Abstract
The use of blood lactate concentrations as a prognostic indicator and therapeutic gauge in feline medicine has been hindered by the inability to obtain values in a timely manner with minimal quantities of blood. Recently, hand-held point-of-care (POC) lactate meters have become commercially available. The objective of this prospective study was to determine if lactate values produced by three commercially available and one medical grade POC meter were in agreement with a laboratory blood analyzer. Blood samples from 47 cats were collected on presentation to an emergency service and processed on four POC meters and a Stat Profile Critical Care Xpress blood analyzer. The results were analyzed using the Bland–Altman method. The blood lactate values produced by the hospital grade POC meter and one of the commercially POC meters were in good agreement with the Critical Care Xpress blood analyzer. Other commercially available POC meters produced acceptable agreement.
Elevations in blood lactate concentrations have been used as an indication of tissue hypoxia in critically ill animals and people (Mizock and Falk 1992, Slomovitz et al 1998, Hughes 1999). In addition, some medical conditions and drug complications have been associated with increases in blood lactate resulting from disordered cellular metabolism (James et al 1999, Holloway et al 2001, Neale et al 2004). In some conditions, the measurement of blood lactate has proven to have prognostic as well as diagnostic value. In human studies, the survival of patients suffering from shock was shown to decrease from 90% to 10% as blood lactate increased from 2.0 to 8.0 nmol/l (Weil and Afifi 1970). In veterinary medicine, blood lactate levels have proven to be prognostic in several canine disease conditions (Lagutchik et al 1998, de Papp et al 1999, Nel et al 2004).
Clinical use of blood lactate concentrations in feline medicine has been hampered by the inability to obtain results in a timely manner and the relatively large amounts of blood required for each analysis. The measurement of blood lactate concentration in clinical practice has often required sending blood to a diagnostic laboratory for analysis. This is impractical due to the time need to obtain results (Slomovitz et al 1998) and the effect of latency on samples (Hughes 1999). In order for lactate concentration to be a meaningful part of the diagnostic plan, the clinician needs the ability to obtain results immediately (Slomovitz et al 1998). Monitoring patient response to treatment and adjusting therapeutics requires serial measurements (Boldt et al 2001). Both of these necessitate the use of readily available tests that require minimal amounts of blood.
Recently, three hand-held points of care meters capable of measuring blood lactate levels have become available: Lactate Pro (Arkray, Inc., Kyoto, Japan), Lactate Scout (SensLab GmbH Lepzig, Germany) and Accutrend (Roche Diagnostics Basel, Switzerland).
These hand-held units are simple to operate, inexpensive to maintain, and require only a single drop of whole blood. Although these meters have been advocated for use in veterinary clinical practice, they were all designed for use by athletes to ensure that their training is at the ‘anabolic threshold’ (Myers and Ashley 1997). These units are calibrated for use with human capillary blood and none of them has been validated for use in cats. In addition to these units, a hospital grade POC blood analyzer (iSTAT; Abbott Point of Care Abbott Park, IL) is available for measuring blood lactate concentrations. This unit, which requires slightly more blood to analyze and is significantly more expensive to operate, has also not been validated for use in cats. The goal of this study was to determine if the blood lactate concentrations generated by these four POC meters were in good agreement with a laboratory blood analyzer (Stat Profile Critical Care Xpress; Nova Biomedical Waltham, MA) when analyzing blood from feline patients.
Methods and materials
Subjects
Fifty cats, which were evaluated by the hospital's emergency service during a 3-month period, were admitted to the study. The only selection criteria used for recruitment of patients was that blood would otherwise have to be drawn for testing purposes, no fluids containing lactate could have been given in the 24 h prior to presentation and that owners had given written consent. No attempt was made to screen patients on the basis of age, sex, or severity of illness. Patients for which lactate measurements could not be determined on all five analyzers were later removed form the study. The school of veterinary medicine clinical study protocol review committee approved the protocol for this study.
Blood samples
All blood samples were collected via direct venepuncture using minimal restraint or an intravenous catheter. The blood sample was divided and 1.0 ml was placed in a lithium heparin tube, as required for the Stat Profile Critical Care Xpress. The remainder of the sample was used to operate the POC meters. In order to minimize variations attributed to blood handling and meter operation, all samples were processed by one of two authors (MJA, MEJ). In every case, one these authors were present when the sample was collected. All lactate measurements were performed immediately and in a simultaneous manner. All meters were calibrated and maintained in accordance with the manufacturer's specifications.
