Abstract
Purpose of review
This review comments on the current guidelines for the treatment of wound infections under definition of acute bacterial skin and skin structure infections (ABSSSI). However, wound infections around a catheter, such as driveline infections of a left ventricular assist device (LVAD) are not specifically listed under this definition in any of the existing guidelines.
Recent findings
Definitions and classification of LVAD infections may vary across countries, and the existing guidelines and recommendations may not be equally interpreted among physicians, making it unclear if these infections can be considered as ABSSSI. Consequently, the use of certain antibiotics that are approved for ABSSSI may be considered as ‘off-label’ for LVAD infections, leading to rejection of reimbursement applications in some countries, affecting treatment strategies, and hence, patients’ outcomes. However, we believe driveline exit site infections related to LVAD can be included within the ABSSSI definition.
Summary
We argue that driveline infections meet the criteria for ABSSSI which would enlarge the ‘on-label’ antibiotic armamentarium for treating these severe infections, thereby improving the patients’ quality of life.
Keywords: acute bacterial skin and skin structure infection, driveline infections, heart assist devices, skin and soft tissue infections, wound infections
INTRODUCTION
Heart failure is a leading cause of morbidity and mortality worldwide, affecting 1–3% of the adult population in industrialized countries, and its prevalence is expected to increase substantially in the future [1]. The use of a left ventricular assist device (LVAD) has revolutionized the treatment of end-stage heart failure. LVADs are used as a bridge to recovery or bridge to transplantation for patients waiting for a donor heart, or as a destination therapy for patients who are ineligible for heart transplant [2,3▪]. A major complication can be LVAD-related infections. However, current treatment guidelines and regulations for these infections are not always clear, and can be improved. As patients should receive the best possible care, especially in palliative care settings, access to appropriate and effective treatment is of crucial importance.
An LVAD is an electromechanical pump, which assists cardiac circulation and can partially or completely replace a failing ventricle. The device consists of an internal pump and an extracorporeal controller unit with batteries. A percutaneous wire, known as the driveline, connects both components [4]. Infections affecting the soft tissues around the driveline outlet, so-called driveline infections (DLIs), are the most common complications, with a reported incidence range among LVAD recipients of 10–52% [3▪,4–6,7▪,8–10,11▪]. Although most DLIs are local, they may also extend into the pump pocket, heart or chest wall, or spread into the bloodstream, leading to potentially life-threatening infections [4]. DLIs are also a major risk factor for additional complications such as sepsis or stroke, hospital readmissions and mortality [4]. Since the number of patients who require LVAD support for longer periods of time is rising and the incidence of DLIs increases with the duration of support [5,9,12], the need for outpatient care of LVAD patients with DLIs is increasing.
The majority of LVAD infections are caused by Gram-positive bacteria. Staphylococcus aureus and S. epidermidis are the most commonly identified organisms, together accounting for approximately 50% of LVAD infections [4,13]. The spectrum of pathogens involved in DLIs is described in detail below (see section Microbiological profile of ABSSSI and LVAD DLI).
Clinical management of DLIs consists of a 2–4-week antibiotics course combined with surgical debridement [8,14,15]. However, recurrent infections often require a prolonged course of antibiotics which evolves toward parenteral therapy when resistant organisms develop [16]. Long-lasting, suppressive antimicrobial therapy may also be required for device or pocket infections to prevent clinical relapse [5].
Box 1.
no caption available
CURRENT GUIDELINES
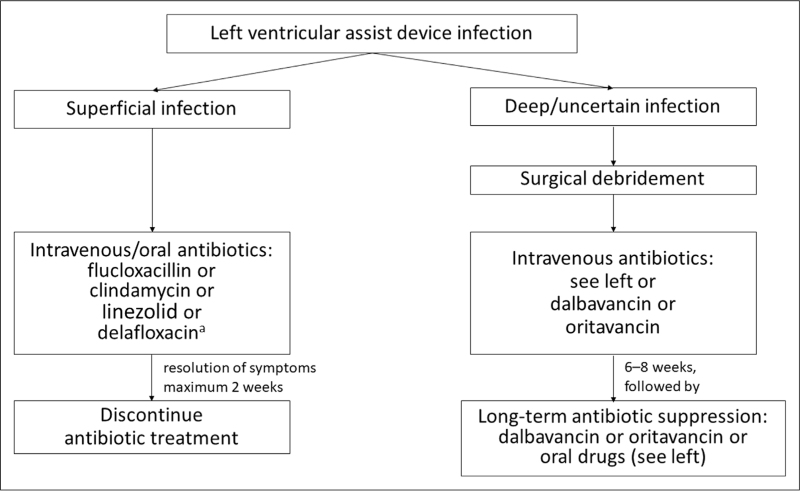
The guidelines of the Infectious Disease Council of the International Society for Heart and Lung Transplantation (ISHLT) recommend basing the treatment strategies on the identification of the responsible pathogen, the location and depth of infection (though the latter is sometimes difficult to assess without surgical exploration), and the transplant candidacy status of the patient [15]. As a first-line treatment for local superficial DLIs, the ISHLT recommends empiric therapy with oral or intravenous (i.v.) antibiotics for a minimum of 2 weeks, or until infection has resolved. DLIs of uncertain depth or deep DLIs should be treated with surgical source control (i.e., debridement and removal of infected and necrotic tissue, if necessary) as well as i.v. antibiotics, until clinical stabilization and improvement of infection (usually 6–8 weeks), followed by a long-term (oral) antibiotic suppression therapy [15]. The guidelines refrain from specifying antibiotic agents but recommend to carefully consider local and institutional epidemiology as well as antibiotic susceptibility patterns, paying special attention to methicillin-resistant S. aureus (MRSA) [15]. The optimal empiric therapy for DLIs as well as a standard definition for early localized infection have not yet been established [14]. In general, broad-spectrum antibiotics are initiated and adjusted to targeted antibiotic treatment upon identification of the specific pathogen [14,15].
