Abstract
The objective of this study was to examine clinical signs, laboratory parameters, and course of disease in Abyssinian and Somali cats with pyruvate kinase (PK) deficiency. The clinical course of 25 PK-deficient cats was followed over a time period of 0.8–11.3 years (median 4.3). Eleven cats (age 0.8–7.8 years, median 4.4) did not show signs according to the owners. In 14 cats (age 0.1–5 years, median 1.7) the owners noted lethargy (10), diarrhoea (seven), pale mucous membranes (six), inappetence (six), poor coat quality (six), weight loss (four), icterus (four), and pica (two). Sixteen cats had been used for breeding at least once before diagnosis. Laboratory abnormalities included anaemia (70%), increased aggregated reticulocyte counts (94%), hyperglobulinaemia (80%), hyperbilirubinaemia (53%), and increased liver enzymes (47%). Six of 25 affected cats died (four) or were euthanased (two) at ages ranging from 1.3 to 11.3 years (median 4.1) presumably because of PK-deficiency. These findings emphasise that PK deficiency shows variation in age of onset and severity of signs. As PK-deficient cats can be asymptomatic testing for PK deficiency before breeding is strongly recommended.
Erythrocytes generate energy almost exclusively through anaerobic glycolysis. Pyruvate kinase (PK) is one of the key regulatory enzymes of this metabolic pathway and catalyses the transformation of phosphoenolpyruvate to pyruvate, the last energy providing step of the anaerobic glycolysis. Four homotetramer isoenzymes of PK coded by two different genes (PKLR and PKM) are expressed depending on the developmental stage and tissue. Specific isoenzymes are present in the liver (L type), muscles, or brain (M1), spleen, leukocytes and thrombocytes (M2), or erythrocytes (R) (Beutler 1983, Miwa and Fujii 1985). A deficiency of the R-PK isoenzyme leads to energy deprivation within the red blood cells (RBCs), resulting in a shortened survival time (haemolysis).
In humans, PK deficiency is one of the most common hereditary forms of haemolytic anaemia caused by an enzymopathy (Paglia 1995). Until now, more than 180 different mutations have been identified (Pissard et al 2006). PK deficiency is transmitted as an autosomal recessive trait. PK deficiency has also been described in several canine breeds such as Basenji (Tasker et al 1969, Giger and Noble 1991), West Highland White Terrier (Chapman and Giger 1990, Skelly et al 1999), Beagle (Giger et al 1991), Cairn Terrier, Eskimo Toy dog, Miniature Poodle, Chihuahua, Pug (Giger 2000a), and Dachshound (Kohn et al 1999). As measurement of the enzyme activity is diagnostically not useful in dogs, a polymerase chain reaction (PCR)-based PK test was developed for certain breeds (Whitney et al 1994, Skelly et al 1999, Giger 2000b).
In 1992, the first case of feline PK deficiency was described in the United States in an Abyssinian male cat (Ford et al 1992). Since then, the disease was identified in other Abyssinians, Somalis, and a few domestic shorthair cats in the United States, Australia, and Europe (Giger 2000a, Kohn et al 2005, Mansfield and Clark 2005, van Geffen et al 2005, Harvey et al 2007). Erythrocytic PK activity was severely decreased in affected cats. Asymptomatic carriers expressed approximately half normal PK activity (Ford et al 1992, Giger et al 1997). Several years ago, the molecular defect was identified as a splicing defect at the 3′-end of exon-6 causing a 13-base pair deletion. Thus, a molecular screening test for PK deficiency in Abyssinian and Somali cats could be developed (Giger 2001).
As only few clinical data have been published until now (Ford et al 1992, Giger 2001, Kohn et al 2005, Mansfield and Clark 2005, Harvey et al 2007), the objective of this study was to evaluate clinical signs, laboratory parameters, and the course of disease in PK-deficient cats.
Materials and methods
During a time period of 4.5 years (May 2002–October 2006), PK DNA test results and pedigrees of 263 Somali and 239 Abyssinian cats (328 from Germany, 174 from other European countries) were collected with the assistance from breeders and owners. The PK PCR testing was performed at the Josephine Deubler Genetic Disease Testing Laboratory, University of Pennsylvania, United States, and since 2006 at Laboklin, Bad Kissingen, Germany. Out of these 502 cats, 37 were homozygous affected (7.4%), and 117 were heterozygous carriers (23.3%). The owners and the veterinarians of 25 of 37 homozygous affected cats could be contacted, and information on the origin of the cat, the breeding history, clinical signs, laboratory results, treatment, and course of disease was collected. Twenty-five cats included 12 Abyssinians (four male/male-neutered and eight female/female-spayed) and 13 Somalis (five male/male-neutered and eight female/female-spayed). From all these 25 cats certificates of the PK test results were available. Owners were encouraged to send ethylenediamine tetra acetic acid (EDTA)-anticoagulated blood and heparin plasma to the Clinic for Small Animals of the Free University (FU) of Berlin.
