Abstract
Three cats were examined because of acute dyspnoea and sudden abdominal enlargement. In all cats, radiographs revealed gastric dilatation–volvulus (GDV) and diaphragmatic hernia. Cardiovascular shock and dyspnoea were treated by intravenous fluid-therapy, oxygen administration and relief of diaphragmatic pressure by means of stomach decompression and in one case placing the patient in an inclined position. Gastric decompression was performed by needle gastrocentesis, placement of a rhino-gastric tube, or a combination of these. Diaphragmatic herniorrhaphy was performed in either case; one cat also underwent gastropexy. The immediate postoperative period resolved uneventfully and the cats were doing well at follow-up. Feline GDV is a rare event in which diaphragmatic hernia may be a predisposing factor.
Case 1: instead, a 3-year-old castrated male domestic shorthair (DSH) cat was presented because of respiratory distress and abdominal distension of sudden onset. The owner reported a motor vehicle accident 6 months before referral.
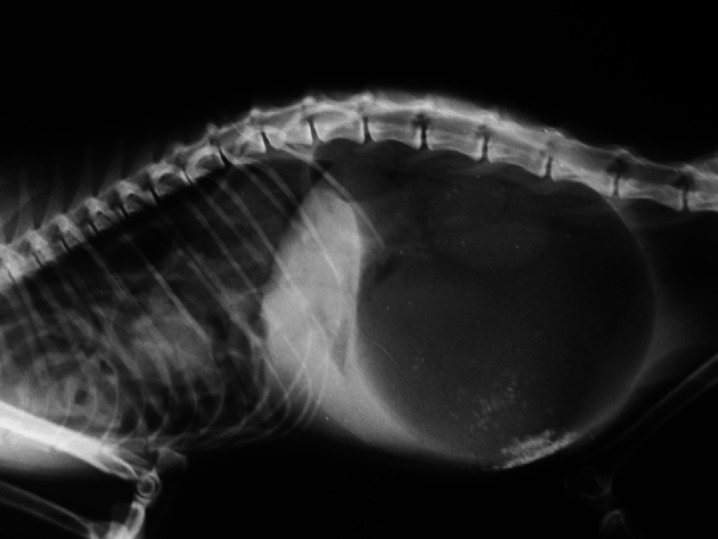
Upon initial admission, the cat showed respiratory distress with asymmetrical abdominal distension, especially on the left side. The abdomen was tympanic at percussion. At clinical examination, neither signs of hypovolaemic shock nor hypoperfusion were detected. Initial laboratory findings (haematocrit, total proteins, glycaemia, and urine specific gravity) were within normal ranges. The cat was initially managed by oxygen-therapy and intravenous administration of lactate-Ringer's solution (LRS) at maintenance rate. A total-body lateral radiograph revealed intra-abdominal dilated stomach displacing the pylorus dorsally, and diaphragmatic hernia. A diagnosis of GDV was formulated on the basis of radiographic signs (Fig 1).
Fig 1.
Radiographic right latero/lateral total-body view of case 1: a presumptive diagnosis of GDV was formulated, while diaphragmatic hernia is clearly visible. At celiotomy, stomach was found 180° clockwise rotated.
To relieve the symptoms related to restrictive dyspnoea, the stomach was decompressed by needle gastrocentesis. Frequent attempts to vomit resulted in recurrence of gastric dilatation (GD), thus urging to place a rhino-gastric tube (diameter 1.2 mm) to maintain decompression. Approximately 12 h after presentation, exploratory surgery was undertaken. The diaphragm presented a left radial laceration, whereas the left lateral hepatic lobe, omentum, small intestine and spleen were herniated into the thoracic cavity. The stomach was found in abdomen and 180° clockwise rotated. The gastric fundus showed a necrotic area. Herniorrhaphy was performed using polidioxanone 4/0 in a simple continuous suture pattern. The necrotic gastric wall was invaginated (MacCoy et al 1986); gastropexy was not performed. A thoracic drainage was inserted for 48 h. Feeding through the rhino-gastric tube began 6 h postoperatively. Within 10 h, the cat began to ingest food by mouth and was discharged 24 h thereafter. GDV did not recur, and at the time of writing (2 years after surgery) the cat is doing well.
