Abstract
Practical relevance Weakness is a relatively common clinical presentation in feline medicine and can be caused by primary neuromuscular disease or by diseases of other body systems affecting the neuromuscular system secondarily. Successful work-up relies on a thorough clinical and neurological examination, and logical problem solving, based on an understanding of the underlying neuroanatomical and pathophysiological mechanisms.
Clinical challenges Feline neuromuscular diseases can be a diagnostic challenge. On initial inspection, the presenting signs can mimic disorders of other body systems, particularly cardiovascular, pulmonary and orthopaedic disease, or may be confused with systemic illnesses. Additionally, because many different pathologies of the feline neuromuscular system converge to a similar clinical phenotype, further diagnostic steps such as electrodiagnostics, cerebrospinal fluid analysis, and muscle and nerve biopsies must be considered even after neuromuscular dysfunction has been identified.
Audience This review provides a framework for the clinical approach to the weak cat and gives a practical summary of neuromuscular diseases for the general practitioner and specialist alike.
Evidence base Many diseases affecting the feline neuromuscular system have been well described in the veterinary literature, mostly based on retrospective case reports and series. The evidence base for the treatment of feline neuromuscular diseases remains very limited.
MULTIMEDIA
A video recording showing a Bengal cat with polyneuropathy is included in the online version of this article doi:10.1016/j.jfms.2011.09.005
The workings of the feline motor system
Weakness in cats secondary to a neuromuscular disorder can be a diagnostic challenge. A basic understanding of the underlying neuroanatomical and pathophysiological mechanisms is important for a successful diagnostic work-up, prognostication and treatment.
It is essential to identify whether the source of a motor abnormality lies in the brain or spinal cord, affects the upper or lower motor neuron and resembles a CNS disease or a neuromuscular problem.
As in other quadrupeds, locomotion in cats is coordinated by the extrapyramidal system. Voluntary movements are initiated in the premotor and motor areas of the cerebral cortex but all signals, apart from those for skilful movements of the front paws, are translated into upper motor neuron signals within the motor centres of the brainstem. Hence, cortical lesions do not readily lead to upper motor neuron signs.
From these centres, the motor signals target on segmentally organised lower motor neurons of the brainstem (for cranial nerve action) and spinal cord (for trunk and limb movement). The lower motor neuron releases its fast conducting axons to the periphery. The term neuromuscular disorder refers to a condition affecting the lower motor neuron, its ensheathed axon in the nerve roots and peripheral nerve, the neuromuscular junction and the excitable muscle fibre, which together form the so-called motor unit.
Motor signals rely on saltatory impulse conduction. Demyelination delays, desynchronises or even blocks the signal propagation, whereas loss of neurons or axons primarily decreases the number of functional units. A delay in nerve conduction in axonal diseases does not occur before a significant number of fast conducting fibres have been lost. The coupling between the nerve impulse and muscle contraction is mediated by acetylcholine, which is released into the neuromuscular cleft upon arrival of the action potential.
Lesions to the upper motor neurons cause reduced voluntary muscle responses but they also render the lower motor neuron ‘excited’, which results in hyperreflexia or spastic paresis …
Damage to the extrapyramidal circuits of the forebrain can result in hyperkinetic or hypokinetic syndromes, as well as dystonia. However, it does not usually lead to upper motor neuron weakness. Lesions to the upper motor neurons in the brainstem and below cause reduced voluntary muscle responses but they also render the lower motor neuron ‘excited’, which results in hyperreflexia or spastic paresis. In contrast, lower motor neuron damage disrupts the efferent myotactic reflex pathway and, therefore, leads to hyporeflexia, decreased muscle tone and flaccid paresis.
Movement analysis is an essential part of the neuromuscular work-up. As indicated above, the specific gait pattern for felid locomotion is encoded by the extrapyramidal forebrain nuclei and the spinal pattern generators, which provide a seemingly automated coordination between front and hind limbs. 1,2 In newborn cats, motor activity is restricted mainly to respiration and feeding-related behaviour. 3 The tonus of postural and antigravity extensor muscles is low due to the immaturity of the myotactic reflex pathways. In the absence of spinocerebellar proprioceptive inputs, newborn kittens physiologically show a degree of intention tremor and hypermetria. Proper standing and walking is not possible before days 30 and 40 postpartum, respectively, although proprioceptive placing may be observed from birth in about 70% of newborn kittens. 4 Both motor skills and muscle strength are considered immature until at least 3 months of life. 2,4
… In contrast, lower motor neuron damage disrupts the efferent myotactic reflex pathway and leads to hyporeflexia, decreased muscle tone and flaccid paresis.
The adult cat is a quick and forceful predator but a poor endurance athlete. Notably, cats rarely stand for a long period of time without moving. Mild neuromuscular weakness most often is identified by reluctance to move or stand. Moreover, absence of the nuchal ligament frequently triggers low head carriage as seen in hypokalaemia. The limb muscles, more so than in dogs, are dominated by energy demanding fast twitch type II fibres, which are mainly glycolytic and many of which are fatigable. 5 As all the muscles appear to be ‘exercise muscles’, there are no muscles that would mask the clinical phenotype at low activity. Due to the rather flexed neutral angles of the distal limb joints in the cat, advanced muscle weakness during stance and walking becomes most obvious by the palmigrade/ plantigrade carriage of carpal and tarsal joints.
It is essential to identify whether the source of a motor abnormality lies in the brain or spinal cord, affects the upper or lower motor neuron and resembles a CNS disease or a neuromuscular problem.
Episodic weakness

Problem solving in feline neuromuscular disease
Define the problem
When establishing the problem list it is important to include the onset of the presenting complaint and the clinical course, as this will help later when considering the differential diagnoses. With suspected neuromuscular disease it is also important to ascertain whether the cat is persistently or episodically weak (ie, normal between episodes) (see box below and on page 838). This information may subsequently help to establish which body system is primarily affected. Cats, in contrast to dogs, tend not to present with episodic weakness — they will usually limit their activity and more commonly show persistent weakness. This usually manifests as ventroflexion of the neck, and laying with their head on their paws (ie, looking really relaxed) even in the middle of a consulting room or other strange and stressful environment (Fig 1).
FIG 1.
Generalised polyneuropathy in a kitten. The patient is tetraparetic. Note the raised scapulae and inability to lift the head
Persistent weakness
Differentials to consider

Cats, in contrast to dogs, more commonly show persistent weakness.
Define the system and location within that system
Once the problem has been defined the next step is to consider which body system is involved. As diseases of several body systems mimic the presenting complaints seen with neuromuscular disease, it is vital to perform a full general physical examination, in particular to determine the presence of cardiopulmonary or orthopaedic disease (eg, polyarthritis, degenerative joint disease), and to identify the presence of systemic illness.
