Abstract
Background:
Multiple studies have reported brain lipidomic abnormalities in Alzheimer’s disease (AD) that affect glycerophospholipids, sphingolipids and fatty acids. However, there is no consensus regarding the nature of these abnormalities, and it is unclear if they relate to disease progression.
Objective:
Monogalactosyl diglycerides (MGDGs) are a class of lipids which have been recently detected in the human brain. We sought to measure their levels in postmortem human brain and determine if these levels correlate with the progression of the AD-related traits.
Methods:
We measured MGDGs by ultraperformance liquid chromatography tandem mass spectrometry in postmortem dorsolateral prefrontal cortex gray matter and subcortical corona radiata white matter samples derived from three cohorts of participants: the Framingham Heart Study, the Boston University Alzheimer’s Disease Research Center and the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program (total n=288).
Results:
We detected 40 molecular species of MGDGs (including diacyl and alkyl/acyl compounds) and found that the levels of 29 of them, as well as total MGDG levels, are positively associated with AD-related traits including pathologically confirmed AD diagnosis, clinical dementia rating, Braak and Braak stage, neuritic plaque score, phospho-Tau AT8 immunostaining density, levels of phospho-Tau396 and levels of Aβ40. Increased MGDG levels were present in both gray and white matter, indicating that they are widespread and likely associated with myelin-producing oligodendrocytes – the principal cell type of white matter.
Conclusion:
Our data implicate the MGDG metabolic defect as a central correlate of clinical and pathological progression in AD.
Keywords: Alzheimer’s disease, lipidomics, cerebral cortex, gray matter, white matter, monogalactosyldiacylglycerol, cerebrosides
Introduction
Alzheimer’s disease (AD) – the most common form of age-related dementia – is caused by a neurodegenerative process that results in the loss of brain synapses and neurons leading to cognitive deficits [1, 2]. While the mechanisms that drive this process remain to be elucidated, analyses of postmortem human brains indicate that the pathophysiology of AD is characterized by abnormal metabolism of major classes of lipids including choline, ethanolamine, serine diacyl- and alkylacyl glycerophospholipids [3-16], several classes of sphingolipids [17] (ceramides [18-22], cerebrosides [23, 24], sulfatides [18, 23-25], gangliosides [26-31], sphingomyelins [11, 17, 32-34]) and free and esterified fatty acids [12, 13, 35-39]. However, there is controversy in the field and inconsistencies in the literature, likely due to the diversity of analytical techniques employed, the variety of brain areas analyzed, the different stages of the disease used and possibly stemming from the fact that AD as a biological entity is complex and specific AD-related neuropathological traits may be associated with different lipidomic abnormalities. Technical advances in lipidomic assays, including ultraperformance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) have enabled the detection and quantification of low abundance, understudied lipids within complex samples, such as brain extracts.
Monogalactosyl diglycerides (MGDGs) are a class of neutral glycerolipids found in small amounts in mammalian nervous system [40-42]. MGDG synthesis has been observed in brain white and gray matter [42, 43] in a reaction catalyzed by hydroxyceramide galactosyltransferase [44] and, because the rate of their biosynthesis, as well as their levels, are highest during the peak of myelin production during postnatal development [41, 45], they have been proposed as markers of myelin and myelination [41, 42]. In contrast to the brain, MGDG levels and turnover are high in male germ cells where they are enzymatically sulfated to form the highly abundant sulfogalactosylglycerolipid, known as seminolipid, which constitutes 10 mole% of the total lipids of the tissue [46]. Seminolipid is necessary for spermatogenesis and has a role in the mechanisms of fertilization [46]. Current understanding of MGDG biochemistry is based on studies of seminolipid and it appears that the metabolic pathways that govern MGDG synthesis and degradation include the same enzymes that synthesize and degrade the relatively abundant brain lipid, galactosylceramide (GC, cerebroside) [46]. Recently, MGDGs were also identified by mass spectrometry in the human brain white and gray matter [47]. Because of the paucity of data on the presence of MGDGs (both diacyl and alkylacyl) in the brain, we verified their chemical identity and quantified their levels in postmortem human dorsolateral prefrontal cortex (DLPFC) gray matter and subcortical corona radiata white matter using samples derived from biorepositories at the Framingham Heart Study (FHS), the Boston University Alzheimer’s Disease Research Center (BUADRC) and the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND)/Brain and Body Donation Program at Banner Sun Health Research Institute. We then assessed the association of MGDG levels with several AD-related traits including pathologically-confirmed AD diagnosis, clinical dementia rating (CDR), Braak and Braak stage, neuritic plaque (NP) score, phospho Tau (Ser202/Thr205) immunostaining density with the AT8 antibody, levels of Tau phosphorylated on serine 396 (pTau396) measured by ELISA, and levels of beta amyloid peptide (Aβ40) measured by ELISA. We found that the levels of multiple molecular species of MGDG as well as the total MGDG amounts were positively associated with these traits, suggesting that the pathophysiology of advancing AD is characterized by abnormal metabolism of these lipids in the brain.
Materials and Methods
Brain donors and common neuropathological procedures.
The postmortem brain samples were derived from the participants of the FHS, BUADRC and AZSAND biorepositories. FHS is a longitudinal community-based cohort based in Framingham, Massachusetts which has enrolled 3 generations of participants since its inception in 1948. The FHS brain donation program, which started in 1997, currently has 568 subjects enrolled, of whom 241 have come to autopsy. Of significance is that 32% of the autopsy cases were deemed cognitively intact clinically at the time of death (CDR 0), 20% were diagnosed with mild cognitive impairment and 48% were diagnosed with dementia (note a majority of FHS cases are non-AD in Table 1). The program has developed a highly standardized set of neuropathology protocols which include the relevant histological and immunohistochemical stains and quantification of AD and other neurological disease-related lesions [48]. The BUADRC serves the population of Boston Massachusetts and is one of more than 30 ADRCs in the US that is funded by the NIA to contribute standardized data (i.e., Uniform Data Set, UDS; Neuropathology Data Set, NDS) to the National Alzheimer’s Coordinating Centers (NACC). The BUADRC has a brain donation program available to all participants. A standard neuropathological analysis on all donated brains that follows NACC guidelines is conducted [49]. Each year, the BU ADRC contributes clinical, neurological, neuropsychological, neuropsychiatric, functional, MRI, and neuropathological data to NACC. The FHS and BUADRC brain biorepositories are housed at one location, are physically integrated, staffed by a common team of professionals and follow common procedures of neuropathological assessment of the postmortem brain tissues. All brains derived from the FHS and BUADRC biorepositories were assessed for AD- and other neurodegenerative disorder-related changes using previously published criteria [50]. Specifically, brains were hemisected and the fixed hemibrain was used for pathological assessment; the other hemibrain was frozen and subsequently used for biochemical assays, including proteins and lipids. The AD diagnosis was performed using National Institute on Aging (NIA) Reagan criteria, including low, intermediate, or high probability of dementia caused by AD [51]. Because the majority of cases were previously evaluated with NIA-Reagan criteria and recent studies suggest that the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) neuritic plaque score and Braak neurofibrillary tangle stage used by NIA-Reagan are the best predictors of cognitive impairment in AD [52], the NIA-Reagan criteria were used instead of the more recent NIA-AA (Alzheimer's Association) AD criteria. The neuropathological, histological and immunohistochemical examination as well as protein analyses (pTau and Aβ) of the brains in the FHS and BUADRC biorepositories are conducted by a single professional team with common protocols. AZSAND study population includes communities of northwest greater Phoenix, Arizona [53] (https://www.brainandbodydonationregistration.org/). The Brain and Body Donation Program at AZSAND started in 1987. One of its priorities and accomplishments is rapid autopsy with a 3-hour median postmortem interval (PMI) (Table 1). The participants are assessed clinically and the brains undergo neuropathologic examination with multiple relevant diagnostic staining procedures and lesion quantitation to arrive at a diagnosis based on standard criteria [53]. Brains with the diagnosis of frontotemporal lobar degeneration, chronic traumatic encephalopathy, amyotrophic lateral sclerosis, motor neuron disease, multiple sclerosis, glioblastoma and traumatic lesions were excluded. Brains with common pathologies such as Lewy bodies, primary age-related tauopathy and vascular disease were included. Each study (i.e. FHS, BUADRC and AZSAND) was approved by their respective institutional review boards.
