Abstract
Background:
Prenatal household air pollution impairs birth weight and increases pneumonia risk however time-varying associations have not been elucidated and may have implications for the timing of public health interventions.
Methods:
The Ghana Randomized Air Pollution and Health Study (GRAPHS) enrolled 1,414 pregnant women from Kintampo, Ghana and measured personal carbon monoxide (CO) exposure four times over pregnancy. Birth weight was measured within 72-hours of birth. Fieldworkers performed weekly pneumonia surveillance and referred sick children to study physicians. The primary pneumonia outcome was one or more physician-diagnosed severe pneumonia episode in the first year of life. We employed reverse distributed lag models to examine time-varying associations between prenatal CO exposure and birth weight and infant pneumonia risk.
Results:
Analyses included n=1,196 mother-infant pairs. In models adjusting for child sex; maternal age, body mass index (BMI), ethnicity and parity at enrollment; household wealth index; number of antenatal visits; and evidence of placental malaria, prenatal CO exposures from 15 to 20 weeks gestation were inversely associated with birth weight. Sex-stratified models identified a similar sensitive window in males and a window at 10-weeks gestation in females. In models adjusting for child sex, maternal age, BMI and ethnicity, household wealth index, gestational age at delivery and average postnatal child CO exposure, CO exposure during 34–39 weeks gestation were positively associated with severe pneumonia risk, especially in females.
Conclusions:
Household air pollution exposures in mid- and late- gestation are associated with lower birth weight and higher pneumonia risk, respectively. These findings support the urgent need for deployment of clean fuel stove interventions beginning in early pregnancy.
Keywords: household air pollution, biomass, prenatal, birth weight, pneumonia, sex-specific effects
1. Background
Worldwide, nearly 2 million deaths are attributed to infants born preterm or with low birth weight(1). Nearly 920,000 deaths are attributed to acute lower respiratory infection (ALRI) in children under five(2), with a disproportionate burden in sub-Saharan Africa(3). Household air pollution (HAP) resulting from the burning of solid fuels on inefficient, traditional cook stoves (4), is a leading risk factor for both reduced birth weight and ALRI risk in children under five(1, 2). Despite this incredible disease burden, stove intervention trials to date, with heterogeneity in stove intervention type and timing of intervention (e.g., prenatal vs early childhood), have largely been unable to demonstrate health benefits. Emerging evidence supports an association between higher prenatal HAP exposure and lower birth weight (5) and higher pneumonia risk(6) suggesting that the prenatal period is a critical window. A better understanding of time-varying associations and sensitive windows of exposure has implications for the timing of public health interventions, such as cleaner fuel stove interventions, and could improve our understanding of underlying mechanistic pathways.
We lack evidence that a stove intervention to reduce HAP can increase birth weight in a susceptible population. Our study, the Ghana Randomized Air Pollution and Health Study (GRAPHS) randomized N=1414 pregnant women to a liquefied petroleum gas (LPG) stove, an improved efficiency biomass stove, or traditional open fire stove (control) and intention-to-treat analyses failed to demonstrate an improvement in birth weight in either intervention arm(7). The Randomized Exposure Study of Pollution Indoors and Respiratory Effects (RESPIRE) study in Guatemala randomized n=534 households to a plancha-type stove with chimney ventilation or open fire (control) for 12 months. A subset set of RESPIRE households included infants for whom birth weight was measured within 48-hours of birth (n=174); no difference in birth weight was observed(8). An ethanol stove intervention in Nigeria in which n=324 pregnant women were randomized to CleanCook ethanol stove or control in a ratio of 1:1 similarly found no improvement in birth weight in the intervention arm as compared to control, although adjusted analyses suggested an effect (9). More recently, the multi-country Household Air Pollution Intervention Network (HAPIN, n=3200) trial reported no improvement in birth weight in the LPG arm as compared to control (10).
However, exposure-response analyses consistently demonstrate an association between higher air pollution and worse birth weight (11). Exposure-response data from HAP pregnancy cohorts are sparse. Analyses from a Tanzanian pregnancy cohort found a 270 gram reduction in birth weight per 1 unit log fine particulate matter (PM2.5) measured once in pregnancy; prenatal carbon monoxide (CO) exposure was not associated with birth weight(12). GRAPHS found a 39-gram lower birth weight and 14% higher odds of low birth weight per 1 part per million (ppm) increase in average prenatal CO exposure(5). Questionnaire-based analyses examining associations between fuel use surveys and birth weight further support these findings (13–15).
Evidence that a stove intervention to reduce HAP can lower ALRI risk is inconsistent (7, 9, 16, 17). GRAPHS failed to demonstrate an improvement in pneumonia risk in either intervention arm (7). The Cooking and Pneumonia Study (CAPS) in Malawi randomized 4339 households to a clean cooking biomass stove arm versus control and found no effect on pneumonia in children under five years(17). However, the RESPIRE study randomized n=534 households to a plancha-type stove with chimney ventilation or open fire (control) for 12 months and found a 33% (95% CI 2, 55) reduction in risk for physician-diagnosed severe pneumonia(16).
Emerging evidence from GRAPHS suggests that HAP exposure may increase pneumonia risk beginning in utero. GRAPHS found a 10% and 15% higher risk for physician-diagnosed pneumonia and severe pneumonia in the first year of life, respectively, per 1ppm higher average prenatal CO(6). RESPIRE found that 1.1 parts per million (ppm) reduction in postnatal personal carbon monoxide (CO) exposure was associated with an 18% (2–30%) and 28% (8–41%) reduction in physician-diagnosed pneumonia and severe pneumonia, respectively(16). To our knowledge, a secondary analysis to understand the influence of prenatal HAP on infant pneumonia within RESPIRE has not been performed. In contrast, the Cooking and Pneumonia Study (CAPS) found no evidence of an exposure-response relationship between childhood HAP and pneumonia risk (18).
Fetal development progresses across gestation and we hypothesize that sensitive windows may exist that are critical for programming future birth weight and pneumonia risk. These sensitive windows represent critical periods of development during which an exposure may modify the structure or function of developing organ systems and predispose the individual to future disease risk. For example, Hansen et al found that exposure to ambient air pollution in early pregnancy was associated with reduced abdominal circumference in mid-gestation(19). Given that the fetus accumulates most body fat during the second half of pregnancy, air pollution-associated decrease in abdominal circumference may reflect impaired development of abdominal organs such as the liver during early pregnancy and, ultimately, result in low birth weight with its attendant effects on childhood mortality, growth and increased risk of chronic disease later in life(1, 19–21).
Similarly, lung development occurs progressively across gestation starting with the pseudoglandular phase in early gestation. The canalicular phase begins in mid-gestation (17–26 weeks) during which development of the airway branching pattern is completed and gas-exchange regions begin to develop. During this period, the respiratory bronchioles begin to appear and type II pneumocytes differentiate. In late gestation (24–36 weeks), the connective tissue between airspaces becomes thinner with further surfactant system maturation and alveoli formation(22, 23). Exposure to household air pollution during these critical phases may disrupt lung development resulting in worse postnatal lung function and higher risk of respiratory infections in childhood.
We hypothesize that deployment of a stove intervention prior to a critical window may be required to observe health benefits. Indeed, HAPIN subgroup analyses suggested that the LPG intervention deployment in earlier gestation (<18-weeks) may be associated with an improvement in birth weight (10). An understanding of time-varying exposure-response relationships between prenatal HAP exposure and birth weight and pneumonia risk to identify sensitive windows of exposure will inform public health interventions.
Herein we leveraged the GRAPHS cohort with maternal repeated, personal CO exposure measurements over pregnancy to examine temporal associations between prenatal HAP exposure and birth weight and physician-diagnosed pneumonia in the first year of life, considered separately. We employed distributed lag models to identify sensitive windows of exposure between prenatal HAP exposure and birth weight and pneumonia risk in the first year of life, considered separately. Effect modification by child sex was also explored.