Determining whether the presenting complaint of weakness is persistent and whether there is a temporal relation to exercise will further assist in defining the affected system (see box below and on page 838).
Following the physical examination, a neurological examination should be performed with the aims of confirming the existence of a neurological lesion and further identifying the lesion location within the nervous system. For a detailed discussion of the neurological examination, with consideration of the needs of the feline patient, the reader is referred to a recent review in this journal. 6 The findings typically seen in feline patients with neuromuscular disease are summarised in the box on page 840.
Two of the most important features to assess in these cats are their gait and posture. The gait of cats with neuromuscular dysfunction is often characterised by stiff, stilted, choppy limb movements. They commonly display, as mentioned, ventroflexion of the neck; when weightbearing, dorsal displacement of the scapulae is seen (Fig 1). It is also important to examine for autonomic dysfunction, which may be present in some disorders.
Define and refine the lesion
Once the lesion has been localised to the neuromuscular system the next key step is to define the pathology affecting the neuromuscular system. A helpful way of recalling the broad types of pathology that can occur is to refer to the DAMNIT-V mnemonic (ie, degenerative, anomalous, metabolic, neoplastic/nutritional, inflammatory/ infectious/idiopathic, traumatic/toxic or vascular). Which type of pathology is most likely will depend on the onset and course of the disease, symmetry of the deficits, pain involvement and the signalment of the patient.
Diagnostic tests
The choice of diagnostic tests in neuromuscular diseases should be guided by the clinical decision-making process. 7
Neurological deficits
Grouped according to location within the neuromuscular system

Primary diagnostics
Laboratory tests
Routine haematology, serum biochemistry and urinalysis are important for identifying systemic abnormalities that may be associated with neuromuscular dysfunction, such as electrolyte disturbances and hypo/hyperglycaemia. These routine tests may also provide evidence of muscle damage (eg, raised creatine kinase [CK] activity or myoglobinuria). When indicated, dependent on compatible clinical findings and the results of routine blood and urine analysis, more specific laboratory tests can be performed. These may include endocrine testing, serology for infectious diseases, and tests for specific neuromuscular disease such as acetylcholine receptor antibody testing.
Imaging
As discussed later, the neuromuscular system may be directly affected by a neoplastic condition, such as lymphoma or peripheral nerve sheath tumour, or as a result of a paraneoplastic condition. Imaging of the thoracic and abdominal cavities can therefore be performed to look for evidence of primary or metastatic neoplasms. Conscious thoracic radiography is also essential to identify megaoesophagus or aspiration pneumonia, albeit these conditions are seen more often in dogs than in cats with generalised neuromuscular disease.
Many different pathologies of the feline neuromuscular system converge to a similar clinical phenotype. Thus, further diagnostic steps must be considered even after neuromuscular dysfunction has been identified.
Secondary diagnostics
Electrodiagnostics
Electrodiagnostics such as electromyography (EMG) and nerve conduction studies (NCSs) can be used variously to confirm suspicions that a neuromuscular dysfunction exists; to demonstrate the distribution of the disease process as a neuropathy, junctionopathy (ie, disorder of neuromuscular transmission) or myopathy; and, in some cases, to determine disease severity.
Cerebrospinal fluid analysis
Cerebrospinal fluid (CSF) may be altered if there is disease associated with the lower motor neuron cell bodies or when nerve roots are involved. Polyradiculoneuritis can result in increased protein in the CSF, often without an associated increase in nucleated cell count (cytoalbuminological dissociation). In some conditions there may be concurrent involvement of the central nervous system (CNS) — for example, lymphoma, where CSF analysis may reveal a neoplastic cell population. When there is a suspicion of infectious disease, both serology and PCR can be performed on CSF; infectious polyradiculoneuritis secondary to toxoplasmosis is one example where this would be useful.
Muscle and nerve biopsy
Due to the convergent clinical picture associated with feline neuromuscular disease, histological examination often remains the only option for providing a conclusive diagnosis and, from there, basing treatment selection on evidence rather than probabilities. The increasing prevalence of immune-mediated diseases in small animals, in particular, has shifted the value of muscle and nerve biopsies from a diagnostic tool towards a procedure with a direct impact on clinical management (Fig 2). In muscle, more than in nerve tissue, microscopy may also provide valuable information regarding the expected recovery or persistence of a dysfunction.
FIG 2.
Immunemediated conditions responsive to immunosuppressive or immunomodulatory treatment. (a) Teased nerve fibres affected by inflammatory demyelinating polyneuropathy in a domestic shorthair cat. (b, c) Polymyositis. (b) Invasion of myofibres (*) and endomysial interstitium by mononuclear cells (haematoxylin and eosin [HE]). (c) Confirmation of an immune-mediated pathology is based on absence of infectious organisms, negative titres and PCR, and evidence of invasion of predegenerate fibres (arrow; modified Gomori stain)
An algorithm for biopsy taking is shown on the right. As a rule of thumb, samples should be harvested from the affected muscle or nerve. If an electrophysiological device is available, an abnormal EMG pattern would be good indication for a muscle biopsy. It should be ensured, however, that biopsy interpretation is not hampered by artificial changes due to needle electrode puncture. The associated motor nerve should also be biopsied if NCSs reveal abnormal features.
Biopsy planning, furthermore, requires consideration of the co-morbidity associated with the surgery. Decision criteria and procedures have been nicely described elsewhere; 8 suffice to say that the laboratory should be contacted in advance in order to match the specific shipping requirements of any additional tests (eg, electron microscopy, respiratory chain analysis, PCR) that are being considered (Figs 3 and 4).
FIG 3.