Other recommendations, such as the Driveline Expert STagINg and carE (DESTINE) consensus, focus more on the prevention, early detection, and stage-related management of DLIs, including surgical source control measures [3▪], without referring to MRSA, although this can be problematic in some regions or institutions [17].
PROBLEMS WITH CURRENT GUIDELINES
In addition to the lack of specific guidance for the choice of antibiotics for treatment of DLIs, definitions and classification of LVAD infections vary across countries and studies, and the existing guidelines and recommendations are not construed equally among physicians. Differences in understanding and interpretation of the definitions and respective guidelines, especially outside the United States, may lead to misunderstanding or misclassification of the infections and impede evidence-based recommendations or comparisons. For example, in the Anglo-Saxon literature, the term ‘cellulitis’ is used as a generic term for erysipelas or for limited (or sometimes severe) soft tissue infections, while in the German terminology, the term ‘limited phlegmon’ (limited soft tissue infection/limited cellulitis) is used. National guidelines, such as the German guideline for parenteral therapy of soft tissue infections (under the auspices of the Paul-Ehrlich-Gesellschaft für Infektionstherapie e.V.) [18▪,19] have only recently tried to clarify the definitions, but misunderstandings may still occur.
An additional source of confusion arises from the term ‘acute bacterial skin and skin structure infection’ (ABSSSI, see details below), which uses the extent of erythema as a quantifiable criterion for evaluation of therapeutic response [20,21]. Guidelines or recommendations for treatment of wound or soft tissue infections under the definition of ABSSSIs have been published [18▪,19–22,23▪▪], but since DLIs are not explicitly included in the definition of ABSSSIs, it can be unclear to the treating physician or the regulatory authorities whether these treatment guidelines also apply to DLIs. Consequently, the use of certain antibiotics that are approved for ABSSSIs may be considered ‘off-label’ when used for treating DLIs, leading to rejection of reimbursement applications in some countries, thereby, affecting treatment strategies and patients’ outcomes.
Historically, most antimicrobials with broad activity against Gram-positive pathogens have been approved for specific indications (only). From the perspective of the infectious disease specialist, this limits the available therapeutic options. The choice of substances active against Gram-positive bacteria should mainly be driven by the rules of microbiological susceptibility testing, pharmacodynamics and pharmacokinetics, as is common practice for so-called reserve antimicrobials that are active against Gram-negative pathogens (e.g., cefiderocol, ceftazidime/avibactam, cetolozane/tazobactam, imipenem/relebactam).
CURRENT DEFINITIONS
Skin and soft tissue infections (SSTIs) include a variety of bacterial infections of the skin and subcutaneous tissue, muscle, and fascia and range from mild to serious life-threatening infections [18▪,22,24,25]. Older age, immunosenescence or immunocompromising conditions, obesity, trauma, and comorbidities such as diabetes mellitus or cardiopulmonary or hepatorenal disease are the main risk factors for SSTIs [26] and a substantial proportion of SSTIs is associated with hospitalization and significant morbidity [18▪,22,24–28]. SSTIs are usually classified according to the causative pathogen with its associated toxins or enzymes, and their clinical presentation and severity [26]. SSTIs have been further classified into simple (uncomplicated) or complicated (can be necrotizing or nonnecrotizing) infections, with the distinction depending on several factors, including comorbidities. Simple infections are restricted to the skin and underlying superficial soft tissues, and typically respond well to outpatient management. Complicated infections extend into the underlying deep tissues and may be associated with systemic inflammatory response syndrome or sepsis, and ischemic necrosis in rare cases [29].
ABSSSI is a term introduced by the Food and Drug Administration (FDA) to assist the drug development industry in designing clinical trials for the treatment of skin infections. ABSSSIs constitute a subset of SSTIs that is usually treated with parenteral antibiotics [20]. According to the FDA definition, ABSSSIs include cellulitis/erysipelas, wound infection, and major cutaneous abscess, and have a minimum lesion surface area of approximately 75 cm2[21]. Although not explicitly mentioned in the FDA guidance, subsequent guidelines have clarified the term ‘wound infection’ as infection resulting from minor injuries that break the skin, or from other causes such as animal bites or foreign objects such as gunshot and knife wounds [20]. This quantitative parameter was primarily intended to enable quantification of the response to therapy. In trial designs, a sufficiently large lesion size would allow a more reliable, quantitative estimate of treatment effects in patients having a surgical incision and drainage for a minor cutaneous abscess [21].
However, ‘ABSSSI’ is an artificial term that was introduced to improve standardization of clinical studies and is a valid inclusion criterion for clinical trials, but in clinical practice, size is not a relevant diagnostic criterion. It was not meant as a term for a particular diagnosis and does not, for example, distinguish between Streptococcus pyogenes-induced erysipelas and S. aureus-caused cellulitis which require different narrow-spectrum antibiotics [18▪,19]. When the term ‘ABSSSI’ is used in drug approval dossiers (since it is the term used in inclusion criteria of the respective clinical studies), one must consider which clinically defined soft tissue infections would fall under this term. Thus, we believe there is a need to clarify and harmonize the different definitions.
