Abstract
Case series summary
Cats with ionized hypercalcemia that were fed diets with either more than 200 mg calcium per 100 kilocalories (kcal), a calcium:phosphorus (Ca:P) ratio greater than 1.4:1 or both, based on diet history, were included in this case series. Ionized hypercalcemia was documented at least twice in all cats before enrollment. Cats were referred for evaluation of ionized hypercalcemia (n = 5) or were incidentally found to have ionized hypercalcemia (n = 5). After medical workups, cats were diagnosed with either idiopathic hypercalcemia (IHC; n = 7) or chronic kidney disease (n = 3). Cats receiving medications to treat IHC (eg, alendronate, corticosteroids) were excluded. Nutritional recommendations were made to transition the cats to diets with less than 200 mg calcium per 100 kcal and a Ca:P ratio less than 1.4:1. Ionized calcium (iCa) concentrations were rechecked in all cats, with a median recheck time of 9 weeks (range 3–20). Of the 10 cats, nine (90%) had a decrease in iCa. Of the 10 cats, six (60%) became normocalcemic after the diet change, three (30%) had a partial response and one (10%) did not respond. Of the four cats that did not achieve normocalcemia with a change in diet, two (50%) received chia seeds (1–2 g per day), and at the next recheck, both cats’ iCa concentrations had normalized. Three cats had a long-term follow-up. Ionized normocalcemia was maintained for at least two consecutive follow-up visits over a median follow-up period of 33 weeks (range 12–34).
Relevance and novel information
Dietary calcium concentrations and the dietary Ca:P ratio appear to be important variables in considering nutritional approaches for hypercalcemic cats.
Keywords: Calcium, phosphate, diet, chia seeds
Introduction
Hypercalcemia in cats is most commonly associated with kidney disease, idiopathic hypercalcemia (IHC) and neoplasia.1–3 Clinical signs of hypercalcemia are often vague, and may include anorexia, weight loss, gastrointestinal signs and urinary signs. Some cats will have no owner-reported clinical signs and hypercalcemia may be diagnosed incidentally.
The complete pathophysiology for the development of ionized hypercalcemia with kidney disease and IHC is poorly understood. Possible mechanisms of hypercalcemia with chronic kidney disease (CKD) include the following: (1) decreased glomerular filtration; (2) increased renal tubular reabsorption; (3) increased intestinal absorption; and (4) increased bone resorption.4,5 For hypercalcemia with IHC, it has been suggested that there may be genetic factors or dietary factors involved, and possibly vitamin D enzymopathies.6,7
It is conceivable that intestinal absorption likely plays a substantial role either directly or indirectly. Calcium is absorbed via the gastrointestinal tract via two mechanisms: (1) a transcellular, active transport mechanism that primarily functions when dietary calcium intake is low or normal and is upregulated by calcitriol; and (2) a paracellular, passive pathway that depends on concentration and electric gradients across tight junctions of the intestinal epithelium. 8 While high dietary calcium intake should theoretically downregulate intestinal absorption, a meta-analysis demonstrated a linear relationship between dietary calcium intake and fecal calcium excretion in cats, suggesting a lack of intestinal adaptation to high dietary calcium loads. 9 Rather than the non-linear relationship between calcium intake and fecal excretion (and thus calcium absorption) that is expected to occur as the intestinal tract adapts to either low or high dietary calcium intake, it appears that in the cat, the gut does not provide a rate- or quantity-limiting step in calcium absorption. 9
Dietary calcium:phosphate (Ca:P) ratios have been demonstrated to influence calcium and phosphate digestibility, as well as phosphate and parathyroid hormone (PTH) concentrations, in cats.9,10 It has been demonstrated in mice that increased dietary Ca:P ratios increase intestinal calcium absorption and serum calcium concentrations and decrease intestinal phosphate absorption. 11
One commonality between IHC and kidney disease is the effect that diet may exert on calcium homeostasis. Development of hypercalcemia in some cats with CKD has been observed during the transition to a low-phosphate veterinary therapeutic renal diet providing 80 mg phosphate per 100 kilocalories (kcal).4,5,12 This diet-associated ionized hypercalcemia was reported to resolve in 8/10 cats after transitioning to a higher-phosphate diet providing 150 mg phosphate per 100 kcal. Another crucial difference between the diets in this study was the dietary Ca:P ratio; the Ca:P ratio was 1.8:1 in the low-phosphate diet vs 1.3:1 in the moderate-phosphate diet. 13
There are several dietary management strategies that have been proposed for the management of IHC in cats, including feeding a low-calcium diet, a high-fiber diet, a renal diet, a urinary diet and the addition of chia seeds.14 –16 Many of these recommendations are based on anecdotal evidence and small case series.