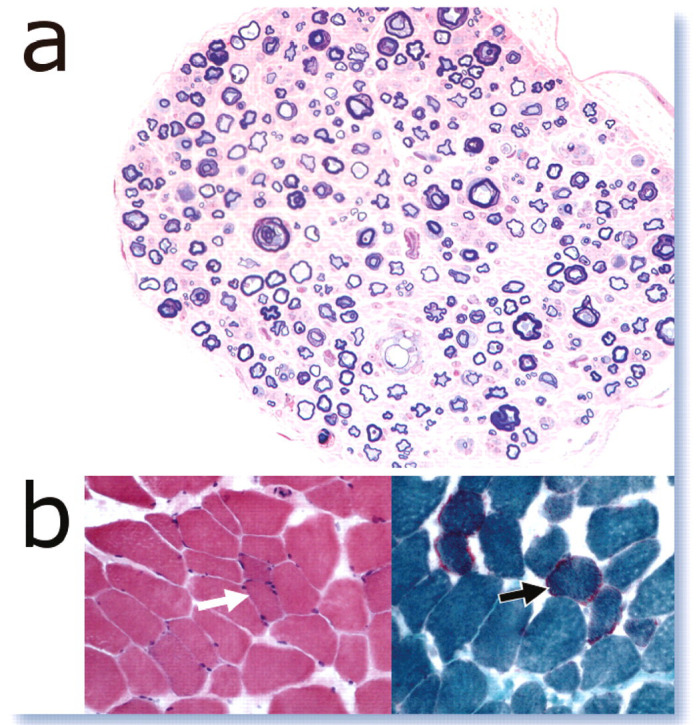
Muscle processing. Qualitative studies enrol standard HE staining (a), but also special stains such as modified Gomori stain to highlight mitochondrial features (b; arrow indicates subsarcolemmal mitochondrial aggregates). This latter stain, as well as the stains for polysaccharide and lipid storage, do not work in formalin-fixed paraffin-embedded tissue. Hence, special care should be taken to ensure the correct handling and shipping of fresh samples. Quantitative investigations are accomplished by myofibre typing (c). In the feline limb muscles type II fibres predominate (inset image; green fibres). Muscle diseases may also lead to changes in myofibre diameter, confirmed by myofibre histograms (d)
FIG 4.

Morphological analysis of peripheral nerves. Routinely, examination for nerve fibre alterations, and vascular and infiltrative diseases is carried out on semi-thin sections (a) (paraphenylenediamine). (b) Small fibre diseases and lysosomal storage disorders require investigation at electron microscopic level (this shows an ultrathin section). (c) Nerve fibre teasing preparations are superior for the detection of demyelinating diseases; this image shows teasing with micropincette after osmium impregnation. All these procedures rely on proper handling of samples, for which guidance should be sought from the specialised laboratory
Specialised metabolic and genetic tests
Further specialised assays may be indicated to investigate the possibility of metabolic disorders. These may be inherited or at least ‘inborn’ errors of metabolism that result in neuromuscular dysfunction. With disorders that result from specific enzyme deficiency (eg, glycogen storage disease), the diagnosis may be based on demonstration of low enzyme activity. Several neuromuscular diseases that are inherited or breed-associated may be confirmed by a DNA-based test (eg, PCR screening tests exist for glycogenosis type IV in Norwegian Forest cats).
Muscle and nerve biopsy is increasingly having an impact on clinical management.
Decision-making criteria for nerve and muscle biopsy
TABLE 1.
Clinical characteristics of neuromuscular diseases in the cat
| Clinical onset | Clinical course | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Disease | Age of onset | Acute | Chronic | Deteriorating | Improving | Episodic | Symmetrical | Pain | ||
|
| ||||||||||
| Neuropathies | Degenerative inherited disorders | |||||||||
| Birman cat distal polyneuropathy | 2 months | ✓ | ✓ | ✓ | ||||||
| Axonal polyneuropathy of Snowshoe cats | 4–6 months | ✓ | ✓ | ✓ | ||||||
| Bengal cat polyneuropathy | 10 months | ✓ | ✓ Initially | ✓ | ✓ | |||||
| Spinal muscular atrophy | 3–4 months | ✓ | ✓ | ✓ | ||||||
| Hypertrophic neuropathy | 7–12 months | ✓ | ✓ | ✓ | ||||||
| Lysosomal storage diseases | ||||||||||
| Glycogenosis type IV (also a myopathy) | 5 months | ✓ | ✓ | ✓ | ||||||
| Niemann-Pick disease type A | 4 months | ✓ | ✓ | |||||||
| Inherited metabolic disorders | ||||||||||
| Hyperchylomicronaemia | 1–2 months | ✓ | ✓ | ✓ | ||||||
| Hyperoxaluria | 6 months | ✓ | ✓ | ✓ | ||||||
| Endocrine/metabolic disorders | ||||||||||
| Diabetic neuropathy | Adult | ✓ | ✓ | ✓ | ||||||
| Infectious/inflammatory | ||||||||||
| Idiopathic polyradiculoneuritis | Any | ✓ | ✓ Initially | ✓ | ✓ | ✓ | ||||
| Chronic inflammatory demyelinating polyneuropathy | Adult | ✓ | ✓ | ✓ | ||||||
| Brachial plexus neuropathy/neuritis | Any | ✓ | ✓ | ✓ | ||||||
| Infectious neuropathies/polyradiculoneuritis | Any | ✓ | ✓ | ✓ | ||||||
| Toxicity | ||||||||||
| Salinomycin/vincristine toxicity | Any | ✓ | ✓ | ✓ | ||||||
| Chronic organophosphate/lead toxicity | Any | ✓ | ✓ | ✓ | ||||||
| Neoplastic | ||||||||||
| Peripheral nerve sheath tumour, lymphoma, etc | Any | ✓ | ✓ | (✓) | ||||||
| Nutritional | ||||||||||
| Tyrosine and phenylalanine deficiency | Any | ✓ | ✓ | ✓ | ||||||
| Traumatic | ||||||||||
| Brachial plexus avulsion, sacrocaudal luxation/fracture, iatrogenic | Any | ✓ | (✓) | (✓) | ||||||
| Vascular | ||||||||||
| Ischaemic neuromyopathy (also a myopathy) | Adult | ✓ | ✓ | (✓) | ✓ | |||||
| J'pathies | Myasthenia gravis (acquired/congenital) | Any | ✓ | ✓ | ✓ | |||||
| Botulism | Any | ✓ | (✓) | ✓ | ||||||
| Tick paralysis | Any | ✓ | (✓) | ✓ | ||||||
| Myopathies | Degenerative inherited disorders | |||||||||
| Nemaline rod myopathy | 6–18 months | ✓ | ✓ | ✓ | ||||||
| Muscular dystrophy | <12 months | ✓ | ✓ | ✓ | ✓ | |||||
| Inherited myopathy of Devon Rex cats | 1–5 months | ✓ | ✓ | ✓ | ||||||
| Metabolic | ||||||||||
| Hypokalaemic myopathy | Any | ✓ | ✓ | ✓ | ✓ | |||||
| Hyperthyroid myopathy | Adult | ✓ | ✓ | ✓ | ✓ | |||||
| Nutritional | ||||||||||
| Vitamin E/selenium-responsive myopathy | Any | ✓ | ✓ | ✓ | ✓ | |||||
| Infectious/inflammatory | ||||||||||
| Polymyositis | Any | ✓ | ✓ | (✓) | ||||||
= present; (✓) = can be present. J'pathies = Junctionopathies
Neuropathies
Degenerative Inherited Disorders
These degenerative, inherited, often breed-specific disorders are rare and predominantly affect young animals (Fig 5). The cats typically present with slowly progressive symmetrical, non-painful neuropathies although some exceptions exist (eg, the asymmetrical presentation of hyperchylomicronaemia). Some of the disorders described below have been demonstrated to be inherited metabolic disorders.
