Abstract
We expressed δ subspecies of protein kinase C (δ-PKC) fused with green fluorescent protein (GFP) in CHO-K1 cells and observed the movement of this fusion protein in living cells after three different stimulations. The δ-PKC–GFP fusion protein had enzymological characteristics very similar to those of the native δ-PKC and was present throughout the cytoplasm in CHO-K1 cells. ATP at 1 mM caused a transient translocation of δ-PKC–GFP to the plasma membrane approximately 30 s after the stimulation and a sequent retranslocation to the cytoplasm within 3 min. A tumor-promoting phorbol ester, 12-O-tetradecanoylphorbol 13-acetate (TPA; 1 μM), induced a slower translocation of δ-PKC–GFP, and the translocation was unidirectional. Concomitantly, the kinase activity of δ-PKC–GFP was increased by these two stimulations, when the kinase activity of the immunoprecipitated δ-PKC–GFP was measured in vitro in the absence of PKC activators such as phosphatidylserine and diacylglycerol. Hydrogen peroxide (H2O2; 5 mM) failed to translocate δ-PKC–GFP but increased its kinase activity more than threefold. δ-PKC–GFP was strongly tyrosine phosphorylated when treated with H2O2 but was tyrosine phosphorylated not at all by ATP stimulation and only slightly by TPA treatment. Both TPA and ATP induced the translocation of δ-PKC–GFP even after treatment with H2O2. Simultaneous treatment with TPA and H2O2 further activated δ-PKC–GFP up to more than fivefold. TPA treatment of cells overexpressing δ-PKC–GFP led to an increase in the number of cells in G2/M phase and of dikaryons, while stimulation with H2O2 increased the number of cells in S phase and induced no significant change in cell morphology. These results indicate that at least three different mechanisms are involved in the translocation and activation of δ-PKC.
Protein kinase C (PKC) is known to be a key enzyme in signal transduction and is involved in the regulation of numerous cellular functions (30). PKC is activated by diacylglycerol (DG) produced by the receptor-coupled hydrolysis of membrane phosphoinositides (28, 30) and serves as the receptor for tumor-promoting phorbol esters such as 12-O-tetradecanoylphorbol 13-acetate (TPA) (2, 28). The PKC family consists of at least 10 different subspecies that can be classified into three groups, classical, new, and atypical PKC (cPKC, nPKC, and aPKC, respectively), based on the structures of their regulatory domains) (29, 30). The differences in structure, enzymatic properties, and patterns of expression strongly suggest the specific functions of each subspecies of PKC, but the individual functions have not been fully clarified.
The δ subspecies of PKC (δ-PKC) belongs to the nPKC group and is activated by DG in a calcium-independent manner (24, 27, 31, 34). Phorbol ester treatment of NIH 3T3 cells overexpressing δ-PKC produced significant changes in cell morphology and slowed cell growth (25), and TPA induced monocytic differentiation in 32D cells overexpressing δ-PKC (26). Furthermore, treatment with phorbol ester of CHO cells overexpressing δ-PKC induced cell division arrest (40), strongly suggesting that δ-PKC is involved in the regulation of cell proliferation and differentiation. In addition to serine/threonine phosphorylation of δ-PKC (1, 31–33), several extracellular signals induce the tyrosine phosphorylation of δ-PKC (5, 6, 11, 19, 22, 23, 37). Denning et al. (5) observed the tyrosine phosphorylation of δ-PKC among various PKC subspecies in cultured keratinocytes transformed with the Ha-v-ras gene. Stimulation of the platelet-derived growth factor receptor resulted in the tyrosine phosphorylation of δ-PKC in myeloid progenitor cells (23). Treatment with phorbol ester also induced the tyrosine phosphorylation of δ-PKC (22). δ-PKC was tyrosine phosphorylated in vitro by c-Fyn (6, 22), c-Src (6, 11, 41) and growth factor receptors (6, 22); however, the effect of tyrosine phosphorylation on PKC activity has been controversial in these reports (5, 6, 11, 22, 23, 38). Considering that δ-PKC is tyrosine phosphorylated by TPA (22), which induced cell division arrest of CHO cells overexpressing δ-PKC (40), the tyrosine phosphorylation of δ-PKC seems to be related to the cell proliferation and differentiation. Recently, it was shown that H2O2 treatment induces the tyrosine phosphorylation of δ-PKC and that δ-PKC is recovered as an activator-independent form from H2O2-treated cells (19). The physiological role of tyrosine phosphorylation of δ-PKC by H2O2, however, has not been elucidated, and the functional differences between TPA- and H2O2-induced activation of δ-PKC have not been clarified.
The PKC subspecies, especially of the cPKC and nPKC groups, are known to translocate from the cytosol to the membrane fraction upon activation (20). The translocation of another subspecies, γ-PKC, was visualized in living cells by using green fluorescent protein (GFP) as a marker protein, and it was revealed that the translocation of γ-PKC is different in response to various stimulations (35). The new method for monitoring PKC translocation is useful for understanding the interaction of PKC and substrates. In the present study, the translocation process of δ-PKC and its involvement in cell cycle regulation were examined by using cultured cells expressing δ-PKC fused to GFP after three different stimulations: (i) with ATP, which causes G-protein-coupled hydrolysis of phosphoinositides in CHO-K1 cells (13), (ii) with TPA, which activates PKC directly (2), and (iii) with H2O2, which causes tyrosine phosphorylation of δ-PKC (19).
MATERIALS AND METHODS
Materials.
TPA was purchased from Sigma (St. Louis, Mo.). Calf thymus H1 histone was from Boehringer GmbH (Mannheim, Germany), and ATP was from Nacalai Tesque (Kyoto, Japan). All other chemicals were of analytical grade.
Cell culture.
COS-7 cells were purchased from Riken Cell Bank (Tsukuba, Japan). Strain CHO-K1 (ATCC CCL 61) was from the American Type Culture Collection. COS-7 cells were cultured in Dulbecco’s modified Eagle medium supplemented with 44 mM NaHCO3 and 10% fetal bovine serum (FBS) in a humidified atmosphere containing 5% CO2 at 37°C. CHO-K1 cells were cultured in Ham’s F-12 medium supplemented with 10% FBS and 14 mM NaHCO3. All media were supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml), and the FBS used was not heat inactivated.
Construction of plasmids encoding δ-PKC–GFP fusion protein.
