Abstract
Objective
Erythematotelangiectatic rosacea (ETR) is recognized by flushing, persistent centrofacial erythema, and telangiectasia. Many lines of topical treatments have been used for ETR with variable outcomes. We aimed to evaluate the efficacy of 10% topical tranexamic acid (TXA) with and without microneedling in treating ETR.
Methods
All patients received treatment on both sides of the face, the right side was treated with microneedling combined with 10% topical TXA, and the left side was treated with 10% topical TXA only. All patients received three sessions at two weeks intervals. The final evaluation was done three months after the last treatment session.
Results
The study included 45 females. Their age ranged between 20 and 48 years. The duration of the disease ranged from two months to five years. Both sides of the face showed improvement after treatment. There was a clinically and dermoscopic significant improvement in the side treated with microneedling + TXA compared to the side of the face treated with TXA alone.
Limitations
The small sample size and the lack of long-term follow-up.
Conclusion
This study showed that TXA is an effective and safe treatment modality for ETR. Microneedling can enhance the delivery of TXA and lead to better outcomes regarding erythema and telangiectasia.
Keywords: Rosacea, tranexamic acid, erythema, flushing, telangiectasia
Rosacea is a chronic inflammatory disease predominantly affecting the centrofacial region (cheeks, chin, nose, and forehead) and the eyes. It is the most common diagnosis of facial erythema and is categorized into four types based on its morphology: erythematotelangiectatic, papulopustular, phymatous, and ocular.1 There are several theories regarding the cause of rosacea, including genetic, environmental, vascular, and inflammatory factors.2
In a recent systematic review, the global prevalence of rosacea was estimated at 5.5 percent of the adult population.3 The disease usually starts between 30 to 50 years of age but can occur at any age, and women are more often affected than men.4,5 The most significant risk for rosacea appears among individuals with fair skin types (I and II). However, darker phototypes can also be affected.6,7
Erythematotelangiectatic rosacea (ETR) is distinguished by frequent episodes of flushing against underlying constant erythema, with telangiectasia present in the majority of patients.8 Traditional options for treating ETR include topical brimonidine tartrate, oxymetazoline with high recurrence rates, and pulsed dye laser or intense pulsed light (IPL), which are expensive.9–11
Tranexamic acid (TXA), trans-4-(Aminomethyl) cyclohexanecarboxylic acid, is an antifibrinolytic agent that competitively inhibits the activation of plasminogen to plasmin, hence suppressing angiogenesis. It also suppresses proinflammatory cytokines and thus decreases erythema. Moreover, it can restore the compromised epidermal permeability barrier function.12 TXA can be combined with microneedling as a transdermal drug delivery method.13
In this study, we aimed to evaluate the efficacy of a 10% topical tranexamic acid solution in treating ETR with and without microneedling.
METHODS
This split-face study was carried out on 45 patients with ETR. The patients were selected from those attending the Dermatology Outpatient Clinics of the University Hospital between August 2020 and August 2022. This study was carried out after being approved by the local Ethics Committee of the Faculty of Medicine. Informed consent was obtained from all participants.
Patients younger than 18 years, patients who were receiving any form of treatment within four weeks before the study, those with active infection or recurrent herpes, patients with a history of bleeding disorders or anticoagulant medications, patients with a history of scar and keloid formation, and pregnant or lactating females were excluded from the study.
All study participants underwent a thorough history taking, including their age, sex, occupation, residence, duration of the disease, and previous treatments. Then, a thorough general and local examination was performed to evaluate the underlying causes of facial erythema and telangiectasia, skin photo type, and exclusion of any other skin problem. Also, dermoscopy was used to confirm the diagnosis of rosacea via the characteristic dermoscopic findings (dark red colored background and arborizing or linear vessels with network-like pattern).
Each patient received treatment on both sides of the face. The right side was treated by microneedling combined with 10% topical TXA, whereas the left side was treated with 10% topical TXA alone. All patients received three sessions at two weeks intervals. Topical 10% TXA was prepared using TXA solution 0.05% from the currently marketed formulation Kapron® (500mg/5mL) ampoules.
