Abstract
Background:
Giardia intestinalis is an intestinal protozoan which commonly causes parasitic gastroenteritis globally. It is a species complex consisting of at least eight assemblages (genotypes). In India, Giardia is mostly underreported and missed in asymptomatic cases.
Aim:
The aim of this study was to genotype the G. intestinalis isolates from stool samples of patients at a tertiary care center in Rajasthan, India, and to clinically correlate it.
Methods:
This prospective pilot cross-sectional study was conducted from 2019 to 2021 in a tertiary care center in western India. Patients who were microscopically positive for giardiasis were enrolled. DNA was extracted from their stool samples and amplified by polymerase chain reaction (PCR) using 4E1-HP as the target sequence. Anthropometric measurements and analysis were done for children by using Anthrocal application.
Results:
A total of 50 patients were enrolled. Diarrhea was present in 18 patients (36%). Among these, 6 were immunocompromised and had different comorbidities. Among the children <12 years of age, 55.17% (n = 16/29) were stunted (<−2 S.D.), and among <5 years, 44.4% (n = 4/9) showed wasting (<−2 S.D.). A PCR product corresponding to assemblage B of G. intestinalis was amplified in 47 stool specimens. Only three stool samples were negative for both assemblages A and B and posed an interesting enigma.
Conclusion:
In this study, a predominance of assemblage B of G. intestinalis was detected in 94% of the isolates. Furthermore, the possibility of zoonotic transmission could not be ruled out.
Keywords: Anthropometric indices, genotype, Giardia, growth retardation, India, zoonoses
INTRODUCTION
Giardia intestinalis (synonym: Giardia duodenalis and Giardia lamblia) is an intestinal organisms flagellate protozoan parasite that causes giardiasis in humans. This is the most common cause of parasitic gastroenteritis in India and globally. Developing countries have reported 2.5 million cases of diarrhea[1] and nutritional deficiencies in children due to giardiasis. It is a World Health Organization (WHO) Neglected Disease since 2004.
The latest research on G. intestinalis surmises it to be a species complex comprising at least eight assemblages (genotypes), based on phylogenetic studies of different isolates.[2] Target genes which have been used for its genotyping are glutamate dehydrogenase (gdh) gene, small subunit ribosomal ribonucleic acid (SSU rRNA), triose phosphate isomerase (tpi) gene, and 4E1HP sequence.[3] The DNA sequence and alloenzyme analysis of G. intestinalis isolates have revealed two major genotypes referred to as assemblage A and assemblage B. These predominantly infect humans. In India, Giardia is mostly underreported and frequently missed in asymptomatic cases.
The aim of this study was to genotype the G. intestinalis isolates from stool samples of patients at a tertiary care center in Rajasthan, India, and to clinically correlate it.
METHODS
This prospective pilot cross-sectional study was conducted from May 2019 to May 2021. This study was performed on patients who consulted the clinicians and/or were admitted to various clinical wards with complaints of diarrhea and gastrointestinal (GI) problems. Among these patients, whoever were positive for giardiasis by light microscopic examination were enrolled and the study was further done to investigate the assemblage of Giardia spp. isolates from the enrolled study population. All the enrolled patients for the study were given detailed clear instructions to submit about 1–2 g of fecal specimens in a wide mouth-clean screw-capped plastic container without any preservatives.
Inclusion criteria of patients were clinical presentation of diarrhea and/or GI symptoms, irrespective of age group, with microscopically positive stool samples for giardiasis and consent of participation in this study. Patients without microscopically positive stool samples for Giardia were excluded. The study was approved by the Intuitional Ethics Committee vide letter number (AIIMS/IEC/2019-20/811).
DNA was extracted directly from the positive stool samples by QIAamp® Fast DNA Stool Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) amplification was performed using 4E1-HP as the target sequence in Eppendorf Mastercycler X50s (Eppendorf) thermocycler using the Taq PCR Master Mix Kit (Qiagen) and the HotStarTaq® Plus Master Mix Kit (Qiagen) as described by Vanni et al.[3] The 4E1-HP sequence of assemblages A and B was designated as 4E1-HPA and 4E1-HPB, respectively. At the end of the reaction, a 272-bp fragment of 4E-1HP sequence of assemblage B was obtained using oligonucleotide primer 4E1-HP B for GAAGTCATCTCTGGGGCAAG as forward primer and oligonucleotide primer 4E1-HP B Rev GAAGTCTAGATAAACGTGTCGG as reverse primer, respectively. Assemblage A using primers 4E1-HP A for AAAGAGATAGTTCGCGATGTC and 4E1-HP A Rev ATTAACAAACAGGGAGACGTATG remained undetected. Both positive and negative controls were included in each batch of the tests. The positive control DNA for assemblage B of G. intestinalis was provided by Dr. Sitara Swarna Rao from CMC, Vellore, India.
Agarose gel (1.3%, HiMedia, India) was prepared in 1X TAE buffer, and the PCR products were loaded with the help of 2l of 6X loading buffer in the wells. Electrophoresis was performed for about 90 min at a constant voltage of 90 mV. A PCR product corresponding to 272 bp for assemblage B was visualized in a gel documentation system (Bio-Rad, USA). Descriptive statistical analysis was done using IBM SPSS software (International Business Machines, Armonk, New York, USA).
RESULTS
A total of 50 patients (males 30, females 20) were enrolled in the study. Among these, 21 (42%) patients were adults (males 14, females 7) and 29 (58%) patients were children (males 16, females 13), as presented in Table 1.
Table 1.
