Abstract
Practical relevance Neurological disease is a relatively common reason for referral, constituting approximately 10% of the feline referral caseload. Nearly one-third to one-half of these cases may be infectious in origin. As such, an awareness of infectious diseases causing central nervous system (CNS) signs in cats, and their clinical diagnosis and management, is relevant to anyone dealing with cats on a regular basis.
Global importance Some conditions (eg, rabies) are more common in certain countries than others. Conditions such as feline infectious peritonitis (FIP) and toxoplasmosis are of global significance.
Patient group Many infectious diseases may affect any feline population. Some, such as FIP, are more common in pedigree households, whereas others such as toxoplasmosis, feline immunodeficiency virus (FIV) or feline leukaemia virus (FeLV) infections, are more likely to affect a single cat with an outdoor lifestyle.
Equipment All patients benefit from thorough history taking and clinical, neurological and ophthalmic examinations, which all require minimal equipment. Infectious diseases may often be diagnosed on blood samples; however, definitive diagnosis may require more extensive investigation involving cerebrospinal fluid analysis or advanced imaging necessitating access to computed tomography or magnetic resonance imaging.
Evidence base The information in this review, which summarises current knowledge of infectious diseases affecting the CNS, is collated from publications on the infectious diseases comprising previous research papers, review articles, case series, case reports and textbooks, supplemented by the clinical experience of the authors.
How significant is infectious CNS disease?
Neurological disease is common in cats — accounting, for example, for approximately 10% of cases in two separate UK-based feline medicine referral clinics. 1,2 There are many different causes of central nervous system (CNS) disease in cats (see box, page 825) and about 30–45% of cases are believed to be infectious in origin. 1,3 However, a specific infectious agent cannot be identified in up to 40% of these cases; which holds true when looking at either clinical cases 4 –6 or histopathological data (Table 1). 1,3 Most of these cases are found to have histopathological changes suggestive of viral infection that is not consistent with feline infectious peritonitis (FIP) (so-called viral non-FIP encephalitides), but no causal agent can be identified. 1 –3 This group becomes even more significant if cats with particular clinical signs are considered. For example, of 30 cats investigated for recurrent seizures, all were found to have structural brain disease and 14 (47%) had non-suppurative meningoencephalitis, suggestive of a viral infection, but no infectious agent could be identified. 5
TABLE 1.
Diagnoses in 92 feline neurological cases found to have CNS histopathology consistent with inflammation and/or infection 1
| Cause | Number | Percentage |
|---|---|---|
|
| ||
| Feline infectious peritonitis (FIP) * | 47 | 51 |
| Viral non-FIP encephalitides † | 32 | 35 |
| Protozoal cysts (eg, toxoplasmosis) | 8 | 9 |
| Bacterial infection | 3 | 3 |
| Feline immunodeficiency virus (FIV) infection | 1 | 1 |
| Cryptococcosis | 1 | 1 |
One of the cats was also found to have an incidental nematode larva (Toxocara) within its lateral ventricle.
Also called non-suppurative (meningo)encephalitides (of unknown cause)
FIP and viral non-FIP encephalitides (ie, non-suppurative [meningo]encephalitides of unknown, although probable viral, cause) are the two most commonly recognised (confirmed or probable) infectious CNS disorders of cats. Toxoplasmosis is the third most commonly identified cause, while other infections such as bacterial infections, feline immunodeficiency virus (FIV), feline leukaemia virus (FeLV), feline panleukopenia virus (FPV), and fungal and parasitic infections only rarely manifest primarily as neurological conditions.
Naturally occurring infectious causes of CNS disease in domestic cats
Diagnostic work-up
The complete diagnostic work-up comprises a careful history and clinical examination, ophthalmic, otoscopic and neurological examinations, haematology, serum biochemistry, tests for specific infectious agents, advanced imaging (magnetic resonance imaging [MRI] or computed tomography [CT]) and cerebrospinal fluid (CSF) analysis (Tables 2 and 3). 7,8 Unfortunately, the lack of pathognomonic clinical signs and the limited availability of many diagnostic tests, including CT/MRI and tests to detect specific infectious agents (see box, page 827), means that it is impossible to confirm the cause in many cases of infectious CNS disease.
TABLE 2.
Information that may be useful in the diagnosis of infectious causes of CNS disease in cats
| Disease | Signalment and history | Common clinical signs | Useful diagnostic tests/findings | Frequency |
|---|---|---|---|---|
|
| ||||
| Feline infectious peritonitis (FIP) | Pedigree cats under 4 years of age that have often come from a large breeding colony Kittens from rescue centres Recent stress (eg, rehoming) | Systemic and ocular signs, multifocal (occasionally focal) CNS signs, altered mentation, ataxia, cranial nerve abnormalities, seizures | Non-regenerative anaemia, increased neutrophils, decreased lymphocytes; reduced serum albumin/globulin ratio; increased serum alpha-1 acid glycoprotein and serum bilirubin; increased serum and CSF FCoV titre; abdominal ultrasound examination; head MRI | Common |
| Other viral encephalitis (eg, Borna disease virus) or viral non-FIP encephalitides | Young to middle-aged cats | Behavioural and/or motor changes | Head MRI | Probably common |
| Protozoal meningoencephalitis (eg, toxoplasmosis) | Adult cats that hunt Occasionally in young cats as a congenital infection | Systemic and ocular signs, multifocal CNS signs, altered mentation, ataxia, cranial nerve abnormalities, seizures | Non-regenerative anaemia, increased neutrophils and lymphocytes; serum biochemistry (resulting from systemic disease); serum and CSF antibody titres; detection of organisms in aspirates or on histopathology; head MRI | Relatively common |
| Fungal meningoencephalitis (eg, cryptococcosis) | Adult cats with a history of upper respiratory tract disease | Nasal and ocular signs, multifocal or focal CNS disease, altered mentation, ataxia, cranial nerve abnormalities, seizures | Demonstration of organisms by India ink stain; serum latex agglutination antigen titre; head CT/MRI | Rare — but may be geographical location dependent |
| Bacterial meningoencephalitis | Any age, history of ear infections, or bacterial focus elsewhere in the body (eg, myocarditis) | Severe depression, pyrexia, neck pain, neurological deficits, ocular changes | Neutrophilia with a left shift; CSF and/or blood culture; head CT/MRI | Rare |
| FeLV-induced lymphoma | Young cats | Multifocal signs, cranial nerve deficits, seizures, altered mentation, spinal pain, paresis/paralysis | Blood FeLV antigen or PCR tests; lymphoma cells in CSF; head and spine MRI | Rare |
TABLE 3.
Infectious diseases of the feline CNS with typical CSF changes
| Disease | CSF pressure | CSF appearance | WBC count a | WBC type b | Total protein (mg/dl) c | Albumin | Globulin | CSF antibodies detectable | Organisms visible |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Feline infectious peritonitis (FIP) | WNL or ↑ | Clear or turbid | +++ (WNL/++) | PMN/mono/mixed | +++ (WNL/+) | ++ | ++ | Yes | No |
| Other viral encephalitis (eg, Borna diseased) | WNL | Clear (turbid) | + (++) | Mono | + (++) | WNL | ? | No | No |
| Protozoal meningoencephalitis (eg, toxoplasmosis) | WNL or | Xanthochromic | + (++) | Mixed/mono/PMN/eos | + (++) | + | + | Variable | Rarely |
| Fungal meningoencephalitis (eg, cryptococcosis) | or viscous | Turbid, xanthochromic | ++ | Mixed/PMN/mono/eos | ++ | ++ | + (++) | Variable | Variable |
| Bacterial meningoencephalitis | WNL or | Turbid | ++ (+++) | PMN (mixed) | ++ (+++) | ++ | ++ | Variable | Yes (variable) |
Reference interval = <4/μl; + = 5–80/μl; ++ = 81–500/μl; +++ = >500/μl
Mono = mononuclear cells (ie, lymphocytes, monocytes and macrophages); PMN = neutrophils predominate; Eos = eosinophils predominate; Mixed = mono, PMNs and eos
Reference interval = <25 mg/dl; + = 25–100 mg/dl; ++ = 100–300 mg/dl; +++ = >300 mg/dl
WNL = within normal limits; = increased. Symbols in parentheses indicate less frequently seen variations
Some viral infections cause neuropathology without inflammation, and these may alter the CSF very little 59
Data from references 2, 5, 13, 61, 66
Signalment and history
As with all clinical investigations, the cat's age, gender, breed and history may help to suggest a diagnosis. For example, cerebellar hypoplasia secondary to in utero FPV infection is seen in young kittens; FIP is seen most commonly in pedigree cats of under 4 years of age that have often come from a large breeding colony; viral non-FIP encephalitides occur most commonly in young to middle-aged cats; toxoplasmosis is seen most commonly in adult cats that hunt (although it can also be seen as a congenital infection in young cats); FIV and FeLV are seen most commonly in older cats that fight; and a cat that is known to have had a chronic suppurative middle ear infection may suffer from its extension into the CNS. In practical terms, however, as most infectious CNS disease is seen in young adult cats, their age may not be that helpful in differentiating this group. 7
Most causes of infectious CNS disease result in neurological signs that are acute in onset and progressive. Signs are usually symmetrical.