Table 1.
Demographic characteristics of the autopsy specimens by study cohorts
| FHS | BUADRC | AZSAND | |
|---|---|---|---|
| Sample size, n | 135 | 103 | 50 |
| Men/Women, n | 57/78 | 54/49 | 32/18 |
| Age y mean ± SD | 87.4 ± 9.2 | 84.1 ± 8.8 | 83.0 ± 7.9 |
| AD/Non-AD (AD%) | 49/86 (36%) | 52/51 (49%) | 37/13 (74%) |
| PMI median (range), h | 7.3 (1.5-48) | 12.5 (1.3-49) | 3.1 (2.1-5.5) |
| APOE-ε4 allele, n | |||
| 0 | 101 | 57 | 29 |
| 1 | 32 | 39 | 18 |
| 2 | 2 | 7 | 3 |
AD diagnosis by the NIA-Reagan criteria. PMI, postmortem interval.
Lipidomic assays.
Lipids were analyzed in the extracts of frozen brain sections containing the dorsolateral prefrontal cortex (Brodmann area 9). The same blocks were used for the other biochemical assays, including Aβ40 and pTau396 (see below). All of the dissections were performed on a metal platform kept on dry ice. Using the FHS and BUADRC brain sections, the corona radiata white matter and cortical gray matter were dissected. Similar AZSAND-derived small cortical samples were carefully dissected to obtain the cortical gray matter. The tissue samples (approximately 15 mg accurately weighed) were extracted by a modified Bligh-Dyer method [53] using a 2:2:2 ratio volume of methanol/ water/ dichloromethane at room temperature after spiking internal standards. Extracted lipids were analyzed on an ABSciex Quadrupole Time of Flight-5600 equipped with a Turbo V ion source (AB Sciex, Concord, Canada) mass spectrometer, using a Shimadzu CTO-20A Nexera 32 UHPLC with Waters Acquity UPLC HSS T3 1.8-mm column (Waters, Milford, MA) as described [54-56]. As controls, an equimolar mixture of 13 authentic internal standards (Supplementary Table 1) and a characterized pool of human plasma and test pool (a pool of small aliquots from all brain tissue extracts used in this study) were analyzed along with the brain samples. Each of these controls were included several times in the randomization scheme such that sample preparation and analytical variability could be monitored.
Neurofibrillary tangle AT8 staining density.
All brain tissue was fixed in periodate-lysine-paraformaldehyde and stored at 4 °C. A tissue block from the dorsolateral prefrontal cortex contralateral to the one used for the biochemical measures was taken perpendicular to the superior frontal sulcus, embedded in paraffin and cut into 10 μm sections. Antigen retrieval was achieved by boiling sections in citrate buffer (pH 6.0) for 10 min. Sections were incubated overnight with the AT8 anti-PHF-tau antibody (Pierce Endogen, 1:2000) at 4 °C and subsequently incubated with biotinylated secondary antibody and then labeled with a 3-amino-9-ethylcarbazol HRP substrate kit (Vector Laboratories). Sections were counter stained with Gill’s Hematoxylin (Vector Laboratories H-3401) and cover-slipped with Permount mounting medium. Immunostained slides were scanned and digitized at 20× magnification with the Aperio ScanScope (Leica). The white matter/gray matter boundary was used as the outer edge of the region of interest to highlight gray matter only. The Aperio nuclear algorithm (Version 9) set to recognize and count AT8 immunoreactive neurofibrillary tangles restricted to the highlighted areas was implemented on Leica image analysis and automated counting software. A counting algorithm was created to recognize cell shape, size, and staining intensity. The Aperio positive pixel count (Version 9) was also used to determine the area of immunoreactivity. All quantifications were normalized to the area measured and are expressed as density per analyzed area.
Measurements of Aβ40 and pTau396.
Aβ40 and pTau396 were measured by ELISA in homogenates of the dorsolateral prefrontal cortex gyral crest of the frozen hemibrains as described previously [57]. Briefly, for Aβ40 the MSD #K15200E kit was used. For pTau396 quantitation, a capturing antibody against pTau396 (Abcam, ab156623; rabbit monoclonal) was used and for the detection the biotinylated HT7 antibody that recognizes residue 159-163 of Tau (Thermo Fisher Scientific) was used.
Data analyses.
The combined negative and positive MS/MS raw lipid abundance counts were used for analyses. In a typical sample approximately 1400 compounds were detected. The raw data were normalized per tissue weight, the relative abundance of each compound in each sample was expressed as a percentage and used for statistical analyses. Linear regression models and ANOVAs were used to examine the associations between MGDG levels and the AD-related traits. Depending on the analyzed cohort (see figures), the traits included AD diagnosis by the NIA-Reagan criteria; CDR; Braak and Braak (BB) stage or compressed BB stage such that stages 0,I,II=1; III,IV=2 and V,VI=3; Neuritic plaque (NP) score (expressed as 0-3 per brain region or as a sum total of 5 regions analyzed in AZSAND or as 0-3 in DLPFC of the FHS and BUADRC samples); Neurofibrillary tangle score expressed as 0-3 per brain region or as a sum total of 5 regions analyzed in AZSAND; LogAT8 antibody staining density; LogpTau396 level; and normalized Aβ40 levels. All analyses included sex and age as covariates. Including the APOE-ε4 gene dosage as a covariate did not appreciably affect the results. The results of these analyses are presented without adjusting for multiple testing with the p-value ≤ 0.05 as significant. Bonferroni-corrected results are also shown in Supplementary Table 3.