Distribution of age and gender among study subjects (n=50)
| Clinical presentation | Adults (n=21) | Children (n=29) | ||
|---|---|---|---|---|
|
|
|
|||
| Male (n=14) | Female (n=07) | Male (n=16) | Female (n=13) | |
| Acute diarrhea (n=12) | 2 | 2 | 5 | 3 |
| Chronic diarrhea (n=06) | 1 | - | 3 | 2 |
| No diarrhea (n=32) | 11 | 5 | 8 | 8 |
Overall, diarrhea was present in 18 patients (n = 18, 36%) including 11 males and 7 females. Among the diarrhea cohort, 6 (33.3%) had chronic diarrhea and 12 (66.7%) had acute diarrhea. Among adults, 5 patients presented with diarrhea (n = 5, 23.8%). Among children, 13 patients presented with diarrhea (n = 13, 44.8%).
Among the total 50 patients enrolled, 44 (88%) were immunocompetent (males 27, females 17), whereas 6 (12%) were immunocompromised (males 3, females 3) and had different underlying comorbidities besides giardiasis. Among these immunocompromised patients, only 1 was adult (male, 1/21 or 4.76%) and 5 were pediatric (males 2, females 3, 5/29 or 17.24%). The division of the study population on the basis of immune status is summarized in Table 2.
Table 2.
Distribution of study subjects (n=50) based on immune status
| Immune status | Total (n=50) | Adults (n=21) | Children (n=29) |
|---|---|---|---|
| Immunocompetent patients | 44 (male: female=27:17) | 20 (male: female=13:7) | 24 (male: female=14:10) |
| Immunocompromised patients | 6 (male: female=3:3) | 1 (male=1) | 5 (male: female=2:3) |
Immunocompetent patients suffered from giardiasis (31, 70.45%), celiac disease (1, 2.27%), iron deficiency anemia (2, 4.55%), intertrochanteric fracture (1, 2.27%), hepatic abscess (2, 4.55%), and gastroenteritis (7, 15.91%). Immunocompromised patients suffered from Bruton’s agammaglobulinemia (1), IgA nephropathy (1), nephrotic syndrome (1), Type 2 congenital pulmonary airway malformation (CPAM) (1), and SAM (severe acute malnutrition) (1), as depicted in Figure 1. One patient became immunocompromised due to prolonged steroid therapy.
Figure 1.
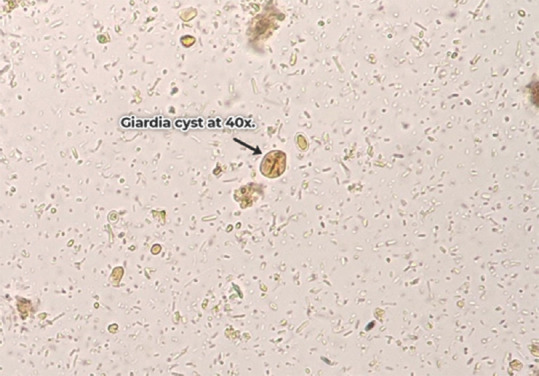
Giardia cyst at 400× magnification under light microscope
The mean age of the total study population (n = 50) was 18.62 + 16.55 years. A male preponderance of 60% (30/50) was observed. Thirty-six percent (18/50) of the study subjects complained of diarrhea. Seventy-eight percent (39/50) of patients complained of abdominal cramps. Constipation was seen in 32% (n = 16) of patients. Postprandial hurry was reported by 30% (15/50) of patients and loss of weight by 20% (n = 10). Forty-eight percent (n = 24) of patients had taken treatment in the past for their symptoms. Consumption of uncooked vegetables was seen in 34% (n = 17), consumption of unhygienic food within 7 days was seen in 30% (n = 15), and consumption of unhygienic water was observed in 50% (n = 25) among the enrolled patients. Two (4%) patients reported contact with pet animals within the last 30 days. Fever was only present in 3 patients. Patients with acute exacerbations of chronic diarrhea (n = 2) and chronic abdominal pain (n = 2) and 1 case of acute abdominal pain were immunocompromised, whereas immunocompetent patients presented with acute diarrhea (n = 12) mainly followed by chronic diarrhea (n = 4). Patients presenting with diarrhea presented with frequency of >6 stools/day (83.3%, 15/18). A complete analysis of the clinical features of patients and other histories is summarized, respectively, in Tables 3 and 4.
Table 3.
Analysis of clinical parameters of total study subjects (n=50)
| Clinical parameters | Total (n=50), n (%) |
|---|---|
| Age (years) | 18.62±16.55 |
| Sex (male:female) | 30:20 |
| Diarrhea | 18 (36) |
| Abdominal cramp | 39 (78) |
| Fever | 3 (6) |
| Loss of appetite | 15 (30) |
| Loss of weight | 10 (20) |
| Vomiting | 9 (18) |
| Malaise/fatiguability | 10 (20) |
| Postprandial hurry | 15 (30) |
| Constipation | 16 (32) |
| Pallor | 10 (20) |
Table 4.
Other relevant clinical parameters (n=50)
| Clinical parameter (n=50) | Present | Absent |
|---|---|---|
| Weight loss | 10 | 40 |
| Safe drinking water | 25 | 25 |
| Treatment taken previously | 24 | 26 |
| Contact with animals within 30 days | 2 | 48 |
| Consumption of uncooked vegetables | 17 | 33 |
| Consumption of unhygienic food | 15 | 35 |
| Poor appetite | 15 | 35 |
| Pallor | 10 | 40 |
| Skin turgor decreased | 4 | 46 |
In adults, abdominal cramp (90.48%, 19/21) was the most common clinical presentation followed by constipation (42.86%, 9/21) and diarrhea (25%, 5/21) and postprandial hurry (28.57%, 6/21). However, in children, abdominal cramp (69%, 20/29) was followed by diarrhea (44.8%, 13/29) and poor appetite (34.5%, 10/29) presentation.