The reported success in cats with CKD in resolution of their hypercalcemia after dietary change prompted us to consider whether the dietary Ca:P ratio could be used as a targeted nutritional approach in hypercalcemic cases, including cats with IHC in addition to those with CKD. Consequently, we developed a new nutritional approach to address hypercalcemia in cats. Our hypothesis was that feeding a diet with a low–moderate calcium concentration (<200 mg calcium per 100 kcal) and a dietary Ca:P ratio <1.4:1 would decrease ionized calcium (iCa) concentrations in cats with either IHC or CKD.
Case series description
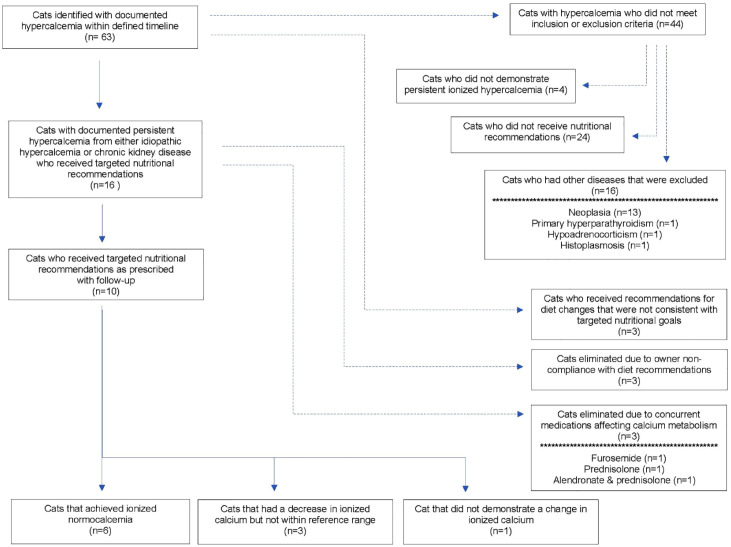
This was a case series of cats presented to The Ohio State University Veterinary Medical Center (OSU-VMC) for the evaluation of ionized hypercalcemia or cats that were incidentally found to have ionized hypercalcemia between August 2020 and August 2022. To identify cases, the electronic medical record was searched for cats that had been assigned a diagnosis of hypercalcemia. In total, 63 cats were initially identified. Cats were included in this study if they had at least two recorded instances of ionized hypercalcemia, if a diet history had been obtained, if specific nutritional recommendations for a diet meeting the goal of controlled calcium and Ca:P ratio had been made, and if at least one follow-up iCa was obtained after the diet change. Cats were excluded if they had diseases known to affect calcium concentrations (eg, neoplasia, primary hyperparathyroidism, histoplasmosis, hypoadrenocorticism) or if they were receiving medications known to affect calcium concentrations (eg, alendronate, corticosteroids, furosemide). Figure 1 shows a CONSORT diagram of case selection.
Figure 1.
CONSORT diagram showing case selection
Ionized hypercalcemia was defined as having an iCa higher than the respective reference laboratory’s reference interval (RI). Four laboratories provided iCa data for this study: the OSU-VMC (Lab A), Michigan State University Veterinary Diagnostic Laboratory (MSU; Lab B), IDEXX (Lab C) and Antech (Lab D) (Table 1). Labs A, B and D utilized the Nova methodology and Lab C utilized VetStat to report iCa concentrations.
Table 1.