2. Methods
GRAPHS was a cluster-randomized controlled cookstove intervention trial in communities from the Kintampo North Municipality and Kintampo South District of Ghana which has been described in detail elsewhere (7, 24, 25). Briefly, pregnant women residing in study communities were eligible for study inclusion if they were the primary household cook, pregnant with a live singleton fetus at gestational age ≤24 weeks and non-smokers. Gestational age at enrollment was determined by ultrasound and estimated date of delivery calculated (26). Following a baseline CO exposure assessment, n=1414 pregnant women were randomized to the LPG stove, improved biomass stove, or traditional, open-fire stove (control) arms. Stove use compliance was 87% in the LPG arm and 69% in the improved biomass arm (7). Procedures were approved by human studies and institutional ethics committees at Kintampo Health Research Centre (KHRC, IRB0004854), the Columbia University Mailman School of Public Health (IRB AAAR4373) and the Icahn School of Medicine at Mount Sinai (IRB HSM-14–00572) and written consent was obtained by all pregnant women. Participants included in these analyses had at least one valid prenatal CO measurement, valid birth weight and at least one fieldworker pneumonia assessment in infancy.
2.1. Prenatal CO Exposures
As described elsewhere(27), we measured 72-hour personal CO exposures (Lascar EL-USB-CO sensor, Lascar Electronics, PA, USA) in pregnant women at four time points. Pregnant women performed personal CO measurements once upon enrollment prior to cookstove intervention deployment, once three weeks after study arm assignment and twice evenly spaced over the remaining antenatal period (27). Participants were instructed to wear the monitors except while asleep or bathing, during which times they were to keep the monitors nearby and off the floor. The CO monitors were housed in a protective plastic case and secured to clothing at the level of the participant’s breathing zone. The gestational age at each prenatal exposure measurement was determined by the ultrasound-confirmed estimated date of delivery and the date of exposure assessment.
The Lascar monitors measured CO in parts per million (ppm) every 10 seconds over a 72-hour period. As previously described, quality assurance/quality control measures included exposing all monitors to certified span gas (50 ppm CO in zero air) at a central laboratory every six weeks, reviewing deployment run time and visual inspection of each deployment per protocol. Data used in these analyses passed all QA/QC checks. Each 72-hour deployment was truncated to the first 48-hours to avoid cases where field pick-up schedules and battery issues may have resulted in missing data for a cooking event and then CO measurements over that 48-hour period were averaged and centered. Forty-eight-hour data completeness was >90% (27).
2.2. Birth Weight
Birth weight was recorded within 72 hours of delivery by community-based field workers. As previously described, birth weight was measured to the nearest 0.1 kg (Tanita BD 585 digital baby scale, Tokyo, Japan) (5, 7). Any birth weight not measured within 72 hours of birth was considered missing.
2.3. Physician-assessed pneumonia and severe-pneumonia
As previously described, GRAPHS pneumonia surveillance protocol included both weekly community-based fieldworker surveillance with self-referrals(6). Community-based fieldworkers were trained in the WHO Integrated Management of Childhood Illness (IMCI) guidelines and made at least weekly visits to each study household over the index child’s first year of life. Any child who was deemed unwell by fieldworker or parent was referred to a central clinic for evaluation by a study physician. Study physicians diagnosed pneumonia and severe pneumonia following WHO IMCI guidelines without chest radiograph or ultrasound as these were not widely available at the time of the study. Pneumonia is defined by the IMCI as cough or difficulty breathing plus an elevated respiratory rate (60 breaths/min in children aged under two months or 50 breaths/min in children aged two to 12 months). Any child diagnosed with pneumonia under age two months or who additionally had hypoxemia, defined as pulse oximetry oxygen saturation less than 90%, or stridor, chest wall in-drawing, or any danger sign (convulsions, inability to drink or breastfeed, vomiting, lethargy, or unconscious) was diagnosed as having severe pneumonia. The primary pneumonia outcome was any physician-diagnosed severe pneumonia episode in the first year of life (yes/no). The secondary pneumonia outcome was any physician-diagnosed pneumonia episode (yes/no).
2.4. Covariates
Maternal age, self-reported ethnicity, parity and wealth index (28) a measure of socioeconomic status relative to other study participants, were determined by questionnaire at enrollment. Parity was collapsed to include 0, 1, 2, 3 or ≥4 prior pregnancies. Maternal height and weight were measured at enrollment and body mass index calculated. The number of antenatal visits over gestation was abstracted from maternal antenatal care records and dichotomized around the study median (≥4 versus <4). Gestational age at delivery was determined using ultrasound dating. Child biological sex was reported at birth. As previously reported, placenta samples were obtained at delivery and underwent histopathological analyses for evidence of prenatal malaria infection(5). Infant postnatal CO exposure measurements following the same procedures as above were assessed at infant age one, three, and nine months of life. As previously reported, a composite measure of average exposure over the first year of life was developed using a linear interpolation approach (5, 25, 29).
2.5. Statistical Analysis
Each pregnant woman had up to four valid CO measurements. We first examined the distribution of gestational age at each prenatal CO measurement to determine the range of available gestational age of prenatal exposure assessments (Supplemental Figure 1).
We employed an extension of the distributed lag model (DLM) to identify sensitive windows for birth weight, physician-diagnosed severe pneumonia and physician-diagnosed pneumonia, considered independently (30, 31). DLMs estimate time-varying associations of an exposure while adjusting for covariates and exposures at other times and have been applied previously in environmental health research by our group (32–35). A traditional DLM requires each participant to have an exposure measurement at each time interval over pregnancy. The GRAPHS data structure includes repeated personal exposure measurements, providing an unbiased estimate of exposure during each measurement period, at random gestational ages per pregnant woman. We therefore employed a previously-reported interchange of the outcome and exposure and used a functional spline model with time-varying coefficients, i.e., a reversed DLM (rDLM) (35–37). All models include a random effect for participant to account for the repeated CO measurements. To our knowledge, this is the only data-driven framework available to examine sensitive windows with our exposure data structure as described. Using this framework, we examined time-varying associations between maternal CO exposures over pregnancy and birth weight and pneumonia risk, considered separately. We then plotted the time-varying associations of estimates, confidence intervals and Holm-Bonferroni-adjusted confidence intervals. A sensitive window is identified when the estimate and confidence intervals do not cross zero for birth weight, or one for pneumonia risk.
For birth weight, multivariable models adjusted for child sex; maternal age, BMI, ethnicity and parity at enrollment; household wealth index; number of antenatal visits; and evidence of placental malaria. For pneumonia outcomes, multivariable models adjusted for child sex; maternal age, BMI at enrollment and ethnicity; household wealth index; gestational age at delivery and average postnatal child CO exposure. Sex-specific effects were explored in stratified analyses. Sensitivity analyses were performed excluding participants with only one prenatal CO measurement. Statistical modeling was performed in SAS v9.4 and R version 4.2.2.
3. Results
As previously reported, GRAPHS enrolled n=1,414 pregnant women, with a mean gestational age at enrollment of 15.7 weeks (range: 6.0–26.0 weeks). The cohort resulted in N=1,306 live births at 28+ weeks gestation. Of these, n=1,196 newborns had at least one valid prenatal CO measurement, valid birth weight data and pneumonia surveillance data. Three hundred and three (25%) children had at least one episode of physician-diagnosed pneumonia and 111 (9%) children had at least one episode of physician-diagnosed severe pneumonia. Participant characteristics are shown in Table 1.
Table 1.