Cysts of G. intestinalis were detected in stool samples of 47/50 (94%) study subjects. Trophozoites of G. intestinalis along with the cysts were observed in stool specimens of 18 (63%) patients. Only 3/50 (6%) patients demonstrated only trophozoites in their stool samples. The microscopic photographs of cysts and trophozoite of G. intestinalis are depicted in Figures 2 and 3.
Figure 2.
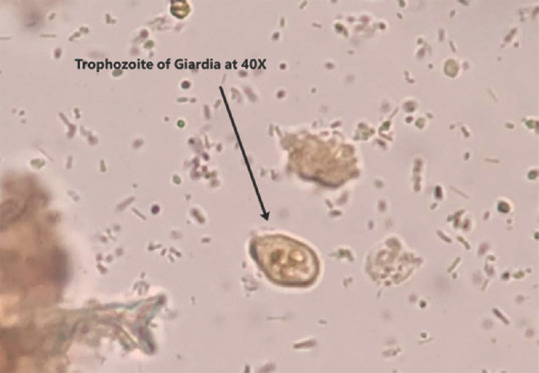
Giardia trophozoite at 400× magnification under light microscope
Figure 3.
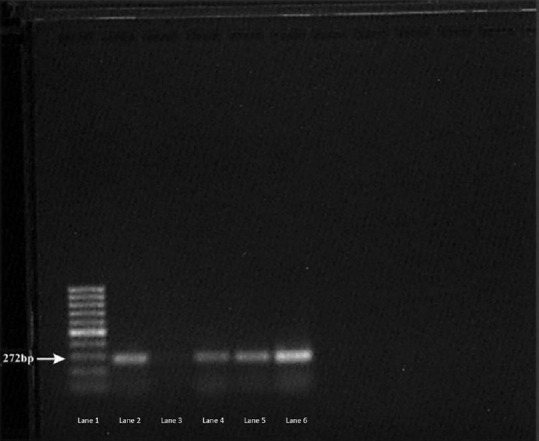
Agarose gel photograph of positive control and some of the positive samples by polymerase chain reaction assay for assemblage B. Lane 1-100 bp Ladder, Lane 2: Positive control, Lane 3: Negative Control, Lane 4, 5, 6: Positive samples
In 12 (24%) of the 50 patients, stool examination also revealed co-infection with other parasites. Among the 6 (28.57%) adults, only 3 (50%) were co-infected with pathogenic parasites. Among the 6 pediatric patients, only 1 (16.67%) had a pathogenic parasite. Entamoeba histolytica (3/12, 25%) was the most common pathogenic parasite, followed by Hymenolepis nana (1/12, 8.33%), whereas Blastocystis hominis (7/12, 58.3%) and Entamoeba coli (1/12, 8.33%) were the most common nonpathogenic parasites. Upper gastrointestinal endoscopy was performed in 4 patients, out of which 2 (50%) showed scalloped folds.
Weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) z scores for the pediatric cases were calculated based on the WHO Child Growth Standards[4] for children up to 12 years of age, as per age criteria. Majority of the children (n = 16/29, 55.17%) were found to be stunted (<−2 S.D.) on the basis of their height for age. Among these, 6.9% (n = 02) were between − 3 S.D. and − 2 S.D., 48.3% (n = 14) were between − 2 S.D. and − 1 S.D., and none were below − 3 S.D. Among the children aged <10 years (n = 20), majority (n = 8, 40%) were of normal weight for their age (<−1 S.D). Only 5% (n = 1) had severe malnutrition (<−3 S.D.), 25% (n = 5) had moderate malnourishment (≥ −3 S.D. to < −2 S.D.), and 30% (n = 6) had mild malnutrition. Among the children aged <5 years of age (n = 9), wasting (<−2 S.D.) was present in 44.4% (n = 04). Only one (11.1%, n = 01) child was below − 3 S.D. and 33.3% (n = 03) children were between − 3 S.D. and -2 S.D.
A PCR product of 272 bp in size corresponding to assemblage B of G. intestinalis indicated positive amplification. Stool specimens microscopically positive for G. intestinalis cyst and/or trophozoites and successfully amplified using the 4E1-HP sequence PCR assay were 47 in number. Figure 4 shows the agarose gel photograph of positive control and some of the samples positive by the PCR assay for assemblage B. The analysis as per assemblage is summarized in Table 5.
Figure 4.
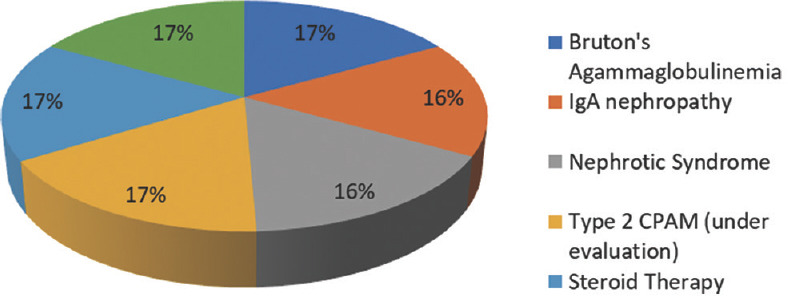
Distribution of immunocompromised patients
Table 5.