Considering that LVAD DLIs are soft tissue infections or wound infections around a percutaneous wire (i.e., a foreign body leading to a break of the skin which may lead to the entry of bacteria with potential for systemic infection), they should be classified as ABSSSIs, even though neither the existing guidelines, nor the ABSSSI definition for drug approvals, explicitly list DLIs.
MICROBIOLOGICAL PROFILE OF ACUTE BACTERIAL SKIN AND SKIN STRUCTURE INFECTION AND LEFT VENTRICULAR ASSIST DEVICE DRIVELINE INFECTIONS
S. aureus (including MRSA) and coagulase-negative staphylococci (CoNS) are the most common pathogens associated with ABSSSIs, followed by Pseudomonas aeruginosa and S. pyogenes. Less common bacteria include other Streptococcus species, other Gram-negative bacteria such as Enterobacterales, and fungal pathogens [20,27].
In LVAD infections, staphylococci are the most frequently isolated bacteria, but other bacteria and fungi have also been reported (Table 1). Polymicrobial infections may account for more than 50% of LVAD infections [4,12,14]. These infections often occur due to superinfection of an existing DLI while the patient is still being treated for the initial pathogen. In such infections, the most common secondary pathogen is P. aeruginosa[14,30].
Table 1.
Overview of commonly identified pathogens associated with left ventricular assist device infections
| Pathogen | Reported proportion of infections (%) | References |
| Gram-positive bacteria | ||
| Staphylococcus aureus | 0–56 | [6,9,12,31,32] |
| Methicillin-susceptible | 8–43 | [7▪,9,12,31,32] |
| Methicillin-resistant | 0–30 | [7▪,8,12,31,33] |
| Coagulase-negative staphylococci | 2–56 | [6,7▪,8,9,12,31,32] |
| Enterococcus spp. | 0.9–29 | [6,7▪,8,12,31,32] |
| Corynebacterium spp. | 0–20 | [7▪,12,31,32] |
| Streptococcus spp. | 0–6 | [7▪,32] |
| Gram-negative bacteria | ||
| Pseudomonas aeruginosa | 2–28 | [6,7▪,8,9,12,31,32] |
| Serratia spp. | 2–9 | [7▪,8,32] |
| Klebsiella pneumoniae | 2–13 | [7▪,8,12,31,32] |
| Enterobacter spp. | 0–5 | [7▪,32] |
| Escherichia coli | 0–11 | [7▪,8,12,32] |
| Acinetobacter baumannii | 1.8 | [7▪] |
| Fungi | ||
| Candida spp. | 0–45 | [6,8,12,32] |
| Aspergillus spp. | 28 | [12] |
DLIs and ABSSSIs are similar with regards to the spectrum of pathogens that cause the infections: both are mainly associated with Gram-positive species which colonize the skin, such as S. aureus and CoNS, while Gram-negative infections are less common, and fungal infections are observed occasionally. These similarities provide evidence to justify classifying DLIs as ABSSSIs.
LEFT VENTRICULAR ASSIST DEVICE DRIVELINE INFECTION AS ACUTE BACTERIAL SKIN AND SKIN STRUCTURE INFECTION, IMPLICATIONS FOR PATIENTS AND TREATMENT OPTIONS
Although LVAD is a temporary option for patients awaiting a heart transplant, it is increasingly being used as a destination therapy for patients who are ineligible for transplant [31,34]. Many of the latter patients also require end-of-life care, and severe complications leading to repeated hospitalizations put these patients at higher risk of acquiring nosocomial infections, including those by multiresistant bacteria such as MRSA; therefore, in settings where removal of an infected device (or parts thereof) is not feasible, life-long antimicrobial therapy is often needed [35].
Guidelines for treatment of ABSSSIs and SSTIs [20,23▪▪] are similar to the guidelines for treatment of DLIs, but – in contrast to the guidelines for DLIs – also include recommendations for specific antibiotics to be used. Recently approved novel antibiotics (such as dalbavancin, oritavancin, and delafloxacin) add to the available treatment options for ABSSSIs [27,36]. Considering the similarities between DLI and ABSSSI with regards to the pathogens involved, these antibiotics could also be beneficial for patients with DLIs [37,38].
Treatment should be tailored to the susceptibility profile after the pathogen has been identified. Based on the current guidelines, the literature, and the personal experience of the authors, a list of recommended antibiotics, focusing on the most common Gram-positive pathogens, was compiled. Details on dosage, frequency, administration, pharmacodynamics, and interactions can be found in Table 2. A proposal for the treatment of superficial and deep-seated LVAD infections is shown in Fig. 1.
Table 2.