Results
MGDG composition of human brain
We first analyzed the dorsolateral prefrontal cortex (DLPFC) gray matter and subcortical corona radiata white matter samples from 135 postmortem FHS biorepository brains (discovery cohort). We subsequently validated our findings in DLPFC gray matter AZSAND samples (n=50) and DLPFC subcortical corona radiata white matter BUADRC samples (n=103). Using liquid chromatography – electrospray ionization mass spectrometry (LC-ESI-MS), we identified 40 unique MGDG species in total (14 MGDG diacyl and 26 MGDG O alkyl/acyl), where 11, 20, 24 and 27 MGDG species were present in AZSAND gray matter, FHS gray matter, BUADRC white matter and FHS white matter, respectively (Supplementary Text, Supplementary Figure 1 and Supplementary Table 2). Brain MGDGs were mostly saturated or monounsaturated (12 saturated, and 16, 6, 4, and 2 species with 1, 2, 3, and 4 double bonds, respectively) (Supplementary Table 2). There were 13 MGDG molecular species present in at least 3 of these sample groups comprising 6 diacyl and 7 alkyl/acyl compounds. The following compounds were found in all of the samples: MGDG 30:0, MGDG 34:1, MGDG 36:1, MGDG 42:1, MGDG O-36:1 and MGDG O-38:1. In the FHS samples, the total amount of MGDGs constituted 0.28 ± 0.08 % and 0.22 ± 0.11 % (mean ± SD) of all lipids, in the white and gray matter, respectively – a value consistent with data from animal studies [41]. In both brain regions, the ether lipids (MGDG O) constituted the majority (72-73%) of these compounds.
The levels of MGDG rise with the progression of AD
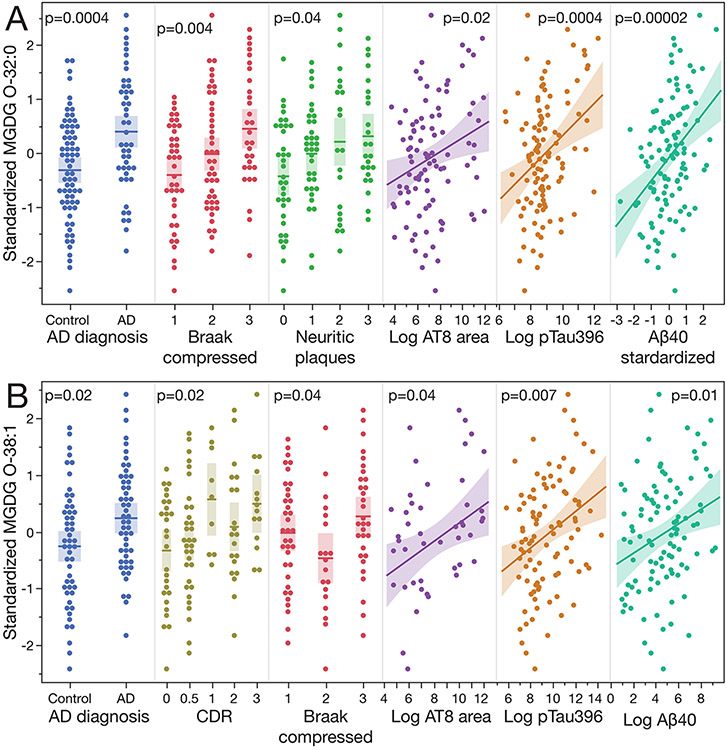
We used the FHS biorepository-derived brains as our discovery cohort to perform lipidomic analyses of the cortical gray matter – a brain tissue that, in AD, is characterized by the hallmarks of AD-related neuronal abnormalities, as well as the subcortical white matter – a tissue rich in lipids but rarely studied in the context of AD. We found that the levels of multiple MGDG species, as well as their total amounts (i.e., the sum of the diacyl MGDG species, the alkyl/acyl MGDG O species, and the sum of all of the MGDGs) were higher in AD as compared to non-AD participants and rose with AD progression as assessed by their associations with CDR, Braak stage, neuritic plaque scores, AT8 area staining, and pTau396 and Aβ40 levels (Figure 1, and Supplementary Table 3). The rise of MGDG levels in association with AD-related traits was apparent both in the cortical gray matter (Figs. 1, 2A) and in the subcortical corona radiata white matter (Fig. 1). To validate these results, we used the white matter samples derived from the BUADRC biorepository. The data were highly concordant with the FHS results (Fig. 1, 2B) and showed an even more significant association of MGDG levels with CDR and pTau396 in this cohort as compared to that in the FHS data set. To validate the gray matter results, we used brains derived from the Arizona-based AZSAND biorepository which is characterized by a short post-mortem interval (PMI) and whose participants are geographically different from the Massachusetts-based FHS cohort. Note that while the available AZSAND clinical and neuropathologic phenotypes do not fully recapitulate all AD-related traits captured in FHS, they do include the Braak stage and neuritic plaque scores. Though we identified fewer gray matter MGDG species in the AZSAND samples as compared to the FHS samples (Supplementary Table 2), many results were concordant between the 2 cohorts (Fig. 1). Remarkably the sums of MGDG and MGDG O compounds as well as the total MGDG levels were positively associated with all of the AD-related traits. The associations tended to be less significant in the AZSAND as compared to the FHS, likely due to the lower statistical power as there were only 50 AZSAND brains analyzed compared to 135 FHS samples. Figure 2 presents 2 examples of MGDG species whose levels were associated with multiple AD-related traits showing all of the data points available for the analyses. In the gray matter the levels of MGDG O-32:0 rose in association with almost all of the measured traits (except for CDR) in the FHS samples (Fig. 2A) and all of the traits assessed in the AZSAND samples. (Fig. 1). Increased levels of MGDG O-32:0 were also found in association with at least one AD-related trait in all of the analyzed sample groups. In the white matter, the levels of alkyl/acyl MGDG O-38:1 were higher in association with almost all (except for the neuritic plaque score) of the AD-related traits examined in the BUADRC samples (Fig. 2B).
Figure 1.