Analytical summary of results as per assemblage identified
| Variables | Genotype assemblage (4E1HP sequence), count (%) | |
|---|---|---|
|
| ||
| Assemblage B (n=47) | Unidentified (n=3) | |
| Age | ||
| Adult | 20 (95.2) | 1 (5.0) |
| Pediatric | 27 (93.3) | 2 (6.9) |
| Sex | ||
| Male | 28 (93.3) | 2 (6.7) |
| Female | 19 (95.0) | 1 (5.0) |
| Diarrhea | ||
| Present | 17 (94.4) | 1 (5.6) |
| Absent | 29 (90.6) | 3 (9.4) |
| Duration of disease | ||
| Acute | 24 (92.3) | 2 (7.7) |
| Chronic | 23 (95.8) | 1 (4.2) |
| Abdominal cramps | ||
| Present | 36 (92.3) | 3 (7.7) |
| Absent | 11 (100.0) | 0 |
| Postprandial hurry | ||
| Present | 13 (92.9) | 1 (7.1) |
| Absent | 34 (94.4) | 2 (5.6) |
| Fever | ||
| Present | 3 (100) | 0 |
| Absent | 44 (93.6) | 3 (6.4) |
| Nausea/vomiting | ||
| Present | 9 (100.0) | 0 |
| Absent | 38 (92.7) | 3 (7.3) |
| Weight loss | ||
| Present | 10 (100.0) | 0 |
| Absent | 37 (92.5) | 3 (7.5) |
| Constipation | ||
| Present | 15 (93.8) | 1 (6.2) |
| Absent | 33 (94.3) | 2 (5.7) |
| Poor appetite | ||
| Present | 14 (93.3) | 1 (6.7) |
| Absent | 33 (94.3) | 2 (5.7) |
| Diarrhea within family | ||
| Absent | 47 (94.0) | 3 (6.0) |
| Contact with animals within 30 days | ||
| Present | 2 (100.0) | 0 |
| Absent | 45 (93.8) | 3 (6.2) |
| Consumption of uncooked vegetables within 7 days | ||
| Present | 15 (88.2) | 2 (11.8) |
| Absent | 32 (97.0) | 1 (3.0) |
| Consumption of unhygienic food within 7 days | ||
| Present | 15 (100.0) | 0 |
| Absent | 32 (91.4) | 3 (8.6) |
| Pallor | ||
| Present | 9 (90.0) | 1 (10.0) |
| Absent | 38 (95.0) | 2 (5.0) |
| Trophozoite of giardia intestinalis | ||
| Present 0–2/hpf | 9 (100.0) | 0 |
| Absent | 27 (93.1) | 2 (6.9) |
| Present >2/hpf | 11 (91.7) | 1 (8.3) |
| Cyst of giardia intestinalis | ||
| Present 0–2/hpf | 34 (100.0) | 0 |
| Absent | 3 (100.0) | 0 |
| Present >2/hpf | 10 (76.9) | 3 (23.1) |
| Plenty | 1 (100.0) | 0 |
| Occult blood | ||
| Positive | 1 (50.0) | 1 (50.0) |
| Negative | 7 (100.0) | 0 |
| Not performed | 39 (95.1) | 2 (4.9) |
| Coccidian parasites | ||
| Absent | 47 (94.0) | 3 (6.0) |
DISCUSSION
G. intestinalis (synonym: G. duodenalis, G. lamblia) is one of the most common causes of parasitic gastroenteritis throughout the world.[1] In humans, clinically, Giardia infection ranges from the asymptomatic carrier state to severe malabsorption syndrome. It is a species complex constituting at least eight assemblages, based on phylogenetic studies.
Three genotypes, namely AI, AII, and B, have been associated with human giardiasis.[2] The association between different genotypes with different clinical manifestations of giardiasis is also established to some extent in different regions of the world.[5] In India, Giardia often remains undiagnosed and not genotyped because a majority of infected individuals are unaware due to a lack of knowledge and proper diagnostic techniques.[6] No studies pertaining to genotypes of Giardia exist in Rajasthan, India. Most of the patients in this study are from in and around Jodhpur, Rajasthan.
G. intestinalis is known to cause frequent infection in pediatric age group. There were 12 children below 5 years of age in our study. Similar observations were made by Roy et al.[6] As children have young immune systems and play outdoors and are exposed to different surroundings and pets, the risk of transmission of infection increases manifold. In our study, adults presented more with constipation (42.86%, 9/21), diarrhea (25%, 5/21), and postprandial hurry (28.57%, 6/21), whereas children presented more with diarrhea (44.8%, 13/29) and poor appetite (34.5%, 10/29). This could be due to quicker communication and treatment before diagnosis in adults. A larger number of cohorts and further studies can help to differentiate between adult and pediatric presentation of giardiasis. However, growth retardation and a prolonged history with or without acute exacerbations are exclusively seen in children.
In our study, 88% (44/50) of study subjects were immunocompetent and 12% (06/50) were immunocompromised. Among immunocompetent individuals, only 1 (2.3%) patient had celiac disease with concomitant giardiasis. Association of celiac disease with giardiasis has also been reported. Studies conducted by Behera et al.[7] revealed both celiac disease and giardiasis present in 8% of children, respectively.
