on cytotoxic memory T cells.
Conclusion: IL-2, a Seemingly Simple Cytokine, Takes Center Stage in Immunoregulation and Immunological Research
Since its discovery in the 1970s, IL-2 takes center stage in biomedical research to harness diverging biological functions which have proven to be very important for adaptive immune responses. IL-2 displays features of both an important immuno-regulatory but also of a potent immunostimulatory cytokine. Which feature is more prominent in a given situation likely depends on the amount of IL-2 present at the respective site and the IL-2 receptor expression profile(s) of the surrounding cell types. The functional activity of IL-2 can be designed not only by mutation of the molecule itself but also by attaching it to distinct binding reagents (mAbs), which, in most instances, steer its receptor specificity and longevity. The delicate functions of IL-2 have the potential to interfere with and improve the management of autoimmune diseases, allergies, and also cancer. The constant improvements of IL-2 derivatives of different kinds hold strong promise that this cytokine will have a number of different clinical applications in the near future. The extent to which autoantibody-based anti-IL-2 activity contributes to the modulation of various IL-2 functions, and whether such activity predisposes to, or even protects from disease, will have to be determined by future studies.
Conflict of Interest Statement
With regard to the authors’ disclosure of potential conflicts of interest, we would like to indicate that Winfried F. Pickl has received honoraria from Novartis, AstraZeneca, and Roche. The other authors have no conflict of interest to declare.
Funding Sources
This work was supported by the Medical University of Vienna and the Danube Allergy Research Cluster (Danube ARC) supported by the State of Lower Austria.
Author Contributions
A.N.A.S, P.A.T, and W.F.P. conceived the concept for this review. A.N.A.S, P.A.T., R.B.S., B.K., and W.F.P wrote the paper. All authors critically read the paper and approved the manuscript.
Funding Statement
This work was supported by the Medical University of Vienna and the Danube Allergy Research Cluster (Danube ARC) supported by the State of Lower Austria.
References
- 1. Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–8. [DOI] [PubMed] [Google Scholar]
- 2. Fujita T, Takaoka C, Matsui H, Taniguchi T. Structure of the human interleukin 2 gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark SC, Arya SK, Wong-Staal F, Matsumoto-Kobayashi M, Kay RM, Kaufman RJ, et al. Human T-cell growth factor: partial amino acid sequence, cDNA cloning, and organization and expression in normal and leukemic cells. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robb RJ. Interleukin 2: the molecule and its function. Immunol Today. 1984 Jul;5(7):203–9. [DOI] [PubMed] [Google Scholar]
- 5. Robb RJ, Kutny RM, Panico M, Morris HR, Chowdhry V. Amino acid sequence and post-translational modification of human interleukin 2. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ju G, Collins L, Kaffka KL, Tsien WH, Chizzonite R, Crowl R, et al. Structure-function analysis of human interleukin-2. Identification of amino acid residues required for biological activity. J Biol Chem. 1987 Apr 25;262(12):5723–31. [PubMed] [Google Scholar]
- 7. Wang A, Lu SD, Mark DF. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–3. [DOI] [PubMed] [Google Scholar]
- 8. Liang SM, Thatcher DR, Liang CM, Allet B. Studies of structure-activity relationships of human interleukin-2. J Biol Chem. 1986 Jan 5;261(1):334–7. [PubMed] [Google Scholar]