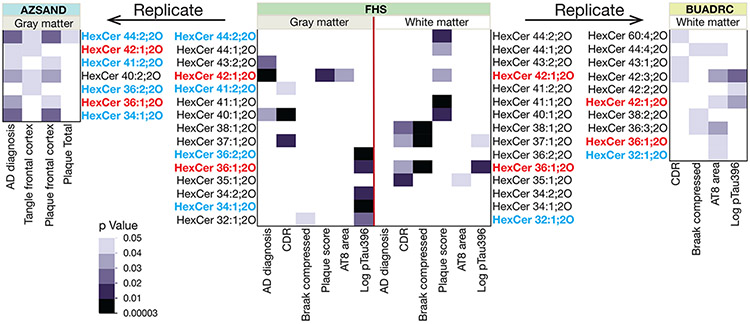
Increased levels of individual MGDG species and the sum of all MGDGs are associated with AD-related phenotypes. Untargeted lipidomic assays were performed as described in Methods and the detected and quantified compounds are annotated by the class shorthand abbreviation: MGDG indicates a diacyl compound and MGDG O indicates an alkyl/acyl compound. The numeric values separated by a colon indicate the total number of carbon atoms of the acyl/alkyl residues linked to the glycerol backbone and the number of double bonds, respectively. for age and sex. The p value of 0.05 was considered as significant. Only the individual compounds that meet this threshold are shown, whereas the sums include all of the MGDG compounds detected in the samples. The central panel shows the data for the gray and white matter of the FHS discovery cohort. The replication cohort data are AZSAND gray matter and BUADRC white matter in the left and right panels, as indicated. The compounds highlighted in blue are common to the discovery and replication cohorts and the compounds highlighted in red are common to all cohorts. The p value color coding is maintained for all of the panels for ease of comparison.
Figure 2.
A) The levels of MGDG O-32:0 in the cortical gray matter increase with the progression of AD. Data are derived from the analyses of the FHS samples. B) The levels of MGDG O-38:1 in the subcortical white matter increase with the progression of AD. Data are derived from the analyses of the BUADRC samples. The p values on the graphs derive from analyses adjusted for age and sex. The shaded areas show 95% confidence intervals. See also Fig. 1.
Interestingly, pTau396 was the trait associated with the largest number of MGDG species (Fig. 1) both in the gray matter, in which the pTau396 measures were performed, as well as in subcortical corona radiata white matter of the same brains (Fig. 1, FHS and BUADRC data sets). These consistent associations between the apparent MGDG lipidomic abnormality in AD and pTau396 levels are in line with our recent observations showing that one of the most reliable and robust brain biochemical correlates of clinical AD and AD-associated neuropathologies is the high tissue level of pTau396 [57] (Supplementary Figure 2, which illustrates these associations determined in our FHS and BUADRC DLPFC samples). Though AD risk, prevalence and certain aspects of brain pathology are sexually dimorphic [58], we did not find an association between MGDG levels and subjects’ sex. This result was consistent with our data showing no association between sex and brain levels of pTau396 (p=0.65) – the AD-related trait most strongly correlated with MGDG levels. Because APOE-ε4 allele is associated with increased risk of AD, and APOE protein modulates lipid metabolism in brain [59], we tested if brain MGDG levels were associated with the APOE-ε4 dosage (APOE-ε4 negative individuals vs APOE-ε4 carriers with 1 or 2 ε4 alleles) and included APOE-ε4 as a covariate in the above analyses. We found that the APOE-ε4 dosage had no appreciable effect on any of measures of these associations.
Because MGDG metabolism is catalyzed by the same enzymes that synthesize and degrade GCs [46] we measured the latter compounds using our untargeted lipidomics assay platform (Fig. 3). We identified 37 species of unmodified GCs in our data. Nineteen of these compounds were found in all four of our data sets and the levels of 23 of these compounds were associated with at least one trait in one of these data sets. Two GC species (HexCer 36:1;2O and HexCer 42:1;2O; Fig. 3 highlighted in red) were associated with at least one AD-related trait in all of the data sets. As was the case for the MGDG compounds, all of these associations were positive; however, the p values of all of these associations tended to be less significant (higher) than those observed for MGDGs and there was no significant association observed between the total amounts of these compounds and any AD-related trait.
Figure 3.
Increased levels of hexosylceramides (HexCer, GC) species are associated with AD-related phenotypes. Untargeted lipidomic assays were performed as described in Methods and the detected and quantified compounds are annotated by the class shorthand abbreviation: HexCer with the numeric values separated by a colon indicate the total number of carbon atoms of the ceramide acyl residues and the number of double bonds, respectively. The ;2O designation indicates that all of these compounds were hexosylceramide non-hydroxyfatty acid-dihydrosphingosines. The cell plot data are presented as p values of linear model analyses adjusted for age and sex as in Figure 1. The p value of 0.05 was considered as significant. Only the individual compounds that meet this threshold are shown. The p value color coding is maintained for all of the panels for ease of comparison. The compounds are highlighted in blue and red as in Figure 1.
Discussion
MGDGs have only recently been identified in human gray and white matter by Wood et al. who detected MGDG 34:1, MGDG 36:1 and MGDG 36:2 in a study that employed an orbitrap mass spectrometer with flow infusion and electrospray ionization [47]. Through the use of UPLC separation prior to the identification and quantitation by MS/MS our data extend these observations, providing evidence for the presence of 40 MGDG molecular species in human brain. Using these methods, we show that the brain MGDG levels rise significantly with AD progression. Importantly, the individual MGDG- and the total MGDG levels were associated with clinical (CDR) as well as neuropathological AD-related abnormalities including tauopathy (Braak stage, AT8 staining, and pTau396 levels) and amyloidosis (plaque score and Aβ40 levels). This consistent pattern of AD clinical symptom- and neuropathology-associated MGDG changes seen in the gray and white matter suggests that abnormal turnover of this lipid class may reflect a fundamental pathophysiologic process of AD and that it is unlikely to be driven by neuronal loss and/or reflects shifts in cellular composition of those tissues that accompany this disease. The robust involvement of the white matter supports previous lipidomic studies [23, 24, 28, 60-62] and points to abnormalities of MGDG turnover in oligodendrocytes – the myelin-producing principal cells of this tissue. Accumulating evidence indicates that oligodendrocytes are indeed vulnerable to AD pathophysiology [63-67]. Given that MGDG synthesis is highly associated with myelination during development [41, 42, 45], it is possible that the rise of MGDG levels in AD reflects a reactivation of the myelination program in response to the neurodegenerative process in this disease.