Comparison of ionized calcium reference ranges among the four reference laboratories used in this study
| Reference laboratory | Ionized calcium reference interval (mmol/l) |
|---|---|
| Lab A: OSU-VMC | 1.18–1.35 |
| Lab B: MSU | 1.00–1.40 |
| Lab C: IDEXX | 1.13–1.38 |
| Lab D: Antech | 1.16–1.34 |
MSU = Michigan State University; OSU-VMC = The Ohio State University Veterinary Medical Center
In total, 16 cats met study inclusion criteria and received targeted nutritional recommendations. Three cats were removed due to owner non-compliance and three cats were removed due to concurrent use of medications that can affect calcium metabolism. The remaining 10 cats were included in this study (Figure 1). The median age at diagnosis was 5.7 years (range 3.1–17.8), and there were six male castrated cats and four female spayed cats. There were seven domestic shorthair (DSH) cats, one domestic longhair cat, one Persian and one Manx. The median body weight was 3.8 kg (range 2.6–6.5), and the cats had a median body condition score of 6/9 (range 4–8/9). Most (6/10, 60%) cats had a normal muscle condition, 3/10 (30%) had mild muscle atrophy and 1/10 (10%) had moderate muscle atrophy.
At diagnosis, four cats had no owner-reported clinical signs, one cat was presented for a wellness examination with occasional vomiting and weight loss, three cats were presented for constipation, one cat was undergoing a perineal urethrostomy after urethral blockage secondary to calcium oxalate stones and one cat was shaking. Table 2 shows the clinical signs of each cat as well as comorbid conditions.
Table 2.
Clinical characteristics of study cats with ionized hypercalcemia, including signalment, clinical signs and comorbid conditions
| Cat | Signalment (age, y) | Clinical signs | Comorbid conditions |
|---|---|---|---|
| 1 (IHC) | MC Persian (5) | Intermittent vomiting, weight loss | Hypertrophic cardiomyopathy |
| 2 (IHC) | MC DSH (6) | None reported | Otitis media/otitis externa, vestibular disease, suspect nasopharyngeal abscess, obesity, calcium oxalate cystolithiasis |
| 3 (IHC) | MC DSH (9) | None reported | Cholecystolithiasis, urate urolithiasis |
| 4 (IHC) | MC DSH (3) | Urethral obstruction secondary to cystolithiasis/urethrolith | Calcium oxalate cystolithiasis, seizures, inappropriate mentation, obesity |
| 5 (IHC) | FS DSH (5) | None reported | Hypertrophic cardiomyopathy |
| 6 (IHC) | MC DSH (4) | None reported | None |
| 7 (IHC) | FS DSH (8) | Constipation | Historical herpes conjunctivitis |
| 8 (CKD) | FS DSH (10) | Shaking | Chronic enteropathy |
| 9 (CKD) | FS Manx (4) | None reported | Chronic enteropathy |
| 10 (CKD) | MC DLH (17) | Constipation | None |
CKD = chronic kidney disease; DLH = domestic longhair; DSH = domestic shorthair; FS = female spayed; IHC = idiopathic hypercalcemia; MC = male castrated
In addition to the measurement of iCa, all 10 cats had a minimum database that included a complete blood count, routine biochemistry and urinalysis. Selected variables are presented in Table 3. The median iCa at baseline was 1.54 mmol/l (range 1.48–1.65). Although there was some variability in specific RIs for the four laboratories, the highest cutoff for demonstration of ionized hypercalcemia was 1.40 mmol/l. All cats were hypercalcemic based on laboratory-specific RIs.
Table 3.