Clinical signs
Most infectious causes of CNS disease result in neurological signs that are acute in onset and progressive, with some cases becoming chronic. There may be a history of past or present systemic disease, particularly in the case of FIP or toxoplasmosis. The clinical signs of meningoencephalitis may help to reflect the location of the lesion. Clinical signs are usually, but not always, symmetrical, and while FIP and toxoplasmosis tend to cause signs of multifocal CNS involvement, some cats with viral non-FIP encephalitides may have focal disease. 3 Few of the infectious agents give rise to specific findings, although in the case of Aujeszky's disease REVIEW / Infectious causes of CNS disease virus (pseudorabies) there is always a history of exposure to pigs from an endemic area, and affected cats usually show intense facial pruritus.
A detailed clinical examination is essential. With toxoplasmosis, FIP, FeLV and FIV, there are usually concurrent systemic signs, and cryptococcosis usually starts with upper respiratory tract signs. Local or systemic lymphadenopathy may also be present. In cats with neurological FIP, abdominal examination often reveals mesenteric lymphadenopathy, and/or irregular kidneys, liver or spleen. 9 A full ocular examination is imperative in all cases and the nature of the changes seen can often help in suggesting which infection is most likely. Inflammatory CNS disease can be associated with uveitis, and fundoscopic examination may reveal papilloedema, optic neuritis, chorioretinitis, and/or retinal haemorrhages (Figs 1—3). Otoscopic examination is needed to look for possible infectious ear disease that could have extended into the CNS. Neurological examination is essential to localise the area of CNS dysfunction, and has been well described elsewhere, 10,11 albeit is not always easy to perform in cats.
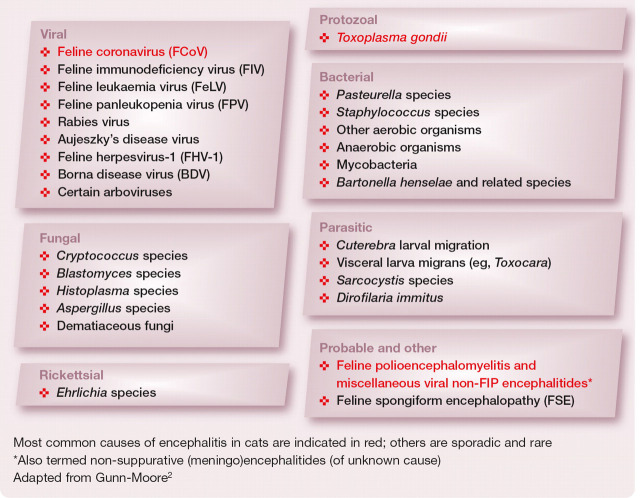
FIG 1.
Retina of a cat with FeLV infection, showing lymphomatous infiltration of the optic disc. Courtesy of Sheila Crispin
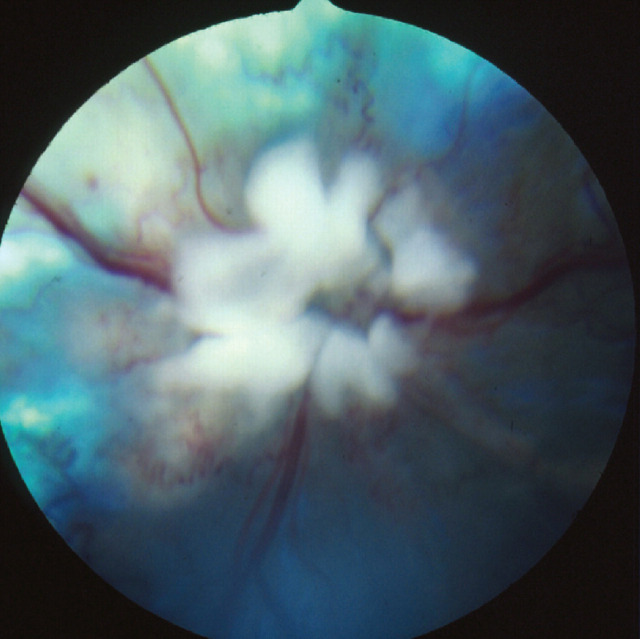
FIG 3.
Mycobacterial granulomatous chorioretinitis. Courtesy of David Gould
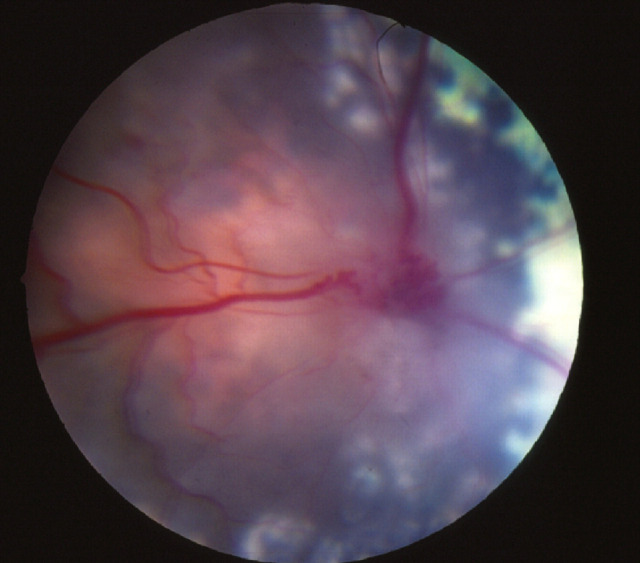
FIG 2.
Granulomatous tissue in the anterior chamber of an eye associated with Mycobacterium microti infection. Courtesy of Philip Hanlon
A full ocular examination is imperative in all cases.
Diagnostic tests
Biochemistry and haematology
Serum biochemistry and routine haematology may help in the diagnosis of toxoplasmosis, FIP, FeLV and FIV, but none of the changes are specific for any of these infections. All four infections can cause non-regenerative anaemia, increased numbers of neutrophils and/or monocytes, and increased serum globulins. While viral infections tend to result in lymphopenia, toxoplasmosis more commonly causes lymphocytosis. Other haematological and serum biochemical changes will vary depending on the extent of systemic involvement. When systemic changes are detected they should be investigated further. Haematology and serum biochemistry are rarely rewarding in cases of viral non-FIP encephalitides or CNS lymphoma. 7
Advanced imaging
For diseases confined to the CNS, MRI/CT (and CSF analysis — see below) are needed to enable diagnosis. MRI is considered superior to CT in the investigation of most cases of neurological disease in cats. 7 Potential MRI changes are mentioned in each of the relevant sections of the text, and discussed in detail elsewhere. 12 Unfortunately, these changes are rarely pathognomonic for a specific type of infection.
CSF analysis
CSF analysis may be non-specific, but it can be helpful in some cases (Table 3). Methods of performing this procedure have been well described elsewhere. 8,13 Standard tests performed on CSF include assessment of its gross physical appearance (colour, turbidity), quantitative analysis (red blood cell count, total nucleated cell count, protein concentration), and cytological analysis (leukocyte distribution and characterisation, in addition to the presence of other cells or infectious agents). CSF can also be assessed by further biochemical tests, microbiology (aerobic, anaerobic and fungal culture), and serological or polymerase chain reaction (PCR) testing for specific infections (see below). Unfortunately, a spinal tap is not without possible hazard as the presence of inflammatory CNS disease increases the risk of raised intracranial pressure, and thus of brain herniation. For this reason, where possible, it is best to perform MRI prior to CSF collection to try to evaluate this risk.
Serology and PCR
Before diagnosing an infectious cause of CNS disease it is necessary to identify that the cat has been infected with a specific infectious agent. Unfortunately, while tests may show that a cat is, or has previously been, infected with a particular agent, this does not necessarily mean that the infection is the cause of the current CNS signs. Antibody, antigen and/or PCR tests are readily available for a number of infectious agents in cats, including FeLV (antigen/PCR), FIV (anti-body/PCR), FIP (antibody/PCR), Toxoplasma species (IgG and IgM titres) and Cryptococcus species (latex cryptococcal antigen test). Although antibody titres reflect direct exposure to the organism, a positive titre does not confirm active infection. An elevated IgM titre reflects acute infection, whereas an elevated IgG titre may be indicative of chronic infection or previous exposure to a pathogen.