G. intestinalis is commonly associated with impaired cellular immunity. In this study, immunocompromised patients presented with either malabsorption or chronic diarrhea. In our study, 2 (4%) patients with Giardia infection were found to be suffering from Type 2 CPAM and Bruton’s agammaglobulinemia. Giardiasis in CPAM patients is often refractory to treatment and is characterized by chronicity and frequent relapse. A recent study on hypergammaglobulinemic patients has revealed opportunistic infections with G. intestinalis in 31.8% of patients.[8] Serum globulins were low in all patients. Duodenal biopsy showed a paucity of plasma cells in 45%, villous atrophy in 35%, and nodular lymphoid hyperplasia in 30% of patients. Although uncommon, hypogammaglobulinemia is associated with GI disease. The possibility of a primary immunodeficiency should be considered in patients presenting with GI symptoms and low serum globulin.
In our study, one patient (2%) had nephrotic syndrome and another IgA nephropathy with prolonged steroid therapy along with giardiasis. Copelovitch et al.[9] also found 4% (3/82) of their patients with nephrotic syndrome suffering from giardiasis. The use of prolonged steroid therapy in such diseases leads to a state of immunosuppression, thus increasing the susceptibility to opportunistic infections including G. intestinalis.
Patients of giardiasis experience variable symptoms such as loose stools, abdominal pain, vomiting, fever, malaise, weight loss, and malabsorption. Abdominal pain was the most common presentation (78%) in our study subjects, followed by diarrhea (36%), constipation (32%), postprandial hurry (30%), poor appetite (30%), loss of weight (20%), and vomiting (18%). The role of G. intestinalis in causing diarrhea has been considered controversial, whereas malnutrition and loss of weight were some of the common symptoms found by Akgun and Celik.[10] Tak et al.[11] reported diarrhea (85%) in comparison to our study which revealed 36% diarrhea with more acute than chronic cases. A new observation after review of other studies in comparison to ours has revealed that in a majority, acute giardiasis manifests with abdominal cramps and chronic giardiasis is associated with recurrent bouts of diarrhea and related chronic symptomatology such as weight loss and malnutrition. This could possibly be due to prolonged diarrhea and loss of appetite in these patients leading to reduced food intake and malabsorption of nutrients. Similar findings were reported by Roy et al.[6] where some of the affected individuals did not have any symptoms of diarrhea, but most of them complained about having an abdominal cramp. Most of the children were found to be suffering from malnutrition and growth retardation similar to our study.
The WHO defines anemia as a hemoglobin level <130 g/L (13 g/dL) in men and <120 g/L (12 g/dL) in women.[12] In giardiasis, damage to the small intestinal mucosa leads to decreased absorption of iron and other micronutrients and thus iron deficiency anemia. Iron deficiency anemia was seen in 20% of our study subjects. Ajjampur et al.[13] also found that G. intestinalis infection had an adverse impact on linear growth of children as well as in levels of hemoglobin.
Growth failure is associated with increased morbidity and mortality in children. Although the etiology of growth failure is multifactorial, malnutrition and repeated infections in children are documented predisposing factors. Apart from diarrhea and abdominal cramps, Giardia infection in children can also result in faltering of long-term growth and impairment of cognitive function.[1]
Growth failure in children, which is indicated by stunting, wasting, and underweight conditions, can be assessed by anthropometric indices (z-scores) of HAZ, WAZ, and WHZ. Stunting was seen in 1 child in our study, whereas wasting was seen in 6 children. 55.17% of children in our study were malnourished and underweight as determined by their weight for age z-scores. Ajjampur et al.[13] from Vellore also reported stunted growth in 62% of children, wasting in 20% of children, and malnourished, underweight growth in 44% of children. Almeida et al.,[14] however, observed stunting and wasting in 44% and 6% of children, respectively. They also found 12% of the children underweight. Ratanapo et al.[15] in Thailand observed 8.5% of children with giardiasis underweight in primary schools. Treatment of giardiasis resulted in increased weight and height gain, allowing catch-up growth in preschool children. Psychomotor development and cognitive development were also adversely affected by early childhood Giardia infections.[13]
In this study, two diagnostic techniques were used: microscopy and PCR-based genotype detection. Microscopic analysis is the most primitive, cost-effective, and reliable method of diagnosis depending only on the morphology. However, it cannot differentiate between different members of the Giardia species complex. Furthermore, sometimes the severity in a patient might be too minuscule; then, the causative agent may not be detected in that specific mount microscopically. In addition, microscopy has a low sensitivity due to the intermittent shedding of the parasite. Detection of Giardia by coproantigen is also used for diagnosis. In a study, 2992 patients were screened for Giardia by microscopy and/or enzyme-linked immunosorbent assay (ELISA). ELISA was performed in 264 patients; of them, 127 were positive by microscopy. The sensitivity and specificity of ELISA were 91 and 91%, respectively.[16]
DNA-based assays have gained popularity due to their precision in discriminating both inter-species and intra-species variations among Giardia spp. The use of molecular techniques such as PCR and further analysis of the amplicon by restriction fragment length polymorphism (RFLP), i.e., PCR-RFLP analysis, have provided a clear distinction between different assemblages (genotypes) of G. intestinalis isolates.[17] In another Indian study, stool microscopy detected 65%, stool PCR detected an additional 27%, and duodenal biopsy PCR detected an additional 8% of giardiasis cases in chronically ill patients. Stool-nested PCR had a sensitivity and specificity of 100% and 94%, respectively, compared to stool microscopy.[18] Recently, a study in India has proved a multiplex loop-mediated isothermal amplification assay suitable for the rapid detection of DNA of G. duodenalis (elongation factor 1 alpha gene) from standard strains in 10–15 min, with high sensitivity and no cross reactivity.[19]
Assemblages A and B are known causes of human giardiasis. Overall assemblage B seems to be more common (60%), followed by assemblage A (35%) and mixed assemblage A + B (5%).[2] So far, there are no reports of homogeneous distribution of the prevailing assemblages globally. Recently, Garcia et al. from New Zealand reported the presence of assemblage E and sub-assemblage AIII in human isolates for the first time.[20] Different assemblages have different distributions in different regions within a country itself. This study provides, for the first time, information on the distribution of the genotypes of G. intestinalis from patients with giardiasis in Rajasthan. We determined G. intestinalis genotypes by PCR using Giardia-specific 4E1-HP sequence like Vanni et al.[3] A band of 272 bp was seen in 47 clinical isolates and identified as assemblage B (94%) similar to several authors like Ghoshal et al.[16] (62%) and Traub et al.[21] (55%). The rest three samples remained unidentified so far.