The biochemical mechanism of the abnormal MGDG turnover in AD brain remains to be elucidated. However, similar abnormalities previously shown among hexosylceramides [21, 25, 68], which, together with MGDGs, are synthesized and degraded by a common set of enzymes, suggest a more general defect in galactolipid turnover in AD. In brain, this pathway predominantly utilizes ceramide backbone lipids, and thus, we propose that the accumulation of MGDG in AD brain may be the result of altered specificity of the MGDG synthesizing or degrading enzymes whose predominant function in the adult brain is the turnover of GCs, such that in AD, in addition to their normal ceramide-based substrates, they are able to utilize the lipids with a glycerol backbone. Synthesis of MGDGs is catalyzed by UDP-galactose:ceramide galactosyltransferase (CGT, UGT8A) which has been postulated as a key regulator of the oligodendrocyte/myelin-enriched genetic networks dysregulated in AD [63]. The breakdown of MGDGs is catalyzed by a complex of galactosylceramidase (GALC) and saposin A (SapA) [69]. Mutations in either GALC or SapA cause a neurodegenerative disorder, Krabbe disease (globoid cell leukodystrophy) [70]. We recently reported that the SapA precursor, prosaposin (PSAP), interacts with amyloid plaques [71], and others have found that its concentrations are elevated in the CSF of AD patients and individuals with preclinical AD [72]. While these data suggest that PSAP-mediated functions may be abnormal in AD, no information is available about the levels or localization of SapA specifically in the human brain. Interestingly, though the levels of both the diacyl and the alkyl/acyl MGDG species were elevated in AD, the latter compounds are more abundant. These MGDG O lipids may function as antioxidants [73, 74] and the increase in their levels might protect against oxidative stress. Moreover, the synthesis of the MGDG O compounds requires a supply of alkyl/acyl glycerol derived from peroxisomes, which exhibit multiple and complex abnormalities in AD [75-77].
In conclusion, our data show that abnormal metabolism of the MGDG class of glycerolipids is a striking feature of AD pathophysiology and raise key questions for future mechanistic investigations, namely what are the enzymatic alterations which cause the accumulation of MGDGs in AD brain and which cell types are vulnerable to this pathophysiologic process. Overall, the MGDG metabolic defect is a novel lipid abnormality associated with AD progression.
Supplementary Material
Supplementary Figure 1. Identification of MGDG in human brain by UHPLC MS/MS: representative examples. A) The chemical structure of MGDG 34:0, and the fragmentation product spectrum showing the addition and loss of acetate, the 16:0 and 18:0 fatty acids, and galactosylglycerol. B) The structure of the MGDG O-32:0 ether compound and its fragmentation product spectrum showing the addition and loss of acetate, loss of the 16:0 fatty acid, and the 16:0 fatty acid itself. The purple lines in A and B on the chemical structures illustrate fragmentation.
Supplementary Figure 2. Positive association of the cortical tissue levels of the tau protein phosphorylated on serine 396 (pTau396) with AD diagnosis, CDR, Braak stage, neuritic plaque score phospho-tau staining (AT8) area and Aβ40 levels. Cortical pTau396 levels were measured by an ELISA in the gray matter samples of the FHS and BUADRC cohorts as indicated. −Log(10)p values adjusted for age and sex are shown.
Acknowledgments
The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract No. N01-HC-25195, HHSN268201500001I and 75N92019D00031). We are grateful to the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of human biological materials. The Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 and P30AG072980, Arizona Alzheimer’s Disease Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Funding
National Institutes of Health grant RF1AG057768 (JKB, TDS)
National Institutes of Health grant RF1AG078299 (JKB)
National Institutes of Health grant U01AG068221 (HL)
National Institutes of Health grant P30AG072978 Boston University AD Research Center (TDS)
National Institutes of Health grant U19AG068753 (TDS, JKB)
National Heart, Lung and Blood Institute (N01-HC-25195, 75N92019D00031 and HHSN2682015000011) (TDS)
United States Department of Veterans Affairs, Veterans Health Administration, BLRD Merit Award I01BX005933 (TDS)
National Institute of Neurological Disorders and Stroke U24 NS072026 (TB)
National Institute on Aging P30AG19610 (TB)
National Institute on Aging P30AG072980 (TB)
Arizona Department of Health Services contract 211002 (TB)
Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001) (TB)
Michael J. Fox Foundation for Parkinson's Research (TB)
Footnotes
Conflict of interest
The authors have no conflict of interest to report.
Data Availability
The data supporting the findings of this study are available within the article and/or its supplementary material. Additional datasets generated and/or analyzed during the study are available from the corresponding authors on reasonable request.
References
- [1].Knopman DS, Amieva H, Petersen RC, Chetelat G, Holtzman DM, Hyman BT, Nixon RA, Jones DT (2021) Alzheimer disease. Nat Rev Dis Primers 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yu M, Sporns O, Saykin AJ (2021) The human connectome in Alzheimer disease - relationship to biomarkers and genetics. Nat Rev Neurol 17, 545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barany M, Chang YC, Arus C, Rustan T, Frey WH (1985) Increased glycerol-3-phosphorylcholine in post-mortem Alzheimer's brain. Lancet 1, 517. [DOI] [PubMed] [Google Scholar]
- [4].Pettegrew JW, Panchalingam K, Moossy J, Martinez J, Rao G, Boller F (1988) Correlation of phosphorus-31 magnetic resonance spectroscopy and morphologic findings in Alzheimer's disease. Arch Neurol 45, 1093–1096. [DOI] [PubMed] [Google Scholar]
- [5].Blusztajn JK, Lopez Gonzalez-Coviella I, Logue M, Growdon JH, Wurtman RJ (1990) Levels of phospholipid catabolic intermediates, glycerophosphocholine and glycerophosphoethanolamine, are elevated in brains of Alzheimer's disease but not of Down's syndrome patients. Brain Res 536, 240–244. [DOI] [PubMed] [Google Scholar]
- [6].Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ (1992) Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci USA 89, 1671–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL (1995) Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer's disease brain. Brain Res 698, 223–226. [DOI] [PubMed] [Google Scholar]
