Abstract
Cerebellar vascular hamartoma was diagnosed in a 16-month-old cat following magnetic resonance imaging and incisional biopsy. The clinical features were consistent with the cerebellar site of the lesion accompanied by signs attributable to cerebellar herniation through the foramen magnum and increased intra-cranial pressure. A lesion of this type represents a previously unreported differential diagnosis for central nervous system lesions in young cats.
A 16-month-old female neutered British Shorthair cat presented to the Queen's Veterinary School Hospital of the Department of Veterinary Medicine, Cambridge, with a 3-month history of mild high-stepping gait in the pelvic limbs, that had more recently progressed to profound ataxia.
On examination the cat was unable to walk without falling to the left and appeared to be non-painful but did resent elevation of the head. Conscious proprioception was reduced in all limbs but was consistently most depressed in the left thoracic limb. Hemi-walking was successfully carried out on the right side limbs but was not initiated, or executed, on the left. Flexor and myotatic reflexes in all limbs were normal. Cranial nerve reflexes were normal but nystagmus was evident when the cat was placed in dorsal recumbency – the direction of which could not be unequivocally defined.
The presence of nystagmus with absence of a head tilt was suggestive of a cerebellar lesion affecting the vestibular tracts and is consistent with the history of ataxia and high-stepping gait. However, animals with purely cerebellar lesions are not paretic, suggesting concomitant deficits in the motor system. It was considered possible that this could have been caused by compression of the brainstem by herniation of the caudal cerebellum through the foramen magnum, or, alternatively by multi-focal disease involving motor pathways in addition to the cerebellum.
Differential diagnoses for a young cat with progressive central nervous system signs include inflammatory disease, either infectious (feline infectious peritonitis, toxoplasma, feline immunodeficiency virus, feline leukaemia virus, bacterial, rickettsial, and fungal) or non-infectious (granulomatous meningoencephalitis), degenerative disorders (lysosomal storage diseases, neuronal abiotrophies, and neuronal dystrophies), malformations, or, more rarely, neoplasia.
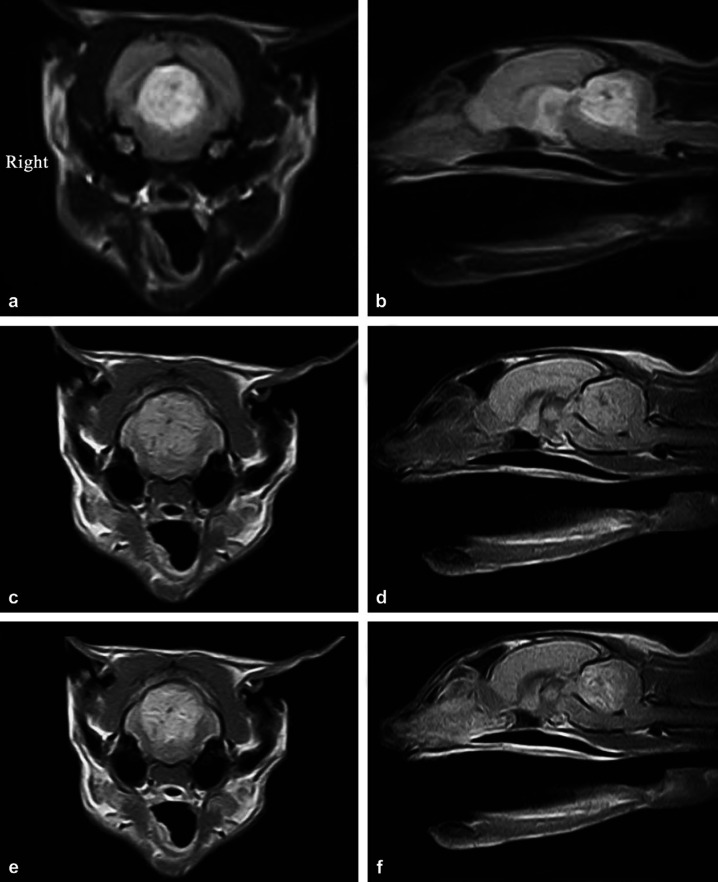
A complete blood count and biochemistry were unremarkable. In view of the likely intra-cranial location of the lesion magnetic resonance imaging (MRI) of the brain was carried out (Esaote Vet MRI 0.2 T permanent magnet). Transverse T2-weighted (T2W) images (Fig 1a) showed a heterogeneously hyperintense lesion extending from the caudal colliculi to the middle of the cerebellum. In some areas the brainstem appeared flattened dorsoventrally, suggestive of compression. The mesencephalic aqueduct was not evident on all sections and there was dilation of the supratentorial ventricular system suggestive of secondary obstructive hydrocephalus. Sagittal T2W images (Fig 1b) revealed a lesion in the cranial portion of the cerebellum with a poorly defined margin between lesion and normal parenchyma. There was mild herniation of the caudal cerebellar vermis sufficient to cause mild compression of the caudal brainstem. On T1-weighted (T1W) images the cerebellar lesion was mildly hyperintense with some hypointense patches when compared with the adjacent parenchyma (Fig 1c, d). Post-gadolinium T1W images showed heterogenous enhancement (Fig 1e, f). Cerebrospinal fluid was not taken because of the risk of herniation from a cisternal tap.