An anesthetic cream was applied for 30 minutes, then wiped with a cotton pad soaked in 70% alcohol. Microneedling with (0.5-1.5mm) depth on automated needle pen devices (dr. pen®; A6S wireless) was used on the right side of the face. The skin was stretched, and 10% TXA solution was applied circularly. A maximum of 1mL of TXA solution was applied to each side of the face. Two hours after treatment, the skin was cleaned with distilled water, and sterile gauze was applied. Patients were instructed to use moisturizer and sunblock and avoid scrubbing the face.
Follow-up of the patients was done before and after treatment by clinical examination and dermoscopy photographs. All patients were photographed (using Canon Power Shot A3400 IS 16MP digital camera) before and three months after the last treatment session. Evaluation of clinical response included the extent of improvement and possible adverse effects, including bleeding, oozing, bruises, erythema, and hyperpigmentation.
The erythema improvement rate was evaluated clinically before and after treatment for the number and diameter of the vessels, based on serial images by the Clinician Erythema Assessment (CEA) score.14
Telangiectasia was evaluated using the Clinician Telangiectasia Assessment (CTA) grades, a 5-point scale where 0=no response, 1=poor response, 2=fair response, 3=good response, and 4=excellent response.15
Flushing was evaluated using visual analogue scales (VAS), a 0 to 10 scale where zero represents the best response and 10 represents the worst.16
Patients were asked to evaluate their level of satisfaction using a quartile grading system (0=no response, 1=fair response, 2=moderate response, or 3=very good response).
Statistical analysis was performed using Statistical Program for Social Science (SPSS) version 24. Quantitative data were expressed as mean±SD. Qualitative data were expressed as frequency and percentage. Mann-Whitney (MW) was used for comparing two groups (for normally distributed data). The chi-square test was used when comparing non-parametric data. P-value <0.05 was considered significant.
RESULTS
This study included 45 female participants. The mean age of all studied patients was 32.4 ± 8.6 years, with a minimum age of 20 and a maximum of 48 years. The mean duration of the disease was 10.8 ± 10 months with a minimum duration of two months and a maximum of five years. Three patients (6.7%) were of Fitzpatrick Skin Type II, 23 patients (53.3%) were of Type III, and 18 patients (40%) were of Type IV. Regarding telangiectasia, 44 patients (97.8%) were of the arborizing type, and only one patient (2.5%) was of the linear type.
Regarding erythema, there was no statistically significant difference in the mean baseline clinical erythema assessment score (CEA) on both sides of the face before treatment. However, there was a highly statistically significant improvement on the CEA scale (p-value <0.001) on both sides of the face after treatment with either modality. Yet, when both modalities were compared, the side treated with microneedling + TXA showed better outcomes (Tables 1–3).
TABLE 1.
Comparisons of CEA and VAS scores before and after treatment with microneedling combined with TXA.
| GRADING SCALES | BEFORE TXA WITH MICRONEEDLING (N = 45) | AFTER TXA WITH MICRONEEDLING (N = 45) | STATISTICAL TEST | P-VALUE | ||
|---|---|---|---|---|---|---|
| CEA | ||||||
| Clear skin | 0 | 0% | 7 | 15.6% | X2 = 68.4 | < 0.001 HS |
| Slight redness | 0 | 0% | 31 | 68.9% | ||
| Definite redness | 17 | 37.8% | 6 | 13.3% | ||
| Marked redness | 22 | 48.9% | 1 | 2.2% | ||
| Fiery redness | 6 | 13.3% | 0 | 0% | ||
| VAS | ||||||
| Mean ± SD | 2.75 ± 0.85 | 1.1 ± 0.3 | MW = 52.5 | < 0.001 HS | ||
TXA: tranexamic acid; CEA: clinician erythema assessment score; VAS: visual analogue scales; SD: standard deviation; X2: Chi square test; MW: Mann-Whitney test; HS: p-value < 0.001 is considered highly significant.