In India, giardiasis has been found to be prevalent throughout the country. In studies from northern India, prevalence rates ranged from 5.5% to 70%, with the highest rates reported in Chandigarh. In studies from southern India, the prevalence rates of giardiasis in children with diarrhea and adults with GI symptoms were 8%–10% and 37.1%, respectively.[22] Tak et al.[11] found 100% (82) study subjects to be infected with assemblage B of Giardia. 85% of patients had diarrhea, out of which 61 (87%) had chronic diarrhea, 8 (11.5%) had acute diarrhea, and 1 (1.5%) had persistent diarrhea. Similarly, Thakur et al.,[23] also found assemblage B III to be predominant (84.37%) among a total study population of 40 patients from the low socioeconomic strata of Chandigarh. In south eastern West Bengal, the overall prevalence was found to be 6.8%. Giardiasis was significantly common in pediatric age group <12 years, with 30.8% assemblage A, 63.5% assemblage B, and 5.7% combined assemblage A + B. Sub-assemblage AII was 17.3%, followed by sub-assemblage AI (13.5%). Assemblage B showed a lot of genetic diversity and balancing selection as per multilocus genotyping.[24] Langbang et al.,[25] from Pondicherry, studied the rural and urban assemblage distribution in 1006 stool samples of children. They found that 500 rural samples had 108 (21%) assemblage A, 116 (23.2%) assemblage B, and 5 (1%) mixed assemblages (A + B) whereas 506 urban samples had 92 (18.1%) assemblage A, 93 (18.3%) assemblage B, and 10 (1.9%) mixed assemblages (A + B) of Giardia. Ajjampur et al.[13] from Vellore studied the genotype in 50 diarrheic and 51 asymptomatic children. They found assemblage B in 40, assemblage A in 5, and mixed assemblage A + B in 5 children presenting with diarrhea. In asymptomatic children, assemblage B was seen in 48, assemblage A in 2, and mixed assemblage A + B in 1 child. Hence, in their study, assemblage B was more common. Laishram et al.,[26] from South India, reported assemblage B as predominant in both children and adults (82·4%) followed by assemblage AII (9·4%). Mixed assemblage A and B infections were observed exclusively in children. In another study from North India,[16] assemblages A and B were found among 44 (38%) and 72 (62%) patients, respectively.
Although assemblage B is most likely transmitted from human to human, it has been reported in some animals and may represent a zoonotic potential as well. The prevalence of bovine giardiasis in dairy farms in West Bengal was 12.2% (22/180) on the basis of the β-giardin gene, with younger calves being more affected. Zoonotic G. duodenalis assemblage A1 was identified in both calves and workers in this study, although the novel assemblage E was found to be the most prevalent genotype detected in cattle.[27] These findings clearly suggest the potential risk of zoonotic transmission of G. duodenalis infections from cattle to humans on dairy farms in India. In another study, Traub et al.[21] looked for zoonotic transmission of G. intestinalis from the state of Assam in India. They found both assemblages A and B in both humans and their pet dogs. Interestingly, they also found assemblages C and D in human beings at the SSU-rRNA gene locus only, but similar finding could not be confirmed at other gene loci such as the tpi and ef-1. They confirmed 19 isolates from human subjects as 9 assemblage B (47%), 6 assemblage A (32%), and 4 mixed assemblage A + B (21%). In a study in a tea tribe in Assam with some evidence of zoonotic transmission from dogs, a high prevalence of dogs harboring human-associated assemblages B and AII was seen while the human samples showed assemblage B and A as well as mixed infections. This study also identified a few assemblage AI in both human and dog samples.[21] In another recent study from the UK,[28] 17.4% (8/46) of feline-derived Giardia strains, from companion animals, were potentially zoonotic, namely assemblages B and AI.
Assemblage B has been correlated with severe giardiasis or symptomatic diarrhea in Ethiopia by Gelanew et al.[29] In contrast, other studies reported a significant association between assemblage A and the presence of severe symptoms.[14,30] In another Indian study, assemblage B was more often associated with malnutrition and loss of appetite than A.[16] Fantinatti et al.[31] found that G. lamblia causes growth delays in preschool children independent of infecting assemblage (A, B, or E). Although we did not have any transplant recipients among the immunocompromised cohort, assemblage B has been commonly found among such patients also.[32] As most of our cases were found to be assemblage B, the correlation of different assemblages in symptomatic and asymptomatic giardiasis could not be ascertained.