Fig 1.
(a) Transverse T2W image at the level of the cranial cerebellum showing a symmetrical, circular heterogeneously hyperintense lesion with ill-defined margins. (b) Sagittal T2W image showing a heterogeneously hyperintense area in the cranial two thirds of the cerebellum with some hypointense streaking. Note also the dorsal compression of the brainstem and slight protrusion of the cerebellum through the caudal foramen. (c) Transverse T1W image showing a mildly hyperintense lesion with some hypointense streaking when compared with the adjacent parenchyma. (d) Sagittal T1W image showing the cranial cerebellum to be mildly hyperintense compared with the surrounding parenchyma and including some hypointense patches. There is also mild cerebellar herniation through the foramen magnum and compression of the brainstem. (e) Post-gadolinium T1W image showing heterogenous enhancement. Within the lesion there are smaller isointense ring enhanced areas. (f) Post-gadolinium T1W sagittal image demonstrating heterogenous enhancement of the lesion with poorly defined margins.
Exploratory craniotomy was selected to expose the lesion, obtain biopsies and allow the possibility of attempting complete excision. The risk–benefit ratio of this procedure was considered superior to that for a ‘bore-hole’ biopsy using a ‘Trucut’ needle. The cerebellum was exposed via a left sided temporal craniectomy combined with excision of the membranous component of the tentorium cerebelli to expose the rostral aspect of the cerebellum. On retraction of the cerebellum ventrally and caudally from the bony tentorium cerebelli a dark red area of tissue beneath the rostral surface was revealed.
A fine needle aspirate of the abnormal tissue was non-diagnostic yielding only red blood cells with a few reactive fibrocytes. Therefore, an incision was made through the dura in order to take a biopsy for tissue smears. Grossly the lesion appeared dark and haemorrhagic and contained numerous white granules. Intra-operative squash preparation and impression smears of the mass stained with Wright's–Giemsa contained capillaries and small uniform rafts of cells with abundant pale cytoplasm and central round smooth chromatin and appeared ‘oligodendrocyte-like’. There were also a few larger mesenchymal-type (meningeal) cells and in highly cellular areas, there was extra cellular eosinophilic material. The cells did not have any criteria of malignancy and there was no evidence of inflammatory disease or infectious agents.
The aim of intra-operative cytology was to rule out diseases that could be treated medically such as Toxoplasma species infection or lymphoma. Surgical resection was not possible because of infiltration of the mass into normal parenchyma. In view of its poor pre-operative neurological status and the fact that neither surgical nor medical treatments were viable, the cat was euthanased while still under anaesthesia. The brain was immediately removed and fixed in 10% formalin and the remaining body underwent post-mortem examination.