TABLE 3.
Comparisons of both treatment modalities after treatment regarding CEA, VAS, and CTA scores, as well as patients’ satisfaction and side effects.
| GRADING SCALES | AFTER TXA WITH MICRONEEDLING (N = 45) | AFTER TXA ONLY (N = 45) | STATISTICAL TEST | p-VALUE | ||
|---|---|---|---|---|---|---|
| CEA | ||||||
| Clear skin | 7 | 15.6% | 0 | 0% | X2 = 43.5 | < 0.001 HS |
| Slight redness | 31 | 68.9% | 7 | 15.6% | ||
| Definite redness | 6 | 13.3% | 31 | 68.9% | ||
| Marked redness | 1 | 2.2% | 7 | 15.6% | ||
| VAS | ||||||
| Mean ± SD | 1.11 ± 0.3 | 1.93 ± 0.68 | MW = 52.5 | < 0.001 HS | ||
| CTA | ||||||
| No response | 2 | 4.4% | 20 | 44.4% | X2 = 51.3 | < 0.001 HS |
| Poor response | 5 | 11.1% | 17 | 37.8% | ||
| Fair response | 8 | 17.8% | 8 | 17.8% | ||
| Good response | 19 | 42.2% | 0 | 0% | ||
| Excellent response | 11 | 24.4% | 0 | 0% | ||
| Patients’ satisfaction | ||||||
| Fair | 7 | 15.6% | 35 | 77.8% | X2 = 39.2 | < 0.001 HS |
| Moderate | 22 | 48.9% | 10 | 22.2% | ||
| Very good | 16 | 35.6% | 0 | 0% | ||
| Side effects | ||||||
| No | 36 | 80% | 45 | 100% | X2 = 10 | 0.002 S |
| Yes | 9 | 20% | 0 | 0% | ||
TXA: tranexamic acid; CEA: clinician erythema assessment score; VAS: visual analogue scales; CTA: clinician telangiectasia assessment grades; SD: standard deviation; X2: Chi square test; MW: Mann-Whitney test; HS: p-value < 0.001 is considered highly significant. S: p-value < 0.05 is considered significant.
TABLE 2.
Comparisons of CEA and VAS scores before and after treatment with TXA only.
| GRADING SCALES | BEFORE TXA ONLY (N = 45) | AFTER TXA ONLY (N = 45) | STATISTICAL TEST | p-VALUE | ||
|---|---|---|---|---|---|---|
| CEA | ||||||
| Slight redness | 0 | 0% | 7 | 15.6% | X2 = 20.1 | < 0.001 HS |
| Definite redness | 20 | 44.4% | 31 | 68.9% | ||
| Marked redness | 22 | 48.9% | 7 | 15.6% | ||
| Fiery redness | 3 | 6.7% | 0 | 0% | ||
| VAS | ||||||
| Mean ± SD | 2.75 ± 0.8 | 1.93 ± 0.68 | MW = 481.5 | < 0.001 HS | ||
TXA: tranexamic acid; CEA: clinician erythema assessment score; VAS: visual analogue scales; SD: standard deviation; X2: Chi square test; MW: Mann-Whitney test; HS: p-value < 0.001 is considered highly significant.
Improvement of telangiectasia was superior in the side of the face that was treated with microneedling + TXA, where 30 (66.6%) patients achieved good to excellent responses, while on the other side treated with TXA alone, most of the patients, 37 (82.2%) had poor to no response (Table 3). VAS for evaluating flushing showed that both sides of the face showed reduced scores after treatment; however, there was a highly statistically significant score reduction after microneedling + TXA when compared to TXA alone (Tables 1–3, Figures 1 and 2).