A complete blood count (CBC) (Cell-Dyn 3500, Abbott Diagnostika, Wiesbaden, Germany), a manual counting of aggregated reticulocytes and a manual differential cell count were performed employing standard methods. In several cats the mean osmotic fragility (OF) of erythrocytes was determined. The EDTA-anticoagulated blood samples were sent overnight together with a sample from a control cat. All samples were tested within 24–36 h after blood collection. The OF test measures the stability of RBC in sodium chloride solutions ranging from 0.85 to 0%. Mean OF was determined from the lysis curve as the concentration of sodium chloride, at which 50% of the RBCs were haemolysed (Beutler 1990, Kohn et al 2000). Plasma biochemistry was performed employing standard methods (Cobas Mira Plus, Fa. Roche Diagnostica, Grenzach-Wyhlen, Germany and Konelab 30i, Thermo Electron Corporation, Dreieich, Germany).
The values of median as well as minimum and maximum were calculated for different parameters. Statistical analyses were performed with computer software (SPSS 12, SPSS GmbH Software, Munich, Germany).
Results
Patient evaluation
The course of disease of 25 PK-deficient cats could be followed over a time period of 0.8–11.3 years (median 4.3). In 11 cats, the owners did not notice any signs of disease up to an age of 0.8–7.8 years (median 4.4). Fourteen cats started displaying various clinical signs at ages ranging from 0.1 to 5 years (median 1.7). These included lethargy (n=10), diarrhoea (seven), pale mucous membranes (six), inappetence (six), poor coat quality (six), weight loss (four), icterus (four), and pica (two) ( Table 1). Four owners detected a relationship between stressful situations (eg, exhibitions two, high temperatures one, and stress in general one) and the appearance of clinical signs. One cat not displaying clinical signs until then died after parturition.
Table 1.
Course of disease of 14 cats with PK deficiency showing clinical signs
| Cat | Age of onset of signs (years) | Clinical signs | Laboratory abnormalities | Treatments | Course of disease |
|---|---|---|---|---|---|
| 1 | 1.5 | Lethargy, pale mucous membranes, inappetence, poor coat quality, weight loss | Hct ↓ | Prednisolone | Alive after 4.2 years |
| 2 | 4.3 | Lethargy, inappetence, diarrhoea, poor coat quality | Hct ↓, WBC ↑, bili ↑, liver enzymes ↑, prot ↑, glob ↑ | Prednisolone, vitamin K, antibiotics, fenbendazole | Euthanased after 1 month |
| 3 | 4 | Lethargy, pale mucous membranes, inappetence, icterus | Hct ↓, bili ↑, liver enzymes ↑, prot ↑, glob ↑ | Prednisolone, antibiotics | Died after 7.3 years |
| 4 | 3.3 | Lethargy, pale mucous membranes, diarrhoea, poor coat quality, pica | Hct ↓, bili ↑, liver enzymes ↑, prot ↑, glob ↑ | Prednisolone | Died after 3.2 years |
| 5 | 1.8 | Lethargy, inappetence, diarrhoea, icterus, poor coat quality, pica | Hct ↓, bili ↑, prot ↑, glob ↑ | Prednisolone | Alive after 3 years |
| 6 | 0.1 | Diarrhoea | Hct ↓, prot ↑, glob ↑ | Antibiotics | Alive after 4.1 years |
| 7 | 1.5 | Lethargy, pale mucous membranes, inappetence, weight loss | Hct ↓, bili ↑, liver enzymes ↑ | Antibiotics, fenbendazole, interferon | Died after 1.2 years |
| 8 | 5 | Diarrhoea | Hct ↓ | No | Alive after 7.5 years |
| 9 | 2.5 | Lethargy, diarrhoea | No | No | Aive after 2.8 years |
| 10 | 0.5 | Lethargy, diarrhoea, pale mucous membranes | WBC ↑ | No | Euthanased after 9 months |
| 11 | 1.3 | Lethargy, pale mucous membranes, inappetence, icterus | Hct ↓, prot ↑, glob ↑ | No | Died after 2.5 years |
| 12 | 0.1 | Poor coat quality, weight loss | Not available | No | Alive after 9 months |
| 13 | 0.1 | Poor coat quality, weight loss | Not available | No | Alive after 9 months |
| 14 | 4.5 | Lethargy, icterus | Not available | No information | Alive after 9 months |
Hct=haematocrit, WBC=white blood cells, bili=plasma bilirubin, prot=plasma protein, glob=plasma globulin, ↑=increased, and ↓=decreased.
Laboratory results
A haematological examination was performed for 16 of 25 PK-deficient cats at the Clinic for Small Animals, FU Berlin. For four cats, only data from laboratory examinations performed by the private veterinarians were available ( Table 2).
Table 2.