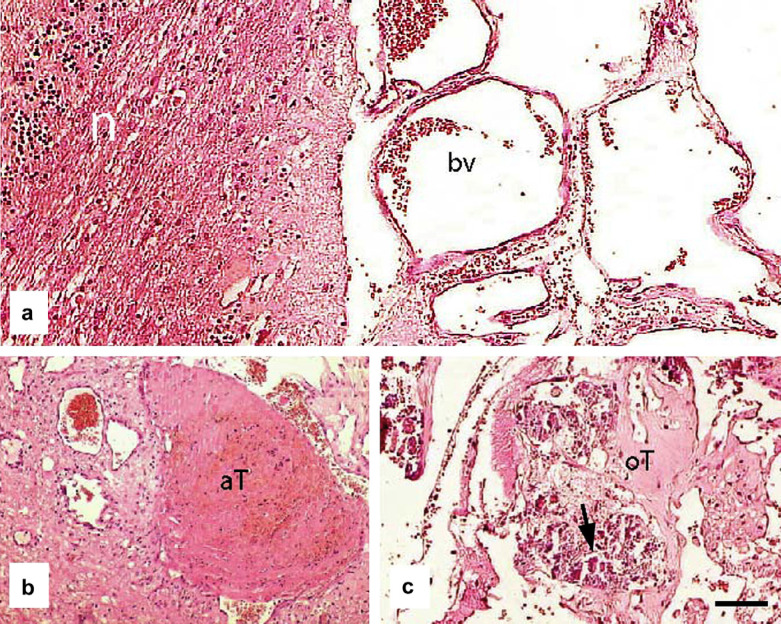
The normal architecture of the central/left part of the cerebellum had been obliterated by a large focal, expansive area of multiple proliferative endothelial cell-lined cavities which contained thrombi and many foci of mineralisation (Fig 2). There were also acute thrombi that contained moderate to large numbers of neutrophils and extravasated erythrocytes with fibrin. There was chromatolysis of some Purkinje cells within adjacent areas of the cerebellum and of neurons within the medulla adjacent to the affected part of the cerebellum. A diagnosis of cerebellar vascular hamartoma was made. No significant lesions were detected within the remainder of the brain or cadaver.
Fig 2.
(a) Photomicrograph showing normal cerebellum (n) adjacent to the large focal, expansive area containing multiple blood vessels (bv). (b) Photomicrograph of section through an acute thrombus (aT); several nearby blood vessels are abnormally thin-walled and dilated. (c) In some regions the thrombi had become organised (oT) and contained small deposits of mineralisation (arrow). Scale bar a–c: 75 μm.
The cat's neurological signs were consistent with a cerebellar lesion with subsequent brainstem compression. Resentment of neck extension may have resulted from raised intra-cranial pressure associated with the mass, obstruction of the mesencephalic aqueduct and compression of the brainstem.
Differential diagnoses from MRI included a neoplastic or inflammatory lesion or possible later stage haematoma. The poor definition of the lesion margin on MR images and lack of cranial–caudal compression of the cerebellum suggested an intra-axial lesion location. Differentials for an intra-axial neoplastic lesion could include a lymphoma, glioma, or medulloblastoma, however, location within the cerebellum is atypical for previously reported neoplastic brain lesions in cats (Troxel et al 2003, 2004, Tomek et al 2006). The signal intensity of the lesion was not consistent with that described for hamartomas in humans, which, when compared with the surrounding grey matter, are typically isointense on T1W images, hyperintense on T2W images and do not enhance on post-contrast T1W images (Barral et al 1988, Lona Soto et al 1991, Shah et al 1999). However, it is suggested that MRI variations should be taken into account in the diagnostic work-up of these lesions (Barral et al 1988) especially as it is likely to be dependent on the type of hamartoma and which structures have proliferated. MRI of an intramedullary spinal cord vascular hamartoma in a Golden Retriever was also described as isointense on T1, and hyperintense on T2 images but did show homogenous enhancement post-contrast (Sanders et al 2002). Contrast enhancement will occur as a result of deficits in the blood brain barrier, which is unsurprising in a lesion containing abnormal blood vessels. The irregular areas of hypointensity seen within this lesion were consistent with the mineralisation detected on histopathology and seen grossly as white granular material.
Cytological evaluation of squash preparations can be considered a fairly accurate and reliable tool in the diagnoses of nervous system lesions and obviously allows rapid intra-operative assessment (De Lorenzi et al 2006). Smear preparation samples also have a good diagnostic accuracy and can rapidly be assessed intraoperatively (Vernau et al 2001, Long et al 2002). The cytological report suggested that the cells were consistent with brain tissue. The cells did not have any criteria of malignancy and were found alongside numerous capillaries. Multi-focal mineralisation found on histology was consistent with the white granular material found at surgery. Various pathological conditions of the brain reported in humans can include calcification, such as neoplasms, granulomatous disease, cerebral calculi, haematoma, vascular malformations such as aneurysms or angiomas and some hereditary disorders (Jun and Burdick 1984, Bertoni et al 1990, Izycka-Swieszewska et al 2000). Histopathology of the whole mass showed an architecture which was consistent with a benign neoplasm such as a haemangioma, however, the very well differentiated nature of the endothelial cells and the young age of the cat suggested a developmental aberration rather than a neoplastic lesion. Therefore, a diagnosis of vascular hamartoma was made. The invasive nature of the lesion, disrupting normal cerebellar parenchyma made it unsuitable for dissection from the surrounding tissue.