CSF antibody titres are thought to be more reliable than serum titres for diagnosing infectious disease within the CNS. To distinguish true intrathecal antibody production from contamination of the CSF with antibodies from the peripheral blood that may be present in the event of a compromised blood—brain barrier, the CSF IgG quotient (or IgG index) can be calculated; this provides a comparison between the concentrations of a specific antibody in the CSF and the same antibody in the serum. 6,14 Looking for evidence of infection within the CNS may be more helpful — for example, performing PCR assays to detect DNA or RNA from an infectious organism within the CSF. However, it should be noted that such tests may be insensitive because they require the presence of the organism in the biological sample under evaluation. Therefore, a negative PCR result does not definitively rule out active infection. Ultimately, a post-mortem examination may be required, often with PCR testing or immunohistochemical staining of sections, to confirm the presence of specific infectious agents.
Feline infectious peritonitis
FIP occurs following infection with a feline coronavirus (FCoV). In up to 10% of cats infected with FCoV, host and viral interactions allow the virus to replicate within macrophages, ultimately resulting in systemic disease manifesting as an immune-mediated vasculitis; this has been reviewed elsewhere. 15 –17
FIP is a common cause of neurological disease in cats, and the most commonly detected infectious cause. It accounts for 45–50% of all cases associated with histopathologically identified inflammatory changes, 1 which equates to 15–20% of all cases of feline neurological disease. 1,5 In young cats (less than 2 years old), it is also the most common histopathologically confirmed cause of spinal cord disease, affecting 24% (28/84) of cases. 18 It is important to realise just how common this infection is; and that, while most clinical cases are seen in young pedigree cats with obvious systemic involvement, this is not invariably the case. 15
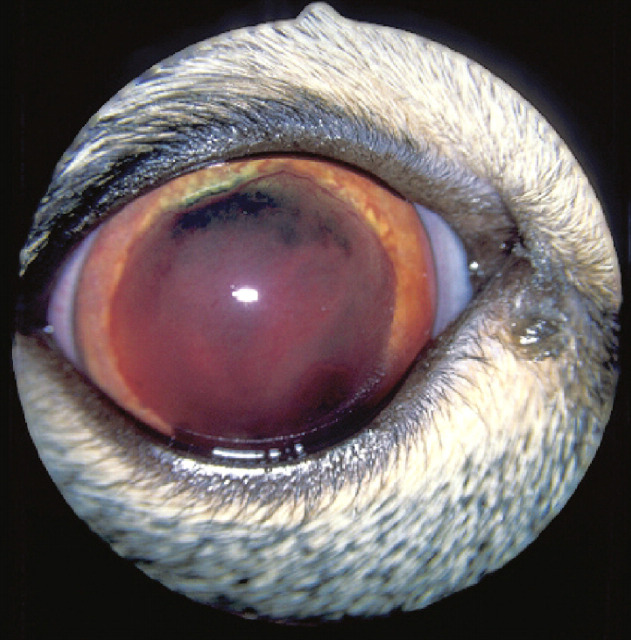
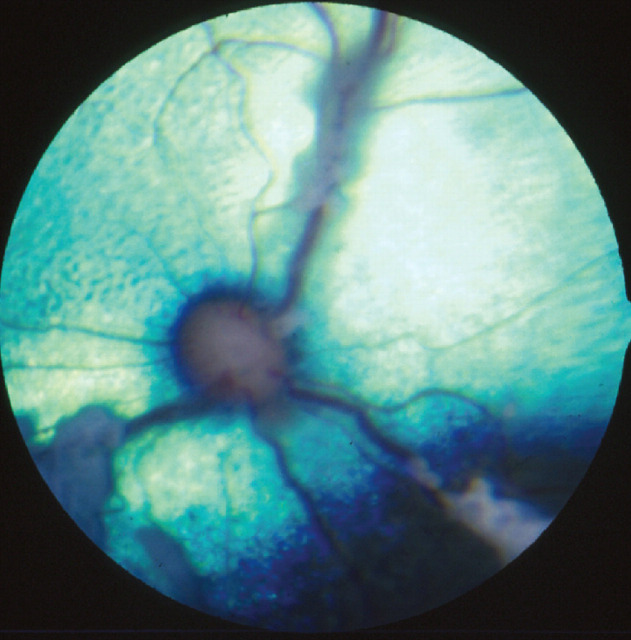
Clinical signs of FIP may be vague, such as weight loss, anorexia or fever. The more frequent, effusive form causes systemic disease secondary to an exuberant humoral immune response, characterised by a fibrinous peritonitis and pleuritis. Affected cats may display abdominal, pleural or pericardial effusion. Neurological involvement (see below) is more common with the non-effusive (or ‘dry’) form of the disease and develops in cats with humoral immunity, but only partial cell-mediated immunity. 9,19,20 Ocular signs are common with neurological FIP, and typically comprise anterior uveitis (often with keratic precipitates), chorioretinitis, anisocoria, and retinal haemorrhage and/or detachment (Figs 4 and 5). 9,15,22
FIG 4.
Anterior chamber haemorrhage and inflammation with marked iris vasculitis in a cat with FIP. Courtesy of Sheila Crispin
FIG 5.
Retina showing perivascular cuffing due to FIP. Courtesy of Sheila Crispin
Neurological signs associated with FIP
Neurological signs are not pathognomonic for FIP. They are usually multifocal but occasionally appear to result from focal disease. They include altered mentation, ataxia, cranial nerve abnormalities, tetraparesis, central vestibular signs, hyperaesthesia and seizures. Seizures have been reported to occur in approximately 25% of cases of neurological FIP, and may be considered a poor prognostic sign, associated with extension of the disease to the forebrain. 21 Generalised seizures and complex focal seizures were reported to affect 64% and 28% of cases, respectively. 21
Diagnosis
Diagnosis of FIP is complex and challenging, with the gold standard being immunofluorescence or immunohistochemistry to detect FCoV antigen within macrophages in body fluids or tissue sections. 15
In practice, antemortem diagnosis is often made based on the combination of signalment (most cases occur in pedigree or rescue cats from multi-cat households that are less than 4 years of age), history (typically of a recent stressful event such as rehoming or neutering), clinical signs, albumin/globulin ratio, FCoV titre, alpha-1 acid glycoprotein level, plus or minus a positive Rivalta's test if ascitic fluid is present. CSF analysis may reveal a predominantly neutrophilic or mixed cell pleocytosis, but can be normal. CSF total protein concentration is often markedly elevated (>200 mg/dl) (Table 3). 3,19,23
Serum tests for FCoV titre are often positive but have a low specificity. Furthermore, a negative serum titre does not exclude the possibility of FIP-associated neurological disease because soluble antibodies can form immune complexes and escape detection by standard tests. There is also considerable overlap in titres in cats with and without FIP (ie, some cats with FIP have low titres, while many cats with high titres never develop FIP). Measurement of FCoV antibodies in CSF has also yielded conflicting results, 9,24 and the value of PCR on CSF has not been fully evaluated. Unfortunately, it is not uncommon for CSF to fail to flow on a spinal tap, due to increased viscosity relating to markedly elevated protein concentrations.
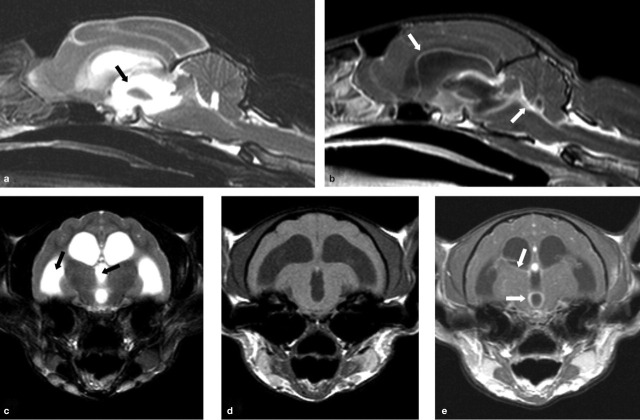
Cats with FIP may show no abnormalities on MRI, but, where abnormalities are detected, meningeal and ependymal lining contrast enhancement, granuloma formation and obstructive hydrocephalus (most commonly due to blockage of the fourth ventricle and subarachnoid space at the level of the foramen magnum) are commonly reported (Fig 6). 9,25
FIG 6.