GRAPHS participant characteristics (n=1,196)
| All Children (n=1,196) | Females (n=597) | Males (n=599) | |
|---|---|---|---|
| Gestational age at delivery, weeks -- median (IQR) † | 39 (39, 40) | 39 (39, 40) | 39 (39, 40) |
| Maternal age, years -- median (IQR) | 27 (22.4, 33.6) | 27.5 (22.8, 33.9) | 26.7 (21.8, 33.6) |
| Maternal body mass index, kg/m2 – median (IQR) | 22.9 (21.1, 25) | 22.7 (20.9, 25) | 23 (21.3, 25) |
| Parity, no. (%) | |||
| 0 | 197 (17) | 89 (15) | 108 (18) |
| 1 | 208 (17) | 108 (19) | 100 (17) |
| 2 | 209 (18) | 111 (19) | 98 (16) |
| 3 | 168 (14) | 76 (13) | 92 (15) |
| ≥4 | 414 (35) | 213 (36) | 201 (34) |
| Asset index – median (IQR) | −0.42 (−1.3, 0.8) | −0.43 (−1.3, 0.7) | −0.41 (−1.3, 0.8) |
| Prenatal CO average, ppm – median (IQR) ± | 1.07 (0.64, 1.67) | 1.03 (0.64, 1.59) | 1.05 (0.64, 1.64) |
| Postnatal CO average, ppm – median (IQR) ± | 0.52 (0.24, 1.02) | 0.54 (0.25, 1.03) | 0.52 (0.23, 1.00) |
| n-miss | 232 | 128 | 104 |
| Ethnicity – no. (%) | |||
| 1 | 203 (17) | 109 (18) | 94 (16) |
| 2 | 155 (13) | 72 (12) | 83 (14) |
| 3 | 786 (66) | 393 (66) | 393 (66) |
| 4 | 52 (4) | 23 (4) | 29 (5) |
| Outcomes | |||
| Birth weight, kilograms – median (IQR) | 2.94 (2.7, 3.2) | 2.9 (2.6, 3.1) | 3 (2.7, 3.2) |
| Any pneumonia, yes – no. (%) | 303 (25) | 134 (22) | 169 (28) |
| Any severe pneumonia, yes – no. (%) | 111 (9) | 53 (9) | 59 (10) |
Abbreviations: CO, carbon monoxide; IQR, interquartile range, kg/m2, kilogram per meter-squared; ppm, parts per million
Gestational age at delivery, established by ultrasound-determined gestational dating
Due to QA/QC, 232 children did not have valid, postnatal (infant) CO data
In total, there were n=3,627 unique, prenatal CO measurements. N=86 (7%), n=264 (22%), n=450 (38%) and n=396 (33%) of women had one, two, three and four valid prenatal CO measurements, respectively. The standard deviation for prenatal and postnatal average CO was 0.86 and 1.68, respectively. The coefficient of variation for prenatal and postnatal average CO was 0.68 and 1.81. Amongst women with >1 measurement, repeated measures are moderately correlated with the average of the participant’s CO measurements (Pearson’s correlation r=0.61, p<0.01). The count of number of measurements available at each week gestation is shown in Supplemental Figure 1. The first exposure assessment occurred at median gestational age of 17 weeks (IQR 13, 20) and the median time between each subsequent measurement was 5 weeks. Due to the nature of pregnant women self-identifying to community-based fieldworkers we, as expected, had few personal exposure assessments prior to ten weeks gestation. We therefore limited the primary analyses to exposure assessments made between 10 to 40 weeks gestation.
3.1. Estimated time-varying association between prenatal CO and newborn birth weight
Examination of the time-varying association between prenatal CO and birth weight demonstrated a sensitive window of exposure between 15 to 20 weeks gestation where prenatal CO was inversely associated with birth weight, following adjustment for child sex; maternal age, BMI, ethnicity and parity at enrollment; household wealth index; number of antenatal visits; and evidence of placental malaria (Figure 1A). For example, at 16 weeks gestation CO exposure was inversely associated with birth weight (β = −0.13, 95% CI −0.18, −0.08, Holm-Bonferroni 95% CI −0.20, −0.06). Exploratory sex-stratified models identified a sensitive window in girls at 10 weeks gestation and a sensitive window in boys from 16–20 weeks where CO exposure is inversely associated with birth weight (Figure 1B, 1C). Sensitivity models excluding participants with one prenatal CO measurement did not substantively change these findings.
Figure 1. Time-varying associations between prenatal household air pollution exposure, as indexed by personal carbon monoxide, and newborn birth weight in A) all children and B) females and C) males.
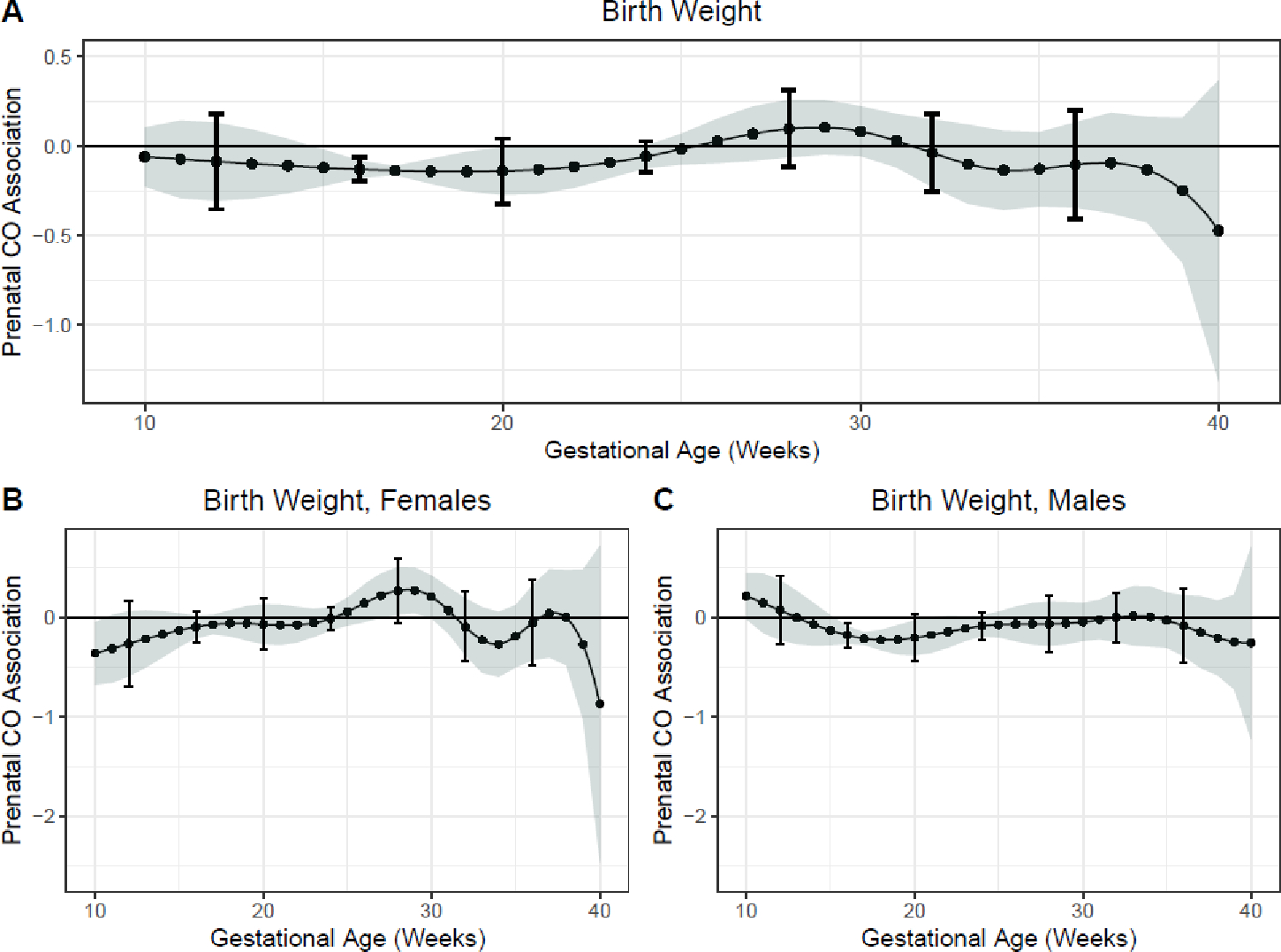
This figure demonstrates the association between carbon monoxide exposure measurements over pregnancy and newborn birth weight measured within 24-hours of life assuming week-specific effects for the overall sample. Models were adjusted for child sex; maternal age, body mass index, ethnicity and parity at enrollment; household wealth index; number of antenatal visits; and evidence of placental malaria. The Y-axis represents the time-varying association between birth weight in kilograms and prenatal CO exposure; the X-axis depicts gestational age in weeks. The solid line shows the predicted estimate and the shaded area represents the 95% confidence interval; the brackets demonstrate the Holm-Bonferroni confidence intervals. A sensitive window is identified when the Holm-Bonferroni confidence intervals do not include zero.
3.2. Estimated time-varying association between prenatal CO and physician-diagnosed severe pneumonia and pneumonia in the first year of life
Examination of the time-varying associations between prenatal CO and physician-diagnosed severe pneumonia in the first year of life demonstrated a sensitive window of exposure between 34–39 weeks gestation where prenatal CO was positively associated with odds of having an episode of physician-diagnosed severe pneumonia in the first year of life, following adjustment for child sex; maternal age, BMI at enrollment and ethnicity; household wealth index; gestational age at delivery and average postnatal child CO exposure (at 36 weeks gestation OR=1.71, 95% CI 1.18, 2.48, Holm-Bonferroni 95% CI 1.02, 2.87, Figure 2A). A statistically insignificant trend for effect was identified in mid-gestation between 22–25 weeks gestation. Amongst females in sex-stratified models, two windows were identified. First, a window at 21 weeks gestation which reached nominal but not Holm-Bonferroni significance (OR 1.35, 95% CI 1.00, 1.82) and a second at 33–38 weeks gestation (at 36 weeks OR 2.25, 95% CI 1.32, 3.84, Holm-Bonferroni 95% CI 1.08, 4.69, Figure 2B, 2C).