A plasmid containing the humanized GFP cDNA (pEGFP-N1) was purchased from Clontech (Palo Alto, Calif.). A cDNA fragment encoding GFP with MunI-EcoRI-BglII sites at the 5′-terminal end and a MunI site at the 3′-terminal end was obtained by PCR using GFP as a template. The sense primer for the GFP was 5′-TTCAATTGAATTCAGATCTATGGTGAGCAAGGGCGAGGAG-3′, and the antisense primer was 5′-GGCAATTGCTAGCTAGCTGGCCAGGATCC-3. The PCR product for GFP was digested by MunI, subcloned into the EcoRI site in the expression vector pTB 701 and named BS340. A cDNA fragment of δ-PKC (34) or its kinase-negative mutant (δ-PKC-KN) (18) with an EcoRI site in the 5′ terminus and a BamHI site in 3′ terminus was also produced by PCR. The δ-PKC-KN (Lys376 mutated to Met) was produced as described previously (18). The sense and antisense primers used were 5′-TTGAATTCATCATGGCACCGTTCCTGCG-3′ and 5′-GCGGATCCTTCCAGGAATTGCTCATAT-3′, respectively. The PCR products for δ-PKC and its kinase-negative mutant were digested with EcoRI and BamHI, then subcloned into EcoRI/BglII sites in BS340, and named BS391 (expressing δ-PKC–GFP) and BS435 (expressing δ-PKC-KN–GFP), respectively. Plasmid pTB801 was used for expression of rat δ-PKC (34).
Expression of δ-PKC–GFP protein in cultured cells.
Transient transfection into COS-7 cells was performed by electroporation. The plasmid (approximately 32 μg) encoding either δ-PKC–GFP or δ-PKC was transfected into 6 × 106 cells by using a Gene Pulser (960 μF, 220 V; Bio-Rad, Hercules, Calif.). Transfection into CHO-K1 cells was carried out by lipofection using TransIT (Takara, Kyoto, Japan) according to the manufacturer’s standard protocol. The fluorescence of δ-PKC–EGFP became detectable 16 h after the transfection in the both cell lines. All experiments were performed 2 days after transfection.
Immunoprecipitation of δ-PKC–GFP and δ-PKC.
Cells expressing δ-PKC–GFP or δ-PKC were harvested with 1 ml of homogenate buffer (250 mM sucrose, 10 mM EGTA, 2 mM EDTA, 20 mM Tris-HCl, 200 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride [pH 7.4]) containing 1% Triton X-100 and were homogenized by sonication (UD-210 TOMY SEIKO Co. Ltd., Tokyo, Japan; output, 5; duty, 50%; 10 times at 4°C). After centrifugation at 19,000 × g for 15 min at 4°C, the supernatant was incubated with either an anti-δ-PKC monoclonal antibody (Transduction Laboratories, Lexington, Ky.) diluted 1:50 for 2 h at 4°C or an anti-GFP polyclonal antibody (diluted 1:50) (Clontech) and then with protein A-Sepharose for an additional 2 h. Samples were centrifuged at 2,000 × g for 5 min at 4°C, and pellets were washed three times with phosphate-buffered saline (PBS) without Ca2+ and Mg2+ [PBS(−)]. Finally, the pellet was suspended in 50 μl of PBS(−) and used for kinase assays or immunoblotting.
Immunoblot analysis.
For detection of translocation, the transfected cells were harvested and homogenized by sonication with homogenate buffer without Triton X-100. After centrifugation at 19,000 × g for 15 min, the supernatant was collected as a cytosol fraction. The pellet was sonicated in homogenate buffer containing 1% Triton X-100 and centrifuged at 19,000 × g for 15 min; then the supernatant was collected as a particulate fraction. For immunoblotting, cytosol samples, particulate samples, or immunoprecipitated samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 7.5% polyacrylamide gel, and the separated proteins were electrophoretically transferred onto polyvinylidiene difluoride (PVDF) filters (Millipore, Bedford, Mass.). Nonspecific binding sites on the PVDF filters were blocked by incubation with 5% skim milk in 0.01 M PBS containing 0.03% Triton X-100 for 18 h. The PVDF filters were then incubated with the anti-δ-PKC monoclonal antibody (diluted 1:1,000), antiphosphotyrosine antibody 4G10 (diluted 1:2,000; Upstate Biotechnology, Lake Placid, N.Y.), or the anti-GFP polyclonal antibody (diluted 1:1,000) for 1 h at 25°C. After washing, the filters were incubated with biotin-labeled horse anti-mouse immunoglobulin G (for δ-PKC antibody or antiphosphotyrosine antibody) or biotin-labeled horse anti-rabbit immunoglobulin G (for GFP antibody) for 30 min and then with the avidin-biotin-peroxidase complex for 30 min. After three rinses, the immunoreactive bands were visualized with an enhanced chemiluminescence detection kit (Amersham, Buckinghamshire, England).
Enzymological properties of δ-PKC–GFP and δ-PKC.
The immunoprecipitated samples (10 μl of suspended pellet) were used for kinase assays. Kinase assays of δ-PKC and δ-PKC–GFP expressed in COS-7 cells were performed as described previously (35). In brief, the kinase activity in 10 μl of each sample was assayed by measuring the incorporation of 32Pi into calf thymus H1 histone from [γ-32P]ATP in the presence of phosphatidylserine (PS; 8 μg/ml), diolein (DO; 0.8 μg/ml), or Ca2+ (5 mM). Basal activity was measured in the presence of 0.5 mM EGTA instead of PS, DO, and Ca2+. The kinase activities of δ-PKC–GFP in CHO-K1 cells after various stimulations were assayed by measuring the incorporation of 32Pi into calf thymus H1 histone from [γ-32P]ATP without any activators such as PS, DO, and Ca2+.
Observation of δ-PKC–GFP translocation.
CHO-K1 cells transfected with δ-PKC–GFP or its mutant were spread onto glass-bottom culture dishes (MatTek Corp., Ashland, Mass.) and cultured for at least 16 h before observation. The culture medium was replaced with Ham’s F-12 medium containing 5 mM HEPES (pH 7.3) instead of FBS.
The fluorescence of δ-PKC–GFP was monitored under a confocal laser scanning fluorescence microscope (Carl Zeiss, Jena, Germany) at 488-nm argon excitation, using a 515-nm-long pass barrier filter. Translocation of δ-PKC–GFP was triggered by a direct application of various stimulants at high concentrations into the medium to obtain the appropriate final concentrations. All experiments involving confocal laser scanning fluorescence microscopy were performed at 37°C.
Cell cycle analysis.