Median (range) values of selected variables in hypercalcemic cats at baseline
| Variable | All cats (n = 10) | Cats with IHC (n = 7) | Cats with CKD (n = 3) |
|---|---|---|---|
| BUN (mg/dl) | 26 (12–77) | 25 (12–31) | 28 (25–77) |
| Creatinine (mg/dl) | 1.5 (1.0–3.6) | 1.4 (1.0–1.8) | 1.7 (1.6–3.6) |
| Phosphorus (mg/dl) | 4.6 (3.7–7.8) | 4.5 (4.0–5.1) | 4.6 (3.7–7.8) |
| Total calcium (mg/dl) | 12.9 (9.5–14.0) | 12.9 (11.5–13.4) | 11.8 (9.5–14.0) |
| Ionized calcium (mmol/l) | 1.54 (1.48–1.65) | 1.53 (1.48–1.64) | 1.57 (1.53–1.65) |
| Albumin (g/dl) | 3.4 (2.9–3.9) | 3.4 (3.3–3.9) | 3.1 (2.9–3.1) |
| Urine specific gravity | 1.050 (1.010–1.050) | 1.048 (1.015–1.054) | 1.013 (1.008–1.017) |
BUN = blood urea nitrogen; CKD = chronic kidney disease; IHC = idiopathic hypercalcemia
Of the 10 cats, seven (70%) had PTH analyzed, and all had values <0.7 pmol/l, which was considered appropriately suppressed given the hypercalcemia. Of the 10 cats, four (40%) had PTH-related protein analyzed, and all had values of 0 pmol/l. Of the cats, 4/10 (40%) had abdominal radiographs performed, 4/10 (40%) had an abdominal ultrasound, 2/10 (20%) had thoracic radiographs and 2/10 (20%) had a cervical ultrasound performed. There were no significant abnormalities identified on diagnostic imaging. The remaining diagnostics were at the discretion of the attending clinicians and owners.
Of the 10 cats, seven (70%) were diagnosed with IHC and three (30%) were diagnosed with CKD. A diagnosis of azotemic CKD was defined as a serum creatinine concentration >1.6 mg/dl with a urine specific gravity of <1.035 on at least two occasions over at least 3 months. Two cats had International Renal Interest Society (IRIS) stage 2 CKD and one cat had IRIS stage 3 CKD.
At the initial evaluation, 6/10 (60%) cats were fed a diet with >200 mg calcium per 100 kcal (range 215–441), 3/10 (30%) cats were fed a diet with a Ca:P ratio >1.4:1 (range 1.5:1–1.7:1) and 1/10 (10%) cat was fed a diet with both >200 mg calcium/100 kcal and a Ca:P ratio >1.4:1 (Table 4). Each cat’s owner was advised to transition the cat to a diet that met the preset therapeutic recommendation of providing a calcium concentration of <200 mg/100 kcal and a Ca:P ratio of <1.4:1. The median calcium concentration of the recommended diets fed was 166 mg/100 kcal (range 120–190) with a median Ca:P of 1.1:1 (range 1.0:1–1.3:1). More detailed nutritional information can be found in Tables 1 and 2 in the supplementary material.
Table 4.
Dietary calcium (mg/100 kcal) and calcium:phosphorus (Ca:P) ratios of diets fed to hypercalcemic cats at baseline and after modified dietary recommendations were made
| Cat | Baseline diet characteristics | Modified dietary recommendation | ||
|---|---|---|---|---|
| Dietary calcium (mg/100 kcal) | Dietary Ca:P ratio | Dietary calcium (mg/100 kcal) | Dietary Ca:P ratio | |
| 1 (IHC) | 441 | 1.2 | 161 | 1.2 |
| 2 (IHC) | 262 | 1.3 | 173 | 1.1 |
| 3 (IHC) | 160 | 1.6 | 170 | 1.0 |
| 4 (IHC) | 230 | 1.0 | 140 | 1.1 |
| 5 (IHC) | 217 | 1.4 | 173 | 1.1 |
| 6 (IHC) | 281 | 1.1 | 187 | 1.2 |
| 7 (IHC) | 343 | 1.2 | 190 | 1.2 |
| 8 (CKD) | 160 | 1.5 | 140 | 1.1 |
| 9 (CKD) | 130 | 1.6 | 158 | 1.0 |
| 10 (CKD) | 215 | 1.7 | 120 | 1.2 |
Cats were all fed recommended diets that provided <200 mg calcium per 100 kcal and a Ca:P ratio <1.4
CKD = chronic kidney disease; IHC = idiopathic hypercalcemia
The median time from baseline to the first recheck of iCa was 9 weeks (range 3–20). iCa decreased in 9/10 (90%) cats. At recheck, the median iCa was 1.35 mmol/l (range 1.18–1.50). Ionized hypercalcemia was restored to normocalcemia (ie, within the respective RI) in 6/10 (60%) cats. There was a partial response, as the iCa decreased a little but not to within the RI, in 3/10 (30%) cats, and no response in 1/10 (10%) cat. Table 5 and Figure 2 demonstrate changes in iCa in cats after their diets were modified.