Various vascular malformations of the central nervous system have been described in both humans and animals including hamartomas, haemangiomas, meningioangiomatosis and arteriovenous malformations (Cho et al 1979, Cordy 1979, Warkany and Lemire 1984, Pumarola et al 1996, Thomas et al 1997, Lorenzo et al 1998, Middleton et al 1999, Sanders et al 2002, Bishop et al 2004, Wang et al 2006). Hamartomas are focal malformations that resemble neoplasms and are formed by disorderly overgrowth of tissue elements normally at that site. The vascular hamartoma diagnosed in this case had localised proliferation of mature blood vessels within normal cerebellar parenchyma. There have been no previous reports of vascular hamartomas within the central nervous system of cats, however, several have been reported in dogs within the spinal cord, cerebrum and hypothalamus (Cook 1977, Smith and Van Winkle 2001, Sanders et al 2002). The hamartomas within the brain consisted of a proliferation of thin-walled vessels of varying calibre. Antibodies to actin and factor VIII were used to assess for endothelial and smooth muscle actin to help further distinguish vessel-type.
Hamartomas can be regarded as a link between neoplasms and malformations (Sanders et al 2002). The aberrant growth causes problems due to adherence to the adjacent tissue and secondary compression. It is, however, important to recognise them as a differential diagnosis for intra-cranial lesions in cats because, depending on the site of the lesion and the degree of invasiveness, surgical resection may be a viable treatment option.
Acknowledgements
Catherine Stalin is funded by the RCVS Trust and the Animal Medical Centre, Manchester, UK.
References
- Barral V., Brunelle F., Brauner R., Rappaport R., Lallemand D. MRI of hypothalamic hamartomas in children, Pediatric Radiology 18, 1988, 449–452. [DOI] [PubMed] [Google Scholar]
- Bertoni F., Unni K.K., Dahlin D.C., Beabout J.W., Onofrio B.M. Calcifying pseudoneoplasms of the neural axis, Journal of Neurosurgery 72, 1990, 42–48. [DOI] [PubMed] [Google Scholar]
- Bishop T.M., Morrison J., Summers B.A., deLahunta A., Schatzberg S.J. Meningioangiomatosis in young dogs: a case series and literature review, Journal of Veterinary Internal Medicine 18, 2004, 522–528. [DOI] [PubMed] [Google Scholar]
- Cho C.Y., Cook J.E., Leipold H.W. Angiomatous vascular malformation in the spinal cord of a Hereford calf, Veterinary Pathology 16, 1979, 613–616. [DOI] [PubMed] [Google Scholar]
- Cook R.W. Hypothalamic hamartoma in a dog, Veterinary Pathology 14, 1977, 138–145. [DOI] [PubMed] [Google Scholar]
- Cordy D.R. Vascular malformations and haemangiomas of the canine spinal cord, Veterinary Pathology 16, 1979, 275–282. [DOI] [PubMed] [Google Scholar]
- De Lorenzi D., Mandara M.T., Tranquillo M., Baroni M., Gasparinetti N., Gandini G., Masserdotti C., Bonfanti U., Bertolini G., Vian P., Bernardini M. Squash-prep cytology in the diagnosis of canine and feline nervous system lesions: a study of 42 cases, Veterinary Clinical Pathology 35, 2006, 208–214. [DOI] [PubMed] [Google Scholar]
- Izycka-Swieszewska E., Rzepko R., Kopczynski S., Franc Z., Szurowska E., Borowska-Lehman J. Meningioangiomatosis with a predominant fibrocalcifying component, Neuropathology 20, 2000, 44–48. [DOI] [PubMed] [Google Scholar]
- Jun C., Burdick B. An unusual fibro-osseous lesion of the brain. Case report, Journal of Neurosurgery 60, 1984, 1308–1311. [DOI] [PubMed] [Google Scholar]