- [8].Farooqui AA, Rapoport SI, Horrocks LA (1997) Membrane phospholipid alterations in Alzheimer's disease: Deficiency of ethanolamine plasmalogens. Neurochem Res 22, 523–527. [DOI] [PubMed] [Google Scholar]
- [9].Guan ZZ, Wang YA, Cairns NJ, Lantos PL, Dallner G, Sindelar PJ (1999) Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol 58, 740–747. [DOI] [PubMed] [Google Scholar]
- [10].Han XL, Holtzman DM, McKeel DW Jr., (2001) Plasmalogen deficiency in early Alzheimer's disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem 77, 1168–1180. [DOI] [PubMed] [Google Scholar]
- [11].Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ (2001) Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem Res 26, 771–782. [DOI] [PubMed] [Google Scholar]
- [12].Igarashi M, Ma K, Gao F, Kim HW, Rapoport SI, Rao JS (2011) Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer's disease prefrontal cortex. J Alzheimers Dis 24, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yuki D, Sugiura Y, Zaima N, Akatsu H, Takei S, Yao I, Maesako M, Kinoshita A, Yamamoto T, Kon R, Sugiyama K, Setou M (2014) DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer's disease. Sci Rep 4, 7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Otoki Y, Kato S, Nakagawa K, Harvey DJ, Jin LW, Dugger BN, Taha AY (2021) Lipidomic Analysis of Postmortem Prefrontal Cortex Phospholipids Reveals Changes in Choline Plasmalogen Containing Docosahexaenoic Acid and Stearic Acid Between Cases With and Without Alzheimer's Disease. Neuromolecular Med 23, 161–175. [DOI] [PubMed] [Google Scholar]
- [15].Batra R, Arnold M, Worheide MA, Allen M, Wang X, Blach C, Levey AI, Seyfried NT, Ertekin-Taner N, Bennett DA, Kastenmuller G, Kaddurah-Daouk RF, Krumsiek J, Alzheimer's Disease Metabolomics C (2022) The landscape of metabolic brain alterations in Alzheimer's disease. Alzheimers Dement 19, 980–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wood PL, Woltjer RL (2022) Serine ether glycerophospholipids: Decrements in the frontal cortex associated with mild cognitive impairment and Alzheimer's disease. Front Aging Neurosci 14, 981868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Varma VR, Oommen AM, Varma S, Casanova R, An Y, Andrews RM, O'Brien R, Pletnikova O, Troncoso JC, Toledo J, Baillie R, Arnold M, Kastenmueller G, Nho K, Doraiswamy PM, Saykin AJ, Kaddurah-Daouk R, Legido-Quigley C, Thambisetty M (2018) Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med 15, e1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Han XL, Holtzman DM, McKeel DW Jr., Kelley J, Morris JC (2002) Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem 82, 809–818. [DOI] [PubMed] [Google Scholar]
- [19].Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA 101, 2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Katsel P, Li C, Haroutunian V (2007) Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer's disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer's disease? Neurochem Res 32, 845–856. [DOI] [PubMed] [Google Scholar]
- [21].Marks N, Berg MJ, Saito M, Saito M (2008) Glucosylceramide synthase decrease in frontal cortex of Alzheimer brain correlates with abnormal increase in endogenous ceramides: consequences to morphology and viability on enzyme suppression in cultured primary neurons. Brain research 1191, 136–147. [DOI] [PubMed] [Google Scholar]
- [22].Filippov V, Song MA, Zhang K, Vinters HV, Tung S, Kirsch WM, Yang J, Duerksen-Hughes PJ (2012) Increased ceramide in brains with Alzheimer's and other neurodegenerative diseases. J Alzheimers Dis 29, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gottfries CG, Bartfai T, Carlsson A, Eckernas S, Svennerholm L (1986) Multiple biochemical deficits in both gray and white matter of Alzheimer brains. Prog Neuropsychopharmacol Biol Psychiatry 10, 405–413. [DOI] [PubMed] [Google Scholar]
- [24].Wallin A, Gottfries CG, Karlsson I, Svennerholm L (1989) Decreased myelin lipids in Alzheimer's disease and vascular dementia. Acta NeurolScand 80, 319–323. [DOI] [PubMed] [Google Scholar]
- [25].Cheng H, Wang M, Li JL, Cairns NJ, Han X (2013) Specific changes of sulfatide levels in individuals with pre-clinical Alzheimer's disease: an early event in disease pathogenesis. J Neurochem 127, 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brooksbank BWL, Martinez M (1989) Gangliosides in the brain in adult Down's syndrome and Alzheimer's disease. Mol Chem Neuropath 11, 157–185. [DOI] [PubMed] [Google Scholar]
- [27].Kracun I, Kalanj S, Talan-Hranilovic J, Cosovic C (1992) Cortical distribution of gangliosides in Alzheimer's disease. Neurochem Int 20, 433–438. [DOI] [PubMed] [Google Scholar]
- [28].Svennerholm L, Gottfries C-G (1994) Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). JNeurochem 62, 1039–1047. [DOI] [PubMed] [Google Scholar]
- [29].Ng Ying Kin NMK, Pan LH, Louvaris JH, Robitaille Y, Nair NPV (1995) Differential changes in regional brain ganglioside and neutral glycosphingolipid contents in Alzheimer's disease. Adv Exp Med Biol 363, 57–64. [DOI] [PubMed] [Google Scholar]
- [30].Fukami Y, Ariga T, Yamada M, Yuki N (2017) Brain Gangliosides in Alzheimer's Disease: Increased Expression of Cholinergic Neuron-Specific Gangliosides. Curr Alzheimer Res 14, 586–591. [DOI] [PubMed] [Google Scholar]
- [31].Kaya I, Jennische E, Dunevall J, Lange S, Ewing AG, Malmberg P, Baykal AT, Fletcher JS (2020) Spatial Lipidomics Reveals Region and Long Chain Base Specific Accumulations of Monosialogangliosides in Amyloid Plaques in Familial Alzheimer's Disease Mice (5xFAD) Brain. ACS Chem Neurosci 11, 14–24. [DOI] [PubMed] [Google Scholar]
- [32].Söderberg M, Edlund C, Alafuzoff I, Kristensson K, Dallner G (1992) Lipid composition in different regions of the brain in Alzheimer's disease/senile dementia of Alzheimer's type. J Neurochem 59, 1646–1653. [DOI] [PubMed] [Google Scholar]
- [33].Kosicek M, Zetterberg H, Andreasen N, Peter-Katalinic J, Hecimovic S (2012) Elevated cerebrospinal fluid sphingomyelin levels in prodromal Alzheimer's disease. Neuroscience letters 516, 302–305. [DOI] [PubMed] [Google Scholar]
- [34].Baloni P, Arnold M, Buitrago L, Nho K, Moreno H, Huynh K, Brauner B, Louie G, Kueider-Paisley A, Suhre K, Saykin AJ, Ekroos K, Meikle PJ, Hood L, Price ND, Alzheimer's Disease Metabolomics C, Doraiswamy PM, Funk CC, Hernandez AI, Kastenmuller G, Baillie R, Han X, Kaddurah-Daouk R (2022) Multi-Omic analyses characterize the ceramide/sphingomyelin pathway as a therapeutic target in Alzheimer's disease. Commun Biol 5, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Söderberg M, Edlund C, Kristensson K, Dallner G (1991) Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids 26, 421–425. [DOI] [PubMed] [Google Scholar]