MRI scans from a cat with neurological FIP. T2-weighted midline sagittal (a) and transverse (c) images reveal obstructive hydrocephalus of the third and lateral ventricles (black arrows). T1-weighted post-contrast sagittal (b), and pre- and post-contrast transverse (d and e, respectively) images of this same region reveal enhancement around the third and lateral ventricles (white arrows) following administration of an intravenous contrast agent (gadolinium). These features are indicative of FIP in which a pyogranulomatous inflammation is seen on the surface of the brain involving the meninges. Courtesy of Mark Lowrie, Davies Veterinary Specialists
It is important to realise just how common FIP is; and that, while most clinical cases are seen in young pedigree cats with obvious systemic involvement, this is not invariably the case.
Post-mortem examination is often required for confirmation of the diagnosis, with demonstration of the presence of FCoV antigen or identification of viral genetic material using a FCoV-specific PCR assay in inflamed tissues. As with many viral infections affecting the CNS, histopathology reveals nonsuppurative meningoencephalomyelitis, with perivascular cuffing and meningeal infiltration with mononuclear cells, gliosis and variable neuronal degeneration. However, the inflammation is often pyogranulomatous, and located around the lateral ventricles, in the meninges, or affects the choroid plexus. 1,9 Ependymitis and choroiditis may also be a feature, affecting the fourth ventricle more than the lateral ventricles, and giving rise to a secondary hydrocephalus that may be detected by MRI.
Treatment and prognosis
Although various therapeutic modalities have been proposed for the treatment of FIP, at present this remains an invariably fatal disease.
CNS toxoplasmosis is often seen as neurological (and ocular) signs in the absence of systemic disease.
Toxoplasmosis
Cats are the definitive host for Toxoplasma gondii, which is an intracellular coccidian parasite. Infection is usually the result of ingestion of prey harbouring bradyzoites, but faeces containing oocysts are another potential source of infection. Clinical reviews of feline toxoplasmosis are available. 14,17
Toxoplasmosis is typically a multisystemic disease, which may either be acute or chronic. Acute disseminated disease arises due to rapid replication of tachyzoites, often within the liver, CNS, pancreas or lungs. Chronic disease is more typically associated with vague signs, such as fever, anorexia, weight loss and diarrhoea. Dissemination of chronic disease may occur in patients that become immunosuppressed (eg, following administration of ciclosporin or infection with feline retroviruses). 26 CNS disease is most commonly seen following reactivation of latent bradyzoites within the brain, spinal cord and/or skeletal muscles. This often manifests as neurological (and ocular) signs in the absence of systemic disease. Again, this may be a sequela of immunosuppression associated with chemotherapy and/or retroviral infection.
Although neurological involvement is histologically present in nearly all cats with toxoplasmosis, clinical signs relating to the CNS are only apparent in approximately 10% of cases.
Toxoplasmosis is the second most common definitively diagnosed infectious disease of the feline CNS. 1,23,25 Although neurological involvement is histologically present in nearly all cats with toxoplasmosis, 27 clinical signs relating to the CNS are only apparent in approximately 10% of cases. 26 Neurological signs (see right) may be seen alone or in conjunction with systemic signs such as pneumonia or diarrhoea. Concurrent clinical signs may also include lymphadenopathy, pancreatitis, hepatitis, myocardial disease and/or myositis. Ocular examination may reveal chorioretinitis and/or uveitis.
Neurological signs associated with toxoplasmosis
Neurological signs are usually multifocal and include behavioural changes, altered mentation, seizures, ataxia, blindness, anisocoria, torticollis, vestibular signs, muscle hyper aesthesia and paresis/paralysis. 17,28
Diagnosis
Diagnosis of toxoplasmosis is challenging. It commonly relies on assessing antibody titres as changes in routine haematology (typically increased neutrophils, lymphocytes and monocytes) and serum biochemistry (eg, azotaemia, changes consistent with hepatopathy, increased globulins) are not specific and depend on the extent of systemic involvement.
IgM titres are an indicator of recent or active infection. Unfortunately, false-negative results (IgM <1/64) may be seen in cats that have been infected for a sufficient length of time for the IgM to undergo a class switch to IgG. They can also arise where extremely high levels of antibody cause a so-called prozone effect (where false-negative or false-low results occur from excess antigen or antibody in a sample due to inability of the analyte to bind to receptor sites). Conversely, false-positive results may be seen in chronic infections with persistent elevation of IgM. 14 Significantly elevated IgG titres may only represent previous exposure; a fourfold rise in titre over a 2–4 week period is more useful in demonstrating recent or active infection.
Routine CSF analysis is non-specific, typically revealing mild increases in lymphocytes (and occasionally neutrophils), with elevated protein concentrations (Table 3). Detection of T gondii IgG or DNA in the CSF may be more supportive of active infection. However, both IgG and DNA have been detected in the CSF (and aqueous humour) of normal as well as clinically ill cats. T gondii-specific IgM antibodies in the CSF may prove to be diagnostic, as unpublished data 26 has suggested that this class of antibody has not been detected in the CSF of healthy cats. Additionally, immunohistochemistry and PCR can also be performed on tissue samples. The rare presence of tachyzoites in the CSF is also diagnostic.
In patients with neurological signs, MRI findings are non-specific and often reflect multifocal involvement; lesions may have indistinct margins. 25 However, solitary space-occupying lesions attributed to granulomas have also been reported, 29,30 which may make differentiation of toxoplasmosis from neoplasia (eg, meningioma or lymphoma) more difficult. 30
Histopathology typically reveals inflammation and necrosis. Demonstration of protozoal tissue cysts is strongly supportive of a diagnosis, but immunohistochemistry or PCR assay may be required to definitively prove these are due to T gondii.
Treatment of toxoplasmosis
Clindamycin (10–12 mg/kg PO, IM or IV q12h) is the treatment of choice for toxoplasmosis. 14,17 However, trimethoprim sulphonamide (15 mg/kg PO q12h) plus a folic acid supplement (0.5–1.0 mg/cat PO q24h) may also be beneficial. Azithromycin (5 mg/kg PO q12h) may be more effective at entering the CNS in some cases, although in some cats toxoplasmosis may be refractory to this drug.
Some authors recommend that low anti-inflammatory doses of corticosteroids be given at the same time as starting antibiotic therapy, as the death of the organisms can trigger more extensive CNS inflammation. While there are no studies investigating this in cats with toxoplasmosis, positive effects have been documented in humans with bacterial, tuberculous and herpes simplex encephalitis. 31
CNS signs of toxoplasmosis may take longer than other clinical signs to improve. 26
Therapy should be continued for at least 4 weeks, and is unlikely to result in complete elimination of the organism, so owners should be warned of the potential for relapse.
Cryptococcosis
Cryptococcal infections are attributed primarily to Cryptococcus neoformans, although Cryptococcus gattii may be involved in some cases. These saprophytic yeasts are typically found in soil and pigeon faeces, and infection is thought to be by inhalation. 32 In people, the infection appears to spread from the lungs to the CNS by the haematogenous route. 32 While spread via this route may also occur in cats, neurological involvement more typically arises as a result of extension from rhinitis.
Although cryptococcosis is the most common mycotic infection of cats, it is a relatively rare cause of inflammatory CNS disease, with neurological signs occurring in only 1% of cases in a UK study, 1 and 6% of cases in an Australian study. 23 Clinical signs associated with cryptococcal infection of the CNS are highlighted below. Neuroanatomical localisation may indicate focal, multifocal or diffuse disease. Concurrent systemic clinical signs usually include nasal/upper respiratory signs, and/or cutaneous disease most commonly affecting the face.
Diagnosis
Culture of Cryptococcus is required for definitive diagnosis, although finding the encapsulated yeast forms in cytological preparations (urine, nasal discharge, lymph node aspirates or cutaneous masses) is strongly suggestive. Serological tests (latex agglutination or ELISA) may be performed to identify cryptococcal capsular antigen. Although cytology, culture and antigen testing may be performed on CSF, the risks of herniation associated with increased intracranial pressure should be considered. Routine CSF analysis may reveal increased numbers of neutrophils, eosinophils and/or mononuclear cells, plus an increased protein concentration (Table 3). The encapsulated organism can be seen in cytological preparations in the majority of cases, especially if using India ink, new methylene blue or Gram stain.
Neurological signs associated with cryptococcosis
Neurological signs include altered mentation and behaviour, seizures, ataxia, circling, head pressing, vestibular signs, cranial nerve abnormalities, generalised tremors, postural reaction deficits, spinal pain and paresis, dilated unresponsive pupils, chorioretinitis and optic neuritis. 32 –36
MRI in cats with cryptococcosis tends to produce very variable findings, including normal scans, multifocal lesions and solitary lesions. 35 Non-contrast enhancing T2-weighted hyperintense lesions (which had low intensity on T1-weighted images) are compatible with gelatinous pseudocysts. Meningeal enhancement is also a frequent finding, consistent with meningitis. Damage to the cribriform plate, retrobulbar disease extension and cerebellar herniation are also reported.