CBC results for 20 cats with PK deficiency at initial examination
| Parameter | Range | Median | Reference range * | Abnormal values | |
|---|---|---|---|---|---|
| n | % | ||||
| Haematocrit (l/l) | 0.13–0.44 | 0.29 | 0.30–0.49 | 11/20 | 55 |
| Haemoglobin (g/l) | 40–160 | 101.5 | 105–161 | 11/20 | 55 |
| Erythrocytes (×1012/l) | 2.2–11.4 | 5.5 | 7–12 | 15/20 | 75 |
| MCV (fl) | 41–71 | 53 | 36–47 | 14/20 | 70 |
| MCHC (mmol/l) | 17–32 | 20 | 19–22 | 5/20 | 25 |
| Abs aggr retic (×109/l) | 5–936 | 98 | <40 | 16/18 | 89 |
| Leukocytes (×109/l) | 3.6–32.1 | 13.5 | 4.0–16.1 | 7/20 | 35 |
| Bands (×109/l) | 0–0.5 | 0 | 0–5 | 0/11 | 0 |
| Segmented (×109/l) | 3.8–9.2 | 6.2 | 3–9 | 1/11 | 9 |
| Eosinophils (×109/l) | 0.2–0.9 | 0.6 | 0.04–0.6 | 5/11 | 45 |
| Lymphocytes (×109/l) | 0.4–9.6 | 4.2 | 1–4 | 7/11 | 64 |
| Monocytes (×109/l) | 0–0.8 | 0.2 | 0.04–0.5 | 1/11 | 9 |
Abs aggr retic=absolute aggregated reticulocyte counts, MCV=mean corpuscular volume, MCHC=mean corpuscular haemoglobin concentration,
reference range of the Clinic for Small Animals, FU of Berlin.
At initial examination, the CBC revealed a mild to severe anaemia with haematocrit (Hct) values of 0.13–0.29 l/l (median 0.25) in 11 of 20 cats. The age of these cats ranged from 0.9 to 7.6 years (median 1.9). For 13 cats, 1–9 (median 3) follow-up examinations were performed over a time period of 0.2–7.3 years (median 1.5). In three of these 13 cats the Hct was below the reference range at each measurement (median values for each cat: 0.11, 0.21, and 0.28 l/l); seven other cats had periods with normal Hct values and periods with Hct values between 0.14 and 0.29 l/l. Another three cats did not display anaemia at any time and had a stable Hct over a time period of 1, 2.9, and 3.8 years ( Fig 1). Overall, 14 cats suffered from anaemia at least once during the study period; nine of these cats showed various clinical signs according to the owners and five cats displayed no clinical signs. Of the six cats with normal Hct values, four cats had no clinical signs. One cat displayed intermittent diarrhoea and lethargy and another cat was euthanased at an age of 1.3 years after having shown lethargy, pale mucous membranes, and severe intermittent diarrhoea from an age of 6 months onwards. However, for each of these two symptomatic cats only one CBC was performed.
Fig 1.
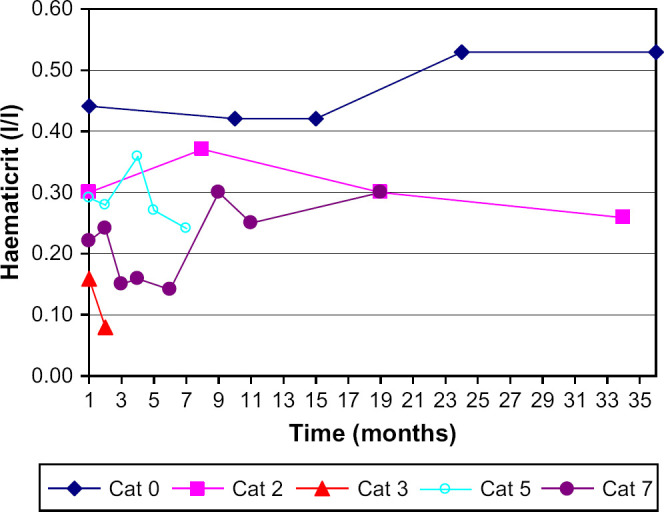
Time course of the Hct in five cats with PK deficiency; cat 0 showed no clinical signs over a time period of 35 months and had a stable Hct.
The absolute number of aggregated reticulocyte counts was increased in 16 of 18 cats (43–936×109/l, median 102), while two cats had values of <40×109/l. One of these two cats displayed a slightly increased number of aggregated reticulocytes (51×109/l) at a follow-up examination. Only eight of the 17 cats with reticulocytosis (55–352×109/l, median 91) were mildly anaemic and had Hct values ranging from 0.22 to 0.29 l/l (median 0.28). The other nine cats with aggregated reticulocyte counts ranging from 43 to 936×109/l (median 195) had Hct values in the reference range.
Six of 20 cats had a leukocytosis (16.6–32.1×109/l,median 20.8). Increased numbers of leukocytes (17.3×109/l and 19.5×109/l) were measured in another two cats 0.9 and 1.5 years after the first examination. In 11 cats, the leukocyte counts were within the reference range at 1–6 measurements (median 2). One cat had leukopenia (3.6×109/l) initially, the white blood cell (WBC) count was in the reference range at three follow-up examinations.