Recommended antibiotics for patients with left ventricular assist device driveline infections with Gram-positive pathogens
| Antimicrobial agent | Spectrum | Dosage | Route | OPAT-ready (Y/N) | Skin and soft tissue permeability (% of serum concentration) | Antibiofilm activity (Y/N) | Frequent interactions | Comments | References |
| β-Lactam antibiotics | |||||||||
| Cefazolin |
Staphylococcus aureus CoNS |
i.v.: 2000–4000 mg every 8h Oral: 1000 mg every 6–8h |
i.v./oral | N | Y | None | [39] | ||
| Ceftaroline |
S. aureus (incl. MRSA) CoNS (incl. MRSE) Streptococcus spp. Enterobacterales |
600–1200 mg every 8h | i.v. | N | Y? | None | Regular monitoring of leukocyte levels required | [40,41] | |
| Ceftobiprole |
S. aureus (incl. MRSA) CoNS (incl. MRSE) Streptococcus spp. Enterobacterales Pseudomonas aeruginosa |
500–1000 mg every 8h | i.v. | N | None | ||||
| Flucloxacillin |
S. aureus CoNS (only MSSE) |
i.v.: 2000–4000 mg every 8h Oral: 1000 mg every 8h |
i.v./oral | N | None | Regular monitoring of liver function parameters required | |||
| Lincosamides and Oxazolidinones | |||||||||
| Clindamycin |
Streptococcus spp. Staphylococcus spp. Anaerobic bacteria |
600–900 mg every 8h | i.v./oral | 95% | N | None | Increased risk of Clostridioides difficile infection | [20,40,42] | |
| Linezolid |
S. aureus (incl. MRSA) CoNS (incl. MRSE) Streptococcus spp. Enterococcus spp. |
600 mg every 8–12h | i.v./oral | 105% | Seldom: Monoamine oxidase inhibitors and selective inhibitors of serotonin reuptake | Regular monitoring of hemoglobin and thrombocytes required | [20,40,42] | ||
| Tedizolid |
S. aureus (incl. MRSA) CoNS Streptococcus spp. Enterococcus spp. |
200 mg every 24h | i.v./oral | 110–120% | ? | In case of ABSSSI, therapy duration >7 days required | [20,40,43] | ||
| Lipoglycopeptides | |||||||||
| Dalbavancin |
S. aureus (incl. MRSA) CoNS (incl. MRSE)a Enterococcus spp. (incl. VanB VRE and vanC-expressing enterococci)a Streptococcus spp. |
1500 mg (single dose, or 1000 mg followed by 500 mg after 1 week) | i.v. infusion during 30 min | Y | 80–85% | Y | None | Alternative dosing: 1500 mg followed by 1000 mg every 15 days |
[20,36,40,44–47] |
| Oritavancin |
S. aureus (incl. MRSA) CoNS (incl. MRSE) Streptococcus pyogenes Enterococcus spp. (incl. VRE, all resistant strains) |
1200 mg followed by 800 mg after 1 week | i.v. infusion during 3 h | Y | Y | Interaction with phospholipid dependent coagulation tests | Active against all vancomycin-resistant enterococci | [20,40] | |
| Teicoplanin |
S. aureus (incl. MRSA) Streptococcus spp. Enterococcus spp. |
2 × 15 mg/kg every 12h for 1–3 days, then 1 × 15 mg/kg 3×/week | i.v. | Y | N | None | Therapeutic drug monitoring necessary: trough level 40–60 μg/ml |
||
| Vancomycin |
S. aureus (incl. MRSA) CoNS Streptococcus spp. Enterococcus spp. |
15–20 mg/kg every 8–12h | i.v. | N | 8–10% | N | Monitoring necessary: trough level 15–20 μg/ml |
[20,40,42] | |
| Quinolones | |||||||||
| Ciprofloxacin |
P. aeruginosa Enterobacterales |
i.v.: 400–600 mg every 12h Oral: 500–750 mg every 8h |
i.v./oral | Y | Typical side effects of quinolones | ||||
| Delafloxacin |
S. aureus (incl. MRSA) CoNS (incl. MRSE) S. pyogenes P. aeruginosa |
i.v.: 300 mg over 60 min every 12h Oral: 450 mg every 12h |
i.v./oral | Y | No association with delayed ventricular repolarization |
Some activity in ciprofloxacin-resistant P. aeruginosa Not a regular treatment for Enterococcus spp | [40] | ||
| Moxifloxacin |
S. aureus CoNS S. pyogenes Enterobacterales |
400 mg every 24h | i.v./oral | Y | Typical side effects of quinolones | ||||
| Various | |||||||||
| Intravenous fosfomycin |
S. aureus (incl. MRSA) Enterococcus spp. Enterobacterales |
i.v.: 12–24 g daily in 2–4 separate infusions | i.v. | 50–91% | Y | None | Must be co-administered with another antibiotic Regular monitoring of the electrolytes required |
[42,48] | |
| Rifampicin |
Staphylococcus spp. (incl. MRSA and MRSE) Streptococcus spp. |
300–450 mg every 12h for at least 6 weeks | i.v. infusion during 30 min/oral | NA | Y | Interactions with many drugs such as methadone, oral hypoglycemics, hormonal contraceptives, anticoagulants, protease inhibitors, phenytoin, theophylline, cardiac glycosides | Must be co-administered with another antibiotic Maximum daily dose 900 mg |
[42] | |
Information specified in this table is based on the mentioned references, as well as the authors’ personal experience.
Dalbavancin was shown to inhibit all VanB-type enterococci (E. faecalis and E. faecium) and 95.5% of teicoplanin-resistant CoNS isolated from hospitalized patients with a proven or suspected infection [47].
ABSSSI, acute bacterial skin and skin structure infection; CoNS, coagulase-negative staphylococci; i.v., intravenous; MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; MSSE, methicillin-susceptible Staphylococcus epidermidis; OPAT, outpatient parenteral antimicrobial therapy; VRE, vancomycin-resistant enterococci.