- 9. Bazan JF. Unraveling the structure of IL-2. Science. 1992 Jul 17;257(5068):410–3. [DOI] [PubMed] [Google Scholar]
- 10. Collins L, Tsien WH, Seals C, Hakimi J, Weber D, Bailon P, et al. Identification of specific residues of human interleukin 2 that affect binding to the 70 kDa subunit (p70) of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grant AJ, Roessler E, Ju G, Tsudo M, Sugamura K, Waldmann TA. The interleukin 2 receptor (IL-2R): the IL-2R alpha subunit alters the function of the IL-2R beta subunit to enhance IL-2 binding and signaling by mechanisms that do not require binding of IL-2 to IL-2R alpha subunit. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernandez R, Poder J, LaPorte KM, Malek TR. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. 2022 Oct;22(10):614–28. [DOI] [PubMed] [Google Scholar]
- 13. Engel P, Boumsell L, Balderas R, Bensussan A, Gattei V, Horejsi V, et al. CD nomenclature 2015: human leukocyte differentiation antigen workshops as a driving force in immunology. J Immunol. 2015 Nov 15;195(10):4555–63. [DOI] [PubMed] [Google Scholar]
- 14. Leonard WJ, Depper JM, Kanehisa M, Kronke M, Peffer NJ, Svetlik PB, et al. Structure of the human interleukin-2 receptor gene. Science. 1985 Nov 8;230(4726):633–9. [DOI] [PubMed] [Google Scholar]
- 15. Rubin LA, Kurman CC, Fritz ME, Biddison WE, Boutin B, Yarchoan R, et al. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–7. [PubMed] [Google Scholar]
- 16. Cornaby AJ, Simpson MA, Madras PN, Dempsey RA, Clowes GH, Monaco AP. Pre-operative interleukin 2 and interleukin 2 receptor levels may predict subsequent renal allograft rejection. Transpl Proc. 1989 Feb;21(1 Pt 2):1861–2. [PubMed] [Google Scholar]
- 17. Perkins JD, Nelson DL, Rakela J, Grambsch PM, Krom RA. Soluble interleukin 2 receptor level in liver allograft recipients: an indicator of rejection. Transpl Proc. 1989 Feb;21(1 Pt 2):2275–6. [PubMed] [Google Scholar]
- 18. Chilosi M, Semenzato G, Cetto G, Ambrosetti A, Fiore-Donati L, Perona G, et al. Soluble interleukin-2 receptors in the sera of patients with hairy cell leukemia: relationship with the effect of recombinant alpha-interferon therapy on clinical parameters and natural killer in vitro activity. Blood. 1987 Nov;70(5):1530–5. [PubMed] [Google Scholar]
- 19. Komp DM, McNamara J, Buckley P. Elevated soluble interleukin-2 receptor in childhood hemophagocytic histiocytic syndromes. Blood. 1989 Jun;73(8):2128–32. [PubMed] [Google Scholar]
- 20. Hatakeyama M, Mori H, Doi T, Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–45. [DOI] [PubMed] [Google Scholar]
- 21. Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, et al. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–82. [DOI] [PubMed] [Google Scholar]
- 22. Baumgartner JW, Wells CA, Chen CM, Waters MJ. The role of the WSXWS equivalent motif in growth hormone receptor function. J Biol Chem. 1994 Nov 18;269(46):29094–101. [PubMed] [Google Scholar]
- 23. Hsieh EW, Hernandez JD. Clean up by aisle 2: roles for IL-2 receptors in host defense and tolerance. Curr Opin Immunol. 2021 Oct;72:298–308. [DOI] [PubMed] [Google Scholar]
- 24. Hemar A, Dautry-Varsat A. Cyclosporin A inhibits the interleukin 2 receptor alpha chain gene transcription but not its cell surface expression: the alpha chain stability can explain this discrepancy. Eur J Immunol. 1990 Dec;20(12):2629–35. [DOI] [PubMed] [Google Scholar]