For flow cytometric analysis, the cells were fixed with 70% ethanol on ice for 20 min, treated with RNase A (0.5 μg/ml) for 20 min, and stained with propidium iodide (50 μg/ml). Cell cycles of the transfected cells were analyzed by flow cytometry (Cyto ACE-300; Jasco, Tokyo, Japan), using a 530 ± 37-nm band pass filter for GFP and 640-nm-long pass filter for propidium iodide.
For the observation of DNA, the cells were fixed with 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 7.4) for 30 min. After two washes with phosphate buffer, the cells were stained with 0.5 μg of 4,6-diamino-2-phenylindole (DAPI; Wako, Osaka, Japan) per ml for 30 min. The fluorescence of DAPI was observed under a confocal laser scanning fluorescence microscope at 364-nm UV excitation, using a 397-nm-long pass filter.
RESULTS
Enzymological characteristics of δ-PKC–GFP fusion protein.
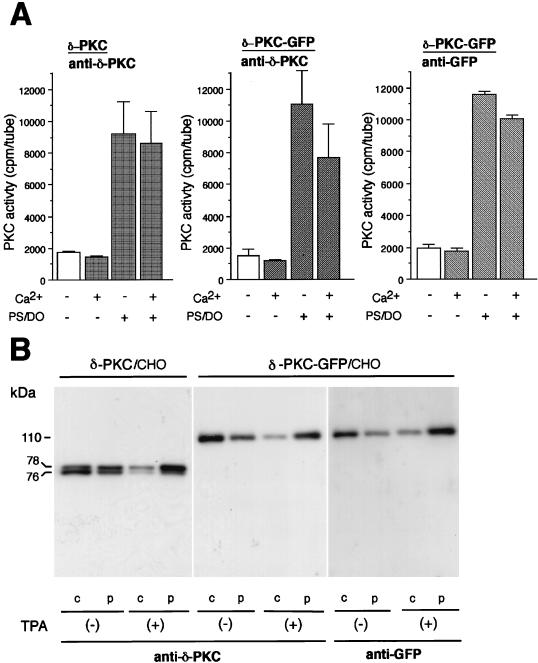
The enzymological characteristics of δ-PKC–GFP were analyzed by kinase assay and by immunoblotting. As shown in Fig. 1A, the kinase assays revealed that both δ-PKC and δ-PKC–GFP which were immunoprecipitated with the anti-δ-PKC antibody from the transfected COS-7 cells were dependent on PS-DO but not on Ca2+. These results indicated that δ-PKC–GFP had enzymological characteristics similar to those of native δ-PKC. δ-PKC–GFP which was immunoprecipitated with the anti-GFP antibody showed similar characteristics. As shown in Fig. 1B, δ-PKC expressed in CHO-K1 cells was detected as doublet bands of 76 and 78 kDa by the monoclonal antibody against the C1 region of δ-PKC. The doublet bands in the cytosol fraction were decreased by TPA treatment, while those in the particulate fraction were increased by the same treatment. The 78-kDa band was more intense than the 76-kDa band in the presence of TPA and vice versa in the absence of TPA. The protein of δ-PKC–GFP was recognized as a single band with the expected molecular mass of 110 kDa by the same antibody. The endogenous δ-PKC was not recognized by immunoblotting under the conditions used, probably because CHO-K1 cells do not express detectable amounts of δ-PKC. In fact, longer exposure enabled us to detect a faint band of endogenous δ-PKC (data not shown). No degradative products of δ-PKC–GFP were found in the control cells or even in the cells treated with TPA. The amounts of membrane-associated δ-PKC–GFP increased after the treatment with TPA as seen in the case of δ-PKC. A single band of 110 kDa was also detected with the antibody against GFP, and TPA-induced translocation from the cytosol to the particulate fraction was similarly observed. No immunoreactive bands were detected by the anti-GFP antibody in the samples from CHO-K1 cells transfected with mock cDNA.
FIG. 1.
Characterization of δ-PKC–GFP transiently expressed in transfected cells. (A) Enzymological characteristics of δ-PKC and δ-PKC–GFP. Kinase activities of δ-PKC and δ-PKC–GFP, which were immunoprecipitated with the anti-δ-PKC antibody or with the anti-GFP antibody from transfected COS-7 cells, were measured in the presence or absence of activators of δ-PKC. Vertical bars represent standard errors of the means. (B) Immunoblot analysis using the anti-δ-PKC and anti-GFP antibodies. CHO-K1 cells transfected with δ-PKC or δ-PKC–GFP were treated with 1 μM TPA for 30 min, and the cytosol (c) and particulate (p) fractions were prepared as described in Materials and Methods. The transferred membranes were stained with the anti-δ-PKC antibody or the anti-GFP antibody.
Visualization of the translocation of δ-PKC–GFP induced by ATP, H2O2, and TPA.
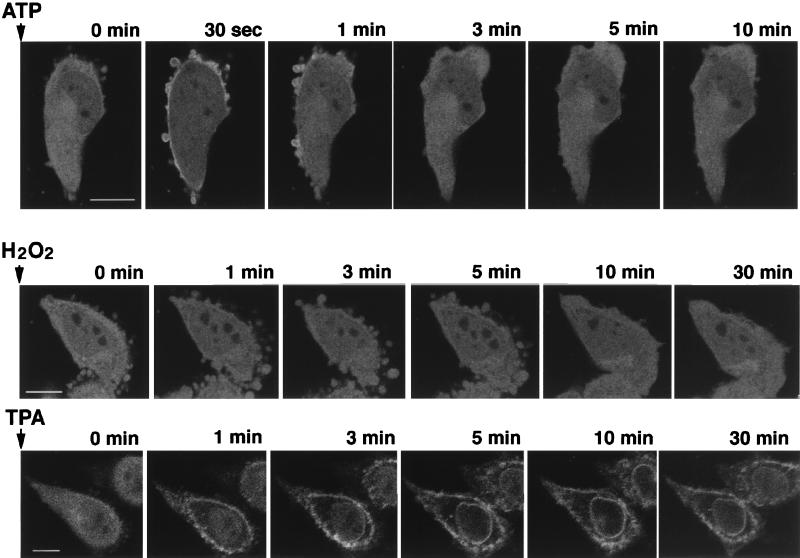
Intense fluorescence of δ-PKC–GFP was found throughout the cytoplasm, including the nucleoplasm of the transfected CHO-K1 cells (Fig. 2). Localization of the fluorescence did not alter at least for 1 h as observed under a confocal laser scanning fluorescence microscope, and the intensity of the fluorescence decreased slightly (data not shown). Activation of δ-PKC–GFP by 1 mM ATP induced a rapid translocation of the fluorescence from the cytosol to the plasma membrane. The translocation was observed within 30 s after stimulation. Thereafter, δ-PKC–GFP was quickly retranslocated from membrane to cytosol again and restored within 3 min to a state similar to that before stimulation. In contrast, no translocation was observed at least 30 min after stimulation with H2O2, even though the intensity of fluorescence appeared to fade slightly. The TPA-induced translocation of δ-PKC–GFP was slower than that by ATP and was unidirectional, from cytoplasm to membrane. Within 3 min after TPA stimulation, δ-PKC–GFP in the perikarya was completely translocated to membrane; then the fluorescence in the nucleoplasm appeared to translocate to the nuclear membrane by 10 min after stimulation. The fluorescence remained on the plasma membrane or the nuclear membrane for at least 60 min after TPA treatment and did not return to the cytoplasm in the cells examined.