Figure 2. Time-varying associations between prenatal household air pollution exposure, as indexed by personal carbon monoxide, and physician-diagnosed severe pneumonia risk over the first year of life in A) all children, B) females, and C) males.
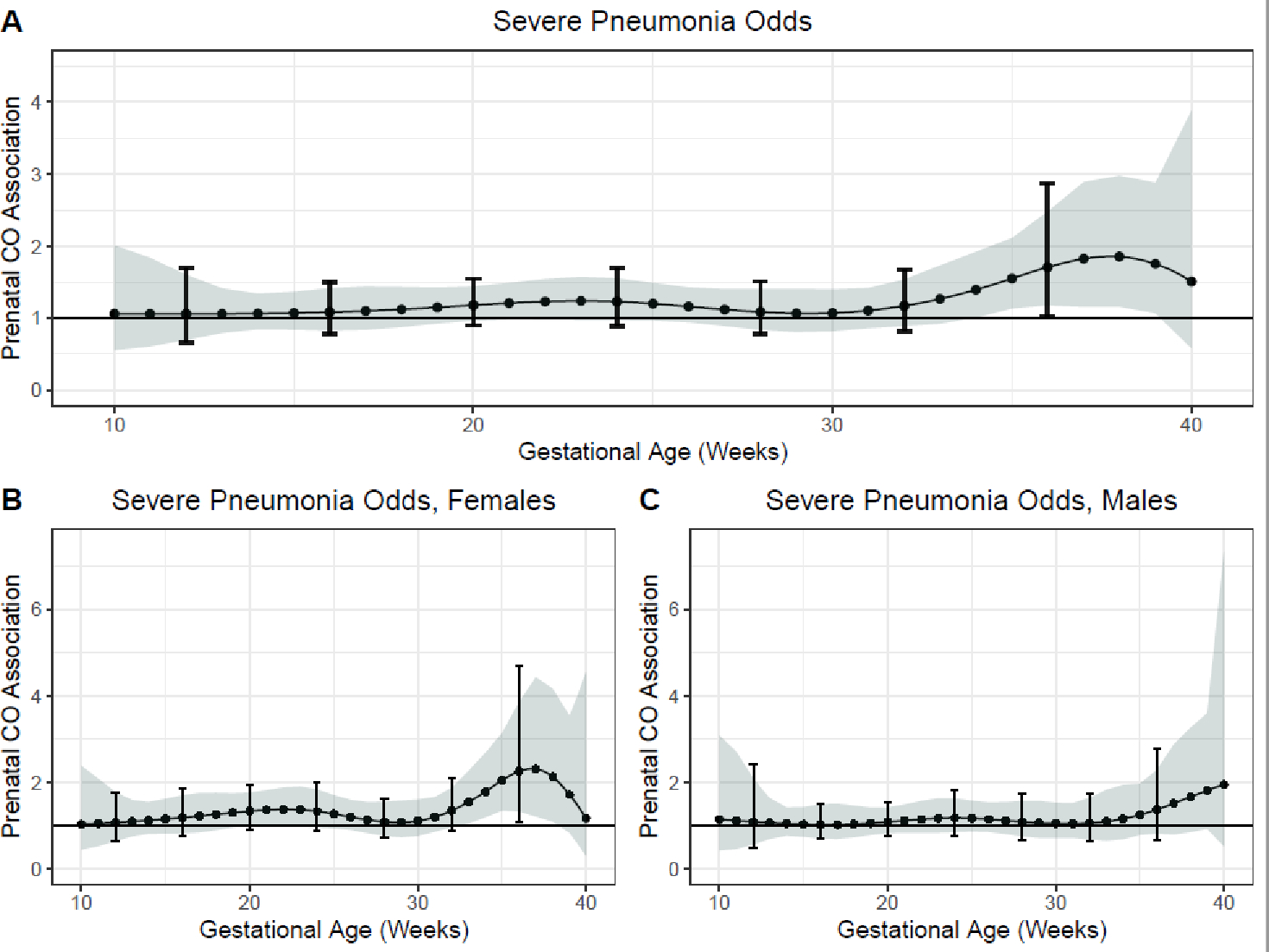
This figure demonstrates the association between repeated personal carbon monoxide exposure measurements over pregnancy and odds of physician-diagnosed severe pneumonia over the first year of life assuming week-specific effects for the overall sample. Models adjusted for child sex; maternal age, BMI at enrollment and ethnicity; household wealth index; gestational age at delivery and average postnatal child CO exposure. The Y-axis represents the association between CO exposure and severe pneumonia odds; the X-axis depicts gestational age. The solid line shows the predicted OR and the shaded area represents the 95% confidence interval; the brackets demonstrate the Holm-Bonferroni confidence intervals. A sensitive window is identified when the Holm-Bonferroni confidence intervals do not include one.
Multivariable models examining the time-varying associations between prenatal CO and physician-diagnosed pneumonia in the first year of life demonstrated a sensitive window of exposure between 26–30 weeks gestation where prenatal CO was positively associated with odds of having an episode of physician-diagnosed pneumonia in the first year of life (Figure 3A). However, the Holm-Bonferroni 95% CI did not reach significance (at 28 weeks gestation, OR 1.23 95% CI 1.03, 1.46, Holm-Bonferroni 95% CI 0.96, 1.56). Amongst females, sex-stratified analyses identified a sensitive window of exposure from 26 to 32 weeks gestation where CO exposure was positively associated with odds of pneumonia (for example, at 28 weeks gestation OR 1.47, 95% CI 1.16, 1.86, Holm-Bonferroni 95% CI 1.06, 2.04). No associations were identified amongst male infants (Figure 3B, 3C). Sensitivity analyses excluding participants with one CO measurement did not substantively change these findings.
Figure 3. Time-varying associations between prenatal household air pollution exposure, as indexed by personal carbon monoxide, and physician-diagnosed pneumonia risk over the first year of life in A) all children, B) females, and C) males.