A differential blood cell count was performed for 11 cats. Six cats displayed mild to severe lymphocytosis with values of 4.2–9.6×109/l (median 6.7). One cat had a lymphopenia (0.4×109/l). In five cats, eosinophilia (0.7–0.9×109/l, median 0.8) was detected. One cat displayed monocytosis (0.8×109/l) at initial examination.
The mean OF of RBC of 13 PK-deficient cats ranged from 0.50 to 0.68% (median 0.58); the value was within the reference range (0.50–0.59%) of our laboratory in 10 cats. The mean OF was mildly elevated in two (0.60 and 0.61%) and moderately elevated in another cat (0.68%). Results of plasma biochemistry were available for 15 cats ( Table 3). In 12 cats, the results of 1–4 (median 2) follow-up examinations were evaluated.
Table 3.
Serum biochemistry results for 15 cats with PK deficiency at initial examination.
| Parameter | Range | Median | Reference range | Abnormal values | |
|---|---|---|---|---|---|
| n | % | ||||
| Protein (g/l) | 59–95 | 74 | 60–75 | 5/15 | 33 |
| Albumin (g/l) | 27–50 | 32 | 25–40 | 1/15 | 7 |
| Globulin (g/l) | 28–62 | 42 | <40 | 8/15 | 53 |
| ALT (U/l) | 10–395 | 46 | <86 | 1/15 | 7 |
| AST (U/l) | 5–77 | 21 | <35 | 2/15 | 13 |
| GLDH (U/l) | 0–33 | 3 | <15 | 2/15 | 13 |
| AP (U/l) | 6–241 | 46 | <76 | 4/15 | 20 |
| Bilirubin (μmol/l) | 2.6–444.7 | 3.9 | <5.1 | 5/15 | 33 |
ALT=alanine aminotransferase, AST=aspartate aminotransferase, GLDH=glutamate dehydrogenase, AP=alkaline phosphatase.
Hyperglobulinaemia (43–62 g/l, median 46) was present in eight cats at initial examination. Another four cats developed an increase in plasma globulins within 0.8–1.8 years. Five cats displayed mild to severe hyperbilirubinaemia (5.1, 8.7, 26, 37, and 445 μmol/l) and another three cats developed hyperbilirubinaemia; at follow-up examinations after 0.1, 0.9, and 1 year the bilirubin values were 5.4, 6.7, and 26 μmol/l, respectively. Five of the eight cats with hyperbilirubinaemia were anaemic (0.13–0.29 l/l). In two of the three non-anaemic cats with increased bilirubin values, the aggregated reticulocyte counts were increased (291 and 452×109/l). In three cats, the bilirubin concentrations were within the reference range (2.6, 3.0, and 3.6 μmol/l) despite suffering from anaemia (Hct values 0.19, 0.26, and 0.29 l/l). One of 15 cats had a moderately increased alanine aminotransferase (ALT) value (395 U/l). Two other cats had mildly increased ALT values (90 and 113 U/l) 0.6 and 2.8 years after first examination. Mildly increased aspartate aminotransferase (AST) values were present in two of 15 cats (2×77 U/l); two other cats showed a mild increase after 0.4 and 1.3 years (37 and 39 U/l). In four cats alkaline phosphatase (AP) was increased at initial examination (78, 105, 108, and 241 U/l). Altogether seven of 15 cats had increased values of one or more liver enzymes at least once.
Therapy and course of disease
Information on the course of disease in 14 cats with clinical signs is given in Table 1. Seven of 14 cats with clinical signs were treated from different veterinarians (cats 1–7). The Hct values of four of these 14 cats during the course of disease are shown in Fig 1.
Eight of 25 affected cats died (five) or were euthanased (three) at ages ranging from 1.3 to 11.3 years (median 3.6). At least in six of these cats the death at an age of 1.3–11.3 years (median 4.1) was presumably a consequence of the PK deficiency. One cat died a few weeks after parturition without clinical signs and one cat was euthanased because of cardiac disease and aortic thromboembolism.
Breeding history
Information on the frequency of breeding could be collected for 25 of 37 PK-deficient cats (15 of them females and 10 males). Eleven of the female cats had littered between the ages of 1.8 and 7.5 years (median 4.4) at least once; four other female cats were not used for breeding. Five of 10 PK-deficient male cats were used for breeding between the age of 4.4 and 11.3 years (median 4.8) at least once.