FIG. 2.
Translocation of δ-PKC induced by various stimuli, as evidenced by: changes in the fluorescence of δ-PKC–GFP expressed in CHO-K1 cells by stimulation with 1 mM ATP at 37°C (bar = 10 μM), 5 mM H2O2 at 37°C (bar = 10 μM), and 1 μM TPA at 37°C (bar = 10 μM).
Kinase activity of δ-PKC–GFP after the treatment with ATP, H2O2, and TPA.
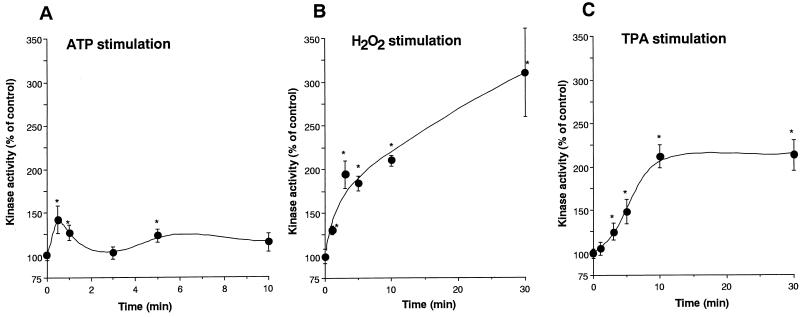
The kinase activity of the immunoprecipitated δ-PKC–GFP was assayed by measuring the incorporation of 32Pi from [γ-32P]ATP into H1 histone without any additional activators such as PS and DO. After treatment with ATP, the kinase activity of the immunoprecipitated δ-PKC–GFP was slightly but significantly increased (Fig. 3A). In contrast, after treatment with H2O2, the kinase activity of δ-PKC–GFP increased continuously up to approximately three times the basal level at 30 min after the treatment (Fig. 3B). TPA also increased the kinase activity to 2.5 times the basal level, and the kinase activity reached a plateau at 5 min after the treatment (Fig. 3C).
FIG. 3.
Changes in kinase activity of δ-PKC–GFP in transfected CHO-K1 cells after stimulation with 1 mM ATP (A), 5 mM H2O2 (B), and 1 μM TPA (C). δ-PKC–GFP was immunoprecipitated with anti-δ-PKC antibody at various time points after stimulation, and kinase activity was assayed with H1 histone as the substrate without any activators such as DO and PS. Data are expressed as percentage of the control level (the kinase activity before stimulation). All results represent the means and standard errors of more than four determinations. Statistical significance: ∗, P < 0.05 versus kinase activity before the stimulation; ∗∗, P < 0.05 between the two indicated points.
Tyrosine phosphorylation of δ-PKC–GFP after treatment with ATP, H2O2, and TPA.
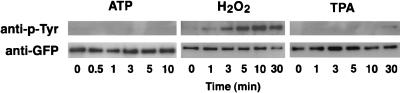
Tyrosine phosphorylation of δ-PKC–GFP after treatment with ATP, H2O2, and TPA was examined by immunoblotting using an antiphosphotyrosine antibody (Fig. 4). The immunoprecipitated samples equivalent to the kinase assay samples were subjected to SDS-PAGE and then transferred to a membrane. ATP did not cause any tyrosine phosphorylation of δ-PKC–GFP, and only slight tyrosine phosphorylation was detected 30 min after treatment with TPA. δ-PKC–GFP, however, was evidently tyrosine phosphorylated by treatment with H2O2. The tyrosine phosphorylation increased gradually after H2O2 treatment. Immunoblotting with the anti-GFP antibody revealed that similar amounts of δ-PKC–GFP were immunoprecipitated in all samples.
FIG. 4.
Tyrosine phosphorylation of δ-PKC–GFP in transfected CHO-K1 cells treated with ATP, H2O2, and TPA. Top row, changes in tyrosine phosphorylation. Tyrosine phosphorylation of δ-PKC–GFP immunoprecipitated with the anti-δ-PKC antibody was analyzed by immunoblotting with the antiphosphotyrosine (anti-p-Tyr) antibody. Bottom row, changes in the amount of δ-PKC–GFP. The amount of δ-PKC–GFP immunoprecipitated with the anti-δ-PKC antibody was measured by immunoblotting with the polyclonal antibody against GFP.
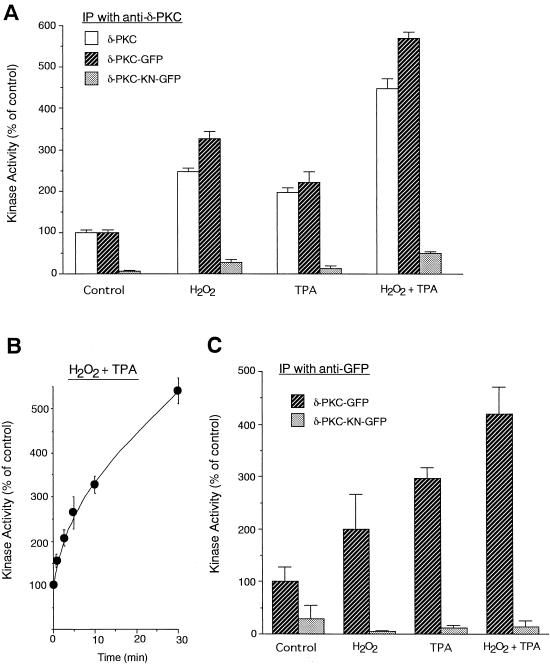
Effect of simultaneous treatment with H2O2 and TPA on the translocation and kinase activity of δ-PKC–GFP.