FIGURE 1.
Proposal for the treatment of superficial and deep/uncertain depth left ventricular assist device infections. Recommended for polymicrobial Gram-positive and Gram-negative infections.
TREATMENT IN AN OUTPATIENT SETTING
To avoid or limit repeated hospitalizations and reduce length of stay, so-called outpatient parenteral antimicrobial therapy (OPAT) should also be considered in patients with DLIs. OPAT is used to treat cardiovascular and surgical wound infections, as well as SSTIs due to S. aureus (including MRSA) and CoNS, when having a predictable course and a low probability of progression [49,50]. The optimal antibiotics for OPAT are those with a long half-life that can be administered 1–2 times daily, or less frequently. Several i.v. antimicrobial agents that could be used in OPAT are available [50].
OPAT can be used for LVAD patients in acute care and for patients in a palliative care setting who may require life-long treatment to control the infection. The use of OPAT in these patients would likely be associated with improved patients’ comfort, cost savings as well as treatment compliance [51].
The implementation of OPAT is associated with several challenges. While delivery of parenteral antimicrobials in an outpatient setting is available in many European countries, some of them lack structured OPAT teams or services [37,52▪], and patients may have difficulties in receiving their treatment regularly, especially on weekends or during holiday periods. In contrast, countries such as Italy, the United Kingdom, or the United States, have widely implemented this approach for treatment of various SSTIs [49,51,52▪,53]. In addition, not all patients may be good candidates for OPAT, since the patient and his/her caregivers must be able to physically and mentally cope with the OPAT. In some cases, social and/or health-system barriers can further complicate the successful use of OPAT [50,54] and without appropriate recommendations the exact indications for treatment can be unclear to the treating physicians.
A growing amount of evidence supports novel antibiotics such as dalbavancin or oritavancin as an effective, long-term therapy for various ‘off-label’ indications, including osteomyelitis and endocarditis [55–58,59▪,60]. Due to their long half-life, these antibiotics appear to be promising options not only for LVAD patients with acute DLIs, but especially for long-term chronic suppression therapy consisting of once-weekly or twice-weekly infusions. Oral treatment is associated with pharmacokinetic limitations. For instance, it has been shown for dicloxacillin in the oral treatment of infective endocarditis that the target antibiotic level of dicloxacillin was attained in only 17% of the patients (LIT POET study) [61▪].
PHARMACO-ECONOMIC ASPECTS OF TREATMENT FOR PATIENTS WITH DLIs
Hospital readmissions are frequently reported for LVAD recipients (over 50%) and are associated with significant healthcare costs in this patient population [62]. The most common causes of rehospitalization after implantation of LVAD include infection, device malfunction, cardiac events, strokes, and bleeding events [62]. Up to 60% of unplanned hospital readmissions are related to DLIs [14,63–67], with a reported median direct hospital cost of approximately $7500 per readmission during an 11-month follow-up [62].
Effective treatment of DLIs in an outpatient setting should contribute to decreasing the rates of hospital readmission due to DLIs and the associated financial burden. It may therefore be beneficial to also consider the ABSSSI guidelines for treatment of DLIs, particularly regarding the use of OPAT.
CONCLUSIONS AND FUTURE DIRECTIONS
In this paper, we have focused on the current definitions and treatment guidelines for ABSSSI and LVAD-associated infections. The wording and terminology used in current guidelines may be interpreted differently across institutions and countries. In our opinion, DLIs are soft tissue infections around indwelling wires that can appear as superficial, deep, or complicated forms of soft tissue infections, and are therefore encompassed by ABSSSIs.
A consequence of the confusion surrounding the definitions of DLI and ABSSSI is that, currently, certain drugs that are approved for ABSSSI and suitable for patients with DLI are considered ‘off-label’ for LVAD infection. We argue that DLIs meet the criteria for ABSSSIs, enlarging the ‘on-label’ antibiotic armamentarium for treating these severe infections. Furthermore, considering DLIs as being ABSSSIs might legally and clinically facilitate future studies of these new drugs with regards to pharmaco-economics and patient comfort.
Acknowledgements
The authors thank Michael Seimetz for fruitful discussions and his support in the publication development. The authors thank Akkodis Belgium for editorial assistance and manuscript coordination, on behalf of Advanz Pharma Services (UK) Ltd. Urszula Miecielica and Geert Behets provided medical writing support, and Sophie Timmery coordinated the manuscript development and provided editorial support.
Contributorship: All authors were involved in the conception of the work and provided substantial input on its content. All authors critically reviewed all versions of the manuscript and approved its final content and submission to the journal.
Instructions to authors: https://journals.lww.com/co-infectiousdiseases/Pages/informationforauthors.aspx
Financial support and sponsorship
Organizational support and publication development support was provided by Advanz Pharma Services (UK) Ltd, a company of the Advanz Pharma group. The authors received no funding for their participation in the development of this manuscript.
Conflicts of interest
Outside of the submitted work, Christian Eckmann received consulting fees from Menarini and Pfizer, honoraria for lectures, presentations and speaker bureaus from Menarini, Pfizer and Shionogi, support for attending meetings and/or travel. He also has a leadership or fiduciary role in the ESGCIP of ESCMID, the Paul-Ehrlich Society (section intra-abdominal and soft tissue infections) and the working group in surgical infections of the German Society for General and Visceral Surgery (DGAV).