- 25. Hemar A, Subtil A, Lieb M, Morelon E, Hellio R, Dautry-Varsat A. Endocytosis of interleukin 2 receptors in human T lymphocytes: distinct intracellular localization and fate of the receptor alpha, beta, and gamma chains. J Cell Biol. 1995 Apr;129(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005 Nov 18;310(5751):1159–63. [DOI] [PubMed] [Google Scholar]
- 27. Arima N, Kamio M, Imada K, Hori T, Hattori T, Tsudo M, et al. Pseudo-high affinity interleukin 2 (IL-2) receptor lacks the third component that is essential for functional IL-2 binding and signaling. J Exp Med. 1992 Nov 1;176(5):1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amit I, Levin I, Wyant T. The computationally designed human antibody, AU-007, mediates human immune activation by endogenous IL-2, while uniquely breaking the IL-2 auto-inhibitory loop and preventing Treg expansion. J ImmunoTherapy Cancer. 2021;9(suppl 2):9. [Google Scholar]
- 29. Kueng HJ, Manta C, Haiderer D, Leb VM, Schmetterer KG, Neunkirchner A, et al. Fluorosomes: a convenient new reagent to detect and block multivalent and complex receptor-ligand interactions. FASEB J. 2010 May;24(5):1572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reem GH, Yeh NH. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–30. [DOI] [PubMed] [Google Scholar]
- 31. Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N Engl J Med. 1998 Apr 30;338(18):1272–8. [DOI] [PubMed] [Google Scholar]
- 32. Rosenberg SA. Interleukin 2 for patients with renal cancer. Nat Clin Pract Oncol. 2007 Sep;4(9):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995 Aug 1;155(3):1151–64. [PubMed] [Google Scholar]
- 34. Toomer KH, Lui JB, Altman NH, Ban Y, Chen X, Malek TR. Essential and non-overlapping IL-2Rα-dependent processes for thymic development and peripheral homeostasis of regulatory T cells. Nat Commun. 2019 Mar 4;10(1):1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007 Dec;8(12):1353–62. [DOI] [PubMed] [Google Scholar]
- 36. Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–61. [DOI] [PubMed] [Google Scholar]
- 37. Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995 Oct;3(4):521–30. [DOI] [PubMed] [Google Scholar]
- 38. Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006 Sep 1;108(5):1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inaba A, Tuong ZK, Zhao TX, Stewart AP, Mathews R, Truman L, et al. Low-dose IL-2 enhances the generation of IL-10-producing immunoregulatory B cells. Nat Commun. 2023 Apr 12;14(1):2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simeonov DR, Gowen BG, Boontanrart M, Roth TL, Gagnon JD, Mumbach MR, et al. Discovery of stimulation-responsive immune enhancers with CRISPR activation. Nature. 2017 Sep 7;549(7670):111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006 Mar 31;311(5769):1924–7. [DOI] [PubMed] [Google Scholar]
- 42. Lotze MT, Matory YL, Ettinghausen SE, Rayner AA, Sharrow SO, Seipp CA, et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–75. [PubMed] [Google Scholar]
- 43. Phelan JD, Orekov T, Finkelman FD. Cutting edge: mechanism of enhancement of in vivo cytokine effects by anti-cytokine monoclonal antibodies. J Immunol. 2008 Jan 1;180(1):44–8. [DOI] [PubMed] [Google Scholar]
- 44. Gibbons JA, Luo ZP, Hannon ER, Braeckman RA, Young JD. Quantitation of the renal clearance of interleukin-2 using nephrectomized and ureter-ligated rats. J Pharmacol Exp Ther. 1995 Jan;272(1):119–25. [PubMed] [Google Scholar]