To elucidate whether H2O2 and TPA activate δ-PKC–GFP through the same pathway, we studied the kinase activity after the simultaneous application of H2O2 and TPA. The kinase activity of δ-PKC–GFP, which was immunoprecipitated with the anti-δ-PKC antibody, was increased 3.2- and 2.4-fold by treatment with H2O2 and TPA, respectively (Fig. 5A). Simultaneous treatment with H2O2 and TPA further activated δ-PKC–GFP, and the kinase activity increased time dependently up to fivefold (Fig. 5B). The cumulative effects of H2O2 and TPA were also found in the cells transfected with the native δ-PKC. No or negligible kinase activity was found in the immunoprecipitated samples from CHO-K1 cells transfected with δ-PKC-KN–GFP). To rule out the possibility that the anti-δ-PKC antibody affected the kinase activity of δ-PKC, δ-PKC–GFP was immunoprecipitated with the anti-GFP antibody. When the anti-GFP antibody was used instead of the anti-δ-PKC antibody, the additive effects of TPA and H2O2 were similarly observed, and no or little kinase activity was immunoprecipitated from the CHO-K1 cells transfected with δ-PKC-KN–GFP (Fig. 5C).
FIG. 5.
Kinase activities of δ-PKC and δ-PKC–GFP after simultaneous stimulation with TPA and H2O2. δ-PKC, δ-PKC–GFP, or δ-PKC-KN–GFP was immunoprecipitated (IP) with the anti-δ-PKC antibody (A and B) or the anti-GFP antibody (C) from transfected CHO-K1 cells which were treated with 5 mM H2O2 alone for 30 min, 1 μM TPA alone for 30 min, or H2O2 and TPA simultaneously for 30 min (A and C) or for 0 to 30 min (B). Data represent the means ± standard errors of more than five experiments.
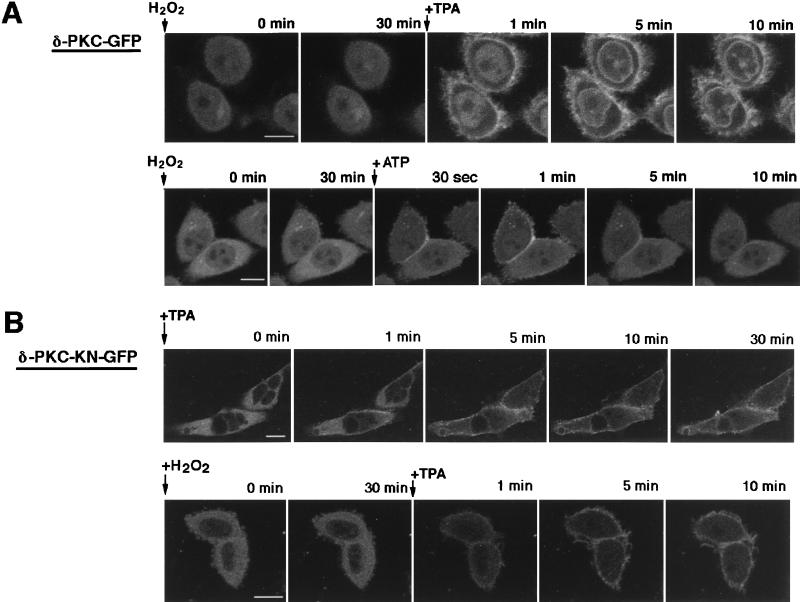
The translocation of δ-PKC–GFP after simultaneous application of TPA and H2O2 was also studied. Thirty-minute treatment with H2O2 did not alter the localization of δ-PKC–GFP as seen in Fig. 2, but sequent treatment with TPA translocated δ-PKC–GFP to the membrane as seen in the absence of H2O2 (Fig. 6A, top row). ATP also induced the transient translocation of δ-PKC–GFP even after treatment of the cells with H2O2 (Fig. 6A, bottom row).
FIG. 6.
Translocation of δ-PKC–GFP and δ-PKC-KN–GFP expressed in CHO-K1 cells. (A) Translocation of δ-PKC–GFP induced by TPA (top) and by ATP (bottom) after treatment with H2O2 (5 mM) for 30 min (bar = 10 μM); (B) translocation of δ-PKC-KN–GFP, as evidenced by changes in the fluorescence of δ-PKC-KN–GFP induced by TPA (δ-PKC–GFP showed similar translocation) (top) and by TPA after treatment with H2O2 (5 mM) for 30 min (bottom) (bar = 10 μM).
When the CHO-K1 cells were transfected with δ-PKC-KN–GFP, translocation by TPA was similarly observed, although the mutant kinase showed no kinase activity (Fig. 6B, top row). Pretreatment with H2O2 did not alter the TPA-induced translocation of δ-PCK-KN–GFP (Fig. 6B, bottom row).
Effects of ATP, TPA, and H2O2 on cell cycle and morphology of CHO cells expressing δ-PKC–GFP.
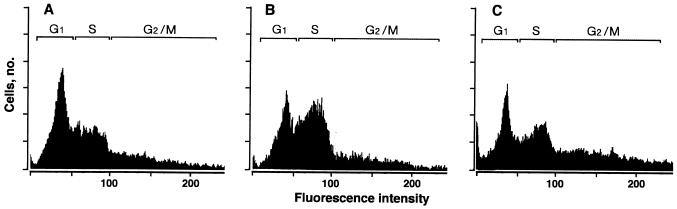
The cell cycles of cells expressing δ-PKC–GFP were analyzed 24 h after transient treatment with ATP, TPA, and H2O2 by flow cytometry. This analysis indicated that 40, 36, and 23% of the CHO cells expressing δ-PKC–GFP growing without stimulation were in G1, S, and G2/M phases, respectively (Fig. 7A). Twenty-four hours after transient treatment with ATP for 15 min, the cell cycle was not significantly altered (data not shown). After transient treatment with TPA (Fig. 7B), however, the number of the cells in G2/M phase was increased (36%) and that in G1 phase was decreased (30%). In contrast, H2O2 treatment (Fig. 7C) increased the number of the cells in S phase (47%) and decreased the number in G1 phase (28%). Cell cycles of untransfected CHO cells were not altered by TPA or H2O2 (data not shown).
FIG. 7.
Cell cycle analysis of CHO-K1 cells expressing δ-PKC–GFP after treatment with H2O2 and TPA. The cell cycle was analyzed by flow cytometry 24 h after transient treatment with 5 mM H2O2 (B) and 1 μM TPA (C) for 15 min. (A) CHO-K1 cells expressing δ-PKC–GFP without stimulation. Percentages of cells in G1, S, and G2/M phases are as follows: (A) 40, 36, and 23, respectively; (B) 28, 47, and 25, respectively; (C) 30, 34, and 36, respectively.