Outside of the submitted work, Cord Sunderkötter received grants from Boehringer Ingelheim, InfectoPharm and Correvio, as well as consulting fees from Biotest, Boehringer Ingelheim and AstraZeneca. He also received support for attending meetings/travel from Janssen and UCB.
Outside of the submitted work, Karsten Becker received grants from the Federal Ministry of Education and Research (BMBF), the European Regional Development Fund (ERDF), the State Ministries of Mecklenburg-Vorpommern, MetaSystems and Pfizer, consulting fees from GenMark Diagnostics and honoraria for lectures, presentations or educational events from Advanz Pharma, Pfizer and Thermo Fisher Scientific. He is inventor of patent applications which are owned by the University of Münster and licensed to Bruker.
Outside of the submitted work, Béatrice Grabein received consulting fees from MSD, Pfizer and Shionogi. She also received honoraria for lectures, presentations and speaker bureaus from Advanz Pharma, InfectoPharm, MSD, Pfizer and Shionogi.
Outside of the submitted work, Stefan Hagel received royalties or licenses from the Federal Ministry of Education and Research (BMBF), as well as honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer, InfectoPharm, Philips, Advanz Pharma, Shionogi, Tillots and Thermo Fisher Scientific. He also received support for attending meetings/travel from Pfizer, InfectoPharm, Philips, Advanz Pharma, Shionogi and Tillots. He participated in Data Safety Monitoring Board and Advisory Board from Advanz Pharma and Shionogi.
Outside of the submitted work, Frank Hanses received consulting fees from MSD, GSK and Sobi. He also received honoraria for lectures, presentations and manuscript writing from Advanz Pharma, InfectoPharm and Akademie für Infektionsmedizin.
Outside of the submitted work, Dominic Wichmann received consulting fees from 3 M, Advanz Pharma, Eumedica, EUSA, Gilead, Kite, Lilly MSD, Novartis, Pfizer and Shionogi. He also received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Advanz Pharma, Gilead, Kite, MSD, Pfizer and Shionogi, as well as support for attending meetings/travel from Shionogi and Gilead.
Outside of the submitted work, Florian Thalhammer received consulting fees from AstraZeneca, Gilead, MSD, Mundipharma, Novartis and Pfizer. He also received honoraria for lectures, presentations and speakers bureaus from Advanz Pharma, DiaSorin, InfectoPharm, Menarini, MSD, Pfizer and Shionogi, as well as support for attending meetings/travel from Menarini.
Footnotes
Christian Eckmann and Cord Sunderkötter are equal contributors to this work and co-first authors.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 2023; 118:3272–3287. [DOI] [PubMed] [Google Scholar]
- 2. Marasco SF, McDonald C, McGiffin DC. Chapter 15 – Surgical implantation. In: Gregory SD, Stevens MC, Fraser JF, editos. Mechanical circulatory and respiratory support. Academic Press; 2017:469–494. [Google Scholar]
- 3▪.Bernhardt AM, Schlöglhofer T, Lauenroth V, et al. Prevention and early treatment of driveline infections in ventricular assist device patients – the destine staging proposal and the first standard of care protocol. J Crit Care 2020; 56:106–112. [DOI] [PubMed] [Google Scholar]; Comprehensive and standardized approach to LVAD infections.
- 4.Qu Y, Peleg AY, McGiffin D. Ventricular assist device-specific infections. J Clin Med 2021; 10:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Horo JC, Abu Saleh OM, Stulak JM, et al. Left ventricular assist device infections: a systematic review. ASAIO J 2018; 64:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon RJ, Weinberg AD, Pagani FD, et al. Prospective, multicenter study of ventricular assist device infections. Circulation 2013; 127:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Lumish HS, Cagliostro B, Braghieri L, et al. Driveline infection in left ventricular assist device patients: effect of standardized protocols, pathogen type, and treatment strategy. ASAIO J 2022; 68:1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]; Results of a pathogen-focused treatment strategy.
- 8.Nienaber JJ, Kusne S, Riaz T, et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 2013; 57:1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma V, Deo SV, Stulak JM, et al. Driveline infections in left ventricular assist devices: implications for destination therapy. Ann Thorac Surg 2012; 94:1381–1386. [DOI] [PubMed] [Google Scholar]
- 10.Topkara VK, Kondareddy S, Malik F, et al. Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg 2010; 90:1270–1277. [DOI] [PubMed] [Google Scholar]
- 11▪.Seretny J, Pidborochynski T, Buchholz H, et al. Decreasing driveline infections in patients supported on ventricular assist devices: a care pathway approach. BMJ Open Qual 2022; 11:e001815. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interesting approach regarding measures to prevent LVAD infections.
- 12.Zinoviev R, Lippincott CK, Keller SC, et al. In full flow: left ventricular assist device infections in the modern era. Open Forum Infect Dis 2020; 7:ofaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toba FA, Akashi H, Arrecubieta C, et al. Role of biofilm in Staphylococcus aureus and Staphylococcus epidermidis ventricular assist device driveline infections. J Thorac Cardiovasc Surg 2011; 141:1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuck A-M. Left ventricular assist device driveline infections: recent advances and future goals. J Thorac Dis 2015; 7:2151–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusne S, Mooney M, Danziger-Isakov L, et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J Heart Lung Transplant 2017; 36:1137–1153. [DOI] [PubMed] [Google Scholar]
- 16.Smith EM, Franzwa J. Chronic outpatient management of patients with a left ventricular assist device. J Thorac Dis 2015; 7:2112–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasanpour AH, Sepidarkish M, Mollalo A, et al. The global prevalence of methicillin-resistant staphylococcus aureus colonization in residents of elderly care centers: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2023; 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Sunderkötter C, Becker K, Eckmann C, et al. Calculated initial parenteral treatment of bacterial infections: skin and soft tissue infections. GMS Infect Dis 2020; 8:Doc11. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive overview of the antibiotic treatment of skin and soft tissue infections.