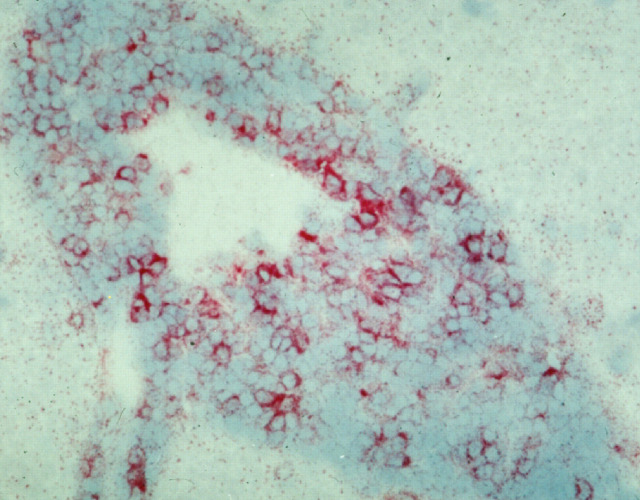
Pathology is compatible with meningitis or meningoencephalitis, with or without pseudocyst formation (Fig 7). The cerebellum and cerebellothalamic region were consistently involved in one study, with the pons, medulla and mid-brain being frequently involved. 35 Cryptococcal granulomas (cryptococcomas) may form in association with a competent cell-mediated immune response. 37
FIG 7.
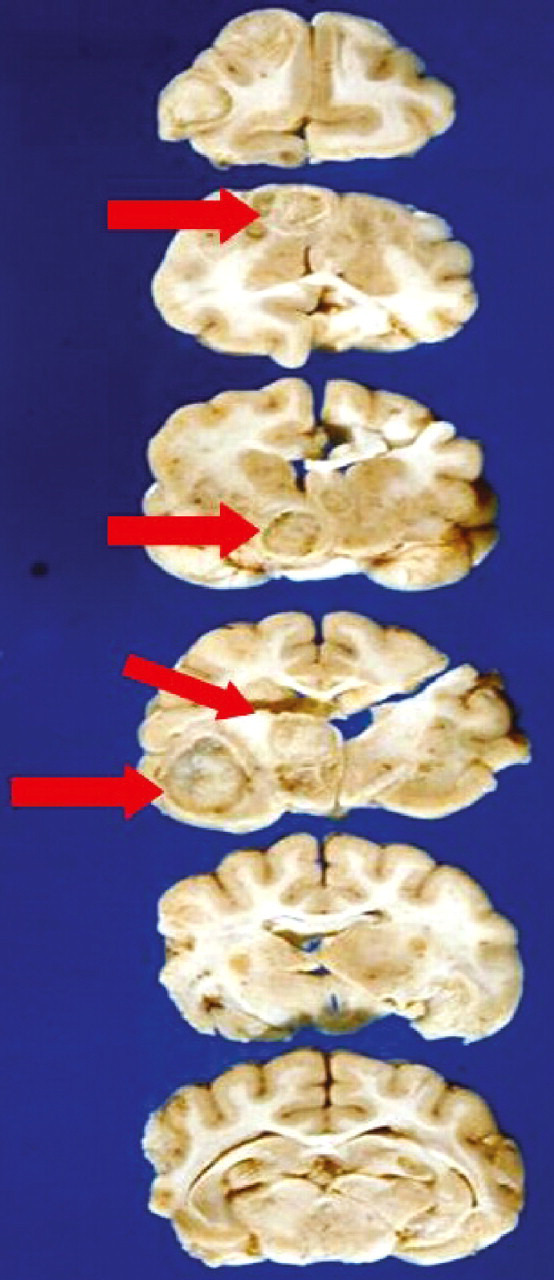
Serial sections through a cat's brain, revealing numerous cryptococcal lesions (arrows). Courtesy of Richard Malik
Prognosis
Median survival time (MST) after diagnosis in one study was only 13 days (range 0–4050 days), with many cats deteriorating rapidly after diagnosis. For those cats that survived more than 3 days after diagnosis, prolonged survival times occurred in some patients. 35 Cats that had altered mentation on presentation had a poorer prognosis, and cats receiving combination therapy survived longer than those receiving single agent treatment.
Although cryptococcosis is the most common mycotic infection of cats, it is a relatively rare cause of inflammatory CNS disease.
Treatment of cryptococcosis
Treatment of cryptococcosis involving the CNS is optimised by using a combination of amphotericin B and flucytosine. 38 Amphotericin B can be administered intravenously as a continuous infusion (0.5 mg/kg q24h) or subcutaneously (0.5–0.8 mg/kg 2–3 times weekly). Flucytosine is given concurrently (250 mg q6–8 h PO). Treatment is required for a minimum of 6 weeks (often 12 weeks) or until the antigen titre declines to zero.
Amphotericin B is nephrotoxic so the development of azotaemia may require suspension of treatment to allow the renal damage to resolve. During suspension of amphotericin B/flucytosine therapy, the fungistatic drugs fluconazole or itraconazole may be used as an alternative. Although a beneficial effect of giving a short course of glucocorticoids at the same time as starting antifungal treatment has been demonstrated in one study, 35 their use is controversial.
Feline retroviral infections
Feline retroviral (FeLV and FIV) infections may have a permissive role in CNS disease by enabling establishment of infections such as FIP, toxoplasmosis and cryptococcosis. 39 –41 In addition, the second most common intracranial neoplasm in cats is lymphoma, which is often associated with FeLV infection. In one study, 18% of cats with intracranial lymphoma were positive for FeLV antigen. 42 Lymphoma is also the most common spinal neoplasm of cats (39% of spinal neoplasms), with many affected individuals being young and FeLV-infected, presenting with spinal pain and paresis/paralysis. 43 Chronic FeLV infection (of greater than 2 years' duration) has been reported to be associated with a chronic myelopathy that may manifest as urinary incontinence, hyperaesthesia, ataxia and paresis progressing to paralysis. 44 Histologically, white matter degeneration was detected diffusely within the spinal cord.
FIV is a neurotropic virus. Up to 20% of experimentally infected cats may develop significant neurological deficits; however, most pet cats are euthanased before these become obvious so CNS changes are only reported in 1–5% of naturally occurring cases. 45 Diagnosis may be hampered as several cases of FIV-induced neurological disease have been serum antibody negative; 46 in one report approximately 20% of cats naturally infected with FIV were antibody negative. 47 It may, therefore, be necessary to perform PCR on blood or CSF to detect the infection, or immunohistochemistry on brain sections collected post mortem. Perivascular inflammatory infiltrate and glial nodules are typical histopathological findings. 45,48
Feline retroviral (FeLV and FIV) infections may have a permissive role in CNS disease by enabling establishment of infections such as FIP, toxoplasmosis and cryptococcosis.
Bacterial meningoencephalitis
Bacterial, typically suppurative, meningoencephalitis may develop as a result of extension of bacterial disease — most commonly from middle ear infection or rhinitis, penetrating injury to the skull or haematogenous spread from distant sites of infection (eg, myocarditis). A wide variety of organisms may be involved, the most common being Staphylococcus species. Bacterial infection of the CNS may result in an abscess and/or, much less frequently, a collection of pus (empyema) in subdural or epidural locations. Brain abscesses are life-threatening due to systemic and local toxicity (in the early stages of cerebritis) and increased intracranial pressure (during/after capsule formation).
Clinical signs, which are largely the result of the inflammatory reaction that the bacteria provoke, are very variable (see left), and usually rapidly progressive and frequently fatal if left untreated. 8,13
Neurological signs associated with FIV infection
Typical signs include behavioural changes (decreased interaction with owners or aggression), anisocoria (Fig 8), altered sleep patterns and paresis. 45 (Note that most pet cats are euthanased before significant neurological deficits become apparent.)
FIG 8.
Three-year-old cat with FIV and static anisocoria (‘static pupil syndrome’). Courtesy of Sheila Crispin
Neurological signs associated with bacterial meningoencephalitis
Affected animals can present with a wide variety of neurological signs of intracranial disease that reflect a focal anatomic diagnosis, suggesting a space-occupying lesion, or a multifocal syndrome associated with many small microabscesses.