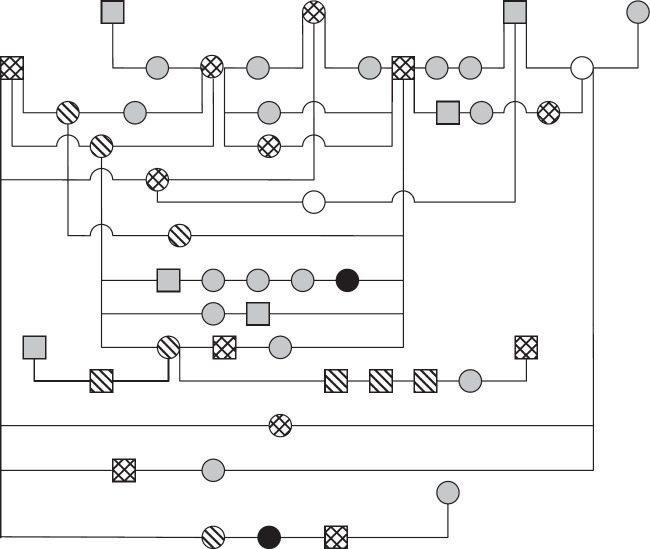
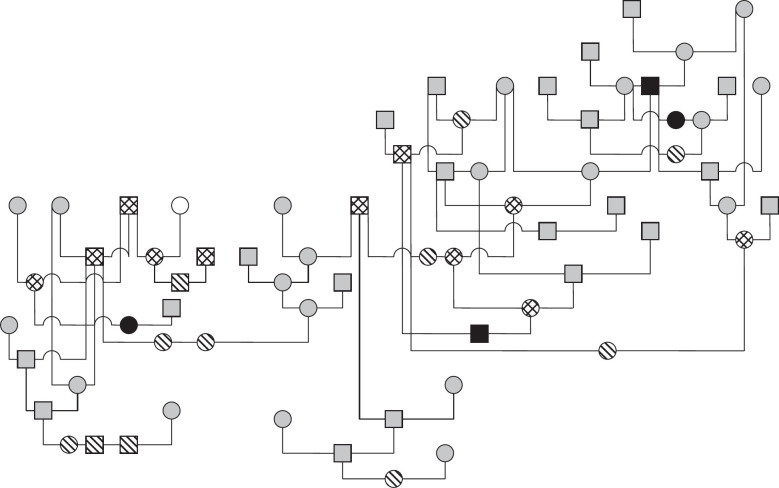
The pedigrees of four generations of 35 of 37 affected cats were available for evaluation. A common ancestor could not be determined. Fifteen affected cats were closely related to each other ( Fig 2). Of the remaining 20 PK-deficient Somali and Abyssinian cats, 15 had common ancestors (Fig 3).
Fig 2.
Pedigree of 11 Abyssinian cats with PK deficiency.  : PK deficiency, alive; ▄: PK deficiency, dead;
: PK deficiency, alive; ▄: PK deficiency, dead;  : PK carrier; □: PK normal;
: PK carrier; □: PK normal;  : no information; □: male; ○: female.
: no information; □: male; ○: female.
Fig 3.
Pedigree of 15 cats with PK deficiency (14 Somalis and one Abyssinian).  : PK deficiency, alive; ▄: PK deficiency, dead;
: PK deficiency, alive; ▄: PK deficiency, dead;  : PK carrier; ○: PK normal;
: PK carrier; ○: PK normal;  : no information; □: male; ○: female.
: no information; □: male; ○: female.
Discussion
For 25 Abyssinian and Somali cats suffering from PK deficiency information on the course of disease over a time period of 0.8–11.3 years (median 4.3) could be obtained. In accordance with previous reports PK deficiency can be asymptomatic in cats, can cause a chronic anaemia, or haemolytic crises can occur intermittently (Giger et al 1997, 2000b). Only in 14 of 25 affected cats did the owners notice signs of disease, usually starting at an age of less than 3 years. In four out of five Abyssinian and Somali cats suffering from PK deficiency (age 2–10 years), which were examined at the School of Veterinary Medicine, University of Pennsylvania, signs comparable to our study, such as lethargy (n=3), pale mucous membranes (two), pica (two), and occasional vomitus (one), were described. In a 10-year-old female cat used for breeding, the anaemia was diagnosed at a routine health check (Kohn 2001). A case study from Australia (Mansfield and Clark 2005) reported on a 7-year-old male Somali cat, which was presented due to icterus and polydipsia. The cat was healthy according to the owner until then. In another case study a 2-year-old Somali cat was referred because of icterus and vomiting 1 year after diagnosis of PK deficiency (Harvey et al 2007). It is uncertain what might trigger haemolytic crises in affected cats. Stressful situations, such as infectious diseases, parturition or exhibitions, might cause a sudden deterioration of the clinical picture by non-specific activation of the macrophage system, damage of erythrocytic membranes, or suppression of haematopoiesis. The authors of the case study from Australia suspected an infection with Mycoplasma haemominutum and a cholangiohepatitis as the triggering factors for the haemolytic crisis.
As PK deficiency can be asymptomatic in cats, at least 16 cats were used for breeding before establishing a diagnosis thus spreading the mutant allele. Testing of Abyssinian and Somali cats used for breeding is, therefore, strongly recommended.
Even though erythrocytic PK deficiency results in an energy deprivation and thus a shortened survival time of RBC, some cats seem to be able to compensate the accelerated breakdown. In this study 17 of 18 cats displayed mild to severe reticulocytosis. Despite these increased aggregated reticulocyte counts, nine cats were not anaemic, which indicates an increased and efficient turnover of RBC.