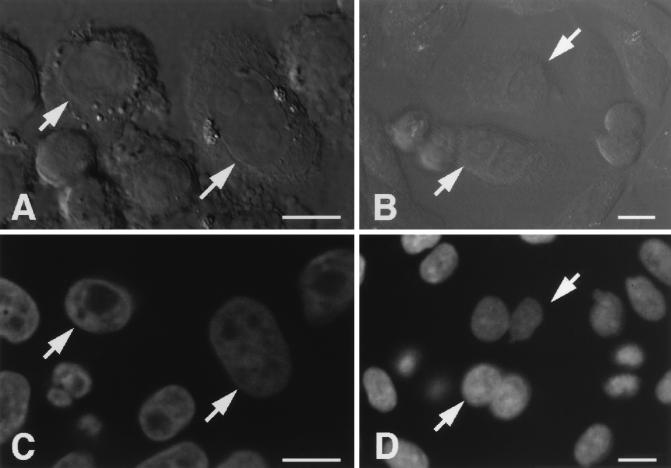
The effect of stimulation on cell morphology was also examined. Treatment with ATP, TPA, and H2O2 for 30 min had no apparent effect on the morphology of CHO cells expressing δ-PKC–GFP (Fig. 2). Twenty-four hours after treatment with TPA for 15 min, most CHO cells expressing δ-PKC–GFP were dikaryons with enlarged and flattened perikarya (Fig. 8B and D) and appeared to be arrested at G2/M phase. In contrast, H2O2 treatment did not appear to inhibit cell cycle progression (Fig. 8A and C). The morphology of the surrounding CHO cells which did not express δ-PKC–GFP was not altered by treatment with TPA or H2O2. Treatment with ATP did not alter the cell morphology 24 h after treatment (data not shown), as described previously (40).
FIG. 8.
Morphology of cells expressing δ-PKC–GFP observed under a Nomarski interference microscope 24 h after treatment with 5 mM H2O2 (A) and 1 μM TPA (B) for 15 min. Cells expressing GFP are indicated by arrows. DNA staining of cells expressing δ-PKC–GFP was observed under a confocal laser scanning fluorescence microscope 24 h after treatment with 5 mM H2O2 (C) or 1 μM TPA (D) for 15 min. The same cells as shown in panels A and B are indicated by arrows. Bars = 10 μM.
DISCUSSION
The role of individual PKC subspecies are thought to be determined through subspecies-specific activation processes or subspecies-specific substrates in the region downstream of the PKC pathway. Although distinct biochemical characteristics among three PKC groups (cPKC, nPKC, and aPKC) have been found (29, 30), the biological effects of each PKC have not been clarified, in part because the substrate specificities among members of the PKC family are not high enough for detection of selective phosphorylation of substrate proteins (4, 16). Therefore, the intracellular localization of individual PKC subspecies has attracted attention for understanding the functional specificity of the PKC family. Distinct cellular and subcellular localizations of PKC subspecies have been demonstrated by light and electron microscopic immunocytochemistry in tissues and cultured cells (10, 39). After treatment with physiological stimuli, however, PKC translocates to different subcellular compartments (14). Therefore, to understand the physiological role of each PKC subspecies, it is necessary to elucidate how the subspecies is translocated after activation.
Direct visualization of a PKC subspecies in living cells has been demonstrated by using GFP fused to the C-terminus of γ-PKC (35), a method by which we can monitor movement in real time and also identify the sites targeted during translocation of each PKC subspecies in living cells in response to various extracellular signals. Although GFP is useful as a marker protein and can be fused to various proteins by recombinant DNA techniques (3), it should be verified that each GFP fusion protein has the same biological properties as its native protein. We have examined the enzymological and immunological properties of δ-PKC–GFP. As shown in Fig. 1, the levels of dependency on PS-DO and Ca2+ were very similar between δ-PKC–GFP and the native δ-PKC, both of which were immunoprecipitated by the anti-δ-PKC antibody, suggesting that δ-PKC–GFP has enzymological properties similar to those of the native δ-PKC. Furthermore, their enzymological properties are also similar to those of δ-PKC–GFP, which was immunoprecipitated with anti-GFP antibody. This finding strongly suggests that both the anti-δ-PKC antibody and the anti-GFP antibody predominantly immunoprecipitate δ-PKC–GFP but not endogenous δ-PKC from cultured cells transfected with the δ-PKC–GFP cDNA. Immunoblotting also indicated that δ-PKC–GFP has the expected molecular weight without obvious degradation, and no degradative product of δ-PKC–GFP was detected even after TPA treatment. δ-PKC is reported to be digested by caspase-3-like proteases (8, 9), but neither δ-PKC nor δ-PKC–GFP was degraded in the transfected cells, probably because the proteins were present in levels too high to be degraded by the proteases. These results suggest that the fluorescence of GFP fused to δ-PKC can be used as a marker for the native δ-PKC.
In this study, we examined the translocation and activation of δ-PKC in CHO-K1 cells after three different types of stimulation: (i) activation of δ-PKC through G protein-coupled receptor by ATP, (ii) direct activation of δ-PKC by TPA (2), and (iii) activation of δ-PKC through tyrosine phosphorylation by H2O2 (19). The rapid and reversible translocation by the stimulation of G-protein-coupled receptor (ATP receptor in this study) and slower translocation by TPA have been reported previously in the case of γ-PKC–GFP. The transient association of δ-PKC–GFP to the membrane after stimulation of the ATP receptor corresponded to the transient increase in kinase activity of the immunoprecipitated δ-PKC–GFP. The slower translocation of δ-PKC–GFP by TPA was also in good agreement with the gradual and irreversible increase in kinase activity. These findings suggest that ATP and TPA activate PKC through different mechanisms, transient and irreversible, respectively, and that the activated form of δ-PKC–GFP is associated with membrane. The transient and irreversible translocation of δ-PKC–GFP may differ in the inactivation mechanism after ATP and TPA treatment; for example, it is possible that ATP activates not only PKC but also DG kinases or DG lipases that can inactivate PKC. Different mechanisms of activation by ATP and TPA are also suggested by the findings that TPA translocated the δ-PKC–GFP in the nucleoplasm to the nuclear membrane, while ATP did not influence the intranuclear localization of δ-PKC–GFP. The difference in translocation between the intracellular and perikaryal δ-PKC–GFP when activated by TPA also suggests that δ-PKC–GFP in the nucleus may be modified differently from that in the perikaryon. As TPA is not a physiological activator of PKC, data obtained with TPA used as a surrogate stimulator for hormone should be interpreted with great caution, as TPA is likely to result in cell responses different from those induced by hormones.