Meters
There are two different lactate measuring technologies employed by POC analyzers. The most common method involves an amperometric reaction. In these units, whole blood reacts with lactate oxidase and potassium ferricyanide to produce an anodic current (Shimojo et al 1993). The meter measures the resulting current and translates it into a lactate value that is displayed on the unit's liquid crystal display. The Lactate Pro, and Lactate Scout both employ this technology. In these units, 5 μl is automatically drawn into the hollow portion of a test strip where the reaction takes place. Although collection of the blood sample is not automated, the iSTAT POC meter uses a similar technology in which platinum is substituted for potassium ferricyanide.
The Accutrend employs a second POC lactate measuring technology, which is based on a colorimetric reaction (Shimojo et al 1989). With this unit, an unmeasured drop of blood (approximately 25 μl) is placed on a test strip that is comprised of four layers. Blood is applied to a protective meshwork that makes up the top layer. In the second layer, serum is separated from red cells by a glass-fiber material. The serum then diffuses to the third layer, where serum lactate reacts with lactate oxidase resulting in a color change. The degree of change is dependent on the amount of lactate in the serum and is read by reflectance photometry. This information is then translated into a lactate concentration that is displayed on the unit's liquid crystal display. The fourth section of the test strip is a plastic support layer that runs the entire length of the strip.
The Stat Profile Critical Care Xpress employs an amperometric reaction to determine blood lactate concentrations. The unit automatically draws 130 μl of blood from the lithium heparin tube and exposes the blood to a membrane-covered electrode. The membrane, which allows only serum to contact the probe, is imbedded with lactate oxidase. This catalyzes the conversion of lactate and oxygen to pyruvate and hydrogen peroxide. The electroactive hydrogen peroxide is oxidized at the surface of a platinum electrode and the current generated is proportional to the lactate concentration. Once calculated, the blood lactate concentration is displayed on a screen and printed.
Statistical analysis
Historically, agreement between different methods of measuring the same biologic parameter has been determined on the basis of regression or correlation coefficient; however, neither of these is optimal for agreement analysis (Bland and Altman 1986, 1999, Seed 2000, Glantz 2005). In this study, agreement between each of the four POC meters and the hospital grade blood analyzer was determined by the Bland–Altman method (Bland and Altman 1986, 1999). This statistical method has been endorsed by biostatisticians (Seed 2000, Glantz 2005) and is widely used in the medical literature. In this analysis, bias was defined as the mean difference between two methods and limits of agreement were calculated as the bias ±1.96×SD. Precision was defined as the 95% confidence interval of the limits of agreement with a P value of 0.05. Statistical analysis was performed by use of a commercially available statistical program (GraphPad Prism 4.0 for Macintosh, GraphPad Software, San Diego, CA).
Results
On the basis of the criteria stated above, the final study population consisted of 47 cats. Three of the original 50 were removed from the study population, as lactate measurements were not available from all four meters. There were 20 females and 27 males. Age was known for 43 cats, which ranged in age from 5 months to 19 years (median age 9 years). Twenty-nine were identified as domestic shorthair cats, four domestic medium hair, four domestic longhair, three Siamese, two Scottish folds, two Persians, one Himalayan, one Maine Coon, and one Cornish Rex. Upon presentation to the emergency service each patient was assigned a preliminary assessment which included: anorexia/weight loss (nine), urethral obstruction/cystitis (seven), neoplasia (six), heart failure (three), diarrhea (three), upper respiratory disease (three), liver disease (three), abscess (two), renal failure (two), epistaxis (two), diabetic ketoacidosis (one), seizure (one), foreign body (one), diaphragmatic hernia (one), chylous effusion (one), lower motor neuron disease (one), stomatitis (one).