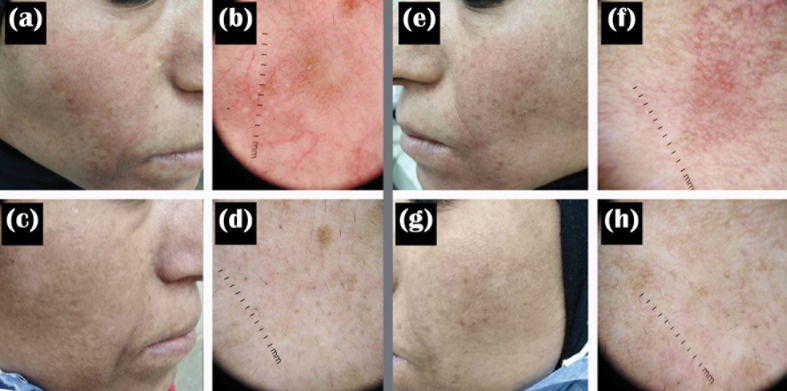
FIGURE 1.
A 43-year-old female patient with ETR. (a, b) Clinical and dermoscopic images of the right side before treatment; (c, d) Clinical and dermoscopic images of the right side after treatment with TXA + microneedling; (e, f) Clinical and dermoscopic images of the left side before treatment; (g, h) Clinical and dermoscopic images of the left side after treatment with TXA alone.
FIGURE 2.
A 38-year-old female patient with ETR. (a) Both sides of the face before treatment; (b) Both sides of the face after treatment; (c) Dermoscopy of the right side before treatment; (d) Dermoscopy of the right side after treatment with TXA + microneedling; (e) Dermoscopy of the left side before treatment; (f) Dermoscopy of the left side after treatment with TXA alone.
At the end of the follow-up period, patients gave the side treated with microneedling + TXA a higher degree of satisfaction when compared to TXA alone, with a highly statistically significant difference. No side effects were reported on the side treated with TXA alone. On the side treated with microneedling + TXA, nine patients (20%) reported side effects in the form of pain in three patients (33.3%), exfoliation in two patients (22.2%), erythema in two patients (22.2%), bruises in one patient (11.1%) and hyperpigmentation in one patient (11.1%) (Table 3).
DISCUSSION
The current approach to rosacea treatment focuses on symptom relief to improve patients' quality of life, minimize disease progression, and maintain remission.17
For mild-to-moderate cases of rosacea, topical therapy is the treatment of choice.18 The FDA has approved brimonidine tartrate 0.33% gel, a selective α2-adrenergic receptor agonist, as the first topical drug for rosacea-associated persistent facial erythema.19 However, reported side effects included contact dermatitis, burning sensation, and rebound erythema.20
Various topical therapies are used as off-label treatments for rosacea, such as macrolides and macrolide analogues, permethrin, retinoids, and topical calcineurin inhibitors.21 None of the currently available topical treatments for rosacea are likely to reduce telangiectasia, and this frequently constitutes a psychological burden and can significantly influence the quality of life of rosacea sufferers.8
TXA was first identified in 1979; however, dermatologists have recently become interested in using it to treat various skin diseases.12
The mechanism of TXA action in the management of rosacea remains unclear. Serine proteases (SPs), including kallikrein, urokinase, and plasmin, have been found in significant concentrations in the skin of rosacea patients.22 SPs are crucial in the inflammatory component of rosacea, angiogenesis, and the impairment of the function of the epidermal barrier.23 TXA hastens barrier repair and protects the skin from epidermal hyperplasia caused by repeated barrier disruption.22
TXA has been shown to reduce erythema by inhibiting proinflammatory cytokines (mainly IL6 and TNFα), angiogenesis, and T cell infiltration caused by keratinocytes, as well as decreasing the quantity of CD31 microvessels and VEGF expression.24,25
Furthermore, TXA suppresses kallikrein-activated protease-activated receptor-2 (PAR-2) activation and subsequent calcium influx in stratum granulosum keratinocytes, producing adequate lamellar body secretion.2
The ease of preparation, application, and cost-effectiveness makes TXA a perfect option for patients presenting with ETR.12
In this study, we tried to evaluate the enhanced delivery of TXA solution through microneedling. The microchannel formation causes a controlled cutaneous injury with minor epidermal damage and enhances drug penetration.26 Following cutaneous injury, a fibronectin network develops, serving as a matrix for the deposition of collagen Type III before Type I collagen eventually takes its place. This transition eventually leads to clinically apparent skin tightening, which gives microneedling an extra advantage.27
Only a few studies have been published that describe the use of topical TXA in the treatment of rosacea.7,22,28 Another study concluded that intradermal TXA microinjections appear to be a safe, effective, and promising alternative method for ETR.29
In this study, the side treated with TXA and microneedling, as well as the side treated with TXA alone, demonstrated a statistically significant difference before and after treatment, but the side treated with the combination, as opposed to the side treated with TXA alone, showed a statistically significant reduction in the clinical erythema assessment score (CEA), visual analogue score and telangiectasia score assessing erythema, flushing and telangiectasia.