In contrast, H2O2 treatment did not translocate δ-PKC–GFP to the membrane, while the same treatment strongly activated δ-PKC–GFP, probably through tyrosine phosphorylation. This finding suggests that the tyrosine-phosphorylated δ-PKC–GFP does not require association with the membrane for its activation. Furthermore, immunoblot analysis also showed that δ-PKC as well as δ-PKC–GFP did not translocate to the particulate fraction after H2O2 treatment (data not shown), suggesting that the H2O2 treatment activates δ-PKC without its translocation to the membrane. Although both TPA and H2O2 induced cumulative activation of δ-PKC–GFP, the effect of TPA and H2O2 on the cell cycle and the cell morphology of the cells overexpressing δ-PKC–GFP differed distinctly. The activation of δ-PKC by TPA may induce the phosphorylation of some proteins that are involved in the G2/M transition of the cell cycle, while such proteins may not be phosphorylated by δ-PKC when activated by H2O2. As TPA is known to be resistant to washing and to bind tightly to PKC, the cell cycle arrest by TPA may be due to the sustained activation of δ-PKC by TPA. However, dikaryons were not increased in the cells treated with H2O2 for 24 h, indicating that the activations of δ-PKC by TPA and H2O2 result in different cell responses.
In addition to H2O2 treatment, there are increasing indications that tyrosine phosphorylation modulates the activity of δ-PKC (6, 23, 38, 41). Among various PKC subspecies, only δ-PKC was tyrosine phosphorylated in keratinocytes expressing the oncogenic Ha-ras gene (5) or by TPA treatment (22). The tyrosine phosphorylation sites of δ-PKC were identified by using site-directed mutagenesis. However, the phosphorylation sites varied among the methods of stimulation; the phosphorylation of tyrosine 52 was induced by IgE antigen (38), tyrosine 187 was phosphorylated by TPA and by platelet-derived growth factor (21), and tyrosine 512 and tyrosine 523 were found to be responsible for the effect of H2O2 (19). Conflicting modes of regulation such as enhancement and inhibition of δ-PKC by tyrosine phosphorylation may derive from the different tyrosine residues phosphorylated by various stimulations. The present study revealed that activation of δ-PKC by H2O2 is via a mechanism different from that by TPA or that by activation of phospholipase C-coupled receptors. Activation of δ-PKC by H2O2 is perhaps mediated by tyrosine phosphorylation of the subspecies, although the involvement of serine/threonine protein kinases is also conceivable. It has been shown that trans- and/or autophosphorylation of PKC is necessary to render the kinase catalytically competent and to regulate the subcellular localization (7, 17). In fact, the serine and threonine residues are strongly phosphorylated after H2O2 treatment (19). Furthermore, it is possible that H2O2 inhibits tyrosine phosphatases by oxidation of their catalytic cysteines (12, 36).
As shown in Fig. 5, TPA further activated δ-PKC after its activation by H2O2. It is unlikely that further activation of δ-PKC by TPA is due to the contamination of other kinases in immunoprecipitates, because no kinase activity was coprecipitated by the anti-δ-PKC antibody or by the anti-GFP antibody from the cells which were transfected with δ-PKC-KN–GFP and stimulated by H2O2 and TPA. This observation indicates that TPA can further activate δ-PKC after its substantial activation by H2O2. It is possible that not all δ-PKC molecules were activated by H2O2 through tyrosine phosphorylation and that the remaining unphosphorylated δ-PKC molecules were further activated by TPA. A part of tyrosine-phosphorylated δ-PKC is still dependent on phospholipid and DG (15), and TPA and ATP still induced the translocation of δ-PKC–GFP to the membrane after H2O2 treatment. These findings raised another possibility, that the tyrosine-phosphorylated δ-PKC can be further activated through its association with the membrane elicited by TPA or ATP. It was reported that δ-PKC was tyrosine phosphorylated after the stimulation of growth factor receptors (6, 23) such as epidermal growth factor receptor and platelet-derived growth factor receptor, suggesting that δ-PKC may be activated by growth factors through a synergistic mechanism which involves receptor tyrosine kinase pathways and phospholipid breakdown by phospholipase C-γ (30).
In conclusion, there are at least three distinct pathways involving δ-PKC which vary in translocation processes and activation mechanisms. The interactions among these distinct pathways and the physiological roles of δ-PKC remain to be clarified.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, Yamanouchi Foundation for Research on Metabolic Disorders, and Kato Memorial Bioscience Foundation.
We thank Yasutomi Nishizuka for helpful discussions.
REFERENCES
- 1.Borner C, Guadagno S N, Fabbro D, Weinstein I B. Expression of four protein kinase C isoforms in rat fibroblasts. Distinct subcellular distribution and regulation by calcium and phorbol esters. J Biol Chem. 1992;267:12892–12899. [PubMed] [Google Scholar]
- 2.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phosbol esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- 3.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 4.Dekker L V. Catalytic specificity of protein kinase C isozymes. In: Parker P J, Dekker L V, editors. Protein kinase C. R. G. Austin, Tex: Landes Company; 1997. pp. 57–74. [Google Scholar]
- 5.Denning M F, Dlugosz A A, Howett M K, Yuspa S H. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C delta. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 6.Denning M F, Dlugosz A A, Threadgill D W, Magnuson T, Yuspa S H. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C δ. J Biol Chem. 1996;271:5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 7.Dutil E M, Keranen L M, DePaoli R A, Newton A C. In vivo regulation of protein kinase C by trans-phosphorylation followed by autophosphorylation. J Biol Chem. 1994;269:29359–29362. [PubMed] [Google Scholar]
- 8.Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong W W, Kamen R, Weichselbaum R, et al. Proteolytic activation of protein kinase C δ by an ICE-like protease in apoptotic cells. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghayur T, Hugunin M, Talanian R V, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase C δ by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodnight J A, Mischak H, Kolch W, Mushinski J F. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem. 1995;270:9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- 11.Gschwendt M, Kielbassa K, Kittstein W, Marks F. Tyrosine phosphorylation and stimulation of protein kinase C δ from porcine spleen by src in vitro. Dependence on the activated state of protein kinase C δ. FEBS Lett. 1994;347:85–89. doi: 10.1016/0014-5793(94)00514-1. [DOI] [PubMed] [Google Scholar]