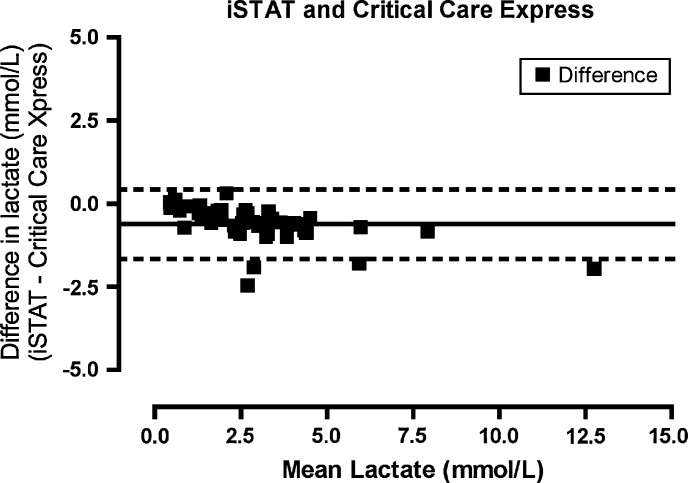
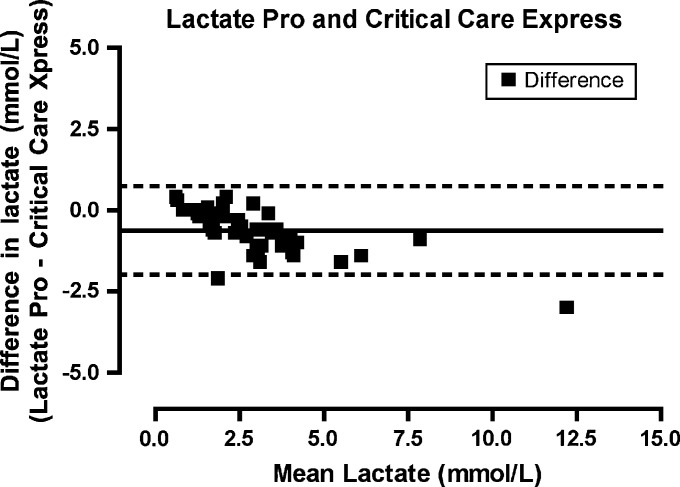
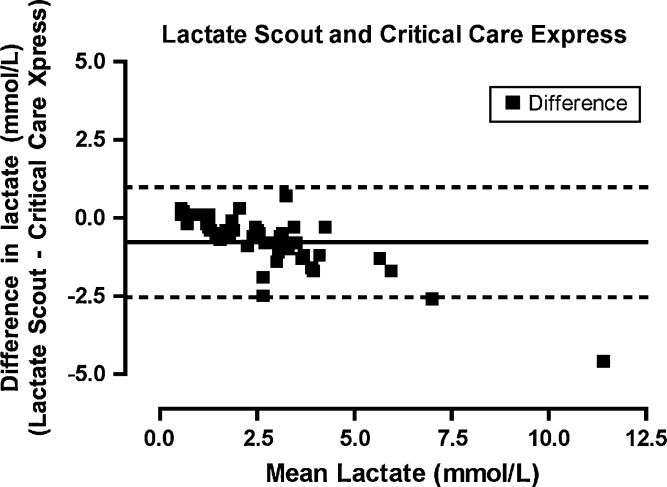
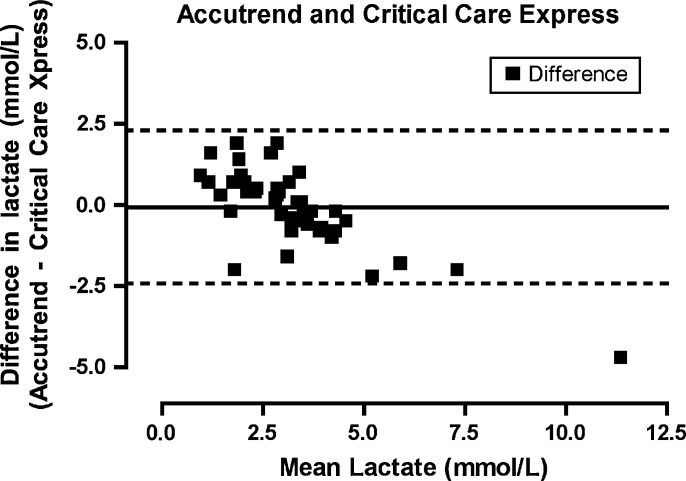
Lactate as measured on the laboratory analyzer ranged from 0.4 to 13.7 mmol/l (mean, 3.06 mmol/l). Agreement between the iSTAT and Stat Profile Critical Care Xpress (Fig 1) was highest for all the POC lactate analyzers (bias −0.617872, limits of agreement −1.66703 to 0.431287, precision −1.82397 to 0.588226). Agreement between the Lactate Pro and Stat Profile Critical Care Xpress (Fig 2) was similar (bias −0.625532, limits of agreement −1.98743 to 0.736368, precision −2.19115 to 0.94008). The Lactate Scout (Fig 3) demonstrated a lower degree of agreement (bias −0.772340, limits of agreement −2.53146 to 0.986777, precision −2.79460 to 1.24991) while the Accutrend (Fig 4) demonstrated the least agreement (bias −0.625532, limits of agreement −2.43216 to 2.29599, precision −2.78597 to 2.64980).
Fig 1.
Bland–Altman plot. The difference between the measurements (iSTAT−Critical Care Express) plotted for the mean of the values measured by the two methods ([iSTAT+Critical Care Express]/2).