FIG 5.
Examples of inherited and breed-related neuropathies. (a) Axonal polyneuropathy in Birman cats. Teased fibre preparations show axonal atrophy with secondary myelin sheath pathology such as widening of the node of Ranvier (upper picture) and formation of bands of Büngner (lower picture). Due to progressive fibre loss the muscle becomes denervated and shows angular atrophied fibres (inset; type II fibres in brown). (b) Bengal cat polyneuropathy. Cats present within the first year of life with axonal changes and loss of fibres (semi-thin section; azure blue/safranin stain). (c) Hypertrophic neuropathy. This unusual type of demyelinating neuropathy is characterised by ‘onion bulb’-like Schwann cell proliferates (semi-thin section; azure blue)
Birman Cat Distal Polyneuropathy
This degenerative disease of Birman cats presents at 8–10 weeks of age with a slowly progressive axonopathy affecting the distal parts of the peripheral nervous system (Fig 5a); in addition, there is evidence of a distal axonopathy of the CNS. 9,10 The disease has a poor prognosis with no known treatment.
Axonal Polyneuropathy of Snowshoe Cats
Another early manifesting neuropathy has been observed in a couple of related Snowshoe kittens that were presented with pelvic limb weakness at 4 and 6.5 months of age. 11 Muscle and nerve biopsies documented an axonal disease. Notably, one of these cats and another recently identified individual showed concurrent CNS abnormalities. 11 In contrast to most known axonal neuropathies, the course was remittent over the observation period of 2 years.
Bengal Cat Polyneuropathy
Another remitting nerve disease has been observed in Bengal cats (see video clip, doi:10.1016/j.jfms.2011.09.005). Nerve biopsies also feature axonal changes (Fig 5b). However, mild lymphohistiocytic infiltrates in distal intramuscular nerve branches render an immune-mediated process possible, which may be facilitated in this breed by certain immune response gene haplotypes.
Spinal Muscular Atrophy (Polyneuronopathy, Motor Neuron Disease)
Spinal muscular atrophy describes the degeneration of motor neuron cell bodies due to premature cell death of unknown cause. This disorder presents as a rapidly progressing tetraparesis with reduced spinal reflexes. 12 –14 It is best characterised in Maine Coon cats, which present at approximately 3–4 months old, and for which there is a DNA-test available.
Hypertrophic Neuropathy
Hypertrophic neuropathy is an inherited disorder better known as a condition of Tibetan mastiffs, which has also been described in cats. This disease is characterised by widespread primary demyelination with hyperplasia of the Schwann cells (Fig 5c). Cats present between 7 and 12 months of age. 12,15 The prognosis is poor due to the progressive nature of the disease.
Lysosomal Storage Diseases
Glycogenosis Type IV
Glycogenosis type IV, a disorder seen in Norwegian Forest cats, results from a deficiency in the glycogen branching enzyme α-1, 4-Dglucan: α-1,4-D-glucan-6-glucosyl transferase. This leads to abnormal glycogen metabolism and deposition, affecting nerve and muscle function. 9,12,16 Cats with this glycogen storage disease present at approximately 5 months of age with a symmetrical, progressive paresis that later progresses to CNS signs. A PCR test is available to screen for this autosomal recessive disorder.
Niemann-Pick Disease Type A
Niemann-Pick disease type A (sphingomyelinosis) has been seen in Siamese and Balinese cats. 17,18 Reduced central lysosomal sphingomyelinase activity results in accumulation of sphingomyelin, cholesterol and gangliosides in neurons and visceral cells of the mononuclear phagocyte system. The onset of clinical signs is at approximately 4 months of age, with kittens showing progressive symmetrical paresis beginning in the pelvic limbs, a plantigrade stance and decreased postural reactions and spinal reflexes. 9 Later, head bobbing and apparent blindness develop. Progression to death is seen before the kitten reaches 1 year of age.
Inherited Metabolic Disorders
Hyperchylomicronaemia
Hyperchylomicronaemia is an autosomal recessive disease that has been seen in Siamese, Persian, Himalayan and domestic short- and longhair cats, presenting at approximately 6 weeks old. 9 A mutation in the gene encoding lipoprotein lipase leads to fasting hyperchylomicronaemia. 19,20 These cats develop lipid granulomas over pressure points and at vertebral foramina. The resulting nerve root compression causes the presenting signs of a progressive, focal or multifocal, asymmetric mononeuropathy.
Hyperoxaluria
Increased urinary oxalate and L-glyceric acid occur as a consequence of reduced activity of the hepatic enzyme D-glycerate dehydrogenase. Cats present acutely at approximately 6 months of age with acute renal failure due to oxalate crystal deposition in the renal tubules. 12,21 These signs are accompanied by a polyneuropathy. There has been no evidence of oxalate deposition outside of the urinary system and the pathogenesis of the accompanying polyneuropathy has not been determined.
Endocrine/Metabolic Disorders
The neuropathy associated with diabetes mellitus in most cases presents as a chronic, progressive, symmetrical lower motor neuron paresis of the pelvic limbs manifesting as a plantigrade stance. 22,23 Occasionally, thoracic limb paresis has been noted, but this is much less common in the cat than the dog. Once good control of the diabetes is achieved there is improvement in signs in most cases. Full recovery is not always achieved, however, and in some cases the neurological deficits remain (Fig 6a). Although the signs of motor dysfunction tend to be readily apparent, electrodiagnostic and pathological studies confirm sensory involvement in cases of diabetic neuropathy, which is probably underdiagnosed clinically.
FIG 6.
Common metabolic causes of neuromuscular disease. (a) Diabetes mellitus is frequently associated with peripheral nerve disease; this specimen is from a 9-year-old British Shorthair cat with chronic diabetic neuropathy. (b) Electrolyte imbalance may culminate in rhabdomyolysis but in the vast majority of cases leads to minor muscle changes, such as occasional fibre atrophy (left picture; HE; white arrow), with or without mitochondrial prominence (right picture; modified Gomori stain; black arrow); these preparations were taken from a domestic shorthair cat with hypokalaemia. Definitive diagnosis requires multimodal testing
Neoplasia
Neoplastic disease is often thought to have a chronic progressive clinical course. In some cases, however, an acute onset is seen when autoregulatory or compensatory mechanisms of the nervous tissue are suddenly overwhelmed.
Neoplastic disease processes such as lymphoma often, but not invariably, have an asymmetrical distribution and may or may not be associated with pain. The most common predilection site for lymphoma in cats is the sciatic nerve due to the proximity of the inguinal lymph nodes and the connection to the epineurium/perineurium via the respective lymphatics. Solitary neoplasms have the potential to cause a symmetrical polyneuropathy as part of a paraneoplastic syndrome; however, this appears to be a very rare occurrence in cats. Very occasionally, tumour-affected cats also may present with a concurrent inflammatory demyelinating polyneuropathy. The causal link is unclear.