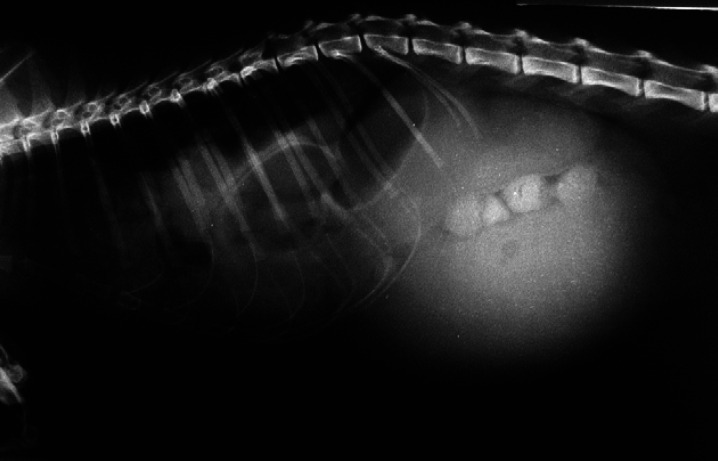
Case 2: instead, a 15-month-old female spayed DSH cat was admitted because of severe restrictive dyspnoea and enlarged abdomen. The cat was hit by car 24 h before presentation. At physical examination, the cat was depressed, and showed signs of hypovolaemia and hypoperfusion. On arrival, increased packed cell volume (58%; range 24–45%), total proteins 8.4 g/dl (range 4.5–8.5 g/dl), hyperglycaemia (208 mg/dl; range 71–159 mg/dl), hypokalaemia (2.5 mEq/l; range 2.9–4.2 mEq/l), hyponatraemia (134 mEq/l; 147–162 mEq/l) and hypochloraemia (87 mEq/l; 112–129 mEq/l) were documented. The right lateral abdominal radiograph revealed GD, abdominal location of the dilated stomach and diaphragmatic hernia (Fig 2).
Fig 2.
Radiographic right latero/lateral total-body view of case 2: liquid distended stomach is evident and is associated to lost details of diaphragmatic line (diaphragmatic hernia). At celiotomy, a 90° clockwise gastric rotation was found.
Medical treatment consisted of oxygen administration and intravenous fluid-therapy with LRS at 20 ml/kg/h rate. A rhino-gastric tube was placed to decompress the stomach. The patient was placed in inclined position in order to further relieving pulmonary compression. Subsequent intermittent aspiration, performed every 2 h, resulted in marked clinical improvement leading to exploratory celiotomy 24 h after initial presentation.
The diaphragm presented a left radial and ventral laceration, with left lateral hepatic lobe, small intestine and spleen herniated into the thoracic cavity. The stomach was found in abdominal location and 90° clockwise rotated. Surgical intervention consisted of gastric derotation and herniorrhaphy; gastropexy was not performed. A thoracic drainage was inserted for 48 h. Postoperative recovery was uneventful and the cat was reportedly normal at 1-year follow-up.
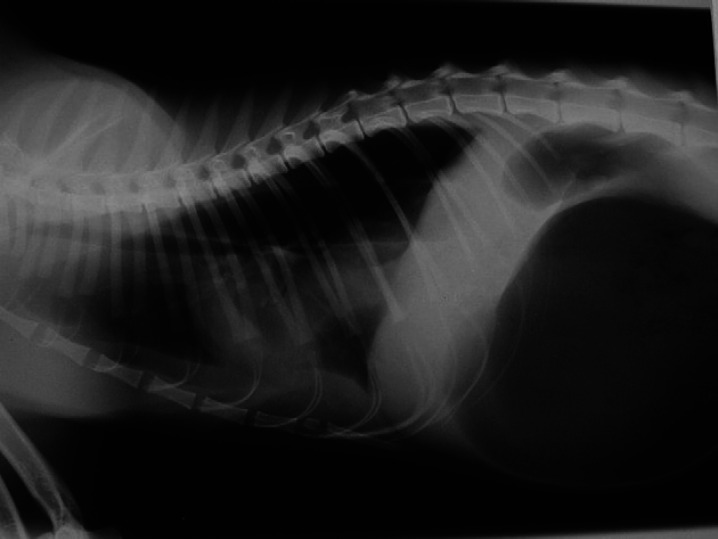
Case 3: instead, a 1-year-old male DSH was presented because of sudden unset of anorexia, vomiting and abdominal distension. The owner reported that 3 months before referral the cat was hit by a car. On physical examination, the cat was lightly depressed, with pale mucosal membranes and capillary refill time mildly increased. Initial laboratory findings included normal packed cell volume (39%; range 24–45%), normal total solids (7.7 g/dl; range 4.5–8.5 g/dl), hyperglycaemia (207 mg/dl; range 71–159 mg/dl), and hypochloraemia (104 mEq/l; 112–129 mEq/l). Immediate stabilisation consisted of oxygen and rapid intravenous fluids with balanced electrolyte solution. A right lateral total-body radiograph showed GDV and loss of the diaphragmatic line (Fig 3). Gastric decompression was achieved by needle gastrocentesis. As soon as the cat was stable, an exploratory celiotomy was performed.