Treatment of bacterial meningoencephalitis
Treatment involves antibacterial drugs, ideally selected according to CSF culture and sensitivity testing. It is best to select bactericidal drugs that cross the blood—brain barrier and achieve high CSF concentrations. 13
Prior to the culture results being known, potentiated sulphonamides are a good choice. 8 With inflammation, some drugs that would not normally gain good brain penetration can cross the blood—brain barrier (eg, clindamycin). More information about specific antibiotic choices is reviewed by Kent. 13
Antibiotic treatment should initially be given intravenously for 3–5 days, and then continued orally until at least 2 weeks beyond clinical resolution. Dying bacteria can trigger an increased inflammatory response so a number of authors recommend a short course of corticosteroids be given at anti-inflammatory doses, starting just before the antibiotics (eg, dexamethasone for 4 days). 8,13,31 Surgical decompression by craniectomy is indicated in cases of brain abscessation that do not respond to medical management. Adjunct therapy may also include analgesia, anticonvulsants and supportive care (eg, intravenous fluids).
Even in people, the mortality rate for bacterial meningoencephalitis is up to 40%, with many of the survivors having permanent neurological deficits.
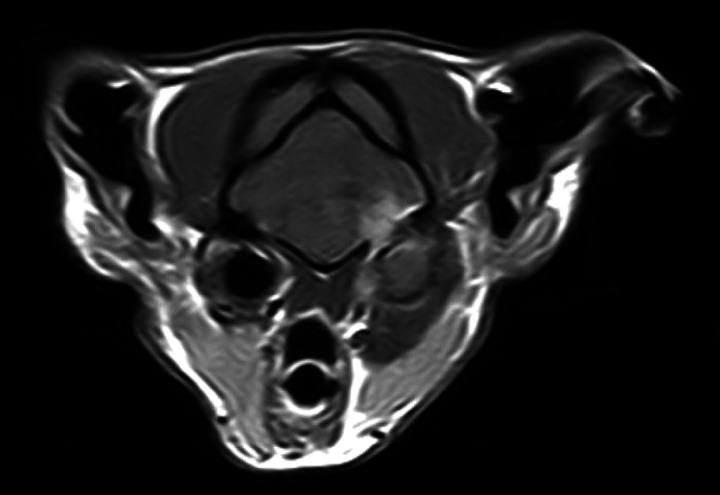
Diagnosis is based on blood and/or CSF culture but false-negative results can occur and the risk of brain herniation following CSF collection is considerable. Gram staining of CSF can be helpful. Routine haematology may show neutrophilia with a left shift, but changes in haematology and serum biochemistry are not always present and are non-specific. CSF cell counts are usually markedly increased, with toxic neutrophils and intracellular bacteria (Table 3). CT or MRI may be useful to reveal the extent of disease and whether or not there is abscessation (Fig 9). 8 Histopathology plus special staining (eg, Gram or Ziehl-Neelsen) may reveal bacteria (Fig 10).
FIG 9.
T1-weighted post-contrast transverse magnetic resonance image of the brain at the level of the tympanic bullae from a cat with otitis media/interna and secondary meningoencephalitis. Courtesy of Cristian Falzone, Davies Veterinary Specialists
FIG 10.
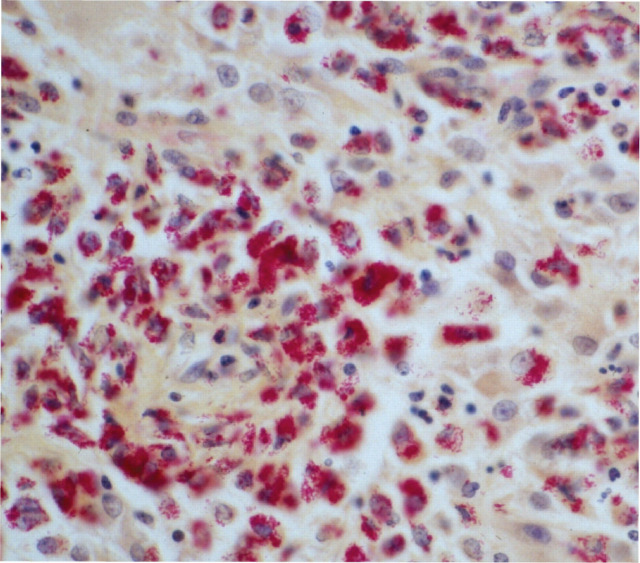
Ziehl-Neelsen-stained histopathological preparation of the brain of a cat with Mycobacterium avium infection, showing inflammation and cells packed with positive-staining bacteria
Other infectious causes of neurological disease in cats
Rabies
The risk of rabies infection out with endemic countries is rare. Cats may also become infected with bat Lyssavirus, but the risk of infection is considered to be extremely low. Rabies infection in cats most typically presents as the furious form and the disease has been reviewed recently in this journal in the context of the ABCD guidelines. 49
Borna disease virus
Borna disease virus (BDV) (reviewed by Gunn-Moore, and Kamhieh and Flower), 2,50 appears to cause a sporadic, progressive polioencephalomalacia, sometimes known as ‘staggering disease’. Serological surveys and surveys looking for BDV RNA within peripheral blood samples show that BDV infection is globally distributed in many different animal (including bird) species and infection is usually asymptomatic. Non-symptomatic cats from northern and central Europe are commonly seropositive, as were 6% of cats in the UK51 and 13–22% of cats in Japan. 52,53 The prevalence of seropositivity increases steadily with the age of the cat 54,55 and appears to be higher in cats that are also retrovirus positive. 51,56,57 Antibodies against BDV and/or BDV RNA have been detected most commonly in cats with neurological signs; 13% in Germany, 58 35% in the UK, 51 and 67% in Japan. 59
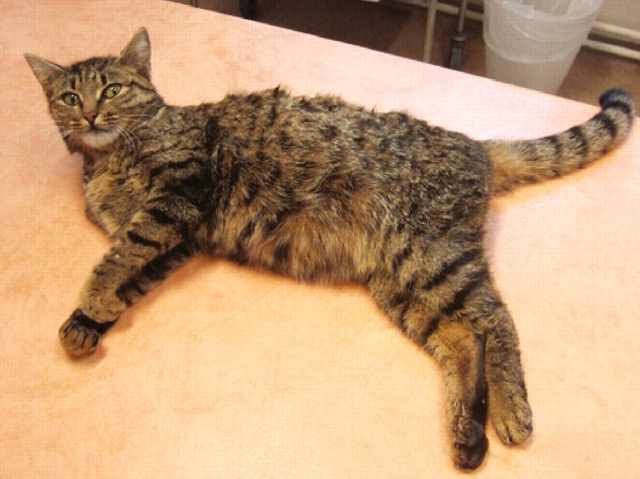
Clinical signs include pyrexia, hindlimb ataxia, behavioural changes, inability to retract the claws and lumbosacral pain (Fig 11). 60 Histopathologically, BDV may result in nonsuppurative meningoencephalomyelitis, with neuronophagia, microgliosis and heavy perivascular cuffing by mononuclear cells (Figs 12 and 13). 54,61 Diagnosis is challenging due to lack of readily available tests, and the difficulty of discerning the significance of positive test results, as they may also be found in clinically normal animals. 2,62
FIG 11.
Two-year-old cat with Borna disease virus infection; she was ataxic and unable to retract her claws. Courtesy of Anna-Lena Berg
FIG 12.
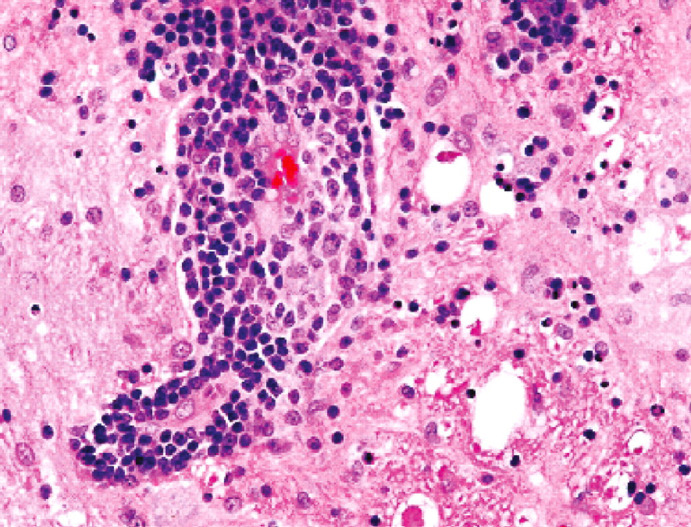
Histopathology of the brain of a cat with BDV, showing inflammation and vacuolation (resulting from neuronal loss). H&E staining. Courtesy of Anna-Lena Berg
FIG 13.