Fig 2.
Bland–Altman plot. The difference between the measurements (Lactate pro−Critical Care Express) plotted for the mean of the values measured by the two methods ([Accutrend+Critical Care Express]/2).
Fig 3.
Bland–Altman plot. The difference between the measurements (Lactate scout−Critical Care Express) plotted for the mean of the values measured by the two methods ([Lactate scout+Critical Care Express]/2).
Fig 4.
Bland–Altman plot. The difference between the measurements (Accutrend−Critical Care Express) plotted for the mean of the values measured by the two methods ([Accutrend+Critical Care Express]/2).
Discussion
Hyperlactemia results when lactate formation occurs at a rate that is faster than its metabolism. It is often categorized into type A and type B based on the underlying cause (Mizock and Falk 1992). Type A occurs when the hyperlactemia is associated with tissue hypoxemia causing a shift from aerobic to anaerobic cellular metabolism (Mizock and Falk 1992). This can be the result of a relative hypoxemia as is seen in exercise and seizures (Hughes 1999) or tissue hypoxemia as occurs with hypo-perfusion or reduced arterial oxygen content (Mizock and Falk 1992). Type B is seen in the absence of hypoxemia and can be the result of disease (James et al 1999), drugs (Mizock and Falk 1992, Neale et al 2004) or inborn errors of metabolism (Mizock and Falk 1992). Some of these conditions may be associated with defects in cellular metabolism resulting in failure of the citric acid cycle to accept pyruvate at an acceptable rate (James et al 1999, Holloway et al 2001). Clinically, the most important cause of hyperlactemia is tissue hypoxia.
Although relevant studies in veterinary medicine are limited, reports in the human literature indicate that serial lactate measurements can be used to estimate the response to treatment and to provide therapy endpoints in hypoperfused, critically ill patients (Kobayashi et al 2001, Husain et al 2003). In some disease conditions, elevations in lactate that decrease in response to appropriate resuscitative therapy have been shown to be associated with a better prognosis than lactate levels that fail to respond (Husain et al 2003, Nguyen et al 2004). These studies suggest that in the critical care setting, measurement of lactate can provide meaningful insight into the adequacy of treatment as well as the prognosis of the patient. New research is needed to determine if serum lactate measurement has a similar utility in feline patients. Such studies would undoubtedly be aided by the availability of POC meters.
In this study, we compared the agreement of four hand-held POC meters with a laboratory blood analyzer. We found that the iSTAT had the highest level of agreement, although one of the commercially available units, the Lactate Pro, also demonstrated a high level of agreement. These results are similar but more pronounced than those reported in a recent study in dogs (Acierno and Mitchell 2007). A difference in the performance of the POC units is likely based on the technology and algorithms used by the various units. While the iSTAT, Lactate Pro and Lactate Scout all use an amperometric reaction to determine blood lactate concentrations; each appears to use a different methodology. The Lactate Scout is able to provide the users with results after only 15 s while the Lactate Pro requires 60 s and the iSTAT takes longer. While the quality of the information is not dependent on the amount of time needed to arrive at a value, the time difference suggests that there are variations in the technology employed. Some appear to be better suited for use in cats. The Accutrend demonstrated the least agreement of all the POC meters and was the only POC unit to use an unmeasured drop of blood and a colorimetric reaction. Interestingly, a study in human medicine found this unit to have the lowest degree of agreement with a laboratory analyzer and determined it be unreliable under certain environmental conditions (Medbo et al 2000).
The prognostic value of measuring blood lactate levels has been demonstrated by several human and canine studies. The inability to measure blood lactate concentration in a timely fashion using minimal amounts of blood has prevented widespread utilization of this important blood parameter in cats. The availability of a commercially available POC meter that is in good agreement with a laboratory blood analyzer brings the promise of cost effective cage-side lactate measurement. Although this study shows that the level of agreement between some of the POC meters and the laboratory analyzer was acceptable, additional studies to further elucidate the accuracy and precision of the assays on clinical samples should be pursued to provide a more complete picture of the ultimate value of these assays for felines. This information will facilitate future studies that will lead to improved monitoring and treatment of critical feline patients.