For differential diagnoses of haemolytic anaemia in cats, infectious diseases (eg, haemoplasmosis and feline leukaemia virus) or immune-mediated haemolysis have to be considered. Haemolytic anaemia caused by PK deficiency is often incorrectly interpreted as immune-mediated haemolysis. The direct Coombs' test, however, was negative for several cats suffering from PK deficiency (Kohn, unpublished). Moreover, haemolysis can be caused by toxins or chemicals or mechanical damage of the RBC (Christopher 2000, Giger 2005, Kohn et al 2006). Increased OF of RBC is an important differential diagnosis for PK deficiency, as this disease has been described in Abyssinian and Somali cats and has a similar clinical picture. As both diseases are hereditary, clinical signs caused by chronic haemolytic anaemia or by haemolytic crises may be noted at a young age (Kohn et al 2000). Thusfar, increased OF of RBC has only been diagnosed in the United States and in one cat from Switzerland (Kohn, unpublished). A hereditary membrane defect has been suggested as a possible cause. In contrast to cats with increased OF of RBC, the mean erythrocytic OF was within the reference range in most cats suffering from PK deficiency examined here. Therefore, the OF test can be useful to differentiate between these two diseases and may also serve to distinguish PK deficiency from immune-mediated haemolysis or haemoplasmosis (Kohn et al 2006).
Only few data on plasma biochemistry values of cats suffering from PK deficiency are available in the literature. In four of 15 cats examined here, plasma bilirubin was increased moderately to severely. It was increased slightly in another four cats. Similarly, in two of three American cats with PK deficiency, serum concentrations of bilirubin were only mildly increased, indicating an efficient compensation of the accelerated breakdown of RBC (Kohn 2001). The Australian cat displayed severe hyperbilirubinaemia and a cholangitis was diagnosed (Mansfield and Clark 2005). Moreover, bilirubin cholelithiasis and the possibility of an extrahepatic bile duct obstruction, which is a frequent complication of chronic haemolysis in man (Wantanabe et al 2002), should be investigated if PK-deficient cats present with jaundice (van Geffen et al 2005, Harvey et al 2007).
Hyperglobulinaemia was present in 12 of 15 cats of our study. Increased globulin concentrations can also be found in cats suffering from increased OF of RBC (Kohn et al 2000) or immune-mediated haemolytic anaemia (Kohn et al 2006). Hyperglobulinaemia can indicate a chronic stimulation of the immune system, but was not described in humans and dogs with PK deficiency.
Six of 15 cats included in this study displayed mildly, one cat moderately increased activities of liver enzymes. Hypoxia in the liver caused by severe anaemia or a circulatory failure can damage hepatocytes. One of seven cats of our study displaying increased activity of liver enzymes suffered from severe anaemia (0.09 l/l). Increased values of liver enzymes have also been described in cats with immune-mediated haemolytic anaemia or increased OF of RBC (Kohn et al 2000, 2006). Moreover, chronic haemolysis is capable of causing significant iron overload. Liver iron concentration over a critical threshold leads to the formation of radicals. Increased redox-active iron present in haemeproteins and cytosolic iron pool catalyses oxidative damage to lipids, proteins, and nucleic acids. Iron-catalysed injury results in damage to cell constituents, including mitochondria, lysosomes, and the sarcolemmal membrane (Schaer et al 1992, Hilgard and Gerken 2005). Thus, chronic haemolysis can lead to hepatocellular damage and finally cirrhosis of the liver (Hilgard and Gerken 2005). This has been described in humans and dogs with PK deficiency (Weiden et al 1981, Schaer et al 1992, Harvey 2006). In the Australian case report the liver showed histologically extramedullary haematopoiesis and moderate bile duct proliferation, with portal areas showing mild lymphocytic accumulations (Mansfield and Clark 2005).
Mild to moderate splenomegaly has been described in cats suffering from PK deficiency (Ford et al 1992, Giger 2000a, Kohn 2001, Mansfield and Clark 2005). Histopathological examination of the spleen of two affected Abyssinian cats showed distinct extramedullary haematopoiesis and haemosiderosis caused by chronic haemolysis (Kohn 2001).
In this study, at least six of 25 PK-deficient cats died or were euthanased presumably as a consequence of PK deficiency at ages between 1.3 and 11.3 years. PK-deficient dogs died at similar ages ranging from 1 to 9 years due to anaemia or liver failure caused by iron overload (Schaer et al 1992, Giger 2000b).