Limitations of the study
Three of our clinical isolates could not be identified which prove to be an interesting enigma. As no assemblage A and/or mixed assemblage/s could be detected in the present study, it was not possible to associate genotype/s distribution with different clinical spectra of giardiasis. Thus, the second objective, i.e. correlation of different genotypes with different clinical scenarios of giardiasis, could not be achieved because only a single genotype was identified. The zoonotic relationship is also not clear in this study, as only the assemblages A and B previously reported as human assemblages were tested; thus, nothing can be commented about zoonotic transmission. However, it could be possible that the unidentified genotypes might be nonhuman genotypes (other than assemblages A and B) or have negligible amounts of Giardia DNA in them. There was also absence of substantial data extrapolating risk factors as pertaining to giardiasis. Therefore, these limitations warrant further studies to understand the molecular epidemiology of giardiasis.
CONCLUSION
This work provides the first information about the prevalence of Giardia assemblage in western India. Majority of our isolates were identified as assemblage B (94%) by molecular characterization. This preliminary study shed an insight about the clinical presentation of patients infected with G. intestinalis assemblage B. Patients infected with assemblage B manifested the entire broad range of clinical symptoms from mild dyspeptic symptoms to abdominal pain, fever, diarrhea, and even sequelae such as malabsorption syndrome, growth retardation, and anemia. Most of our patients presented with abdominal pain (78%) and acute diarrhea (66.7%). Future studies with larger number of clinical or environmental samples from this part of the country are certainly warranted to find the true diversity of this amitochondriate eukaryotic pathogenic protozoan in India.
Ethical statement
The study was approved by Institutional Ethics Committee of AIIMS Jodhpur vide certificate number AIIMS/IEC/2019/1808 dated 20/06/2019.
Financial support and sponsorship
This study was supported by the Research Cell of All India Institute of Medical Sciences, Jodhpur, India (AIIMS/IEC/2019-20/811).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge Dr. Sitara Swarna Rao from CMC, Vellore, India, for providing us with the positive control DNA for assemblage B of Giardia intestinalis.
REFERENCES
- 1.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, et al. Global causes of diarrheal disease mortality in children <5 years of age: A systematic review. PLoS One. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cacci8 SM, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol. 2008;160:75–80. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Vanni I, Cacci SM, van Lith L, Lebbad M, Svbba SG, Pozio E, et al. Detection of Giardia duodenalis assemblages A and B in human feces by simple, assemblage-specific PCR assays. PLoS Negl Trop Dis. 2012;6:e1776. doi: 10.1371/journal.pntd.0001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The WHO Child Growth Standards. 2007. [[Last accessed on 2023 Nov 21]]. Available from: https://www.who.int/tools/child-growth-standards .
- 5.Mohammed Mahdy AK, Surin J, Wan KL, Mohd-Adnan A, Al-Mekhlafi MS, Lim YA. Giardia intestinalis genotypes: Risk factors and correlation with clinical symptoms. Acta Trop. 2009;112:67–70. doi: 10.1016/j.actatropica.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Roy M, Singha B, Dhar D, Roychoudhury S. Prevalence of Giardia intestinalis with other co-infecting parasites in Barak Valley, Assam, India: A molecular approach. J Parasit Dis. 2019;43:426–42. doi: 10.1007/s12639-019-01107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behera B, Mirdha BR, Makharia GK, Bhatnagar S, Dattagupta S, Samantaray JC. Parasites in patients with malabsorption syndrome: A clinical study in children and adults. Dig Dis Sci. 2008;53:672–9. doi: 10.1007/s10620-007-9927-9. [DOI] [PubMed] [Google Scholar]
- 8.Desai L, Kurien RT, Simon EG, Dutta AK, Joseph AJ, Chowdhury SD. Hypogammaglobulinemia-associated gastrointestinal disease cal study in chi. Indian J Gastroenterol. 2014;33:560–3. doi: 10.1007/s12664-014-0514-7. [DOI] [PubMed] [Google Scholar]
- 9.Copelovitch L, Sam Ol O, Taraquinio S, Chanpheaktra N. Childhood nephrotic syndrome in Cambodia: An association with gastrointestinal parasites. J Pediatr. 2010;156:76–81. doi: 10.1016/j.jpeds.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Akgun S, Celik T. Evaluation of Giardia intestinalis, Entamoeba histolytica and Cryptosporidium hominis/Cryptosporidium parvum in human stool samples by the BD MAX (TM) enteric parasite panel. Folia Parasitol (Praha) 2020;67:2020.020. doi: 10.14411/fp.2020.020. [DOI] [PubMed] [Google Scholar]
- 11.Tak V, Mirdha BR, Yadav P, Vyas P, Makharia GK, Bhatnagar S. Molecular characterisation of Giardia intestinalis assemblages from human isolates at a Tertiary Care Centre of India. Indian J Med Microbiol. 2014;32:19–25. doi: 10.4103/0255-0857.124290. [DOI] [PubMed] [Google Scholar]
- 12.Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. WHO. 2011. [[Last accessed on 2023 Nov 21]]. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11 .