- 12.Hecht D, Zick Y. Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate in vitro. Biochem Biophys Res Commun. 1992;188:773–779. doi: 10.1016/0006-291x(92)91123-8. [DOI] [PubMed] [Google Scholar]
- 13.Iredale P A, Hill S J. Increase in intracellular calcium via activation of an endogenous P2-purinoceptor in cultured CHO-K1 cells. Br J Pharmacol. 1993;110:1305–1310. doi: 10.1111/j.1476-5381.1993.tb13960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996;8:168–173. doi: 10.1016/s0955-0674(96)80062-7. [DOI] [PubMed] [Google Scholar]
- 15.Kadotani M, Nishiuma T, Nanahoshi M, Tsujishita Y, Ogita K, Nakamura S, Kikkawa U, Asaoka Y. Characterization of tyrosine-phosphorylated δ isoform of protein kinase C isolated from Chinese hamster ovary cells. J Biochem (Tokyo) 1997;121:1047–1053. doi: 10.1093/oxfordjournals.jbchem.a021693. [DOI] [PubMed] [Google Scholar]
- 16.Kazanietz M G, Areces L B, Bahador A, Mischak H, Goodnight J, Mushinski J F, Blumberg P M. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1993;44:298–307. [PubMed] [Google Scholar]
- 17.Keranen L M, Dutil E M, Newton A C. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- 18.Konishi H, Matsuzaki H, Tanaka M, Ono Y, Tokunaga C, Kuroda S, Kikkawa U. Activation of RAC-protein kinase by heat shock and hyperosmolarity stress through a pathway independent of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996;93:7639–7643. doi: 10.1073/pnas.93.15.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11223–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraft A S, Anderson W B, Cooper H L, Sando J J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of ELA4 thymoma cells. J Biol Chem. 1982;257:13193–13196. [PubMed] [Google Scholar]
- 21.Li W, Chen X H, Kelley C A, Alimandi M, Zhang J, Chen Q, Bottaro D P, Pierce J H. Identification of tyrosine 187 as a protein kinase C-δ phosphorylation site. J Biol Chem. 1996;271:26404–26409. doi: 10.1074/jbc.271.42.26404. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Mischak H, Yu J C, Wang L M, Mushinski J F, Heidaran M A, Pierce J H. Tyrosine phosphorylation of protein kinase C-δ in response to its activation. J Biol Chem. 1994;269:2349–2352. [PubMed] [Google Scholar]
- 23.Li W, Yu J C, Michieli P, Beeler J F, Ellmore N, Heidaran M A, Pierce J H. Stimulation of the platelet-derived growth factor β receptor signaling pathway activates protein kinase C-δ. Mol Cell Biol. 1994;14:6727–6735. doi: 10.1128/mcb.14.10.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mischak H, Bodenteich A, Kolch W, Goodnight J, Hofer F, Mushinski J F. Mouse protein kinase C-δ, the major isoform expressed in mouse hemopoietic cells: sequence of the cDNA, expression patterns, and characterization of the protein. Biochemistry. 1991;30:7925–7931. doi: 10.1021/bi00246a008. [DOI] [PubMed] [Google Scholar]
- 25.Mischak H, Goodnight J A, Kolch W, Martiny B G, Schaechtle C, Kazanietz M G, Blumberg P M, Pierce J H, Mushinski J F. Overexpression of protein kinase C-δ and -ɛ in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 26.Mischak H, Pierce J H, Goodnight J, Kazanietz M G, Blumberg P M, Mushinski J F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-α and -δ and not by protein kinase C-β II, -ɛ, -ζ, and -η. J Biol Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- 27.Mizuno K, Kubo K, Saido T C, Akita Y, Osada S, Kuroki T, Ohno S, Suzuki K. Structure and properties of a ubiquitously expressed protein kinase C, nPKC δ. Eur J Biochem. 1991;202:931–940. doi: 10.1111/j.1432-1033.1991.tb16453.x. [DOI] [PubMed] [Google Scholar]
- 28.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature (London) 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 29.Nishizuka Y. The molecular heterogeneity of protein kinase C and implications for cellular regulation. Nature (London) 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 30.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 31.Ogita K, Miyamoto S, Yamaguchi K, Koide H, Fujisawa N, Kikkawa U, Sahara S, Fukami Y, Nishizuka Y. Isolation and characterization of δ-subspecies of protein kinase C from rat brain. Proc Natl Acad Sci USA. 1992;89:1592–1596. doi: 10.1073/pnas.89.5.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno S, Mizuno K, Adachi Y, Hata A, Akita Y, Akimoto K, Osada S, Hirai S, Suzuki K. Activation of novel protein kinase Cδ and Cɛ upon mitogenic stimulation of quiescent rat 3Y1 fibroblasts. J Biol Chem. 1994;269:17495–17501. [PubMed] [Google Scholar]
- 33.Olivier A R, Parker P J. Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J Biol Chem. 1994;269:2758–2763. [PubMed] [Google Scholar]
- 34.Ono Y, Fujii T, Ogia K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988;263:6927–6932. [PubMed] [Google Scholar]
- 35.Sakai N, Sasaki K, Ikegaki N, Shirai Y, Saito N. Direct visualization of translocation of γ-subspecies of protein kinase C in living cells using fusion proteins with green fluorescent protein. J Cell Biol. 1997;139:1465–1476. doi: 10.1083/jcb.139.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skorey K, Ly H D, Kelly J, Hammond M, Ramachandran C, Huang Z, Gresser M J, Wang Q. How does alendronate inhibit protein-tyrosine phosphatases? J Biol Chem. 1997;272:22472–22480. doi: 10.1074/jbc.272.36.22472. [DOI] [PubMed] [Google Scholar]
- 37.Soltoff S P, Toker A. Carbachol, substance P, and phorbol ester promote the tyrosine phosphorylation of protein kinase C δ in salivary gland epithelial cells. J Biol Chem. 1995;270:13490–13495. doi: 10.1074/jbc.270.22.13490. [DOI] [PubMed] [Google Scholar]
- 38.Szallasi Z, Denning M F, Chang E Y, Rivera J, Yuspa S H, Lehel C, Olah Z, Anderson W B, Blumberg P M. Development of a rapid approach to identification of tyrosine phosphorylation sites: application to PKC δ phosphorylated upon activation of the high affinity receptor for IgE in rat basophilic leukemia cells. Biochem Biophys Res Commun. 1995;214:888–894. doi: 10.1006/bbrc.1995.2370. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, Kikkawa U, Nishizuka Y. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-δ subspecies. Proc Natl Acad Sci USA. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zang Q, Lu Z, Curto M, Barile N, Shalloway D, Foster D A. Association between v-Src and protein kinase C δ in v-Src-transformed fibroblasts. J Biol Chem. 1997;272:13275–13280. doi: 10.1074/jbc.272.20.13275. [DOI] [PubMed] [Google Scholar]