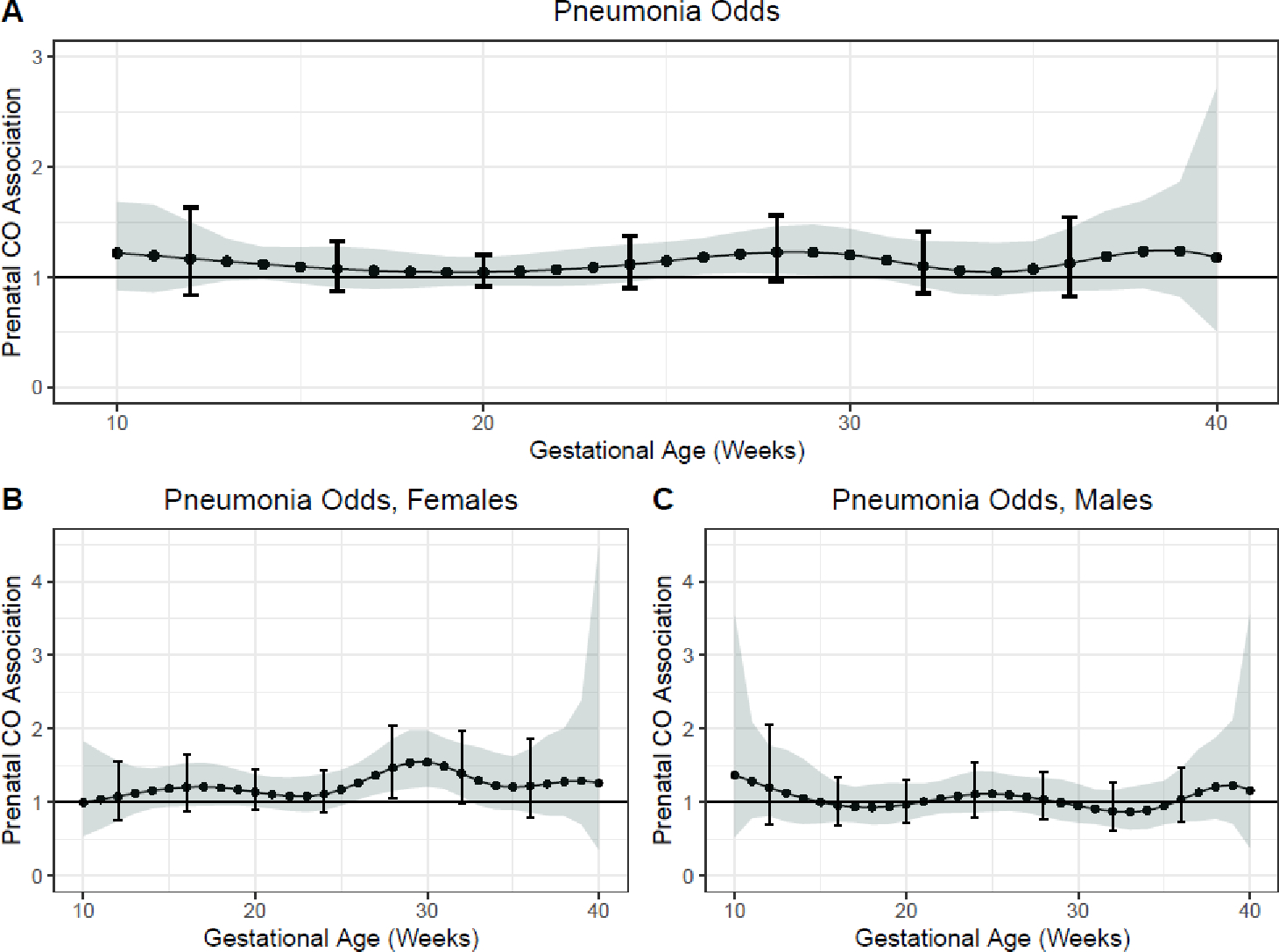
This figure demonstrates the association between repeated personal carbon monoxide exposure measurements over pregnancy and odds of physician-diagnosed pneumonia over the first year of life assuming week-specific effects for the overall sample. Models adjusted for child sex; maternal age, BMI at enrollment and ethnicity; household wealth index; gestational age at delivery and average postnatal child CO exposure. The Y-axis represents the association between CO exposure and pneumonia odds; the X-axis depicts gestational age. The solid line shows the predicted OR and the shaded area represents the 95% confidence interval; the brackets demonstrate the Holm-Bonferroni confidence intervals. A sensitive window is identified when the Holm-Bonferroni confidence intervals do not include one.
4. Discussion
These data add to emerging literature supporting the importance of prenatal HAP exposure on infant health, and specifically birth weight and pneumonia risk in infancy. Herein, we combine repeated, personal CO exposure assessments over gestation and data-driven statistics to identify sensitive windows of exposure. These data suggest that prenatal CO exposure in early to mid-gestation was associated with lower birth weight while prenatal CO exposure in later gestation was associated with increased risk for physician-diagnosed severe pneumonia and, amongst females, pneumonia. Taken together, these data suggest the importance of deployment of cleaner fuel cooking interventions in early pregnancy to improve birth weight and reduce pneumonia risk. Although pneumonia sensitive windows in particular were identified in later gestation, intervening in early pregnancy (i.e., prior to the critical window as opposed to during the critical window) will ensure reduced exposure during the critical window thus maximizing the effect of the intervention.
The Developmental Origins of Health and Disease theory highlights the importance of fetal development on future disease risk (38). Our results find that early to mid-gestation exposure to HAP impairs birth weight. This timing is coincident with the second wave of endovascular trophoblastic invasion and may help explain this as a particularly sensitive window(39). Reductions in birth weight increase risk for neonatal mortality, stunting and chronic disease over the life course. Low birth weight, an extreme where birth weight is less than 2500 grams, is one of the top ten risk factors for lifelong death and is responsible for 178 million disability adjusted life years (DALYs) with a disproportionate burden in sub-Saharan Africa (40). Mid- and late-gestation HAP exposure had an additional impact on infant pneumonia. Pneumonia is one of the leading causes of morbidity and mortality in children under five worldwide and HAP is responsible for 44% of pneumonia-related deaths in children under 5 year(3, 41). Overall, HAP is responsible for 91.5 million DALYs, and 2.3 million deaths annually(41), although these estimates have not previously considered the influence of prenatal exposures. Infants who survive episodes of severe pneumonia may be at increased risk for poor lung health over the life course (42–44). Taken together, these results support the importance of prenatal HAP exposure on fetal development with implications for future, lifelong health.
Although prior exposure-response analyses have demonstrated associations between higher early life HAP exposure and birth weight or pneumonia, cookstove intervention trials have largely failed to deliver on improving these same outcomes (7, 8, 16, 17). While the ability of a stove intervention to reduce HAP exposures is paramount, the interventions must be delivered at time points that are biologically susceptible to intervention. In GRAPHS, pregnant women in the LPG, improved biomass and control arms were enrolled by median GA 15.3 weeks (IQR 12.0, 18.3), 16.9 weeks (IQR 13.1, 20.3), and 16.4 weeks (IQR 13.0, 20.3) respectively. Per protocol, the intervention stoves were deployed following a baseline exposure assessment resulting in stove deployment at median GA 16.7 weeks (IQR 13, 20.3) and 19 weeks (IQR 15.3, 22.0) for the LPG and improved biomass arm, respectively. It is plausible therefore that the cookstove intervention occurred on average too late to have an effect on birth weight and possibly severe pneumonia. Further, work by our group finds heterogeneity in exposure reduction across study arms(27) suggesting that not only does a critical window need to be met but also an exposure reduction must occur prior to that window. The available GRAPHS dataset is underpowered to demonstrate an improvement in outcomes in those who received the intervention prior to these windows.
GRAPHS employed active pregnancy surveillance with resident fieldworkers seeking out pregnant women in addition to community-level education to encourage self-referral – an approach that we hypothesized would recruit pregnant women as early as possible. The delay in identification of pregnancies may have resulted from cultural norms surrounding late disclosure of pregnancy, which is also linked to late initiation of antenatal care in many rural sub-Sahara African settings (45). For example, some women perceive pregnancy as a normal physiological process that requires medical attention only when they are unwell or when the pregnancy becomes visible in the 4th or 5th month. Others, especially unmarried young women may experience stigma when they disclose their pregnancy and many seek advice from elderly family members before disclosing their pregnancy. Some women view early pregnancy as the most vulnerable period and fear making a pregnancy public during this period (45, 46). Young, primigravid women may have difficulty recognizing unplanned or unexpected pregnancies (47). Given these barriers, providing a cleaner stove to women of childbearing age who are likely to conceive in the coming year may be one strategy to ensure that a cleaner stove is used throughout pregnancy. However, the effectiveness of any clean cookstove public health program to increase birthweight and reduce pneumonia may be diminished by socioeconomic factors such as ability to consistently purchase and use clean cooking fuels.
Sensitive windows between prenatal HAP exposure and infant outcomes, such as birth weight and pneumonia, have not previously been identified. These findings may begin to elucidate underlying mechanistic pathways affected by prenatal HAP exposure, as indexed by repeated personal maternal CO exposure assessments. Prior work assessing the effects of prenatal HAP exposure has largely relied on a single time-point exposure assessment or the averaging of exposures or time-weighed exposures in pregnancy and therefore has been unable to assess vulnerable windows(5, 6, 12, 29). This study leverages a data-driven biostatistical approach developed as an extension of distributed lag models, which have been widely used in environmental health, to expand on prior work and understand time-varying associations. Further, use of these data-driven methods reduces bias that results from the assignment of exposure averages such as trimester averages (48).
Previous ambient air pollution research supports an association between mid-gestation exposures and birth weight. For example, Hansen et al measured repeated fetal ultrasonic measurements between 13–26 weeks gestation and analyzed associations between ambient pollutants in the first four months of pregnancy and found a consistent association between increased PM10 from 13–17 weeks gestation and fetal growth, including abdominal circumference, femur length and head circumference suggestive of an overall reduction in fetal size(19). Evidence from the Children’s Health Study suggests an association between ozone and birth weight particularly during the second and third trimester(19). Contrary to our findings, the authors demonstrate an association between CO and birth weight only in the first trimester, a finding that we only replicated amongst females although we note that we lack exposure data from the earlier weeks of pregnancy. A longitudinal cohort from Spain found an association between increased NO2 and neonatal anthropometry, including fetal length, biparietal diameter, abdominal circumference and weight from weeks 20–32 gestation(19).