- 45. Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009 Apr 13;206(4):751–60.In [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, et al. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc Natl Acad Sci U S A. 2010 Feb 2;107(5):2171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neunkirchner A, Kratzer B, Kohler C, Smole U, Mager LF, Schmetterer KG, et al. Genetic restriction of antigen-presentation dictates allergic sensitization and disease in humanized mice. EBioMedicine. 2018 May;31:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smaldini PL, Trejo F, Cohen JL, Piaggio E, Docena GH. Systemic IL-2/anti-IL-2Ab complex combined with sublingual immunotherapy suppresses experimental food allergy in mice through induction of mucosal regulatory T cells. Allergy. 2018 Apr;73(4):885–95. [DOI] [PubMed] [Google Scholar]
- 49. Klein M, Misme-Aucouturier B, Cheminant MA, De Carvalho M, Wauters M, Tranquet O, et al. Engineering a safe monoclonal anti-human IL-2 that is effective in a murine model of food allergy and asthma. Allergy. 2022 Mar;77(3):933–45. [DOI] [PubMed] [Google Scholar]
- 50. Kohler C, Smole U, Kratzer B, Trapin D, Schmetterer KG, Pickl WF. Allergen alters IL-2/αIL-2-based Treg expansion but not tolerance induction in an allergen-specific mouse model. Allergy. 2020 Jul;75(7):1618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trotta E, Bessette PH, Silveria SL, Ely LK, Jude KM, Le DT, et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat Med. 2018 Jul;24(7):1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karakus U, Sahin D, Mittl PRE, Mooij P, Koopman G, Boyman O. Receptor-gated IL-2 delivery by an anti-human IL-2 antibody activates regulatory T cells in three different species. Sci Transl Med. 2020 Dec 16;12(574):12. [DOI] [PubMed] [Google Scholar]
- 53. Lee JY, Lee E, Hong SW, Kim D, Eunju O, Sprent J, et al. TCB2, a new anti-human interleukin-2 antibody, facilitates heterodimeric IL-2 receptor signaling and improves anti-tumor immunity. Oncoimmunology. 2020;9(1):1681869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clinical Trials . Study of AU-007, A monoclonal antibody that binds to IL-2 and inhibits IL-2rα binding, patients with unresectable locally advanced or metastatic cancer; 2022. [Google Scholar]
- 55. Tauber PA, Kratzer B, Schatzlmaier P, Smole U, Kohler C, Rausch L, et al. The small molecule inhibitor BX-795 uncouples IL-2 production from inhibition of Th2 inflammation and induces CD4(+) T cells resembling iTreg. Front Immunol. 2023;14:1094694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996 Dec 6;274(5293):1739–44. [DOI] [PubMed] [Google Scholar]
- 57. Barzilai O, Ram M, Shoenfeld Y. Viral infection can induce the production of autoantibodies. Curr Opin Rheumatol. 2007 Nov;19(6):636–43. [DOI] [PubMed] [Google Scholar]
- 58. Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004 Jan 1;172(1):625–35. [DOI] [PubMed] [Google Scholar]
- 59. Eggleton P, Haigh R, Winyard PG. Consequence of neo-antigenicity of the “altered self”. Rheumatology. 2008 May;47(5):567–71. [DOI] [PubMed] [Google Scholar]
- 60. Meyer S, Woodward M, Hertel C, Vlaicu P, Haque Y, Karner J, et al. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell. 2016 Jul 28;166(3):582–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sadighi Akha AA, Kumanovics A. Anti-cytokine autoantibodies and inborn errors of immunity. J Immunol Methods. 2022 Sep;508:113313. [DOI] [PubMed] [Google Scholar]
- 62. Amendt T, Jumaa H. Adaptive tolerance: protection through self-recognition. Bioessays. 2022 Mar;44(3):e2100236. [DOI] [PubMed] [Google Scholar]