- 19.Sunderkötter C, Becker K, Eckmann C, et al. S2k guidelines for skin and soft tissue infections excerpts from the S2k Guidelines for “calculated initial parenteral treatment of bacterial infections in adults - update 2018”. J Dtsch Dermatol Ges 2019; 17:345–369. [DOI] [PubMed] [Google Scholar]
- 20.Pollack CV, Jr, Amin A, Ford WT, Jr, et al. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med 2015; 48:508–519. [DOI] [PubMed] [Google Scholar]
- 21. US Food and Drug Administration. Guidance for industry: acute bacterial skin and skin structure infections: developing drugs for treatment. 2013. Available at: https://www.fda.gov/files/drugs/published/Acute-Bacterial-Skin-and-Skin-Structure-Infections---Developing-Drugs-for-Treatment.pdf [Accessed 12 December 2022]. [Google Scholar]
- 22.Esposito S, Bassetti M, Concia E, et al. Diagnosis and management of skin and soft-tissue infections (SSTI). a literature review and consensus statement: an update. J Chemother 2017; 29:197–214. [DOI] [PubMed] [Google Scholar]
- 23▪▪.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:147–159. [DOI] [PubMed] [Google Scholar]; Still the gold standard for management of skin and soft tissue infections.
- 24.Moffarah AS, Al Mohajer M, Hurwitz BL, et al. Skin and soft tissue infections. Microbiol Spectr 2016; 4:14–25. [DOI] [PubMed] [Google Scholar]
- 25.Esposito S, Noviello S, Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis 2016; 29:109–115. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan K, Salinas RC, Agudelo Higuita NI. Skin and soft tissue infections. Am Fam Physician 2015; 92:474–483. [PubMed] [Google Scholar]
- 27.Eckmann C, Tulkens PM. Current and future options for treating complicated skin and soft tissue infections: focus on fluoroquinolones and long-acting lipoglycopeptide antibiotics. J Antimicrob Chemother 2021; 76:iv9–iv22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller LG, Eisenberg DF, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings. BMC Infect Dis 2015; 15:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merlino JI, Malangoni MA. Complicated skin and soft-tissue infections: diagnostic approach and empiric treatment options. Cleve Clin J Med 2007; 74: (Suppl 4): S21–28. [DOI] [PubMed] [Google Scholar]
- 30.Koval CE, Thuita L, Moazami N, et al. Evolution and impact of drive-line infection in a large cohort of continuous-flow ventricular assist device recipients. J Heart Lung Transplant 2014; 33:1164–1172. [DOI] [PubMed] [Google Scholar]
- 31.Pavlovic NV, Randell T, Madeira T, et al. Risk of left ventricular assist device driveline infection: a systematic literature review. Heart Lung 2019; 48:90–104. [DOI] [PubMed] [Google Scholar]
- 32.Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2011; 30:164–174. [DOI] [PubMed] [Google Scholar]
- 33.Belz S, Fisquet S, Ahuja A, et al. Incidence of infection and antimicrobial consumption in ventricular assist device (VAD) recipients at the Prince Charles Hospital (Tpch): a retrospective analysis. Heart Lung Circ 2020; 29:1234–1240. [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Nicholson L, Cassidy CJ, et al. Left ventricular assist device: a bridge to transplant or destination therapy? Postgrad Med J 2016; 92:271–281. [DOI] [PubMed] [Google Scholar]
- 35.Kavanagh KT, Cormier LE. Success and failures in MRSA infection control during the COVID-19 pandemic. Antimicrob Resist Infect Control 2022; 11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber RE, Fleige C, Layer F, et al. Determination of a tentative epidemiological cut-off value (Ecoff) for dalbavancin and Enterococcus faecium. Antibiotics 2021; 10:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hitzenbichler F, Mohr A, Camboni D, et al. Dalbavancin as long-term suppressive therapy for patients with gram-positive bacteremia due to an intravascular source—a series of four cases. Infection 2021; 49:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard-Anderson J, Pouch SM, Sexton ME, et al. Left ventricular assist device infections and the potential role for dalbavancin: a case report. Open Forum Infect Dis 2019; 6:ofz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pant N, Wallis SC, Roberts JA, et al. In vitro effect of synovial fluid from patients undergoing arthroplasty surgery on MRSA biofilm formation. J Antimicrob Chemother 2022; 77:1041–1044. [DOI] [PubMed] [Google Scholar]
- 40.Golan Y. Current treatment options for acute skin and skin-structure infections. Clin Infect Dis 2019; 68:S206–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lázaro-Díez M, Remuzgo-Martínez S, Rodríguez-Mirones C, et al. Effects of subinhibitory concentrations of ceftaroline on methicillin-resistant Staphylococcus aureus (MRSA) biofilms. PLoS One 2016; 11:e0147569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckmann C, Dryden M. Treatment of complicated skin and soft-tissue infections caused by resistant bacteria: value of linezolid, tigecycline, daptomycin and vancomycin. Eur J Med Res 2010; 15:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iqbal K, Milioudi A, Wicha SG. Pharmacokinetics and pharmacodynamics of tedizolid. Clin Pharmacokinet 2022; 61:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Drugbank Online. Dalbavancin. Available at: https://go.drugbank.com/drugs/DB06219 [Accessed 12 December 2022]. [Google Scholar]