- [36].Skinner ER, Watt C, Besson JAO, Best PV (1993) Differences in the fatty acid composition of the grey and white matter of different regions of the brains of patients with Alzheimer's disease and control subjects. Brain 116, 717–725. [DOI] [PubMed] [Google Scholar]
- [37].Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, Morris MC (2012) Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 29, 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Snowden SG, Ebshiana AA, Hye A, An Y, Pletnikova O, O'Brien R, Troncoso J, Legido-Quigley C, Thambisetty M (2017) Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med 14, e1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nasaruddin ML, Pan X, McGuinness B, Passmore P, Kehoe PG, Holscher C, Graham SF, Green BD (2018) Evidence That Parietal Lobe Fatty Acids May Be More Profoundly Affected in Moderate Alzheimer's Disease (AD) Pathology Than in Severe AD Pathology. Metabolites 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Steim JM (1967) Monogalactosyl diglyceride: a new neurolipid. Biochim Biophys Acta 144, 118–126. [DOI] [PubMed] [Google Scholar]
- [41].Pieringer RA, Deshmukh DS, Flynn TJ (1973) The association of the galactosyl diglycerides of nerve tissue with myelination. Prog Brain Res 40, 398–405. [PubMed] [Google Scholar]
- [42].Deshmukh DS, Flynn TJ, Pieringer RA (1974) The biosynthesis and concentration of galactosyl diglyceride in glial and neuronal enriched fractions of actively myelinating rat brain. J Neurochem 22, 479–485. [DOI] [PubMed] [Google Scholar]
- [43].Wenger DA, Petitpas JW, Pieringer RA (1968) The metabolism of glyceride glycolipids. II. Biosynthesis of monogalactosyl diglyceride from uridine diphosphate galactose and diglyceride in brain. Biochemistry 7, 3700–3707. [DOI] [PubMed] [Google Scholar]
- [44].van der Bijl P, Strous GJ, Lopes-Cardozo M, Thomas-Oates J, van Meer G (1996) Synthesis of non-hydroxy-galactosylceramides and galactosyldiglycerides by hydroxyceramide galactosyltransferase. Biochem J 317 ( Pt 2), 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wells MA, Dittmer JC (1967) A comprehensive study of the postnatal changes in the concentration of the lipids of developing rat brain. Biochemistry 6, 3169–3175. [DOI] [PubMed] [Google Scholar]
- [46].Tanphaichitr N, Kongmanas K, Faull KF, Whitelegge J, Compostella F, Goto-Inoue N, Linton JJ, Doyle B, Oko R, Xu H, Panza L, Saewu A (2018) Properties, metabolism and roles of sulfogalactosylglycerolipid in male reproduction. Prog Lipid Res 72, 18–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wood PL, Hauther KA, Scarborough JH, Craney DJ, Dudzik B, Cebak JE, Woltjer RL (2021) Human Brain Lipidomics: Utilities of Chloride Adducts in Flow Injection Analysis. Life (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Au R, Seshadri S, Knox K, Beiser A, Himali JJ, Cabral HJ, Auerbach S, Green RC, Wolf PA, McKee AC (2012) The Framingham Brain Donation Program: neuropathology along the cognitive continuum. Curr Alzheimer Res 9, 673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Besser LM, Kukull WA, Teylan MA, Bigio EH, Cairns NJ, Kofler JK, Montine TJ, Schneider JA, Nelson PT (2018) The Revised National Alzheimer's Coordinating Center's Neuropathology Form-Available Data and New Analyses. J Neuropathol Exp Neurol 77, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mez J, Solomon TM, Daneshvar DH, Murphy L, Kiernan PT, Montenigro PH, Kriegel J, Abdolmohammadi B, Fry B, Babcock KJ, Adams JW, Bourlas AP, Papadopoulos Z, McHale L, Ardaugh BM, Martin BR, Dixon D, Nowinski CJ, Chaisson C, Alvarez VE, Tripodis Y, Stein TD, Goldstein LE, Katz DI, Kowall NW, Cantu RC, Stern RA, McKee AC (2015) Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alzheimers Res Ther 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET (1999) Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol 58, 1147–1155. [DOI] [PubMed] [Google Scholar]
- [52].Serrano-Pozo A, Qian J, Muzikansky A, Monsell SE, Montine TJ, Frosch MP, Betensky RA, Hyman BT (2016) Thal Amyloid Stages Do Not Significantly Impact the Correlation Between Neuropathological Change and Cognition in the Alzheimer Disease Continuum. J Neuropathol Exp Neurol 75, 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, Sabbagh MN (2015) Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology 35, 354–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Afshinnia F, Rajendiran TM, Karnovsky A, Soni T, Wang X, Xie D, Yang W, Shafi T, Weir MR, He J, Brecklin CS, Rhee EP, Schelling JR, Ojo A, Feldman H, Michailidis G, Pennathur S (2016) Lipidomic Signature of Progression of Chronic Kidney Disease in the Chronic Renal Insufficiency Cohort. Kidney Int Rep 1, 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Afshinnia F, Rajendiran TM, Soni T, Byun J, Wernisch S, Sas KM, Hawkins J, Bellovich K, Gipson D, Michailidis G, Pennathur S, Michigan Kidney Translational Core CIG (2018) Impaired beta-Oxidation and Altered Complex Lipid Fatty Acid Partitioning with Advancing CKD. J Am Soc Nephrol 29, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Maile MD, Standiford TJ, Engoren MC, Stringer KA, Jewell ES, Rajendiran TM, Soni T, Burant CF (2018) Associations of the plasma lipidome with mortality in the acute respiratory distress syndrome: a longitudinal cohort study. Respir Res 19, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stathas S, Alvarez VE, Xia W, Nicks R, Meng G, Daley S, Pothast M, Shah A, Kelley H, Esnault C, McCormack R, Dixon E, Fishbein L, Cherry JD, Huber BR, Tripodis Y, Alosco ML, Mez J, McKee AC, Stein TD (2021) Tau phosphorylation sites serine202 and serine396 are differently altered in chronic traumatic encephalopathy and Alzheimer's disease. Alzheimers Dement 18, 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, Baracchi F, Girouard H, Misoch S, Giacobini E, Depypere H, Hampel H, Women's Brain P, the Alzheimer Precision Medicine I (2018) Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol 14, 457–469. [DOI] [PubMed] [Google Scholar]
- [59].Martens YA, Zhao N, Liu CC, Kanekiyo T, Yang AJ, Goate AM, Holtzman DM, Bu G (2022) ApoE Cascade Hypothesis in the pathogenesis of Alzheimer's disease and related dementias. Neuron 110, 1304–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Englund E, Brun A, Alling C (1988) White matter changes in dementia of Alzheimer's type. Biochemical and neuropathological correlates. Brain 111, 1425–1439. [DOI] [PubMed] [Google Scholar]