References
- Acierno M., Mitchell M. Evaluation of four point-of-care meters for rapid determination of blood lactate concentrations in dogs, Journal of the American Veterinary Medical Association 230, 2007, 1315–1318. [DOI] [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement, Lancet 1, 1986, 307–310. [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. Measuring agreement in method comparison studies, Statistical Methods in Medical Research 8, 1999, 135–160. [DOI] [PubMed] [Google Scholar]
- Boldt J., Kumle B., Suttner S., Haisch G. Point-of-care (poc) testing of lactate in the intensive care patient. Accuracy, reliability, and costs of different measurement systems, Acta Anaesthesiologia Scandavia 45, 2001, 194–199. [DOI] [PubMed] [Google Scholar]
- de Papp E., Drobatz K.J., Hughes D. Plasma lactate concentration as a predictor of gastric necrosis and survival among dogs with gastric dilatation-volvulus: 102 cases (1995–1998), Journal of the American Veterinary Medical Association 215, 1999, 49–52. [PubMed] [Google Scholar]
- Glantz S. How to Test for Trends Primer of Biostatistics, 2005, McGraw–Hill: New York, 253–320. [Google Scholar]
- Holloway P., Benham S., St John A. The value of blood lactate measurements in icu: an evaluation of the role in the management of patients on haemofiltration, Clinica Chimica Acta 307, 2001, 9–13. [DOI] [PubMed] [Google Scholar]
- Hughes D. Lactate measurement: diagnostic, therapeutic, and prognostic implications. Bonagura J. Kirk's Current Veterinary Therapy xiii, 1999, WB Saunders: Philadelphia, 112–116. [Google Scholar]
- Husain F.A., Martin M.J., Mullenix P.S., Steele S.R., Elliott D.C. Serum lactate and base deficit as predictors of mortality and morbidity, American Journal of Surgery 185, 2003, 485–491. [DOI] [PubMed] [Google Scholar]
- James J.H., Luchette F.A., McCarter F.D., Fischer J.E. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis, Lancet 354, 1999, 505–508. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Gando S., Morimoto Y., Nanzaki S., Kemmotsu O. Serial measurement of arterial lactate concentrations as a prognostic indicator in relation to the incidence of disseminated intravascular coagulation in patients with systemic inflammatory response syndrome, Surgery Today 31, 2001, 853–859. [DOI] [PubMed] [Google Scholar]
- Lagutchik M.S., Ogilvie G.K., Hackett T.B. Increased lactate concentration in ill and injured dogs, Journal of Veterinary Emergency Critical Care 8, 1998, 117–127. [Google Scholar]
- Medbo J.I., Mamen A., Olsen O. Holt, Evertsen F. Examination of four different instruments for measuring blood lactate concentration, Scandinavian Journal of Clinical Laboratory Investigations 60, 2000, 367–380. [DOI] [PubMed] [Google Scholar]
- Mizock B.A., Falk J.L. Lactic acidosis in critical illness, Critical Care Medicine 20, 1992, 80–93. [DOI] [PubMed] [Google Scholar]
- Myers J., Ashley E. Dangerous curves. A perspective on exercise, lactate, and the anaerobic threshold, Chest 111, 1997, 787–795. [DOI] [PubMed] [Google Scholar]
- Neale R., Reynolds T.M., Saweirs W. Statin precipitated lactic acidosis?, Journal of Clinical Pathology 57, 2004, 989–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel M., Lobetti R.G., Keller N., Thompson P.N. Prognostic value of blood lactate, blood glucose, and hematocrit in canine babesiosis, Journal of Veterinary Internal Medicine 18, 2004, 471–476. [DOI] [PubMed] [Google Scholar]
- Nguyen H.B., Rivers E.P., Knoblich B.P., Jacobsen G., Muzzin A., Ressler J.A., Tomlanovich M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock, Critical Care Medicine 32, 2004, 1637–1642. [DOI] [PubMed] [Google Scholar]
- Seed P. Comparing several methods of measuring the same quality, Stata Technical Bulletin 10, 2000, 2–9. [Google Scholar]
- Shimojo N., Naka K., Nakajima C., Yoshikawa C., Okuda K., Okada K. Test-strip method for measuring lactate in whole blood, Clinical Chemistry 35, 1989, 1992–1994. [PubMed] [Google Scholar]
- Shimojo N., Naka K., Uenoyama H., Hamamoto K., Yoshioka K., Okuda K. Electrochemical assay system with single-use electrode strip for measuring lactate in whole blood, Clinical Chemistry 39, 1993, 2312–2314. [PubMed] [Google Scholar]
- Slomovitz B.M., Lavery R.F., Tortella B.J., Siegel J.H., Bachl B.L., Ciccone A. Validation of a hand-held lactate device in determination of blood lactate in critically injured patients, Critical Care Medicine 26, 1998, 1523–1528. [DOI] [PubMed] [Google Scholar]
- Weil M.H., Afifi A.A. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock), Circulation 41, 1970, 989–1001. [DOI] [PubMed] [Google Scholar]