Fig 3.
Radiographic right latero/lateral total-body view of case 3: typical radiographic GDV signs are associated with diaphragmatic hernia.
Midline laparotomy revealed a large right ventral diaphragmatic tear with herniation of the right lateral hepatic lobe, part of the small intestine and spleen. The stomach was still rotated (180° clockwise) in abdominal cavity. Herniorrhaphy was performed. The stomach was repositioned and fixed to the right abdominal wall using an incisional gastropexy technique (MacCoy et al 1982). The recovery after surgery was uneventful and the patient was reportedly normal at 2-and-a-half-year follow-up.
GDV is a syndrome that occurs more often in dogs, and the literature seldom refers to feline cases (Bredal et al 1996). Respiratory compromise, abdominal distension, cardiovascular shock and electrolyte disturbance are common in dogs and cats presenting with GDV. Diagnosis of GDV is easily made on the basis of signs and radiological appearance, including dorso-cranial displacement of the antrus pyloricus. The right lateral recumbency view seems to be sufficient to formulate a definitive diagnosis of GDV in dogs (Hathcock 1984) as well in cats.
In dogs, GDV is multifactorial in origin, and proposed predisposing factors include thoracic depth/width ratio (Glickman et al 1996, 2000a,b) familiarity, stress (Purdue Bloat Research Group 1994–1998, Schaible et al 1997) and concurrent diseases (Millis et al 1995, Braun et al 1996, Marconato 2006).
In cats, the aetiology of GDV remains unresolved; however, concurrent diseases may act as predisposing factors. The diaphragmatic hernia may have a contributory role in development of GDV in cats, being present in three out of 10 in Bredal's report (Bredal et al 1996) and in all cases described here. In dogs, the initiating event for GDV is the accumulation of gas within the stomach by aerophagia. In cats, dyspnoea caused by diaphragmatic hernia may lead to aerophagia, gastric distension and eventually to stomach displacement. Spleen herniation seems to be another predisposing factor leading to stomach malposition. In the cases of this series all cats presented herniation of the liver, small intestine, and spleen, thus causing a continuous and increasing traction on the great curvature of the stomach by either the gastrosplenic ligament and omentum, ultimately evoking various degrees of gastric torsion.
The cornerstone of emergency medical treatment is directed to improve oxygen delivery to the tissues. Surgery is mandatory and should be performed as soon as the patients are stable. In cases 1 and 2, further aspiration of gastric fluids with a rhino-gastric tube contributed to improve tissues perfusion and cardiovascular stabilisation, thereby limiting vascular obstruction and improving the venous return to the heart. Nevertheless, in case 1 a necrotic area at the gastric fundus was found during surgery. In this case the stomach was rotated more than 180°, thus leading to vascular impairment and consequent gastric wall ischaemia; delayed surgical decision may have caused gastric wall necrosis successfully managed by gastric wall invagination. Postoperatively, no complications related to surgical procedure were noted. Unlike dogs, in which surgical gastropexy is strongly suggested to prevent GDV relapse (Glickman et al 1998), in cats surgery could be limited to gastric repositioning with no need for gastropexy. In all cases reported here, surgical intervention was aimed at repositioning herniated organs and restoring diaphragmatic integrity. Because cats are normally not at risk for GDV, and the diaphragmatic herniation is believed to be the predisposing factor for stomach rotation, in two out of three cats in our series herniorrhaphy was the sole surgical procedure. The rarity of GDV in cats and the paucity of literature regarding its treatment and recurrence rate may lead to different opinions in surgical treatment of a bloated cat.