- 13.Ajjampur SS, Sankaran P, Kannan A, Sathyakumar K, Sarkar R, Gladstone BP, et al. Giardia duodenalis assemblages associated with diarrhea in children in South India identified by PCR-RFLP. Am J Trop Med Hyg. 2009;80:16–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida AA, Delgado ML, Soares SC, Castro AO, Moreira MJ, Mendonao CM, et al. Genotype analysis of Giardia isolated from asymptomatic children in Northern Portugal. J Eukaryot Microbiol. 2006;53(Suppl 1):S177–8. doi: 10.1111/j.1550-7408.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 15.Ratanapo S, Mungthin M, Soontrapa S, Faithed C, Siripattanapipong S, Rangsin R, et al. Multiple modes of transmission of giardiasis in primary schoolchildren of a rural community, Thailand. Am J Trop Med Hyg. 2008;78:611–5. [PubMed] [Google Scholar]
- 16.Ghoshal U, Shukla R, Pant P, Ghoshal UC. Frequency, diagnostic performance of coproantigen detection and genotyping of the Giardia among patients referred to a multi-level teaching hospital in northern India. Pathog Glob Health. 2016;110:316–20. doi: 10.1080/20477724.2016.1254141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Y, Li J, Zhang L. Genetic diversity and molecular diagnosis of Giardia. Infect Genet Evol. 2023;113:105482. doi: 10.1016/j.meegid.2023.105482. [DOI] [PubMed] [Google Scholar]
- 18.Jangra M, Dutta U, Shah J, Thapa BR, Nada R, Gupta N, et al. Role of polymerase chain reaction in stool and duodenal biopsy for diagnosis of giardiasis in patients with persistent/chronic diarrhea. Dig Dis Sci. 2020;65:2345–53. doi: 10.1007/s10620-019-06042-2. [DOI] [PubMed] [Google Scholar]
- 19.Mewara A, Khunger S, Sharma C, Krishnamoorthi S, Singh S, Yadav R, et al. Arapid multiplex loop-mediated isothermal amplification (mLAMP) assay for detection of Entamoeba histolytica and Giardia duodenalis. Lett Appl Microbiol. 2023;76:ovad114. doi: 10.1093/lambio/ovad114. [DOI] [PubMed] [Google Scholar]
- 20.Garcia RJ, Ogbuigwe P, Pita AB, Velathanthiri N, Knox MA, Biggs PJ, et al. First report of novel assemblages and mixed infections of Giardia duodenalis in human isolates from New Zealand. Acta Trop. 2021;220:105969. doi: 10.1016/j.actatropica.2021.105969. [DOI] [PubMed] [Google Scholar]
- 21.Traub RJ, Monis PT, Robertson I, Irwin P, Mencke N, Thompson RC. Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitology. 2004;128:253–62. doi: 10.1017/s0031182003004505. [DOI] [PubMed] [Google Scholar]
- 22.Laishram S, Kang G, Ajjampur SS. Giardiasis: A review on assemblage distribution and epidemiology in India. Indian J Gastroenterol. 2012;31:3–12. doi: 10.1007/s12664-012-0161-9. [DOI] [PubMed] [Google Scholar]
- 23.Thakur S, Kaur U, Sehgal R. Genetic diversity of Giardia isolates from patients in Chandigarh region: India. BMC Res Notes. 2021;14:26. doi: 10.1186/s13104-020-05419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosal A, Sardar SK, Haldar T, Maruf M, Saito-Nakano Y, Dutta S, et al. Genotyping and epidemiological distribution of diarrhea-causing isolates of Giardia duodenalis in southeastern part of West Bengal, India. Parasitol Res. 2023;122:2567–84. doi: 10.1007/s00436-023-07956-7. [DOI] [PubMed] [Google Scholar]
- 25.Langbang D, Dhodapkar R, Parija SC, Premarajan KC, Rajkumari N. Molecular characterization of Giardia intestinalis assemblages in children among the rural and urban population of Pondicherry, India. Trop Parasitol. 2022;12:8–14. doi: 10.4103/tp.TP_52_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laishram S, Kannan A, Rajendran P, Kang G, Ajjampur SS. Mixed Giardia duodenalis assemblage infections in children and adults in South India. Epidemiol Infect. 2012;140:2023–7. doi: 10.1017/S0950268811002767. [DOI] [PubMed] [Google Scholar]
- 27.Khan SM, Debnath C, Pramanik AK, Xiao L, Nozaki T, Ganguly S. Molecular evidence for zoonotic transmission of Giardia duodenalis among dairy farm workers in West Bengal, India. Vet Parasitol. 2011;178:342–5. doi: 10.1016/j.vetpar.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Krumrie S, Capewell P, McDonald M, Dunbar D, Panarese R, Katzer F, et al. Molecular characterisation of Giardia duodenalis from human and companion animal sources in the United Kingdom using an improved triosephosphate isomerase molecular marker. Curr Res Parasitol Vector Borne Dis. 2022;2:100105. doi: 10.1016/j.crpvbd.2022.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelanew T, Lalle M, Hailu A, Pozio E, Caccie SM. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102:92–9. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 30.VolotTr AC, Costa-Macedo LM, Haddad FS, BranddM A, Peralta JM, Fernandes O. Genotyping of Giardia duodenalis from human and animal samples from Brazil using beta-giardin gene: A phylogenetic analysis. Acta Trop. 2007;102:10–9. doi: 10.1016/j.actatropica.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Fantinatti M, Cascais-Figueredo T, Austriaco-Teixeira P, Carvalho-Costa FA, Da-Cruz AM. Giardia lamblia-infected preschoolers present growth delays independent of the assemblage A, B or E. Mem Inst Oswaldo Cruz. 2023;118:e230043. doi: 10.1590/0074-02760230043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav P, Khalil S, Mirdha BR. Molecular appraisal of intestinal parasitic infection in transplant recipients. Indian J Med Res. 2016;144:258–63. doi: 10.4103/0971-5916.195041. [DOI] [PMC free article] [PubMed] [Google Scholar]