The mechanisms underpinning the associations between prenatal air pollution and birth weight are unknown. Functioning of the placenta may be impaired by maternal air pollution exposures resulting in poorer fetal growth and development (49–51). Air pollution-induced inflammation may also be important, and sex-differences in intrauterine inflammation suggest higher pro-inflammatory markers in male infants, supporting our exploratory analyses suggesting that boys may be more vulnerable to the effect of prenatal HAP exposure on birth weight (52). Further, a male sex-specific predisposition has been seen in the ambient air pollution literature with a number of child health outcomes (32, 34, 53). Some evidence suggests that prenatal air pollution exposure may affect expression of Sox8, SRY (sex-determining region Y)-box 8, which plays a role in sex-determination(54). Mice deficient in Sox8 have reduced birth weight (55).
Pneumonia analyses suggest that CO exposures in late-gestation with a suggestion of effect in mid-gestation are associated with higher risk for pneumonia. Prior work in GRAPHS found that prenatal HAP exposure was associated with impaired infant lung function, which may increase risk for future pneumonia(29). Supporting ambient air pollution evidence shows that higher mid- and late-gestation exposures impair lung health, specifically asthma risk and poorer lung function (32, 34). The identified mid-gestation window corresponds with the canalicular phase of lung development where complex airway branching patterns emerge, gas-exchange regions develop including vascular development and angiogenesis, and airway epithelium differentiates(22, 23). Mouse models of maternal tobacco smoke exposure found a reduction in fetal lung volume beginning in the canalicular development phase, possibly from a decrease in mesenchymal tissue (56). The late-gestation window corresponds with the saccular and alveolar phase of lung development when the airspaces further mature with thinning of connective tissue and development of surfactant. Septation during the alveolar phase exponentially increases the gas-exchange surface area preparing the fetus for ex-utero life(22). Mouse models of air pollution exposure on lung development demonstrate impaired fetal lung cell proliferation and inflammation and altered alveolarization (57).
Prenatal HAP exposure may also increase pneumonia risk by altering immune function (58). Maternal air pollution exposure during pregnancy may act directly, via translocation through the placenta (59), or indirectly, through induction of maternal and fetal inflammation and oxidative stress (60). Supporting this, prior work in GRAPHS suggests an association with prenatal HAP on cord blood mitochondrial DNA copy number and telomere length, biomarkers of oxidative stress, with potential implications for the developing immune system (61–63). A prolonged shift towards T helper lymphocyte 2 (Th2) and T helper lymphocyte type 17 (Th17) immunity may increase risk for severe respiratory infections (64). While the influence of prenatal air pollution exposures on adaptive immune response predisposition has not been clearly defined, exposure to PM can increase Th2 cytokine secretion (65). Maternal allergen exposure can also result in a stronger offspring Th2 response (66). Prenatal ambient air pollution exposure has been associated with altered lymphocyte and cytokine profiles in cord blood (53, 67).
Sex-stratified analysis suggest that girls were more vulnerable to the effect of prenatal CO compared to boys. This is consistent with previous findings of sex-specific effects of average prenatal CO on infant lung function and pneumonia risk in this cohort, with girls at higher risk compared to boys (6, 29). Although the mechanisms underlying these sex-specific effects are unknown, lung development in girls is distinctively different from that in boys with female fetuses exhibiting earlier and more rapid lung development than male fetuses until 32 weeks gestation(68). Female lungs also have a more mature phospholipid profile compared to male lungs(69, 70) and sex-specific differences have been observed in whole-lung transcriptomic profiles of the fetus (70). Whether these sex-specific differences in lung development account for the vulnerability of female infants to the effects of prenatal CO exposure is not known. Suggestive evidence comes from the tobacco literature where female rats appear differentially affected by exposure to prenatal nicotine exposure(71). In humans, adolescent girls exposed to tobacco smoke had higher risk of wheeze and slower growth in FEV1 compared to boys (72).
We note several study strengths. We assessed personal maternal CO exposure at four time points over pregnancy. Gestational dating of the cohort allowed us to assign a gestational age to each exposure measurement. We then leveraged a data-driven statistical approach to objectively identify sensitive windows without bias for the importance of specific timepoints that do not follow developmental patterns, such as trimester delineation. Our mother-infant cohort from rural Ghana is more likely to be exposed to HAP and more likely to have lower birth weight and more pneumonia, as compared to mother-infant dyads from higher-income regions. Detailed characterization of our cohort beginning at enrollment allowed for adjustment by important covariates and confounders. Finally, we are able to explore sex-specific associations.
We also acknowledge limitations. Due to participant burden and financial constraints, we were unable to assess HAP exposures continuously or even more frequently over the study period. Our results from rural Ghana may not be applicable to other populations exposed to HAP, due to differences in exposure levels or solid fuel choice and stove use. Our primary exposure metric was CO, which is a product of combustion and has been shown to affect placental function and cross the placental to influence fetal development; fine particulate matter was measured in a subset of pregnant women and only once thereby not allowing similar analyses with this important pollutant. It is plausible that sensitive windows may be different for different pollutants. Ongoing studies have more extensive exposure assessments and it will be important to compare findings (73). As we continue to follow this cohort, it will be important to understand how prenatal and early childhood HAP exposures and sensitive windows of exposure in early childhood continue to affect child development, including growth and pneumonia risk. Further, complimentary animal studies are needed to better delineate mechanistic underpinnings and HAP impacts on fetal development.
In summary, we identify time-varying associations between prenatal HAP exposure and lower birth weight and higher pneumonia risk, supporting the importance of HAP exposure during the in utero period. These findings support public health efforts that target early pregnancy for cleaner fuel cooking interventions.
Supplementary Material
Acknowledgement
The authors acknowledge Kintampo Health Research Centre and the other Ghana Health Service facilities in the Kintampo North Municipality and Kintampo South District, study participants and community members in the study area.
Funding
The Ghana Randomized Air Pollution and Health Study was funded by the National Institutes of Health (R21TW010957, R01ES026991, R01ES019547, R01ES034433, P30ES009089, R01ES034433, P30ES023515), the Clean Cooking Alliance, and the Thrasher Research Fund. Ghana Health Service facilities in the Kintampo North Municipality and Kintampo South Districts provided facilities for GRAPHS. AGL was supported by K23HL135349, BJW was supported by K23ES021471.
Footnotes
Conflicts of Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.GBD Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 39610258: 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAllister DA, Liu L, Shi T, Chu Y, Reed C, Burrows J, Adeloye D, Rudan I, Black RE, Campbell H. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. The Lancet Global Health 2019; 7: e47–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization W.H.O. Pneumonia in children. Key facts 11 November 2022 https://wwwwhoint/news-room/fact-sheets/detail/pneumonia Accessed 01 May 2023.