In conclusion, feline GDV remains a rare event and there is suggestion that diaphragmatic hernia may play a role in the development of this disease in cat. As a consequence, cats with diaphragmatic hernia should be treated as soon as possible by repositioning herniated organs and restoring diaphragmatic integrity, even if asymptomatic. If GDV is concurrent to diaphragmatic hernia, gastropexy seems unnecessary, as herniorrhaphy eliminates the predisposing factor. On the other hand, if GDV develops independently, gastropexy may be indicated.
GDV in cats is a new pathology and needs to be studied thoroughly in the future.
Acknowledgement
The authors would like to thank Laura Marconato for meticulous work of revision of this manuscript.
References
- Braun L., Lester S., Kuzma A.B., Hosie S.C. Gastric dilatation–volvulus in the dog with histological evidence of preexisting inflammatory bowel disease: a retrospective study of 23 cases, Journal of the American Animal Hospital Association 32, 1996, 287–290. [DOI] [PubMed] [Google Scholar]
- Bredal W.P., Eggertsdòttir A.V., Austefjord O. Acute gastric dilatation in cats: a case series, Acta Veterinaria Scandinavica 37 (4), 1996, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman L.T., Glickman N.W., Schellemberg D.B., Raghavan M., Lee T.L. Incidence of and breed-related risk factors for gastric dilatation–volvulus in dogs, Journal of the American Veterinary Medical Association 216 (1), 2000a, 40–45. [DOI] [PubMed] [Google Scholar]
- Glickman L.T., Glickman N.W., Schellenberg D.B., Raghavan M., Lee T. Non-dietary risk factors for gastric dilatation–volvulus in large and giant breed dogs, Journal of the American Veterinary Medical Association 217 (10), 2000b, 1492–1499. [DOI] [PubMed] [Google Scholar]
- Glickman L.T., Lantz G.C., Schelemberg D.B., Glickman N.W. A prospective study of survival and recurrence following the acute gastric dilatation–volvulus syndrome in 136 dogs, Journal of the American Animal Hospital Association 34, 1998, 253–259. [DOI] [PubMed] [Google Scholar]
- Glickman L.T., Emerick T., Glickman N.W., Glickman S., Lantz G., Perez C., Schellenberg D., Widmer W., Yi Q. Radiological assessment of the relationship between thoracic conformation and the risk of gastric dilatation–volvulus in dogs, Veterinary Radiology and Ultrasound 37, 1996, 174–180. [Google Scholar]
- Hathcock J.T. Radiographic view of choice for the diagnoses of gastric volvulus: the right lateral recumbent view, Journal of the American Animal Hospital Association 20, 1984, 967. [Google Scholar]
- MacCoy D.M., Kneller S.K., Sundberg J.P., et al. Partial invagination of the canine stomach for treatment of infarction of the gastric wall, Veterinary Surgery 15, 1986, 237–245. [Google Scholar]
- MacCoy D.M., Sykes G.P., Hoffer R.E., Harvey H.J. A gastropexy technique for permanent fixation of the pyloric antrum, Journal of the American Animal Hospital Association 18, 1982, 763–768. [Google Scholar]
- Marconato L. Gastric dilatation–volvulus as complication after surgical removal of a splenic haemangiosarcoma in a dog, Journal of Veterinary Medicine. A, Physiology, Pathology, Clinical Medicine 53 (7), 2006, 371–374. [DOI] [PubMed] [Google Scholar]
- Millis, et al. Gastric dilatation–volvolus after splenic torsion in two dogs, Journal of the American Veterinary Medical Association 207 (3), 1995, 314. [PubMed] [Google Scholar]
- Purdue Bloat Research Group Bloat notes: news from the canine gastric dilatation–volvulus research program, http://www.vet.purdue.edu/epi/bloat.htm, 1994–1998.
- Schaible R.H., Ziech J., Glickman N.W., Schellenberg D., Yi Q., Glickman L.T. Predisposition to gastric dilation–volvulus in relation to genetics of thoracic conformation in Irish setters, Journal of the American Animal Hospital Association 33, 1997, 379–383. [DOI] [PubMed] [Google Scholar]