- 45.Jones RN, Stilwell MG. Comprehensive update of dalbavancin activity when tested against uncommonly isolated Streptococci, Corynebacterium spp., Listeria monocytogenes, and Micrococcus spp. (1357 strains). Diagn Microbiol Infect Dis 2013; 76:239–240. [DOI] [PubMed] [Google Scholar]
- 46.Leighton A, Gottlieb AB, Dorr MB, et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother 2004; 48:940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kresken M, Klare I, Wichelhaus TA, et al. Glycopeptide resistance in Enterococcus spp. and coagulase-negative Staphylococci from hospitalised patients in Germany: occurrence, characteristics and dalbavancin susceptibility. J Glob Antimicrob Resist 2022; 28:102–107. [DOI] [PubMed] [Google Scholar]
- 48.Hashemian SMR, Farhadi Z, Farhadi T. Fosfomycin: the characteristics, activity, and use in critical care. Ther Clin Risk Manag 2019; 15:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell ED, Czoski Murray C, Meads D, et al. Clinical and cost-effectiveness, safety and acceptability of community intravenous antibiotic service models: Civas systematic review. BMJ Open 2017; 7:e013560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Candel FJ, Julián-Jiménez A, González-Del Castillo J. Current status in outpatient parenteral antimicrobial therapy: a practical view. Rev Esp Quimioter 2016; 29:55–68. [PubMed] [Google Scholar]
- 51.Chapman AL, Seaton RA, Cooper MA, et al. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statement. J Antimicrob Chemother 2012; 67:1053–1062. [DOI] [PubMed] [Google Scholar]
- 52▪.Emilie C, de Nocker P, Saïdani N, et al. Survey of delivery of parenteral antimicrobials in non-inpatient settings across Europe. Int J Antimicrob Agents 2022; 59:106559. [DOI] [PubMed] [Google Scholar]; Real-life description of outpatient parenteral antibiotic therapy (OPAT).
- 53.Esposito S, Noviello S, Leone S, et al. Outpatient parenteral antibiotic therapy (OPAT) in different countries: a comparison. Int J Antimicrob Agents 2004; 24:473–478. [DOI] [PubMed] [Google Scholar]
- 54.Ajaka L, Heil E, Schmalzle S. Dalbavancin in the treatment of bacteremia and endocarditis in people with barriers to standard care. Antibiotics (Basel) 2020; 9:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gatti M, Andreoni M, Pea F, et al. Real-world use of dalbavancin in the era of empowerment of outpatient antimicrobial treatment: a careful appraisal beyond approved indications focusing on unmet clinical needs. Drug Des Devel Ther 2021; 15:3349–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scoble PJ, Reilly J, Tillotson GS. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes 2020; 7:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas G, Henao-Martínez AF, Franco-Paredes C, et al. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents 2020; 56:106069. [DOI] [PubMed] [Google Scholar]
- 58.Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the General Hospital of Vienna. Clin Infect Dis 2018; 67:795–798. [DOI] [PubMed] [Google Scholar]
- 59▪.Dryden MS. Alternative clinical indications for novel antibiotics licensed for skin and soft tissue infection? Curr Opin Infect Dis 2015; 28:117–124. [DOI] [PubMed] [Google Scholar]; Innovative approach for usage of novel antibiotics in new indications.
- 60.Hornak JP, Reynoso D. Early clinical experience with delafloxacin: a case series. Am J Med Sci 2022; 363:359–363. [DOI] [PubMed] [Google Scholar]
- 61▪.Bock M, Theut AM, van Hasselt JGC, et al. Attainment of target antibiotic levels by oral treatment of left-sided infective endocarditis: a poet substudy. Clin Infect Dis 2023; 77:242–251. [DOI] [PubMed] [Google Scholar]; Precise documentation how difficult it is to achieve target concentrations with oral drugs.
- 62.Akhter SA, Badami A, Murray M, et al. Hospital readmissions after continuous-flow left ventricular assist device implantation: incidence causes, and cost analysis. Ann Thorac Surg 2015; 100:884–889. [DOI] [PubMed] [Google Scholar]
- 63.Cagliostro B, Levin AP, Fried J, et al. Continuous-flow left ventricular assist devices and usefulness of a standardized strategy to reduce drive-line infections. J Heart Lung Transplant 2016; 35:108–114. [DOI] [PubMed] [Google Scholar]
- 64.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis 2006; 6:426–437. [DOI] [PubMed] [Google Scholar]
- 65.Hernandez GA, Nunez Breton JD, Chaparro SV. Driveline infection in ventricular assist devices and its implication in the present era of destination therapy. Open J Cardiovasc Surg 2017; 9:1179065217714216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernandez RE, Singh SK, Hoang DT, et al. Present-day hospital readmissions after left ventricular assist device implantation: a large single-center study. Tex Heart Inst J 2015; 42:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smedira NG, Hoercher KJ, Lima B, et al. Unplanned hospital readmissions after Heartmate II implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail 2013; 1:31–39. [DOI] [PubMed] [Google Scholar]