Therapeutic options are limited in cases of PK deficiency (Giger 2000b, 2001). Experimentally, bone marrow transplantation has been described in dogs (Weiden et al 1981). In affected cats, splenectomy can be performed in cases of recurrent haemolytic crises or severe splenic enlargement, which can restrict the expansion of the stomach and cause inappetence (Ford et al 1992, Giger 2000a). The objective of a splenectomy is to remove one major site of RBC phagocytosis. In one Abyssinian cat the Hct stabilised after splenectomy. This cat survived at least 10 years (Ford et al 1992, Giger 2001). A stabilisation of the Hct and improvement of the general condition were noted in two other cats after splenectomy (Kohn 2001). Splenectomy is also a therapeutic option in humans suffering from severe transfusion-dependant PK deficiency, hereditary spherocytosis or autoimmune-mediated haemolytic anaemia (Paglia 1995). However, Basenji dogs with PK deficiency did not improve clinically after removal of the spleen (Giger and Noble 1991).
As PK deficiency is often misinterpreted as immune-mediated haemolysis or haemoplasmosis, the cats are frequently treated with prednisolone or antibiotics, as was the case in this study. Positive effects of glucocorticoids might be due to a membrane stabilising effect and due to inhibition of the mononuclear phagocytic system, thus preventing or delaying phagocytosis of damaged RBC. A transient or partial response was found in some cats of this study, however, the effect was difficult to judge due to the waxing and waning course of PK deficiency.
To prevent haemolytic crises, stressful situations should be avoided for PK-deficient cats. Blood transfusions can represent a life saving measure during severe haemolytic crises if the Hct values decrease below 0.10–0.15 l/l (Weingart et al 2004). However, none of the cats included in this study had received a blood transfusion.
Acknowledgements
Foremost, we are grateful to the numerous committed breeders of Abyssinian and Somali cats for their support regarding this study. We thank Laboklin for access to data of PK-deficient cats. We also thank Prof Dr U Giger for thoughtful discussions with regard to interpreting the data.
References
- Beutler E. Hereditary nonspherocytic hemolytic anemia, pyruvate kinase deficiency and other abnormalities. Williams J.W., Beutler E., Erslev A.J., Lichtman M.A. Hematology, 1983, McGraw-Hill Publishing Company: New York, 574–582. [Google Scholar]
- Beutler E. Osmotic fragility. Williams J.W., Beutler E., Erslev A.J., Lichtman M.A. Hematology, 1990, McGraw-Hill Publishing Company: New York, 1726–1728. [Google Scholar]
- Chapman R.L., Giger U. Inherited erythrocyte pyruvate kinase deficiency in the West Highland White Terrier, Journal of Small Animal Practice 31, 1990, 610–616. [Google Scholar]
- Christopher M.M. Disorders of feline red blood cells. Bonagura J.D. Kirk's Current Veterinary Therapy XIII, 2000, WB Saunders: Philadelphia, 421–424. [Google Scholar]
- Ford S., Giger U., Duesberg C., Beutler E., Wang P. Inherited erythrocyte pyruvate kinase (PK) deficiency causing hemolytic anemia in an Abyssinian cat, Journal of Veterinary Internal Medicine 6, 1992, 123. [Google Scholar]
- Giger U., Mason G.D., Wang P. Inherited erythrocyte pyruvate kinase deficiency in a Beagle, Veterinary Clinical Pathology 20, 1991, 83–86. [DOI] [PubMed] [Google Scholar]
- Giger U., Noble N.A. Determination of erythrocyte pyruvate kinase deficiency in Basenjis with chronic hemolytic anemia, Journal of the American Veterinary Medical Association 198 (10), 1991, 1755–1761. [PubMed] [Google Scholar]
- Giger U., Rajpurohit Y., Wang P., Ford S., Kohn B., Patterson D.F., Beutler E., Henthorn P.S. Molecular basis of erythrocyte pyruvate kinase (R-PK) deficiency in cats, Blood 90 (suppl), 1997, 5b. [Google Scholar]
- Giger U. Hereditary erythrocyte disorders. Bonagura J.D. Kirk's Current Veterinary Therapy XIII, 2000a, WB Saunders: Philadelphia, 414–420. [Google Scholar]
- Giger U. Erythrocyte phosphofructokinase and pyruvate kinase deficiencies. Feldman B.F., Zinkl J.G., Jain N.C. Schalm's Veterinary Hematology, 2000b, Lippincott Williams & Wilkins: Baltimore, 1020–1025. [Google Scholar]
- Giger U. Hereditary erythrocyte disorders. August J.R. Consultations in Feline Internal Medicine 4, 2001, WB Saunders: Philadelphia, 484–489. [Google Scholar]
- Giger U. Regenerative anemias caused by blood loss or hemolysis. Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine, 6th edn, 2005, Elsevier Saunders: St. Louis, 1886–1907. [Google Scholar]
- Harvey J.W. Pathogenesis, laboratory diagnosis, and clinical implications of erythrocyte enzyme deficiencies in dogs, cats, and horses, Veterinary Clinical Pathology 35 (2), 2006, 144–156. [DOI] [PubMed] [Google Scholar]
- Harvey A.M., Holt P.E., Barr F.J., Rizzo F., Tasker S. Treatment and long-term follow-up of extrahepatic biliary obstruction with bilirubin cholelithiasis in a Somali cat with pyruvate kinase deficiency, Journal of Feline Medicine and Surgery 9 (5), 2007, 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgard P., Gerken G. Liver cirrhosis as a consequence of iron overload caused by hereditary nonsperocytic hemolytic anemia, World Journal of Gastroenterology 11 (8), 2005, 1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn B., Freistedt R., Pekrun A., Wang P., Giger U. Chronische hämolytische Anämie und Osteosklerose aufgrund einer Erythrozyten-Pyruvatkinase-Defizienz bei einem Langhaardackel, Kleintierpraxis 44 (6), 1999, 437–445. [Google Scholar]
- Kohn B., Goldschmidt M.H., Hohenhaus A.E., Giger U. Anemia, splenomegaly, and increased osmotic fragility of erythrocytes in Abyssinian and Somali cats, Journal of the American Veterinary Medical Association 217 (10), 2000, 1483–1491. [DOI] [PubMed] [Google Scholar]
- Kohn B. (2001) Erythrozytenstudien bei gesunden und anämischen Katzen. PhD thesis, Faculty of Veterinary Medicine, Free University of Berlin.