- 4.Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, Lahiff M, Rehfuess EA, Mishra V, Smith KR. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environmental health perspectives 2013; 121: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn AK, Adjei IA, Ayuurebobi K, Agyei O, Boamah-Kaali EA, Burkart K, Carrión D, Chillrud SN, Gould CF, Gyaase S. Prenatal household air pollutant exposure is associated with reduced size and gestational age at birth among a cohort of Ghanaian infants. Environment international 2021; 155: 106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinney PL AK, Lee AG, Burkart K, Boamah-Kaali E, Twumasi M, Gyaase S, Quinn A, Oppong FB, Wylie BJ, Kaali S. Prenatal and postnatal household air pollution exposures and pneumonia risk: evidence from the Ghana Randomized Air Pollution and Health Study. Chest 2021. Nov 1; 160: 1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack DW, Ayuurebobi K, Gould CF, Boamah-Kaali E, Lee AG, Mujtaba MN, Chillrud S, Kaali S, Quinn AK, Gyaase S. A cluster randomised trial of cookstove interventions to improve infant health in Ghana. BMJ Global Health 2021; 6: e005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environmental health perspectives 2011; 119: 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander DA, Northcross A, Karrison T, Morhasson-Bello O, Wilson N, Atalabi OM, Dutta A, Adu D, Ibigbami T, Olamijulo J. Pregnancy outcomes and ethanol cook stove intervention: a randomized-controlled trial in Ibadan, Nigeria. Environment international 2018; 111: 152–163. [DOI] [PubMed] [Google Scholar]
- 10.Clasen TF, Chang HH, Thompson LM, Kirby MA, Balakrishnan K, Díaz-Artiga A, McCracken JP, Rosa G, Steenland K, Younger A, Aravindalochanan V, Barr DB, Castañaza A, Chen Y, Chiang M, Clark ML, Garg S, Hartinger S, Jabbarzadeh S, Johnson MA, Kim DY, Lovvorn AE, McCollum ED, Monroy L, Moulton LH, Mukeshimana A, Mukhopadhyay K, Naeher LP, Ndagijimana F, Papageorghiou A, Piedrahita R, Pillarisetti A, Puttaswamy N, Quinn A, Ramakrishnan U, Sambandam S, Sinharoy SS, Thangavel G, Underhill LJ, Waller LA, Wang J, Williams KN, Rosenthal JP, Checkley W, Peel JL. Liquefied Petroleum Gas or Biomass for Cooking and Effects on Birth Weight. N Engl J Med 2022; 387: 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Luo X, Zhao C, Zhang B, Tao J, Yang Z, Ma W, Liu T. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: A meta-analysis. Environmental pollution (Barking, Essex : 1987) 2016; 211: 38–47. [DOI] [PubMed] [Google Scholar]
- 12.Wylie BJ, Kishashu Y, Matechi E, Zhou Z, Coull B, Abioye AI, Dionisio KL, Mugusi F, Premji Z, Fawzi W. Maternal exposure to carbon monoxide and fine particulate matter during pregnancy in an urban Tanzanian cohort. Indoor air 2017; 27: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boy E, Bruce N, Delgado H. Birth weight and exposure to kitchen wood smoke during pregnancy in rural Guatemala. Environmental health perspectives 2002; 110: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan MN, CZ BN, Mofizul Islam M, Islam MR, Rahman MM. Household air pollution from cooking and risk of adverse health and birth outcomes in Bangladesh: a nationwide population-based study. Environmental health : a global access science source 2017; 16: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra V, Dai X, Smith KR, Mika L. Maternal exposure to biomass smoke and reduced birth weight in Zimbabwe. Annals of epidemiology 2004; 14: 740–747. [DOI] [PubMed] [Google Scholar]
- 16.Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, Balmes J, Diaz A, Arana B, Bruce N. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. The Lancet 2011; 378: 1717–1726. [DOI] [PubMed] [Google Scholar]
- 17.Mortimer K, Ndamala CB, Naunje AW, Malava J, Katundu C, Weston W, Havens D, Pope D, Bruce NG, Nyirenda M. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. The Lancet 2017; 389: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer K, Lesosky M, Semple S, Malava J, Katundu C, Crampin A, Wang D, Weston W, Pope D, Havens D, Gordon SB, Balmes J. Pneumonia and Exposure to Household Air Pollution in Children Under the Age of 5 Years in Rural Malawi: Findings From the Cooking and Pneumonia Study. Chest 2020; 158: 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen CA, Barnett AG, Pritchard G. The effect of ambient air pollution during early pregnancy on fetal ultrasonic measurements during mid-pregnancy. Environmental health perspectives 2008; 116: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ville Y, Nyberg D. Growth, Doppler and fetal assessment. Diagnostic imaging of fetal anomalies Philadelphia: Lippincott Williams & Wilkins; 2003: 31–58. [Google Scholar]
- 21.Jingjia Liang CX, Liu Qian, Fan Xikang, Xu Jin, Zhang Liye, Hang Dong, Hongcai Shang, Gu Aihua. Association between birth weight and risk of cardiovascular disease: Evidence from UK Biobank. Nutrition, Metabolism and Cardiovascular Diseases 2021; 31: 2637–2643,. [DOI] [PubMed] [Google Scholar]
- 22.Copland I PM. Lung development and fetal lung growth. Paediatric respiratory reviews 2004. Jan 1;5:S259–64. [DOI] [PubMed] [Google Scholar]
- 23.A H. Developmental biology of the pulmonary circulation. Paediatric respiratory reviews 2005. Mar; 1;6(1):35–43. [DOI] [PubMed] [Google Scholar]
- 24.Jack DW, Asante KP, Wylie BJ, Chillrud SN, Whyatt RM, Ae-Ngibise KA, Quinn AK, Yawson AK, Boamah EA, Agyei O. Ghana randomized air pollution and health study (GRAPHS): study protocol for a randomized controlled trial. Trials 2015; 16: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boamah-Kaali E, Jack DW, Ae-Ngibise KA, Quinn A, Kaali S, Dubowski K, Oppong FB, Wylie BJ, Mujtaba MN, Gould CF. Prenatal and postnatal household air pollution exposure and infant growth trajectories: evidence from a rural Ghanaian pregnancy cohort. Environmental health perspectives 2021; 129: 117009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boamah EA, Asante K, Ae-Ngibise K, Kinney PL, Jack DW, Manu G, Azindow IT, Owusu-Agyei S, Wylie BJ. Gestational age assessment in the Ghana Randomized Air Pollution and Health Study (GRAPHS): ultrasound capacity building, fetal biometry protocol development, and ongoing quality control. JMIR research protocols 2014; 3: e3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chillrud SN, Ae-Ngibise KA, Gould CF, Owusu-Agyei S, Mujtaba M, Manu G, Burkart K, Kinney PL, Quinn A, Jack DW. The effect of clean cooking interventions on mother and child personal exposure to air pollution: results from the Ghana Randomized Air Pollution and Health Study (GRAPHS). Journal of exposure science & environmental epidemiology 2021; 31: 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunnsteinsson S, Labrique AB, West KP Jr., Christian P, Mehra S, Shamim AA, Rashid M, Katz J, Klemm RD. Constructing indices of rural living standards in Northwestern Bangladesh. J Health Popul Nutr 2010; 28: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AG KS, Quinn A, Delimini R, Burkart K, Opoku-Mensah J, Wylie BJ, Yawson AK, Kinney PL, Ae-Ngibise KA, Chillrud S. Prenatal household air pollution is associated with impaired infant lung function with sex-specific effects. Evidence from GRAPHS, a cluster randomized cookstove intervention trial. American Journal of Respiratory and Critical Care Medicine 2019. Mar 15; 199(6): 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coull BA, Bobb JF, Wellenius GA, Kioumourtzoglou MA, Mittleman MA, Koutrakis P, Godleski JJ. Part 1. Statistical Learning Methods for the Effects of Multiple Air Pollution Constituents. Res Rep Health Eff Inst 2015: 5–50. [PubMed] [Google Scholar]
- 31.Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Statistics in medicine 2010; 29: 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu HH, Chiu YH, Coull BA, Kloog I, Schwartz J, Lee A, Wright RO, Wright RJ. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. Am J Respir Crit Care Med 2015; 192: 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, Wilson A, Schwartz J, Cohen S, Coull BA, Wright RO, Wright RJ. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J Allergy Clin Immunol 2018; 141: 1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee AG, Le Grand B, Hsu HL, Chiu YM, Brennan KJ, Bose S, Rosa MJ, Brunst KJ, Kloog I, Wilson A, Schwartz J, Morgan W, Coull BA, Wright RO, Baccarelli AA, Wright RJ. Prenatal fine particulate exposure associated with reduced childhood lung function and nasal epithelia GSTP1 hypermethylation: Sex-specific effects. Respir Res 2018; 19: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, Schnaas L, Gennings C, Hu H, Wright R, Rojo MMT, Arora M. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int 2018; 121: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YH, Ferguson KK, Meeker JD, McElrath TF, Mukherjee B. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environ Health 2015; 14: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gennings C, Curtin P, Bello G, Wright R, Arora M, Austin C. Lagged WQS regression for mixtures with many components. Environ Res 2020; 186: 109529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker DJ. Fetal origins of coronary heart disease. BMJ (Clinical research ed) 1995; 311: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato Y Endovascular trophoblast and spiral artery remodeling. Molecular and cellular endocrinology 2020; 503: 110699. [DOI] [PubMed] [Google Scholar]
- 40.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018; 392: 1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization W.H.O. Household air pollution and health. Key facts 28 November 2022 https://wwwwhoint/news-room/fact-sheets/detail/household-air-pollution-and-health Accessed 01 May 2023.