Immunohistochemistry (IgG) of the brain of a cat with BDV, showing perivascular cuffing. Courtesy of Anna-Lena Berg
Highly pathogenic avian influenza
Although this infection is primarily associated with respiratory signs and pyrexia, cats infected with H1N1 influenza frequently show signs attributable to encephalitis prior to death. The index of suspicion is likely to be increased if the cat has contact with birds in an endemic area. 60
Feline panleukopenia virus (FPV; feline parvovirus)
Cerebellar hypoplasia may result from in utero infection, perinatal infection or vaccination of a pregnant queen with a live FPV vaccine. Affected kittens typically have a wide-based gait with an intention tremor that is apparent as soon as they start exploring as young kittens. This is a non-progressive disorder and there are no associated inflammatory changes within the brain. Other, less commonly seen defects include forebrain lesions (which can result in seizures and/or behavioural changes) and/or retinal damage. These can occur with or without concurrent cerebellar hypoplasia.
Other naturally occurring and clinically significant CNS infections
A variety of organisms have been seen very occasionally as a cause of CNS disease in cats, including Bartonella henselae, which typically causes cat scratch disease in humans, but can also cause CNS disease in cats, 63 Mycoplasma felis, 64 feline herpesvirus (FHV-1), feline calicivirus (FCV), Aujeszky's disease virus, Aspergillus species and dematiaceous fungi (Cladophialophora bantiana), and nemotodes (eg, cuterebral larval migration, Sarcocystis neurona). A number of different arboviruses (the arthropod-borne encephalomyelitis group) have also been shown to infect cats (eg, BDV — see above), St Louis encephalitis virus, Japanese encephalitis virus, Yellow fever virus and Rift Valley fever virus, to name just a few. Many of these viruses have a very wide geographic distribution, including the USA and Europe, and some are endemically present in wild mammal populations to which free-living cats may become exposed. However, while studies show that cats are potentially at risk of becoming infected with these organisms, their role in causing significant naturally arising feline neurological disease remains to be determined (reviewed in Gunn-Moore). 2
Viral non-FIP encephalitides
Many cases of neurological disease in cats fall into the category of viral non-FIP encephalitides. While the cause(s) remain unknown, the nature of the histopathological change is highly suggestive of viral infection. These cases classically fall into two groups based on histopathology (reviewed in Gunn-Moore) 2 : group 1 — with non-suppurative encephalomyelitis, and group 2 — with polioencephalomyelitis or polioencephalitis.
Group 1 Cats show a wide age range (from a few months to over 18 years), with young adults being overrepresented. They tend to have an acute duration of illness; and typically develop ataxia, nystagmus, seizures, head tremor, anorexia, apathy and fever, occasionally preceded by vomiting and diarrhoea (although not all cats show all signs). 2,3,6
Group 2 Cats vary in age from a few months old to middle-aged. Affected individuals tend to have a subacute to chronic course that may last for months. Partial recovery may be seen in some cats, which can go on to live for many years. Affected cats present with ataxia, paresis, and depressed postural reactions in all four limbs. These cats may occasionally show hyperaesthesia, or even lower motor neuron signs (muscle atrophy and decreased tendon reflexes). They can also show tremors, pupillary abnormalities, defective vision, nystagmus and seizures. 65 When seizures occur they do so as episodes of multiple short seizures.
Unfortunately, until the cause(s) of these conditions is understood, specific diagnostic tests are not possible and treatment is symptomatic. Some cats may recover, even though they presented with severe signs and/or cluster seizures. MRI may or may not show multifocal areas of enhancement.
KEY POINTS
Clinically significant neurological disease is common in cats.
Infectious causes of central nervous system disease are believed to account for 30–45% of cases.
The two most commonly recognised causes are feline infectious peritonitis (FIP) and viral non-FIP encephalitides (ie, non-suppurative [meningo]encephalitides of unknown, although probable viral, cause), with toxoplasmosis comprising the third most common infectious cause.
Unfortunately, a specific infectious agent cannot be identified in up to 40% of cases.
Bacterial infections, feline immunodeficiency virus, feline leukaemia virus, feline panleukopenia, and fungal and parasitic infections are seen only rarely as a cause of neurological disease.
Biography

References
- 1. Bradshaw JM, Pearson GR, Gruffydd-Jones T. A retrospective study of 286 cases of neurological disorders of the cat. J Comp Pathol 2004; 131: 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gunn-Moore DA. Infectious diseases of the central nervous system. Vet Clin North Am Small Anim Pract 2005; 35: 103–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rand JS, Parent J, Percy D, et al. Clinical, cerebrospinal fluid and histological data from twenty-seven cats with primary inflammatory disease of the central nervous system. Can Vet J 1994; 35: 103–10. [PMC free article] [PubMed] [Google Scholar]
- 4. Hopkins A. Feline neurology. Part 1: Intracranial disorders. In Pract 1992; 14: 59–65. [Google Scholar]
- 5. Quesnel AD, Parent JM, McDonell W, et al. Diagnostic evaluation of cats with seizure disorders: 30 cases (1991–1993). J Am Vet Med Assoc 1997; 210: 65–71. [PubMed] [Google Scholar]
- 6. Munana KR. Inflammatory disorders of the central nervous system. In: August JR, ed. Consultations in feline internal medicine. Vol 4. Philadelphia: WB Saunders, 2001: 425–33. [Google Scholar]
- 7. Parent JM, Quesnel AD. Seizures in cats. Vet Clin North Am Small Anim Pract 1996; 26: 811–25. [PubMed] [Google Scholar]
- 8. Rusbridge C. The nervous system. In: Ramsey I, Tennant B, eds. BSAVA manual of canine and feline infectious diseases. Cheltenham: BSAVA Publications, 2001: 231–49. [Google Scholar]
- 9. Foley JE, Lapointe JM, Koblik P, et al. Diagnostic features of clinical neurologic feline infectious peritonitis. J Vet Intern Med 1998; 12: 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garosi L. Neurological examination of the cat: how to get started. J Feline Med Surg 2009; 11: 340–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Negrin A, Schatzberg S, Platt SR. The paralyzed cat: neuroanatomic diagnosis and specific spinal cord diseases. J Feline Med Surg 2009; 11: 361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hecht S, Adams WH. MRI of brain disease in veterinary patients part 2: Acquired brain disorders. Vet Clin North Am Small Anim Pract 2010; 40: 39–63. [DOI] [PubMed] [Google Scholar]
- 13. Kent M. Bacterial infections of the central nervous system. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd edn. St Louis: Saunders Elsevier, 2006: 962–74. [Google Scholar]
- 14. Dubey JP, Lappin MR. Toxoplasmosis and neosporosis. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd edn. St Louis: Saunders Elsevier, 2006: 754–75. [Google Scholar]
- 15. Hartmann K. Feline infectious peritonitis. Vet Clin North Am Small Anim Pract 2005; 35: 39–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pedersen NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg 2009; 11: 225–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kent M. The cat with neurological manifestations of systemic disease: key conditions impacting on the CNS. J Feline Med Surg 2009; 11: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marioni-Henry K, Vite CH, Newton AL, et al. Prevalence of diseases of the spinal cord of cats. J Vet Intern Med 2004; 18: 851–58. [DOI] [PubMed] [Google Scholar]
- 19. Kline KL, Joseph RJ, Averill DR. Feline infectious peritonitis with neurological involvement: clinical and pathological findings in 24 cats. J Am Anim Hosp Assoc 1994; 30: 111–18. [Google Scholar]
- 20. Foley JE, Leutenegger C. A review of coronavirus infection in the central nervous system of cats and mice. J Vet Intern Med 2001; 15: 438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Timmann D, Cizinauskas S, Tomek A, et al. Retrospective analysis of seizures associated with feline infectious peritonitis in cats. J Feline Med Surg 2008; 10: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diaz JV, Poma R. Diagnosis and clinical signs of feline infectious peritonitis in the central nervous system. Can Vet J 2009; 50: 1091–93. [PMC free article] [PubMed] [Google Scholar]