- Kohn B., Fumi C., Seng A., Giger U. Anämie infolge erythrozytären Pyruvatkinase-Mangels und deren Verbreitung bei Somali- und Abessinierkatzen in Deutschland, Kleintierpraxis 50 (Heft 5), 2005, 305–312. [Google Scholar]
- Kohn B., Weingart C., Eckmann V., Ottenjann M., Leibold W. Primary immune-mediated hemolytic anemia in 19 cats: diagnosis, therapy, and outcome (1998–2004), Journal of Veterinary Internal Medicine 20, 2006, 159–166. [DOI] [PubMed] [Google Scholar]
- Mansfield C.S., Clark P. Pyruvate kinase deficiency in a Somali cat in Australia, Australian Veterinary Journal 83 (8), 2005, 483–485. [DOI] [PubMed] [Google Scholar]
- Miwa S., Fujii H. Molecular aspects of erythroenzymopathies associated with hereditary hemolytic anemia, American Journal of Hematology 19, 1985, 293–305. [DOI] [PubMed] [Google Scholar]
- Paglia D.E. Enzymopathies. Hoffmann R., Benz E.J., Shattil S.J., Furie B., Cohen H.J., Silberstein L.E. Hematology. Basic Principles and Practice, 1995, Churchill Livingstone: New York, 656–667. [Google Scholar]
- Pissard S., Max-Audit I., Skopinski L., Vasson A., Vivien P., Bimet C., Goossens M., Galacteros F., Wajcman H. Pyruvate kinase deficiency in France: a 3-year study reveals 27 new mutations, British Journal of Haematology 133 (6), 2006, 683–689. [DOI] [PubMed] [Google Scholar]
- Schaer M., Harvey J.W., Calderwood-Mays M., Giger U. Pyruvate kinase deficiency causing hemolytic anemia with secondary hemochromatosis in a Cairn Terrier, Journal of the American Animal Hospital Association 28, 1992, 233–239. [Google Scholar]
- Skelly A., Wallace M., Rajpurohit Y., Wang P., Giger U. Identification of a 6 base pair insertion in West Highland White Terriers with erythrocyte pyruvate kinase deficiency, American Journal of Veterinary Research 60, 1999, 1169–1172. [PubMed] [Google Scholar]
- Tasker J.B., Severin G.A., Young S., Gillette E.L. Familial anemia in the Basenji dog, Journal of the American Veterinary Medical Association 154, 1969, 158–165. [PubMed] [Google Scholar]
- van Geffen C, Daminet S, Savary-Bataille K. (2005) Pyruvate kinase deficiency and extrahepatic bile duct obstruction with bilirubin cholelithiasis in a Somali cat and PK status in 15 related cats. In: Proceedings of the 48th Annual British Small Animal Veterinary Association Congress, Birmingham, UK. 596.
- Wantanabe Y., Miyauchi K., Horiuchi A., Kikkawa H., Kusunose H., Kotani T., Kawachi K. Concomitant laparoscopic splenectomy and cholecystectomy as an effective and minimally invasive treatment of pyruvate kinase deficiency with gallstones, Surgical Endoscopy 16 (10), 2002, 1495. [DOI] [PubMed] [Google Scholar]
- Weiden P.L., Hackmann R.C., Deeg H.J., Storb R. Long-term survival and reversal of iron overload after marrow transplantation in dogs with congenital hemolytic anemia, Blood 57, 1981, 66–70. [PubMed] [Google Scholar]
- Weingart C., Giger U., Kohn B. Clinical experience with whole blood transfusions in 91 cats, Journal of Feline Medicine and Surgery 6, 2004, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney K.M., Goodman S.A., Bailey E.M., Lothrop C.D. The molecular basis of canine pyruvate kinase deficiency, Experimental Hematology 22, 1994, 866–874. [PubMed] [Google Scholar]