Infectious/Inflammatory Disorders
Infectious/inflammatory conditions of the neuromuscular system can be symmetrical, progressive and occasionally painful. In some conditions, spontaneous improvement is seen (such as idiopathic polyradiculoneuritis), whereas other conditions show progression until the necessary treatment is instigated.
Idiopathic Polyradiculoneuritis
Feline patients with idiopathic polyradiculoneuritis typically present with an acuteonset paraparesis, progressing rapidly over 2–3 days to non-ambulatory, lower motor neuron tetraparesis and then tetraplegia. 24,25
Often considered a chronic, progressive disease, neoplasia can present acutely when autoregulatory or compensatory mechanisms of nervous tissue are suddenly overwhelmed.
The inflammatory pathology is centred on the nerve roots, with greater involvement of the ventral (motor) root. This is reflected in the relative preservation of sensory function in the clinical patient and the presence of paresis without ataxia. Spinal reflexes are reduced or absent. Electrodiagnostic studies aimed at assessing nerve root function will provide further support for the diagnosis and CSF analysis often reveals elevated protein, reflecting the nerve root involvement. Peripheral nerve biopsy will show non-specific changes secondary to the nerve root pathology.
In spite of the dramatic presenting neurological deficits, feline patients can make a complete recovery over 2–6 weeks with just supportive care, although some severe cases have died due to respiratory failure in the acute stages. A relapsing form has been reported with two to three episodes of this neuropathy with full recovery in between. 26
Chronic Inflammatory Demyelinating Polyneuropathy
Chronic inflammatory demyelinating polyneuropathy (chronic relapsing polyneuropathy) describes an insidiously progressive, presumptive autoimmune lower motor neuron disorder that has been reported in adult cats. 25,27 Nerve biopsy reveals demyelination and remyelination with evidence of inflammatory infiltrates. Most cats show some response to treatment with corticosteroids; however, relapses often occur. 25 In some cases there has been apparent spontaneous improvement.
Brachial Plexus Neuropathy/Neuritis
Brachial plexus neuropathy (neuritis) is a relatively rare disorder that presents as a bilateral lower motor neuron paresis of the thoracic limbs, and is suspected to be the result of an autoimmune pathology. 28,29 There may be pain on manipulation of the thoracic limbs and involvement of additional peripheral nerves. 28 Signs may resolve with treatment, but relapses have been recorded.
Infectious Neuropathies
In young dogs, protozoal infections can produce a polyradiculoneuritis, mostly affecting the lumbosacral nerve roots, 30 and it is possibile that Toxoplasma might cause similar signs in cats. Feline leukaemia virus (FeLV) infection can, in rare cases, be associated with tetraparesis due to a generalised polyneuropathy. 25 Urinary incontinence seen in some cats with FeLV may be the result of a neuropathic syndrome. The potential for feline immunodeficiency virus (FIV) infections to produce a generalised neuropathy in combination with an inflammatory myopathy has been demonstrated experimentally. 31
Infectious/ inflammatory conditions of the neuromuscular system can be symmetrical, progressive and occasionally painful.
Toxic Neuropathy
Salinomycin
Cat food contaminated with salinomycin (a chicken coccidiostat) was responsible for an outbreak of a progressive polyneuropathy involving dysphagia and dyspnoea. 32 Most cats showed recovery with supportive care and removal of the affected feed.
Vincristine
Vincristine has resulted in neuropathies in human patients, probably due to its adverse effects on axon transport. The possibility of toxic neuropathy should be considered in cats receiving vincristine that present with a lower motor neuron paresis and/or sensory deficits. 33,34
Trauma
Trauma-related neuropathy may be seen in association with a fracture (eg, sciatic nerve dysfunction secondary to a pelvic fracture) or in the absence of orthopaedic disease where the nerve suffers a traction injury at the time of the trauma. Traumatic neuropathies tend to be acute in onset and commonly show an improving clinical course; however, when associated with a fracture, subsequent bone remodelling may cause progressive nerve impingement and dysfunction. Lateralising clinical signs are often a feature of traumatic neuropathies (eg, monoparesis seen with a brachial plexus injury), although symmetrical signs do not exclude the possibility of a traumatic injury. In the acute stages these injuries are often painful.
Vascular Disorders
Aortic Thromboembolism/Ischaemic Neuromyopathy
Occlusion of the distal aorta secondary to thromboembolism and associated inflammatory mediator effects on the collateral circulation result in ischaemia to the pelvic limb muscles and peripheral nerves. Although cases resulting from neoplastic emboli or hypercoagulable states have been reported, in cats this syndrome is typically seen secondarily to cardiomyopathy. 35,36
Cats present with an acute onset, painful lower motor neuron paraparesis/paraplegia, with more severe dysfunction often noted in the distal limbs. 25 Distal pelvic limb musculature is often firm and painful. Peripheral pulses will be reduced or absent and the limb extremities may be palpably cold. Diagnosis is based on characteristic clinical findings, including demonstration of reduced pelvic limb blood flow and identification of cardiomyopathy. Ultrasonography can be used to confirm the presence of thromboembolic disease. In cases where neurological function recovers, the prognosis remains guarded due to the likelihood of recurrence.
Junctionopathies
Myasthenia Gravis
Acquired Myasthenia Gravis
Acquired myasthenia gravis is an immunemediated disease associated with the production of antibodies directed against the acetylcholine receptor of the neuromuscular junction. 37 The resultant deficiency in functional receptors reduces the sensitivity of the post-synaptic membrane to acetylcholine. This leads to decreased neurotransmission and clinical signs of paresis. This autoimmune disease may in some cases develop as part of a paraneoplastic syndrome and can also be seen in association with thymic dysfunction (thymoma, thymic cyst or non-neoplastic thymic disease). 25,38 –40 Abyssinians and Somalis seem to be overrepresented compared with other breeds of cat. 39
Myasthenia gravis can be classified according to the clinical signs:
Focal myasthenia gravis presents as weakness of focal muscle groups. Oesophageal, pharyngeal, laryngeal or facial muscle involvement is most commonly seen.
Generalised myasthenia gravis presents with generalised weakness that can be induced or exacerbated by exercise.
Acute fulminating myasthenia gravis is a form of generalised myasthenia gravis characterised by a rapid onset and progression.
Several differences exist in the typical presentation of cats with myasthenia gravis compared with dogs. 37,39 Focal forms are less common in cats, accounting for just 15% of feline cases. In cats, there is also a higher proportion of cases (26%) in which myasthenia gravis occurs in association with thymoma.