In our study, patients gave the side treated with TXA and microneedling a higher rating and degree of satisfaction than TXA alone. Their satisfaction was not only due to the improvement of rosacea but also to the improvement of skin texture.
Limitations. The limitations of this study include the small number of treated cases and the lack of long-term follow-up to assess relapse, necessitating future studies that address these issues.
CONCLUSION
According to our study results, topical TXA solution can be considered a safe and effective therapeutic option for the management of ETR, with no major adverse effects. Further studies are needed to compare its efficacy against other available treatment modalities.
REFERENCES
- van Zuuren EJ, Fedorowicz Z, Tan J et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol. 2019;181(1):65–79. doi: 10.1111/bjd.17590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bageorgou F, Vasalou V, Tzanetakou V, Kontochristopoulos G. The new therapeutic choice of tranexamic acid solution in treatment of erythematotelangiectatic rosacea. J Cosmet Dermatol. 2019;18(2):563–567. doi: 10.1111/jocd.12724. [DOI] [PubMed] [Google Scholar]
- van Zuuren EJ, Arents BWM, van der Linden MMD et al. Rosacea: New Concepts in Classification and Treatment. Am J Clin Dermatol. 2021;22(4):457–465. doi: 10.1007/s40257-021-00595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Schöfer H, Araviiskaia E et al. Prevalence of rosacea in the general population of Germany and Russia - The RISE study. J Eur Acad Dermatol Venereol. 2016;30(3):428–434. doi: 10.1111/jdv.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessinioti C, Antoniou C. The "red face": Not always rosacea. Clin Dermatol. 2017;35(2):201–206. doi: 10.1016/j.clindermatol.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Rainer BM, Fischer AH, Luz Felipe da Silva D et al. Rosacea is associated with chronic systemic diseases in a skin severity-dependent manner: results of a case-control study. J Am Acad Dermatol. 2015;73(4):604–608. doi: 10.1016/j.jaad.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Alexis AF, Callender VD, Baldwin HE et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: Review and clinical practice experience. J Am Acad Dermatol. 2019;80(6):1722–1729.e7. doi: 10.1016/j.jaad.2018.08.049. [DOI] [PubMed] [Google Scholar]
- Rainer BM, Kang S, Chien AL. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(1):e1361574. doi: 10.1080/19381980.2017.1361574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Johnson SM. The Role of Topical Brimonidine Tartrate Gel as a Novel Therapeutic Option for Persistent Facial Erythema Associated with Rosacea. Dermatol Ther (Heidelb). 2015;5(3):171–181. doi: 10.1007/s13555-015-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143(11):1369–1371. doi: 10.1001/archderm.143.11.1369. [DOI] [PubMed] [Google Scholar]
- Chunharas C, Boen M, Alhaddad M, Wu DC. The Efficacy of Pulsed Dye Laser Pretreated With or Without Local Anesthetic on Patients Presenting With Erythema of Face, Neck, Chest, and Extremities. Lasers Surg Med. 2020;52(4):307–314. doi: 10.1002/lsm.23146. [DOI] [PubMed] [Google Scholar]