Table 5.
Baseline and recheck ionized calcium (iCa) concentrations (mmol/l) in cats with ionized hypercalcemia
| Cat | Baseline iCa (mmol/l) | 1st recheck iCa (mmol/l) | Weeks from baseline | Additional treatment | 2nd recheck iCa (weeks from baseline) | 3rd recheck iCa (weeks from baseline) |
|---|---|---|---|---|---|---|
| 1 (IHC) | 1.64 B | 1.18 D | 12 | 1.29 (34) C | ||
| 2 (IHC) | 1.52 A | 1.25 A | 5 | |||
| 3 (IHC) | 1.50 A | 1.41 B | 8 | |||
| 4 (IHC) | 1.60 A | 1.37 D | 15 | |||
| 5 (IHC) | 1.54 B | 1.35 C | 10 | |||
| 6 (IHC) | 1.48 A | 1.29 A | 7 | 1.23 (33) A | ||
| 7 (IHC) | 1.53 B | 1.50 B | 9 | Received 2 g chia seeds per day after 1st recheck | 1.29 (18)C | |
| 8 (CKD) | 1.65 A | 1.35 A | 3 | 1.28 (12) A | 1.28 (32) | |
| 9 (CKD) | 1.57 C | 1.46 C | 17 | Received 1 g chia seeds per day after 1st recheck | 1.17 (43) C | |
| 10 (CKD) | 1.53 A | 1.25 A | 20 |
Reference laboratories are indicated by superscript letters A, B, C, D. Reference intervals for the laboratories are reported in Table 1. iCa concentrations in bold are outside the respective laboratory’s reference interval
CKD = chronic kidney disease; IHC = idiopathic hypercalcemia
Figure 2.
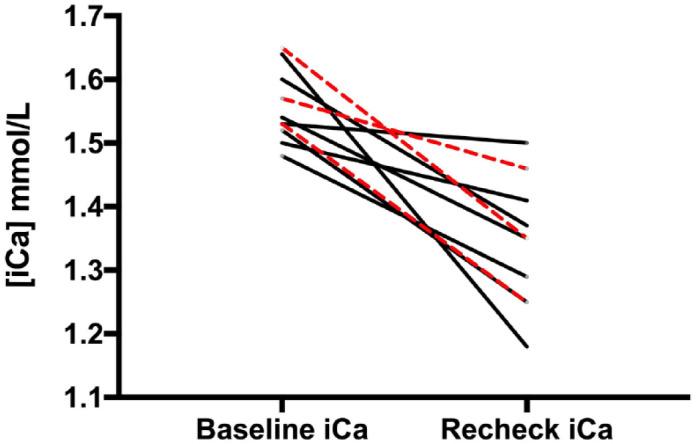
Ionized calcium (iCa) concentrations in hypercalcemic cats (black lines: idiopathic hypercalcemia; red dashed lines: chronic kidney disease) at baseline and recheck evaluation after dietary modification
In 2/4 (50%) cats that did not achieve normocalcemia, chia seed supplementation was provided, as this strategy has been reported to normalize iCa in three cats that did not respond to other nutritional modifications. 16 Both cats that received chia seeds (at 1–2 g per day) achieved normocalcemia. Cat 7 (IHC) received 2 g chia seeds per day, and its iCa decreased from 1.50 mmol/l to 1.29 mmol/l. Cat 9 (CKD) received 1 g chia seeds per day, and its iCa decreased from 1.46 mmol/l to 1.17 mmol/l.