Myasthenia gravis is suspected based on the clinical presentation, response to administration of the short-acting anticholinesterase drug edrophonium chloride (Tensilon) and electrodiagnostic findings. Definitive diagnosis relies on demonstration of acetylcholine receptor antibodies.
Treatment involves medication with the longer acting anticholinesterase pyridostigmine and management of any thymic disease. The use of immunomodulatory drugs should only be considered if absolutely necessary due to the high risk of aspiration pneumonia in patients with dysphagia and regurgitation.
Congenital Myasthenia Gravis
Myasthenia gravis can present as a congenital condition, predominantly due to a congenital lack of functional acetylcholine receptors. 37 Treatment with pyridostigmine can result in clinical improvement. The long-term prognosis for affected cats is uncertain because of the low number of reported cases.
Because the sensory neurons and lower motor neurons are typically unaffected in cats with myopathy, postural reactions and spinal reflexes will typically be normal.
Botulism
Botulism usually occurs following ingestion of preformed toxin produced by Clostridium botulinum in carrion or spoiled food, and can present as a generalised paresis with reduced spinal reflexes, facial muscle paresis, dysphonia, dysphagia, megaoesophagus, and/or autonomic signs such as salivation, urinary dysfunction and mydriasis. Signs may be preceded by gastrointestinal complaints. Cats appear to be relatively resistant to botulinum toxin. 25
Tick Paralysis
Certain tick species release a salivary neurotoxin that interferes with acetylcholine release at the neuromuscular junction. 41 It can also affect the production of action potentials and propagation of both sensory and motor neuron action potentials. These effects result in an acute onset of tetraparesis, often beginning in the pelvic limbs, progressing to paralysis. 25 Spinal reflexes are lost, with occasional involvement of the cranial nerves. Dermacentor ticks in North America and Ixodes ticks in Australia are the notable species associated with disease in dogs and cats. Cats appear to be relatively resistant to tick paralysis in North America; however, severe clinical signs, resulting in death, have been seen in cases in Australia. 41
Myopathies
Cats with myopathy may present with a generalised paresis. Other presenting signs include exercise intolerance, a stiff gait and ventroflexion of the neck. Because the sensory neurons and lower motor neurons are typically unaffected, postural reactions and spinal reflexes will typically be normal on neurological examination, although some exceptions exist where the patellar reflex can be reduced.
Degenerative Inherited Disorders
Nemaline Rod Myopathy
Nemaline rod myopathy has been seen in young cats (6–18 months old) presenting acutely with an apparently hereditary condition manifesting as progressive paresis and exercise intolerance. 42 Diagnosis is based on the clinical signs and presence of rod-shaped nemaline inclusions within myofibres, in the absence of a demonstrable cause for myopathy. The prognosis is poor in these cases, due to progressive muscle atrophy and inappetence.
Dystrophin-deficient (X-linked) Muscular Dystrophy
Dystrophin links the cytoskeleton of the myofibre to the extracellular matrix and is therefore vital in maintaining muscle basement membrane (sarcolemmal) integrity. 43 Cats with dystrophin-deficient muscular dystrophy present with a stiff gait and may bunny hop when running; adduction of the hocks and cervical rigidity may also be seen. A striking feature of X-linked muscular dystrophy in cats is generalised muscle hypertrophy, involving the tongue, laryngeal, cardiac and diaphragmatic muscles. Additional features include multifocal lingual calcification, hepatosplenomegaly and megaoesophagus.
A clinical suspicion of muscular dystrophy is based on signalment (typically young, male cats), clinical presentation, increased CK activity (sometimes markedly increased), and EMG changes with spontaneous activity characterised by complex repetitive discharges. Definitive diagnosis is achieved by demonstrating the absence of dystrophin on immunohistology of muscle biopsy specimens. The prognosis is generally considered poor, due in particular to the lingual and diaphragmatic hypertrophy.
Muscular Dystrophy with Laminin Alpha 2 Deficiency
Laminin alpha 2 (merosin) is a large glycoprotein component of the basement membrane of skeletal muscle fibres. It is also one of the components that links dystrophin to the extracellular matrix and contributes to sarcolemmal stability. Laminin alpha 2 is not exclusive to muscle fibres and is an important component of the Schwann cell basement membrane. In this form of muscular dystrophy, in contrast to X-linked muscular dystrophy, young cats present with muscle weakness and atrophy and, in some cases, muscle contractures. 43 There may be peripheral nerve dysfunction due to adverse effects on the Schwann cell. Definitive diagnosis is made by demonstrating an absence of laminin alpha 2 on immunohistology of muscle biopsy samples.
β-Sarcoglycan-Deficient Muscular Dystrophy
Sarcoglycans are transmembrane glycoproteins that, in humans and dogs, are associated with the so-called limb-girdle or proximal muscular dystrophies. In the cat, one case of β-sarcoglycan deficiency has been described in a male Italian domestic shorthair kitten. At an age of 6 months the animal was presented due to weakness, reluctance to move and dyspnoea. Blood work revealed mild hyperglycaemia, hypertriglyceridaemia, increased alanine transferase and CK, hyperkalaemia and hypercalcaemia. Muscle biopsies showed myofibre dystrophy and the molecular pathogenesis was identified using immunohistochemistry and Western blot. 44
Metabolic Disorders
Hypokalaemic Myopathy
Hypokalaemic myopathy is relatively common in cats (Fig 6b) and usually seen in association with chronic renal disease with excessive urinary potassium loss. 45 Alternative causes of the hypokalaemia include reduced dietary intake of potassium, gastrointestinal loss, hyperthyroidism, diuretic use, hyperaldosteronism and post-obstructive diuresis following treatment of urethral obstruction. 25,46 Affected cats present acutely with a stiff gait and neck ventroflexion. Unlike typical metabolic disease processes, pain (myalgia) is seen in some cases. In severe hypokalaemia, respiratory compromise and rhabdomyolysis can be seen.
Supportive care and oral potassium supplementation can result in notable improvement over the first 2–3 days of treatment. The primary cause of the hypokalaemia should also be addressed. Young Burmese cats (2–6 months old) have been seen with a hereditary periodic hypokalaemic myopathy. 47 The frequency and severity of the transient episodes can be reduced with dietary potassium supplementation.
Nutritional Disorders
Vitamin E deficiency is a notable cause of myopathy in large animals and, although rare, has been seen in feline patients. A confirmed case of vitamin E deficiency myopathy was reported in a young cat fed exclusively on coley. 48 Muscle pain and swelling can be present, with the potential for sudden death given the effects on cardiac muscle. Clinical recovery has been seen following supplementation with vitamin E.