- 63. Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003 Sep 5;301(5638):1374–7. [DOI] [PubMed] [Google Scholar]
- 64. Silverstein AM. Autoimmunity versus horror autotoxicus: the struggle for recognition. Nat Immunol. 2001 Apr;2(4):279–81. [DOI] [PubMed] [Google Scholar]
- 65. Mostbock S. Cytokine/Antibody complexes: an emerging class of immunostimulants. Curr Pharm Des. 2009;15(7):809–25. [DOI] [PubMed] [Google Scholar]
- 66. Watanabe M, Uchida K, Nakagaki K, Trapnell BC, Nakata K. High avidity cytokine autoantibodies in health and disease: pathogenesis and mechanisms. Cytokine Growth Factor Rev. 2010 Aug;21(4):263–73. [DOI] [PubMed] [Google Scholar]
- 67. Ku CL, Chi CY, von Bernuth H, Doffinger R. Autoantibodies against cytokines: phenocopies of primary immunodeficiencies? Hum Genet. 2020 Jun;139(6–7):783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jouvenne P, Fossiez F, Banchereau J, Miossec P. High levels of neutralizing autoantibodies against IL-1 alpha are associated with a better prognosis in chronic polyarthritis: a follow-up study. Scand J Immunol. 1997 Oct;46(4):413–8. [DOI] [PubMed] [Google Scholar]
- 69. Cai W, Qiu C, Zhang H, Chen X, Zhang X, Meng Q, et al. Detection of circulating natural antibodies to inflammatory cytokines in type-2 diabetes and clinical significance. J Inflamm. 2017;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ishizaka S, Tsujii T. IL-2 antibody production in lupus mice. Cell Immunol. 1989 Jan;118(1):100–7. [DOI] [PubMed] [Google Scholar]
- 71. Perol L, Lindner JM, Caudana P, Nunez NG, Baeyens A, Valle A, et al. Loss of immune tolerance to IL-2 in type 1 diabetes. Nat Commun. 2016 Oct 6;7:13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marzinotto I, Liberati D, Brigatti C, Bonfanti R, Stabilini A, Monti P, et al. Autoantibody binding in liquid phase to IL-2 in human sera is not type 1 diabetes specific. Diabetologia. 2017 Sep;60(9):1834–5. [DOI] [PubMed] [Google Scholar]
- 73. Churlaud G, Rosenzwajg M, Cacoub P, Saadoun D, Valteau-Couanet D, Chaput N, et al. IL-2 antibodies in type 1 diabetes and during IL-2 therapy. Diabetologia. 2018 Sep;61(9):2066–8. [DOI] [PubMed] [Google Scholar]
- 74. Whitehead RP, Ward D, Hemingway L, Hemstreet GP 3rd, Bradley E, Konrad M. Subcutaneous recombinant interleukin 2 in a dose escalating regimen in patients with metastatic renal cell adenocarcinoma. Cancer Res. 1990 Oct 15;50(20):6708–15. [PubMed] [Google Scholar]
- 75. Allegretta M, Atkins MB, Dempsey RA, Bradley EC, Konrad MW, Childs A, et al. The development of anti-interleukin-2 antibodies in patients treated with recombinant human interleukin-2 (IL-2). J Clin Immunol. 1986 Nov;6(6):481–90. [DOI] [PubMed] [Google Scholar]
- 76. Bost KL, Hahn BH, Saag MS, Shaw GM, Weigent DA, Blalock JE. Individuals infected with HIV possess antibodies against IL-2. Immunology. 1988 Dec;65(4):611–5. [PMC free article] [PubMed] [Google Scholar]
- 77. Balsari A, Caruso A. Natural antibodies to IL-2. Biotherapy. 1997;10(1):25–8. [DOI] [PubMed] [Google Scholar]
- 78. Tiberio L, Caruso A, Pozzi A, Rivoltini L, Morelli D, Monti E, et al. The detection and biological activity of human antibodies to IL-2 in normal donors. Scand J Immunol. 1993 Nov;38(5):472–6. [DOI] [PubMed] [Google Scholar]
- 79. Monti E, Pozzi A, Tiberio L, Morelli D, Caruso A, Villa ML, et al. Purification of interleukin-2 antibodies from healthy individuals. Immunol Lett. 1993 Jun;36(3):261–6. [DOI] [PubMed] [Google Scholar]
- 80. Watanabe M, Uchida K, Nakagaki K, Kanazawa H, Trapnell BC, Hoshino Y, et al. Anti-cytokine autoantibodies are ubiquitous in healthy individuals. FEBS Lett. 2007 May 15;581(10):2017–21. [DOI] [PubMed] [Google Scholar]