- 23. Singh M, Foster DJ, Child G, et al. Inflammatory cerebrospinal fluid analysis in cats: clinical diagnosis and outcome. J Feline Med Surg 2005; 7: 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boettcher IC, Steinberg T, Matiasek K, et al. Use of anticoronavirus antibody testing of cerebrospinal fluid for diagnosis of feline infectious peritonitis involving the central nervous system in cats. J Am Vet Med Assoc 2007; 230: 199–205. [DOI] [PubMed] [Google Scholar]
- 25. Negrin A, Lamb CR, Cappello R, et al. Results of magnetic resonance imaging in 14 cats with meningoencephalitis. J Feline Med Surg 2007; 9: 109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lappin MR. In: Bonagura JD, Twedt DC, eds. Kirk's current veterinary therapy XIV. St Louis: Saunders Elsevier, 2009: 1254–58. [Google Scholar]
- 27. Dubey JP, Carpenter JL. Histologically confirmed clinical toxo plasmosis in cats: 100 cases (1952–1990). J Am Vet Med Assoc 1993; 203: 1556–66. [PubMed] [Google Scholar]
- 28. Alves L, Gorgas D, Vandevelde M, et al. Segmental meningomyelitis in 2 cats caused by Toxoplasma gondii. J Vet Intern Med 2011; 25: 148–52. [DOI] [PubMed] [Google Scholar]
- 29. Pfohl JC, Dewey CW. Intracranial Toxoplasma gondii granuloma in a cat. J Feline Med Surg 2005; 7: 369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falzone C, Baroni M, de Lorenzi D, et al. Toxoplasma gondii brain granuloma in a cat: diagnosis using cytology from an intraoperative sample and sequential magnetic resonance imaging. J Small Anim Pract 2008; 49: 95–99. [DOI] [PubMed] [Google Scholar]
- 31. Fitch MT, van de Beek D. Drug insight: steroids in CNS infectious diseases — new indications for an old therapy. Nat Clin Pract Neurol 2008; 4: 97–104. [DOI] [PubMed] [Google Scholar]
- 32. Malik R, Krockenberger M, O'Brien CR, et al. Cryptococcosis. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd edn. St Louis: Saunders Elsevier, 2006: 584–98. [Google Scholar]
- 33. Beatty JA, Barrs VR, Swinney GR, et al. Peripheral vestibular disease associated with cryptococcosis in three cats. J Feline Med Surg 2000; 2: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belluco S, Thibaud JL, Guillot J, et al. Spinal cryptococcoma in an immunocompetent cat. J Comp Pathol 2008; 139: 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sykes JE, Sturges BK, Cannon MS, et al. Clinical signs, imaging features, neuropathology, and outcome in cats and dogs with central nervous system cryptococcosis from California. J Vet Intern Med 2010; 24: 1427–38. [DOI] [PubMed] [Google Scholar]
- 36. Trivedi SR, Malik R, Meyer W, Sykes JE. Feline cryptococcosis: impact of current research on clinical management. J Feline Med Surg 2011; 13: 163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Foster SF, Charles JA, Parker G, et al. Cerebral cryptococcal granuloma in a cat. J Feline Med Surg 2001; 3: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malik R, Craig AJ, Wigney DI, et al. Combination chemotherapy of cryptococcosis using subcutaneously administered amphotericin B. Aust Vet J 1996; 73: 124–28. [DOI] [PubMed] [Google Scholar]
- 39. Davidson MG, Rottman JB, English RV, et al. Feline immunodeficiency virus predisposes cats to acute generalised toxoplasmosis. Am J Pathol 1991; 143: 1486–97. [PMC free article] [PubMed] [Google Scholar]
- 40. O'Neil SA, Lappin MR, Reif JS, et al. Clinical and epidemiological aspects of feline immuno deficiency virus and Toxoplasma gondii coinfections in cats. J Am Anim Hosp Assoc 1991; 27: 211–20. [Google Scholar]
- 41. Gerds-Grogan S, Dayrell-Hart B. Feline cryptococcosis: a retrospective evaluation. J Am Anim Hosp Assoc 1997; 33: 118–22. [DOI] [PubMed] [Google Scholar]
- 42. Troxel MT, Vite CH, Van Winkle TJ, et al. Feline intracranial neoplasia: retrospective review of 160 cases (1985–2001). J Vet Intern Med 2003; 17: 850–59. [DOI] [PubMed] [Google Scholar]
- 43. Marioni-Henry K, Van Winkle TJ, Smith SH, et al. Tumors affecting the spinal cord of cats: 85 cases (1980–2005). J Am Vet Med Assoc 2008; 232: 237–43. [DOI] [PubMed] [Google Scholar]
- 44. Carmichael KP, Bienzle D, McDonnell JJ. Feline leukemia virus-associated myelopathy in cats. Vet Pathol 2002; 39: 536–45. [DOI] [PubMed] [Google Scholar]
- 45. Meeker RB. Feline immunodeficiency virus neuropathogenesis: from cats to calcium. J Neuroimmune Pharmacol 2007; 2: 154–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gunn-Moore D, Pearson G, Harbour D, et al. Encephalitis with giant cells in a cat with naturally occurring feline immunodeficiency virus infection demonstrated by in situ hybridization. Vet Pathol 1996; 33: 699–703. [DOI] [PubMed] [Google Scholar]
- 47. Hopper CD, Sparkes AH, Gruffydd-Jones TJ, et al. Clinical and laboratory findings in cats infected with feline immunodeficiency virus. Vet Rec 1989; 125: 341–46. [DOI] [PubMed] [Google Scholar]
- 48. Phillips TR, Prospero-Garcia O, Puaoi DL, et al. Neurological abnormalities associated with feline immunodeficiency virus infection. J Gen Virol 1994; 75: 979–87. [DOI] [PubMed] [Google Scholar]
- 49. Frymus T, Addie D, Belák S, et al. Feline rabies: ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kamhieh S, Flower RL. Borna disease virus (BDV) infection in cats: a concise review based on current knowledge. Vet Q 2006; 28: 66–73. [PubMed] [Google Scholar]
- 51. Reeves NA, Helps CR, Gunn-Moore DA, et al. Natural Borna disease virus infection in cats in the United Kingdom. Vet Rec 1998; 143: 523–26. [DOI] [PubMed] [Google Scholar]
- 52. Nakamura Y, Asahi S, Nakaya T, et al. Demonstration of Borna disease virus RNA in peripheral blood mononuclear cells derived from domestic cats in Japan. J Clin Microbiol 1996; 34: 188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nishino Y, Funaba M, Fukushima R, et al. Borna disease virus infection in domestic cats: evaluation by RNA and antibody detection. J Vet Med Sci 1999; 61: 1167–70. [DOI] [PubMed] [Google Scholar]
- 54. Lundgren AL, Johannisson A, Zimmermann W, et al. Neurological disease and encephalitis in cats experimentally infected with Borna virus. Acta Neuropathol 1997; 93: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ludwig H, Bode L. The neuropathogenesis of Borna disease virus infections. Intervirology 1997; 40: 185–97. [DOI] [PubMed] [Google Scholar]
- 56. Helps CR, Turan N, Bilal T, et al. Detection of antibodies to Borna disease virus in Turkish cats by using recombinant p40. Vet Rec 2001; 149: 647–50. [DOI] [PubMed] [Google Scholar]
- 57. Huebner J, Bode L, Ludwig H. Borna disease virus infection in FIV-positive cats in Germany. Vet Rec 2001; 149: 152. [DOI] [PubMed] [Google Scholar]
- 58. Lundgren AL, Ludwig H. Clinically diseased cats with nonsuppurative meningoencephalomyelitis have Borna disease virus-specific antibodies. Acta Vet Scand 1993; 34: 101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakamura Y, Watanabe M, Kamitani W, et al. High prevalence of Borna disease virus in domestic cats with neurological disorders in Japan. Vet Microbiol 1999; 70: 153–69. [DOI] [PubMed] [Google Scholar]
- 60. Njaa B. Emerging viral encephalitides in dogs and cats. Vet Clin North Am Small Anim Pract 2008; 38: 863–78. [DOI] [PubMed] [Google Scholar]
- 61. Lundgren AL. Feline non-suppurative meningoencephalomyelitis. A clinical and pathological study. J Comp Pathol 1992; 107: 411–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Greene CE, Berg AL. Borna disease meningoencephalomyelitis. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd edn. St Louis: Saunders Elsevier, 2006: 165–67. [Google Scholar]
- 63. Leibovitz K, Pearce L, Brewer M, et al. Bartonella species antibodies and DNA in cerebral spinal fluid of cats with central nervous system disease. J Feline Med Surg 2008; 10: 332–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beauchamp DJ, da Costa RC, Premanandan C, Burns CG, Cui J, Daniels JB. Mycoplasma felis-associated meningoencephalomyelitis in a cat. J Feline Med Surg 2011; 13: 139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vandevelde M. Neurologic diseases of suspected infectious origin. In: Greene CE, ed. Infectious diseases of the dog and cat. Philadelphia: WB Saunders, 1998: 530–39. [Google Scholar]
- 66. Fenner WR. Central nervous system infections. In: Greene CE, ed. Infectious diseases of the dog and cat. Philadelphia: WB Saunders, 1998: 647–57. [Google Scholar]