There was long-term iCa follow-up in three cats in which management was consistent. Cat 1 (IHC) remained within the RI 22 weeks from the first recheck, or 34 weeks from baseline, cat 6 (IHC) remained within the RI 25 weeks from the first recheck, or 33 weeks from baseline, and cat 8 (CKD) remained within the RI 19 weeks from the first recheck, or 32 weeks from baseline.
Discussion
This study demonstrated that simple nutritional modifications affect iCa in cats with hypercalcemia due to either IHC or CKD. In general, the amount of dietary calcium in all feline diets ranges from approximately 130 mg/100 kcal to >600 mg/100 kcal, compared with the Association of American Feed Officials (AAFCO) minimum recommendation of 150 mg/100 kcal for feline adult maintenance. 17 If cats cannot effectively downregulate their calcium uptake from the intestinal tract at high dietary calcium concentrations, this may influence the development of hypercalcemia. 9
Similarly, dietary phosphorus in feline diets ranges from approximately 80 mg/100 kcal to >450 mg/100 kcal, compared with the AAFCO minimum of 125 mg/100 kcal. It is generally recommended to maintain a Ca:P ratio >1.0:1 to reduce the risk of nutritional hyperparathyroidism. Adverse renal effects have been observed in studies where cats were fed high-phosphate diets with Ca:P ratios of <1.0:1.18,19 While there is no maximum dietary Ca:P recommendation for cats, most diets tend to stay below 2.0:1.20,21
It has recently been demonstrated that feeding a low-phosphate diet to cats with CKD increases the risk for the development of hypercalcemia.4,5,12,13 Conversely, many studies have demonstrated the utility of feeding a reduced-phosphate diet in slowing the progression of CKD and decreasing the markers of CKD mineral and bone disorders.22–25 The three cats with CKD in this study were all eating therapeutic renal diets at the time of enrollment. In a review of their records, it appears as though at least one of these cats (cat 8) developed hypercalcemia after transitioning to the renal diet. For the other two cats, the diagnoses of hypercalcemia and CKD were made simultaneously, and it cannot be ascertained how their diets may have influenced the development of hypercalcemia before enrollment.
It is important to note that there is a wide range of both calcium and Ca:P ratios among reduced-phosphate diets designed for cats with CKD. 26 Currently available therapeutic renal diets for cats provide a Ca:P range of 1.1:1–1.9:1. Thus, for cats with CKD prone to hypercalcemia, or those that develop hypercalcemia, specific reduced-phosphate diets may be more appropriate, namely diets that provide a Ca:P ratio <1.4:1. Indeed, 2/3 cats with CKD in this study were transitioned to different reduced-phosphate diets, with lower Ca:P ratios.
It is also possible that other nutrients that were unintentionally modified may have influenced the outcome variable. The most common nutritional approach besides decreasing calcium intake for hypercalcemia management is increasing dietary fiber intake, likely by decreasing intestinal absorption. 15 The crude and total dietary fiber concentrations of diets fed to the cats at baseline and then after transition were reviewed retrospectively. There was no significant difference in crude fiber or total dietary fiber concentrations between diets fed at baseline and diets fed during treatment (P = 0.68 and P = 0.84, respectively) (Tables 1 and 2 in the supplementary material).
There is tremendous variability in the amount of dietary calcium and Ca:P ratios among higher-fiber feline diets. Most higher-fiber diets provide more than 200 mg calcium per 100 kcal, some more than 400 mg/100 kcal. Specific nutrient profiles should be reviewed before simply recommending a high-fiber diet for the management of hypercalcemia in cats. Interestingly, chia seeds are a high-fiber food (approximately 6.6 g/100 kcal) with a low calcium concentration (approximately 115 mg/100 kcal), as well as a low Ca:P ratio (0.7:1). It is possible that all of these nutritional factors influence the resolution of hypercalcemia in cats.