- 42.Castro-Rodriguez JA, Holberg CJ, Wright AL, Halonen M, Taussig LM, Morgan WJ, Martinez FD. Association of radiologically ascertained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: a prospective study. American journal of respiratory and critical care medicine 1999; 159: 1891–1897. [DOI] [PubMed] [Google Scholar]
- 43.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics 2015; 135: 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castleman WL, Sorkness RL, Lemanske RF, Grasee G, Suyemoto MM. Neonatal viral bronchiolitis and pneumonia induces bronchiolar hypoplasia and alveolar dysplasia in rats. Laboratory investigation; a journal of technical methods and pathology 1988; 59: 387–396. [PubMed] [Google Scholar]
- 45.Warri D, George A. Perceptions of pregnant women of reasons for late initiation of antenatal care: a qualitative interview study. BMC Pregnancy and Childbirth 2020; 20: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts J, Hopp Marshak H, Sealy DA, Manda-Taylor L, Mataya R, Gleason P. The role of cultural beliefs in accessing antenatal care in Malawi: a qualitative study. Public Health Nursing 2017; 34: 42–49. [DOI] [PubMed] [Google Scholar]
- 47.Kaswa R, Rupesinghe GF, Longo-Mbenza B. Exploring the pregnant women’s perspective of late booking of antenatal care services at Mbekweni Health Centre in Eastern Cape, South Africa. African journal of primary health care & family medicine 2018; 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. American journal of epidemiology 2017; 186: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clemente DB, Casas M, Vilahur N, Begiristain H, Bustamante M, Carsin AE, Fernández MF, Fierens F, Gyselaers W, Iñiguez C, Janssen BG, Lefebvre W, Llop S, Olea N, Pedersen M, Pieters N, Santa Marina L, Souto A, Tardón A, Vanpoucke C, Vrijheid M, Sunyer J, Nawrot TS. Prenatal Ambient Air Pollution, Placental Mitochondrial DNA Content, and Birth Weight in the INMA (Spain) and ENVIRONAGE (Belgium) Birth Cohorts. Environmental health perspectives 2016; 124: 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C, Yang M, Zhu Z, Sun S, Zhang Q, Cao J, Ding R. Maternal exposure to air pollution and the risk of low birth weight: A meta-analysis of cohort studies. Environmental research 2020; 190: 109970. [DOI] [PubMed] [Google Scholar]
- 51.Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sánchez BN, Rojas-Bracho L, Viveros-Alcaráz M, Castillo-Castrejón M, Beltrán-Montoya J, Brown DG, O’Neill MS. Air pollution, inflammation and preterm birth: a potential mechanistic link. Med Hypotheses 2014; 82: 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cossi M, Zuta S, Padula AM, Gould JB, Stevenson DK, Shaw GM. Role of infant sex in the association between air pollution and preterm birth. Annals of epidemiology 2015; 25: 874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baïz N, Slama R, Béné MC, Charles MA, Kolopp-Sarda MN, Magnan A, Thiebaugeorges O, Faure G, Annesi-Maesano I. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations. The EDEN study cohort. BMC pregnancy and childbirth 2011; 11: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopes TdBM Groth EE, Veras M Furuya TK, Costa NdSX Júnior GR, Lopes FD, de Almeida FM, Cardoso WV, Saldiva PHN. Pre-and postnatal exposure of mice to concentrated urban PM2. 5 decreases the number of alveoli and leads to altered lung function at an early stage of life. Environmental pollution 2018; 241: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sock E, Schmidt K, Hermanns-Borgmeyer I, Bösl MR, Wegner M. Idiopathic weight reduction in mice deficient in the high-mobility-group transcription factor Sox8. Molecular and cellular biology 2001; 21: 6951–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Unachukwu U, Trischler J, Goldklang M, Xiao R, D’Armiento J. Maternal smoke exposure decreases mesenchymal proliferation and modulates Rho-GTPase-dependent actin cytoskeletal signaling in fetal lungs. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2017; 31: 2340–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Barros Mendes Lopes T, Groth EE, Veras M, Furuya TK, de Souza Xavier Costa N, Ribeiro Júnior G, Lopes FD, de Almeida FM, Cardoso WV, Saldiva PHN, Chammas R, Mauad T. Pre- and postnatal exposure of mice to concentrated urban PM(2.5) decreases the number of alveoli and leads to altered lung function at an early stage of life. Environmental pollution (Barking, Essex : 1987) 2018; 241: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martino D, Prescott S. Epigenetics and prenatal influences on asthma and allergic airways disease. Chest 2011; 139: 640–647. [DOI] [PubMed] [Google Scholar]
- 59.Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, Van Eyken P, Plusquin M, Roeffaers MB, Ameloot M. Ambient black carbon particles reach the fetal side of human placenta. Nature communications 2019; 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krusche J, Basse S, Schaub B. Role of early life immune regulation in asthma development. Seminars in immunopathology 2020; 42: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaali S, Jack D, Delimini R, Hu L, Burkart K, Opoku-Mensah J, Quinn A, Ae-Ngibise KA, Wylie B, Boamah-Kaali EA, Chillrud S, Owusu-Agyei S, Kinney PL, Baccarelli AA, Asante KP, Lee A. Prenatal Household Air Pollution Alters Cord Blood Mononuclear Cell Mitochondrial DNA Copy Number: Sex-Specific Associations. Int J Environ Res Public Health 2018; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuo L, Otenbaker NP, Rose BA, Salisbury KS. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Molecular immunology 2013; 56: 57–63. [DOI] [PubMed] [Google Scholar]
- 63.Kaali S, Jack D, Opoku-Mensah J, Bloomquist T, Aanaro J, Quinn A, Boamah-Kaali EA, Kinney P, Mujtaba MN, Agyei O, Yawson AK, Osei-Owusu S, Delimini R, Wylie B, Ae-Ngibise KA, Baccarelli A, Owusu-Agyei S, Chillrud SN, Asante KP, Lee A. Prenatal Household Air Pollution Exposure, Cord Blood Mononuclear Cell Telomere Length and Age Four Blood Pressure: Evidence from a Ghanaian Pregnancy Cohort. Toxics 2021; 9: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glencross DA, Ho TR, Camiña N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free radical biology & medicine 2020; 151: 56–68. [DOI] [PubMed] [Google Scholar]
- 65.Fernvik E, Scharnweber T, Knopp D, Niessner R, Vargaftig BB, Peltre G. Effects of fractions of traffic particulate matter on TH2-cytokines, IgE levels, and bronchial hyperresponsiveness in mice. Journal of toxicology and environmental health Part A 2002; 65: 1025–1045. [DOI] [PubMed] [Google Scholar]
- 66.Warner JA, Jones CA, Jones AC, Warner JO. Prenatal origins of allergic disease. The Journal of allergy and clinical immunology 2000; 105: S493–498. [DOI] [PubMed] [Google Scholar]
- 67.Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, Schaub B. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PloS one 2011; 6: e23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: observations, hypotheses, and future directions. Pediatric pulmonology 2015; 50: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 69.Torday J, Nielsen H. The sex difference in fetal lung surfactant production. Experimental lung research 1987; 12: 1–19. [DOI] [PubMed] [Google Scholar]
- 70.Kho AT, Chhabra D, Sharma S, Qiu W, Carey VJ, Gaedigk R, Vyhlidal CA, Leeder JS, Tantisira KG, Weiss ST. Age, sexual dimorphism, and disease associations in the developing human fetal lung transcriptome. American Journal of Respiratory Cell and Molecular Biology 2016; 54: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Naeem E, Tian J, Lombardi V, Kwong K, Akbari O, Torday JS, Rehan VK. Sex-specific perinatal nicotine-induced asthma in rat offspring. American journal of respiratory cell and molecular biology 2013; 48: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. New England Journal of Medicine 1996; 335: 931–937. [DOI] [PubMed] [Google Scholar]
- 73.Clasen T, Checkley W, Peel JL, Balakrishnan K, McCracken JP, Rosa G, Thompson LM, Barr DB, Clark ML, Johnson MA, Waller LA, Jaacks LM, Steenland K, Miranda JJ, Chang HH, Kim DY, McCollum ED, Davila-Roman VG, Papageorghiou A, Rosenthal JP. Design and Rationale of the HAPIN Study: A Multicountry Randomized Controlled Trial to Assess the Effect of Liquefied Petroleum Gas Stove and Continuous Fuel Distribution. Environmental health perspectives 2020; 128: 47008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