Inflammatory Disorders
Immune-mediated Inflammatory Myopathy (Polymyositis)
This inflammatory disease is less commonly reported in cats than in dogs. 25 The pathogenesis is unknown and not associated with systemic or infectious diseases (see below). Clinical signs are symmetrical and there may be exercise intolerance, and a stiff and uncomfortable gait; demonstrable myalgia may not be present. CK activity will be raised, except at the end stage of disease, and abnormal spontaneous electrical activity can be identified on EMG. Muscle biopsy, demonstrating a mononuclear cell inflammatory infiltrate with myofibre necrosis and regeneration, is required for definitive diagnosis. A lack of identifiable cause, such as an infectious agent, is also required for the diagnosis of immune-mediated polymyositis.
Immunosuppressive therapy with prednisolone has resulted in recovery in some cats, although relapses may occur. It should be noted that an autoimmune polymyopathy can occur as a paraneoplasic complication. Where there is poor response to treatment, or relapses occur, the cat should be evaluated for underlying malignancy.
KEY POINTS
Weakness in cats can be caused by primary neuromuscular disease or by diseases of other body systems affecting the neuromuscular system secondarily.
The diagnostic work-up of cats presenting with weakness can be clinically challenging, and requires an in-depth clinical and neurological examination, and logical problem solving, backed up by an understanding of the underlying neuroanatomical and pathophysiological mechanisms.
Typical clinical signs of neuromuscular dysfunction in cats are mainly gait and postural abnormalities, such as a stiff, stilted, choppy limb movement, ventroflexion of the neck and dorsal displacement of the scapulae when weightbearing.
The onset and clinical course of the presenting complaint, any association with exercise and whether weakness is persistent or episodic should be considered when creating a problem list.
The clinical phenotype of different feline neuromuscular diseases is similar. Further diagnostic steps (eg, electrodiagnostics, cerebrospinal fluid analysis, muscle and nerve biopsies) are, therefore, often warranted in order to reach a final diagnosis.
Infectious Inflammatory Myopathy
The potential for protozoal infection to result in myositis in dogs is well described. 30 Toxoplasma and Sarcocystis species have been identified in feline muscle and Neospora species has been shown experimentally to infect feline muscle; 49 however, any relation to clinical disease is not known. Of the bacterial causes of myositis, clostridial myositis appears to be most common. It presents as a focal lesion with painful, crepitant swelling and may follow muscle injuries or surgical procedures. Viral-induced myositis is rare, although a subclinical myositis has been seen in cats experimentally infected with FIV. 31
Acknowledgements
The authors would like to thank Ms Emma Davies and Dr Kate Chandler for their contribution of image material.
Biography

References
- 1. Duysens J, Van de Crommert HW. Neural control of locomotion; the central pattern generator from cats to humans. Gait Posture 1998; 7: 131–41. [DOI] [PubMed] [Google Scholar]
- 2. Martin JH. The corticospinal system: from development to motor control. Neuroscientist 2005; 11: 161–73. [DOI] [PubMed] [Google Scholar]
- 3. Muir GD. Early ontogeny of locomotor behaviour: a comparison between altricial and precocial animals. Brain Res Bull 2000; 53: 719–26. [DOI] [PubMed] [Google Scholar]
- 4. Villablanca JR, Olmstead CE. Neurological development of kittens. Dev Psychobiol 1979; 12: 101–27. [DOI] [PubMed] [Google Scholar]
- 5. Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 1973; 21: 51–55. [DOI] [PubMed] [Google Scholar]
- 6. Garosi L. Neurological examination of the cat. How to get started. J Feline Med Surg 2009; 11: 340–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shelton GD. Routine and specialized laboratory testing for the diagnosis of neuromuscular diseases in dogs and cats. Vet Clin Pathol 2010; 39: 278–95. [DOI] [PubMed] [Google Scholar]
- 8. Dickinson PJ, LeCouteur RA. Muscle and nerve biopsy. Vet Clin North Am Small Anim Pract 2002; 32: 63–102. [DOI] [PubMed] [Google Scholar]
- 9. Coates JR, O'Brien DP. Inherited peripheral neuropathies in dogs and cats. Vet Clin North Am Small Anim Pract 2004; 34: 1361–401. [DOI] [PubMed] [Google Scholar]
- 10. Moreau PM, Vallat JM, Hugon J, Leboutet MJ, Vandevelde M. Peripheral and central distal axonopathy of suspected inherited origin in Birman cats. Acta Neuropathol 1991; 82: 143–46. [DOI] [PubMed] [Google Scholar]
- 11. Matiasek LA, Lujan Feliu-Pascual A, Shelton DG, De Risio L, Matiasek K. Axonal neuropathy with unusual clinical course in young Snowshoe cats. J Feline Med Surg 2009; 11: 1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chrisman CL. Polyneuropathies of cats. J Small Anim Pract 2000; 41: 384–89. [DOI] [PubMed] [Google Scholar]
- 13. Iannaccone ST. Feline spinal muscular atrophy. Pediatr Res 2005; 57: 322–23. [DOI] [PubMed] [Google Scholar]
- 14. Shelton GD, Hopkins AL, Ginn PE, et al. Adult-onset motor neuron disease in three cats. J Am Vet Med Assoc 1998; 212: 1271–75. [PubMed] [Google Scholar]
- 15. Dahme E, Kraft W, Scabell J. Hypertrophic polyneuropathy in cats [German]. Zentralbl Veterinarmed A 1987; 34: 271–88. [PubMed] [Google Scholar]
- 16. Fyfe JC, Giger U, VanWinkle TJ, et al. Glycogen storage disease type IV: inherited deficiency of branching enzyme activity in cats. Pediatr Res 1992; 32: 719–25. [DOI] [PubMed] [Google Scholar]
- 17. Baker HJ, Wood PA, Wenger DA, et al. Sphingomyelin lipidosis in a cat. Vet Pathol 1987; 24: 386–91. [DOI] [PubMed] [Google Scholar]