- Jakhar D, Kaur I, Yadav S. Topical 10% Tranexamic Acid for Recalcitrant Topical Steroid-Dependent Face. Indian Dermatol Online J. 2020;11(6):1024–1026. doi: 10.4103/idoj.IDOJ_97_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ita K. Transdermal Delivery of Drugs with Microneedles-Potential and Challenges. Pharmaceutics. 2015;7(3):90–105. doi: 10.3390/pharmaceutics7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foolad N, Prakash N, Shi VY et al. The use of facial modeling and analysis to objectively quantify facial redness. J Cosmet Dermatol. 2016;15(1):43–48. doi: 10.1111/jocd.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Liu H, Leyden JJ et al. Reliability of Clinician Erythema Assessment grading scale. J Am Acad Dermatol. 2014;71(4):760–763. doi: 10.1016/j.jaad.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Couper M, Tourangeau R, Conrad F et al. Evaluating the effectiveness of visual analog scales: A web experiment. Soc Sci Comput Rev. 2006;24(2):227–245. [Google Scholar]
- Reinholz M, Ruzicka T, Steinhoff M et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;6:4–15. doi: 10.1111/ddg.13139. 14 Suppl. [DOI] [PubMed] [Google Scholar]
- Del Rosso JQ, Thiboutot D, Gallo R et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92(5):234–240. [PubMed] [Google Scholar]
- Layton AM, Schaller M, Homey B et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29(12):2405–2410. doi: 10.1111/jdv.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe E, Lim S. Paradoxical Erythema Reaction of Long-term Topical Brimonidine Gel for the Treatment of Facial Erythema of Rosacea. J Drugs Dermatol. 2016;15(6):763–765. [PubMed] [Google Scholar]
- Sharma A, Kroumpouzos G, Kassir M et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022;21(5):1895–1904. doi: 10.1111/jocd.14816. [DOI] [PubMed] [Google Scholar]
- Kim MS, Chang SE, Haw S et al. Tranexamic acid solution soaking is an excellent approach for rosacea patients: a preliminary observation in six patients. J Dermatol. 2013;40(1):70–71. doi: 10.1111/j.1346-8138.2012.01515.x. [DOI] [PubMed] [Google Scholar]
- Zhong S, Sun N, Liu H et al. Topical tranexamic acid improves the permeability barrier in rosacea. Dermatol Sin. 2015;33(2):112–117. [Google Scholar]
- Manosroi A, Podjanasoonthon K, Manosroi J. Stability and release of topical tranexamic acid liposome formulations. J Cosmet Sci. 2002;53(6):375–386. [PubMed] [Google Scholar]
- Li Y, Xie H, Deng Z et al. Tranexamic acid ameliorates rosacea symptoms through regulating immune response and angiogenesis. Int Immunopharmacol. 2019;67:326–334. doi: 10.1016/j.intimp.2018.12.031. [DOI] [PubMed] [Google Scholar]
- Aust MC, Reimers K, Kaplan HM et al. Percutaneous collagen induction-regeneration in place of cicatrisation?. J Plast Reconstr Aesthet Surg. 2011;64(1):97–107. doi: 10.1016/j.bjps.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Aust MC, Fernandes D, Kolokythas P et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121(4):1421–1429. doi: 10.1097/01.prs.0000304612.72899.02. [DOI] [PubMed] [Google Scholar]
- Jakhar D, Kaur I, Misri R. Topical 10% tranexamic acid for erythematotelangiectatic steroid-induced rosacea. J Am Acad Dermatol. 2022;86(1):e1–e2. doi: 10.1016/j.jaad.2019.12.067. [DOI] [PubMed] [Google Scholar]
- Daadaa N, Litaiem N, Karray M et al. Intradermal tranexamic acid microinjections: a novel treatment option for erythematotelangiectatic rosacea. J Cosmet Dermatol. 2021;20(10):3324–3329. doi: 10.1111/jocd.14209. [DOI] [PubMed] [Google Scholar]