- 81. Reiher WE 3rd, Blalock JE, Brunck TK. Sequence homology between acquired immunodeficiency syndrome virus envelope protein and interleukin 2. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weigent DA, Hoeprich PD, Bost KL, Brunck TK, Reiher WE 3rd, Blalock JE. The HTLV-III envelope protein contains a hexapeptide homologous to a region of interleukin-2 that binds to the interleukin-2 receptor. Biochem Biophys Res Commun. 1986 Aug 29;139(1):367–74. [DOI] [PubMed] [Google Scholar]
- 83. Prummer O. Treatment-induced antibodies to interleukin-2. Biotherapy. 1997;10(1):15–24. [DOI] [PubMed] [Google Scholar]
- 84. Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R, et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24-30;302(5906):305–10. [DOI] [PubMed] [Google Scholar]
- 85. Lahm HW, Stein S. Characterization of recombinant human interleukin-2 with micromethods. J Chromatogr. 1985 Jun 19;326:357–61. [DOI] [PubMed] [Google Scholar]
- 86. Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–92. [DOI] [PubMed] [Google Scholar]
- 87. Rieger R, Michaelis A, Green MM. A glossary of genetics and cytogenetics: classical and molecular. Heidelberg: Springer Berlin; 1968. [Google Scholar]
- 88. Patents . Oxidation-resistant muteins of Il-2 and other protein. https://patents.google.com/patent/US5116943A. [Google Scholar]
- 89. Weigel U, Meyer M, Sebald W. Mutant proteins of human interleukin 2. Renaturation yield, proliferative activity and receptor binding. Eur J Biochem. 1989 Mar 15;180(2):295–300. [DOI] [PubMed] [Google Scholar]
- 90. Chen X, Ai X, Wu C, Wang H, Zeng G, Yang P, et al. A novel human IL-2 mutein with minimal systemic toxicity exerts greater antitumor efficacy than wild-type IL-2. Cell Death Dis. 2018 Sep 24;9(10):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, et al. Exploiting a natural conformational switch to engineer an interleukin-2’ superkine. Nature. 2012 Mar 25;484(7395):529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rao BM, Girvin AT, Ciardelli T, Lauffenburger DA, Wittrup KD. Interleukin-2 mutants with enhanced alpha-receptor subunit binding affinity. Protein Eng. 2003 Dec;16(12):1081–7. [DOI] [PubMed] [Google Scholar]
- 93. Rao BM, Driver I, Lauffenburger DA, Wittrup KD. Interleukin 2 (IL-2) variants engineered for increased IL-2 receptor alpha-subunit affinity exhibit increased potency arising from a cell surface ligand reservoir effect. Mol Pharmacol. 2004 Oct;66(4):864–9. [DOI] [PubMed] [Google Scholar]
- 94. Rao BM, Driver I, Lauffenburger DA, Wittrup KD. High-affinity CD25-binding IL-2 mutants potently stimulate persistent T cell growth. Biochemistry. 2005 Aug 9;44(31):10696–701. [DOI] [PubMed] [Google Scholar]
- 95. Matthews L, Chapman S, Ramchandani MS, Lane HC, Davey RT Jr, Sereti I. BAY 50-4798, a novel, high-affinity receptor-specific recombinant interleukin-2 analog, induces dose-dependent increases in CD25 expression and proliferation among unstimulated, human peripheral blood mononuclear cells in vitro. Clin Immunol. 2004 Dec;113(3):248–55. [DOI] [PubMed] [Google Scholar]
- 96. Margolin K, Atkins MB, Dutcher JP, Ernstoff MS, Smith JW 2nd, Clark JI, et al. Phase I trial of BAY 50-4798, an interleukin-2-specific agonist in advanced melanoma and renal cancer. Clin Cancer Res. 2007 Jun 1;13(11):3312–9. [DOI] [PubMed] [Google Scholar]