- [61].Wood PL, Barnette BL, Kaye JA, Quinn JF, Woltjer RL (2015) Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer's disease subjects. Acta Neuropsychiatr 27, 270–278. [DOI] [PubMed] [Google Scholar]
- [62].Obis E, Sol J, Andres-Benito P, Martin-Gari M, Mota-Martorell N, Galo-Licona JD, Pinol-Ripoll G, Portero-Otin M, Ferrer I, Jove M, Pamplona R (2023) Lipidomic Alterations in the Cerebral Cortex and White Matter in Sporadic Alzheimer's Disease. Aging Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].McKenzie AT, Moyon S, Wang M, Katsyv I, Song WM, Zhou X, Dammer EB, Duong DM, Aaker J, Zhao Y, Beckmann N, Wang P, Zhu J, Lah JJ, Seyfried NT, Levey AI, Katsel P, Haroutunian V, Schadt EE, Popko B, Casaccia P, Zhang B (2017) Multiscale network modeling of oligodendrocytes reveals molecular components of myelin dysregulation in Alzheimer's disease. Mol Neurodegener 12, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tse KH, Cheng A, Ma F, Herrup K (2018) DNA damage-associated oligodendrocyte degeneration precedes amyloid pathology and contributes to Alzheimer's disease and dementia. Alzheimers Dement 14, 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Allen M, Wang X, Burgess JD, Watzlawik J, Serie DJ, Younkin CS, Nguyen T, Malphrus KG, Lincoln S, Carrasquillo MM, Ho C, Chakrabarty P, Strickland S, Murray ME, Swarup V, Geschwind DH, Seyfried NT, Dammer EB, Lah JJ, Levey AI, Golde TE, Funk C, Li H, Price ND, Petersen RC, Graff-Radford NR, Younkin SG, Dickson DW, Crook JR, Asmann YW, Ertekin-Taner N (2018) Conserved brain myelination networks are altered in Alzheimer's and other neurodegenerative diseases. Alzheimers Dement 14, 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sadick JS, O'Dea MR, Hasel P, Dykstra T, Faustin A, Liddelow SA (2022) Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer's disease. Neuron 110, 1788–1805 e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kenigsbuch M, Bost P, Halevi S, Chang Y, Chen S, Ma Q, Hajbi R, Schwikowski B, Bodenmiller B, Fu H, Schwartz M, Amit I (2022) A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nature neuroscience 25, 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dehghan A, Pinto RC, Karaman I, Huang J, Durainayagam BR, Ghanbari M, Nazeer A, Zhong Q, Liggi S, Whiley L, Mustafa R, Kivipelto M, Solomon A, Ngandu T, Kanekiyo T, Aikawa T, Radulescu CI, Barnes SJ, Graca G, Chekmeneva E, Camuzeaux S, Lewis MR, Kaluarachchi MR, Ikram MA, Holmes E, Tzoulaki I, Matthews PM, Griffin JL, Elliott P (2022) Metabolome-wide association study on ABCA7 indicates a role of ceramide metabolism in Alzheimer's disease. Proc Natl Acad Sci U S A 119, e2206083119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hill CH, Cook GM, Spratley SJ, Fawke S, Graham SC, Deane JE (2018) The mechanism of glycosphingolipid degradation revealed by a GALC-SapA complex structure. Nat Commun 9, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Suzuki K (2003) Globoid cell leukodystrophy (Krabbe's disease): update. J Child Neurol 18, 595–603. [DOI] [PubMed] [Google Scholar]
- [71].Mendsaikhan A, Tooyama I, Bellier JP, Serrano GE, Sue LI, Lue LF, Beach TG, Walker DG (2019) Characterization of lysosomal proteins Progranulin and Prosaposin and their interactions in Alzheimer's disease and aged brains: increased levels correlate with neuropathology. Acta Neuropathol Commun 7, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Andersson A, Remnestal J, Nellgard B, Vunk H, Kotol D, Edfors F, Uhlen M, Schwenk JM, Ilag LL, Zetterberg H, Blennow K, Manberg A, Nilsson P, Fredolini C (2019) Development of parallel reaction monitoring assays for cerebrospinal fluid proteins associated with Alzheimer's disease. Clin Chim Acta 494, 79–93. [DOI] [PubMed] [Google Scholar]
- [73].Paul S, Lancaster GI, Meikle PJ (2019) Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog Lipid Res 74, 186–195. [DOI] [PubMed] [Google Scholar]
- [74].Jove M, Mota-Martorell N, Obis E, Sol J, Martin-Gari M, Ferrer I, Portero-Otin M, Pamplona R (2023) Ether Lipid-Mediated Antioxidant Defense in Alzheimer's Disease. Antioxidants (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kou J, Kovacs GG, Hoftberger R, Kulik W, Brodde A, Forss-Petter S, Honigschnabl S, Gleiss A, Brugger B, Wanders R, Just W, Budka H, Jungwirth S, Fischer P, Berger J (2011) Peroxisomal alterations in Alzheimer's disease. Acta Neuropathol 122, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lizard G, Rouaud O, Demarquoy J, Cherkaoui-Malki M, Iuliano L (2012) Potential roles of peroxisomes in Alzheimer's disease and in dementia of the Alzheimer's type. J Alzheimers Dis 29, 241–254. [DOI] [PubMed] [Google Scholar]
- [77].Semikasev E, Ahlemeyer B, Acker T, Schanzer A, Baumgart-Vogt E (2023) Rise and fall of peroxisomes during Alzheimer s disease: a pilot study in human brains. Acta Neuropathol Commun 11, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kind T, Liu KH, Lee DY, DeFelice B, Meissen JK, Fiehn O (2013) LipidBlast in silico tandem mass spectrometry database for lipid identification. Nature methods 10, 755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yu D, Rupasinghe TWT, Boughton BA, Natera SHA, Hill CB, Tarazona P, Feussner I, Roessner U (2018) A high-resolution HPLC-QqTOF platform using parallel reaction monitoring for in-depth lipid discovery and rapid profiling. Anal Chim Acta 1026, 87–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Identification of MGDG in human brain by UHPLC MS/MS: representative examples. A) The chemical structure of MGDG 34:0, and the fragmentation product spectrum showing the addition and loss of acetate, the 16:0 and 18:0 fatty acids, and galactosylglycerol. B) The structure of the MGDG O-32:0 ether compound and its fragmentation product spectrum showing the addition and loss of acetate, loss of the 16:0 fatty acid, and the 16:0 fatty acid itself. The purple lines in A and B on the chemical structures illustrate fragmentation.
Supplementary Figure 2. Positive association of the cortical tissue levels of the tau protein phosphorylated on serine 396 (pTau396) with AD diagnosis, CDR, Braak stage, neuritic plaque score phospho-tau staining (AT8) area and Aβ40 levels. Cortical pTau396 levels were measured by an ELISA in the gray matter samples of the FHS and BUADRC cohorts as indicated. −Log(10)p values adjusted for age and sex are shown.
Data Availability Statement
The data supporting the findings of this study are available within the article and/or its supplementary material. Additional datasets generated and/or analyzed during the study are available from the corresponding authors on reasonable request.