- 18. Wenger DA, Sattler M, Kudoh T, Snyder SP, Kingston RS. Niemann-Pick disease: a genetic model in Siamese cats. Science 1980; 208: 1471–73. [DOI] [PubMed] [Google Scholar]
- 19. Jones BR, Johnstone AC, Cahill JI, Hancock WS. Peripheral neuropathy in cats with inherited primary hyperchylomicronaemia. Vet Rec 1986; 119: 268–72. [DOI] [PubMed] [Google Scholar]
- 20. Ginzinger DG, Lewis ME, Ma Y, Jones BR, Liu G, Jones SD. A mutation in the lipoprotein lipase gene is the molecular basis of chylomicronemia in a colony of domestic cats. J Clin Invest 1996; 97: 1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McKerrell RE, Blakemore WF, Heath MF, et al. Primary hyperoxaluria (L-glyceric aciduria) in the cat: a newly recognised inherited disease. Vet Rec 1989; 125: 31–34. [DOI] [PubMed] [Google Scholar]
- 22. Kramek BA, Moise NS, Cooper B, Raffe MR. Neuropathy associated with diabetes mellitus in the cat. J Am Vet Med Assoc 1984; 184: 42–45. [PubMed] [Google Scholar]
- 23. Mizisin AP, Shelton GD, Burgers ML, Powell HC, Cuddon PA. Neurological complications associated with spontaneously occurring feline diabetes mellitus. J Neuropathol Exp Neurol 2002; 61: 872–84. [DOI] [PubMed] [Google Scholar]
- 24. Gerritsen RJ, van Nes JJ, van Niel MH, van den Ingh TS, Wijnberg ID. Acute idiopathic polyneuropathy in nine cats. Vet Q 1996; 18: 63–65. [DOI] [PubMed] [Google Scholar]
- 25. Dickinson P, Lecouteur R. Feline neuromuscular disorders. Vet Clin North Am Small Anim Pract 2004; 34: 1307–59. [DOI] [PubMed] [Google Scholar]
- 26. Granger N, Stalin CE, Harcourt Brown TB, Jeffery ND. Idiopathic polyradiculoneuropathy in a Bengal cat: electrophysiological findings and 1 year follow-up. J Feline Med Surg 2008; 10: 603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Braund KG, Vallat JM, Steiss JE, Panangala VS, Zimmer PL. Chronic inflammatory demyelinating polyneuropathy in dogs and cats. J Peripher Nerv Syst 1996; 1: 149–55. [PubMed] [Google Scholar]
- 28. Freeman PM, Harcourt-Brown TR, Jeffery ND, Granger N. Electrophysiologic evidence of polyneuropathy in a cat with signs of bilateral brachial plexus neuropathy. J Am Vet Med Assoc 2009; 234: 240–44. [DOI] [PubMed] [Google Scholar]
- 29. Garosi L, de Lahunta A, Summers B, Dennis R, Scase T. Bilateral, hypertrophic neuritis of the brachial plexus in a cat: magnetic resonance imaging and pathological findings. J Feline Med Surg 2006; 8: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Troxel MT. Infectious neuromuscular diseases of dogs and cats. Top Companion Anim Med 2009; 24: 209–20. [DOI] [PubMed] [Google Scholar]
- 31. Phillips TR, Prospero-Garcia O, Wheeler DW, et al. Neurologic dysfunctions caused by a molecular clone of feline immunodeficiency virus, FIV-PPR. J Neurovirol 1996; 2: 388–96. [DOI] [PubMed] [Google Scholar]
- 32. van der Linde-Sipman JS, van den Ingh TS, van Nes JJ, et al. Salinomycin-induced polyneuropathy in cats: morphologic and epidemiologic data. Vet Pathol 1999; 36: 152–56. [DOI] [PubMed] [Google Scholar]
- 33. Cho ES, Lowndes HE, Goldstein BD. Neurotoxicology of vincristine in the cat. Morphological study. Arch Toxicol 1983; 52: 83–90. [DOI] [PubMed] [Google Scholar]
- 34. Goldstein BD, Lowndes HE, Cho E. Neurotoxicology of vincristine in the cat. Electrophysiological studies. Arch Toxicol 1981; 48: 253–64. [DOI] [PubMed] [Google Scholar]
- 35. Smith SA, Tobias AH, Jacob KA, Fine DM, Grumbles PL. Arterial thromboembolism in cats: acute crisis in 127 cases (1992–2001) and long-term management with low-dose aspirin in 24 cases. J Vet Intern Med 2003; 17: 73–83. [DOI] [PubMed] [Google Scholar]
- 36. Laste NJ, Harpster NK. A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977–1993. J Am Anim Hosp Assoc 1995; 31: 492–500. [DOI] [PubMed] [Google Scholar]
- 37. Shelton GD. Myasthenia gravis and disorders of neuromuscular transmission. Vet Clin North Am Small Anim Pract 2002; 32: 189–206. [DOI] [PubMed] [Google Scholar]
- 38. Day MJ. Review of thymic pathology in 30 cats and 36 dogs. J Small Anim Pract 1997; 38: 393–403. [DOI] [PubMed] [Google Scholar]
- 39. Shelton GD, Ho M, Kass PH. Risk factors for acquired myasthenia gravis in cats: 105 cases (1986–1998). J Am Vet Med Assoc 2000; 216: 55–57. [DOI] [PubMed] [Google Scholar]
- 40. Malik R, Gabor L, Hunt GB, et al. Benign cranial mediastinal lesions in three cats. Aust Vet J 1997; 75: 183–87. [DOI] [PubMed] [Google Scholar]
- 41. Malik R, Farrow BR. Tick paralysis in North America and Australia. Vet Clin North Am Small Anim Pract 1991; 21: 157–71. [DOI] [PubMed] [Google Scholar]
- 42. Cooper BJ, De Lahunta A, Gallagher EA, Valentine BA. Nemaline myopathy of cats. Muscle Nerve 1986; 9: 618–25. [DOI] [PubMed] [Google Scholar]
- 43. Shelton G, Engvall E. Muscular dystrophies and other inherited myopathies. Vet Clin North Am Small Anim Pract 2002; 32: 103–24. [DOI] [PubMed] [Google Scholar]
- 44. Salvadori C, Vattemi G, Lombardo R, Marini M, Cantile C, Shelton GD. Muscular dystrophy with reduced beta-sarcoglycan in a cat. J Comp Pathol 2009; 140: 278–82. [DOI] [PubMed] [Google Scholar]
- 45. Dow SW, Fettman MJ, Curtis CR, LeCouteur RA. Hypokalemia in cats: 186 cases (1984–1987). J Am Vet Med Assoc 1989; 194: 1604–8. [PubMed] [Google Scholar]
- 46. Nemzek JA, Kruger JM, Walshaw R, Hauptman JG. Acute onset of hypokalemia and muscular weakness in four hyperthyroid cats. J Am Vet Med Assoc 1994; 205: 65–68. [PubMed] [Google Scholar]
- 47. Blaxter A, Lievesley P, Gruffydd-Jones T, Wotton P. Periodic muscle weakness in Burmese kittens. Vet Rec 1986; 118: 619–20. [DOI] [PubMed] [Google Scholar]
- 48. Dennis JM, Alexander RW. Nutritional myopathy in a cat. Vet Rec 1982; 111: 195–96. [DOI] [PubMed] [Google Scholar]
- 49. Craig TM. Parasitic myositis of dogs and cats. Semin Vet Med Surg (Small Anim) 1989; 4: 161–67. [PubMed] [Google Scholar]