- 97. Peterson LB, Bell CJM, Howlett SK, Pekalski ML, Brady K, Hinton H, et al. A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J Autoimmun. 2018 Dec;95:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gillies SD, Lan Y, Hettmann T, Brunkhorst B, Sun Y, Mueller SO, et al. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin Cancer Res. 2011 Jun 1;17(11):3673–85. [DOI] [PubMed] [Google Scholar]
- 99. Gillessen S, Gnad-Vogt US, Gallerani E, Beck J, Sessa C, Omlin A, et al. A phase I dose-escalation study of the immunocytokine EMD 521873 (Selectikine) in patients with advanced solid tumours. Eur J Cancer. 2013 Jan;49(1):35–44. [DOI] [PubMed] [Google Scholar]
- 100. VanDyke D, Iglesias M, Tomala J, Young A, Smith J, Perry JA, et al. Engineered human cytokine/antibody fusion proteins expand regulatory T cells and confer autoimmune disease protection. Cell Rep. 2022 Oct 18;41(3):111478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Arenas-Ramirez N, Zou C, Popp S, Zingg D, Brannetti B, Wirth E, et al. Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci Transl Med. 2016 Nov 30;8(367):367ra166. [DOI] [PubMed] [Google Scholar]
- 102. Sahin D, Arenas-Ramirez N, Rath M, Karakus U, Humbelin M, van Gogh M, et al. An IL-2-grafted antibody immunotherapy with potent efficacy against metastatic cancer. Nat Commun. 2020 Dec 22;11(1):6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chang DZ, Tasayco ML, Ciardelli TL. Structural analogs of interleukin-2: a point mutation that facilitates biological response. Mol Pharmacol. 1995 Jan;47(1):206–11. [PubMed] [Google Scholar]
- 104. Chang DZ, Wu Z, Ciardelli TL. A point mutation in interleukin-2 that alters ligand internalization. J Biol Chem. 1996 Jun 7;271(23):13349–55. [DOI] [PubMed] [Google Scholar]
- 105. Clinical Trials . A study to evaluate safety, pharmacokinetics, and efficacy of RO6895882 in participants with advanced and/or metastatic solid tumors. https://clinicaltrials.gov/ct2/show/NCT02004106. [Google Scholar]
- 106. Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology. 2017;6(3):e1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hori T, Uchiyama T, Tsudo M, Umadome H, Ohno H, Fukuhara S, et al. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987 Oct;70(4):1069–72. [PubMed] [Google Scholar]
- 108. Teshigawara K, Wang HM, Kato K, Smith KA. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–76. [DOI] [PubMed] [Google Scholar]
- 110. Atkins MB, Mier JW, Parkinson DR, Gould JA, Berkman EM, Kaplan MM. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988 Jun 16;318(24):1557–63. [DOI] [PubMed] [Google Scholar]
- 111. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999 Jul;17(7):2105–16. [DOI] [PubMed] [Google Scholar]
- 112. Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kuo TT, Aveson VG. Neonatal Fc receptor and IgG-based therapeutics. MAbs. 2011 Sep-Oct;3(5):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fallon EM, Liparoto SF, Lee KJ, Ciardelli TL, Lauffenburger DA. Increased endosomal sorting of ligand to recycling enhances potency of an interleukin-2 analog. J Biol Chem. 2000 Mar 10;275(10):6790–7. [DOI] [PubMed] [Google Scholar]
- 116. Ricci MS, Sarkar CA, Fallon EM, Lauffenburger DA, Brems DN. pH Dependence of structural stability of interleukin-2 and granulocyte colony-stimulating factor. Protein Sci. 2003 May;12(5):1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Epstein AL, Mizokami MM, Li J, Hu P, Khawli LA. Identification of a protein fragment of interleukin 2 responsible for vasopermeability. J Natl Cancer Inst. 2003 May 21;95(10):741–9. [DOI] [PubMed] [Google Scholar]