- Soto A. Lona, Takahashi M., Yamashita Y., Sakamoto Y., Shinzato J., Yoshizumi K. MRI findings of hypothalamic hamartoma: report of five cases and review of the literature, Computerized Medical Imaging and Graphics 15, 1991, 415–421. [DOI] [PubMed] [Google Scholar]
- Long S.N., Anderson T.J., Long F.H., Johnston P.E. Evaluation of rapid staining techniques for cytologic diagnosis of intracranial lesions, American Journal of Veterinary Research 63, 2002, 381–386. [DOI] [PubMed] [Google Scholar]
- Lorenzo V., Pumarola M., Munoz A. Meningioangiomatosis in a dog: magnetic resonance imaging and neuropathological studies, Journal of Small Animal Practice 39, 1998, 486–489. [DOI] [PubMed] [Google Scholar]
- Middleton J.R., Valdez R., Britt L.G., Parish S.M., Tyler J.W. Progressive hindlimb paraparesis in a goat associated with a vascular hamartoma, Veterinary Record 144, 1999, 264–265. [DOI] [PubMed] [Google Scholar]
- Pumarola M., de las M.J. Martin, Vilafranca M., Obach A. Meningioangiomatosis in the brain stem of a dog, Journal of Comparative Pathology 115, 1996, 197–201. [DOI] [PubMed] [Google Scholar]
- Sanders S.G., Bagley R.S., Gavin P.R., Konzik R.L., Cantor G.H. Surgical treatment of an intramedullary spinal cord hamartoma in a dog, Journal of the American Veterinary Medical Association 221, 2002, 659–661. [DOI] [PubMed] [Google Scholar]
- Shah P., Patkar D., Patankar T., Shah J., Srinivasa P., Krishnan A. MR imaging features in hypothalamic hamartoma: a report of three cases and review of literature, Journal of Postgraduate Medicine 45, 1999, 84–86. [PubMed] [Google Scholar]
- Smith S.H., Van Winkle T. Cerebral vascular hamartomas in five dogs, Veterinary Pathology 38, 2001, 108–112. [DOI] [PubMed] [Google Scholar]
- Thomas W.B., Adams W.H., McGavin M.D., Gompf R.E. Magnetic resonance appearance of intracranial haemorrhage secondary to cerebral vascular malformation in a dog, Veterinary Radiology and Ultrasound 38, 1997, 371–375. [DOI] [PubMed] [Google Scholar]
- Tomek A., Cizinauskas S., Doherr M., Gandini G., Jaggy A. Intracranial neoplasia in 61 cats: localisation, tumour types and seizure patterns, Journal of Feline Medicine and Surgery 8, 2006, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel M.T., Vite C.H., Massicotte C., McLear R.C., Van Winkle T.J., Glass E.N., Tiches D., Dayrell-Hart B. Magnetic resonance imaging features of feline intracranial neoplasia: retrospective analysis of 46 cats, Journal of Veterinary Internal Medicine 18, 2004, 176–189. [DOI] [PubMed] [Google Scholar]
- Troxel M.T., Vite C.H., Van Winkle T.J., Newton A.L., Tiches D., Dayrell-Hart B., Kapatkin A.S., Shofer F.S., Steinberg S.A. Feline intracranial neoplasia: retrospective review of 160 cases (1985–2001), Journal of Veterinary Internal Medicine 17, 2003, 850–859. [DOI] [PubMed] [Google Scholar]
- Vernau K.M., Higgins R.J., Bollen A.W., Jimenez D.F., Anderson J.V., Koblik P.D., LeCouteur R.A. Primary canine and feline nervous system tumours: intraoperative diagnosis using the smear technique, Veterinary Pathology 38, 2001, 45–57. [DOI] [PubMed] [Google Scholar]
- Wang Y., Gao X., Yao Z.W., Chen H., Zhu J.J., Wang S.X., Gao M.S., Zhou L.F., Zhang F.L. Histopathological study of five cases with sporadic meningioangiomatosis, Neuropathology 26, 2006, 249–256. [DOI] [PubMed] [Google Scholar]
- Warkany J., Lemire R.J. Arteriovenous malformations of the brain, Teratology 29, 1984, 333–353. [DOI] [PubMed] [Google Scholar]