While it would have been ideal to compare some other nutrients among these diets (eg, vitamin A, vitamin D), given the retrospective nature of this study, data on these nutrients were not readily available. The nutrients reported in this study were obtained from pet food company product guides, which routinely report nutrients such as calcium, phosphorus and fiber, but do not provide complete nutrient profiles. Ultimately, if we can identify a simple nutritional approach to managing hypercalcemia in cats, this will increase the likelihood of clinicians being able to utilize this algorithm in practice.
It appears likely that the nutritional recommendations used in our study can be applied to cats with hypercalcemia and concurrent comorbid conditions (eg, chronic enteropathies, urolithiasis). For example, in cats with hypercalcemia and chronic enteropathy, several limited-ingredient and hydrolyzed diets meet the criteria of providing a dietary calcium concentration of <200 mg/100 kcal and a dietary Ca:P ratio <1.4:1. Several feline diets marketed for the management of calcium oxalate and struvite urolithiasis also meet these nutritional parameters.
The degree of biological or analytical variability in the magnitude of circulating iCa, if any, during disorders associated with hypercalcemia has not yet been studied in cats. It is conceivable that some variability in the measurement of iCa could arise from the time of day that blood was collected and whether the cat was fasted or not before collecting the blood sample, since this was not standardized in this study. The chances of transient hypercalcemia were minimized by requiring two iCa measurements to be above the RI before inclusion in this study. Unexplained transient hypercalcemia was commonly encountered in a recent review of dogs and cats. 1 One-third of the cats in this study had more than the one required follow-up measurement of iCa after dietary change and demonstrated continued normocalcemia. The observation that iCa declined in 9/10 cats in our study argues against random fluctuations in iCa that caused this decrease.
An additional limitation of this study was that iCa was measured on different analyzers at four different laboratories using different sample types and handling conditions. The collection of blood samples was not standardized as to the time of day or fasting vs non-fasting conditions. For prospective studies in the future, it would be best to standardize these conditions, preferably using anerobic handling conditions and the same analyzer.
Using diet to lessen the degree of ionized hypercalcemia in cats may reduce reliance on medications. The oral bisphosphonate alendronate has commonly been recommended to manage IHC.27,28 The long-term use of oral alendronate has been associated with osteonecrosis of the jaw and increased cortical bone thickness leading to pathological fractures.29–32 The long-term use of glucocorticoids can contribute to adverse effects on bone health and may also increase the risk of the development of diabetes mellitus secondary to insulin antagonism.33,34
Conclusions
Cats with ionized hypercalcemia due to either IHC or CKD often returned to normocalcemia in this study when fed diets with less than 200 mg calcium/100 kcal as well as a dietary Ca:P ratio less than 1.4:1. In cats that had partial to no response to this nutritional strategy, the addition of chia seeds (at 1–2 g per day) effectively decreased iCa concentrations. Additional prospective studies are needed to corroborate the results of this retrospective case series.
Supplemental Material
Specific diets and nutrient profiles fed to cats at baseline.
Specific diets and nutrient profiles fed to cats during study.
Acknowledgments
The authors gratefully acknowledge Dr. Louise Murray for her input regarding clinical cases in which this nutritional strategy was implemented.
Footnotes
Accepted: 10 January 2024
Supplementary material: The following files are available online as supplementary material:
Supplementary Table 1: Specific diets and nutrient profiles fed to cats at baseline.
Supplementary Table 2: Specific diets and nutrient profiles fed to cats during study.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Adam J Rudinsky  https://orcid.org/0000-0001-5369-3152
https://orcid.org/0000-0001-5369-3152
Dennis J Chew  https://orcid.org/0000-0001-7063-6803
https://orcid.org/0000-0001-7063-6803
Valerie J Parker  https://orcid.org/0000-0001-6505-8068
https://orcid.org/0000-0001-6505-8068
References
- 1. Coady M, Fletcher DJ, Goggs R. Severity of ionized hypercalcemia and hypocalcemia is associated with etiology in dogs and cats. Front Vet Sci 2019; 6. DOI: 10.3389/fvets.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Savary K, Price GS, Vaden SL. Hypercalcemia in cats: a retrospective study of 71 cases. J Vet Intern Med 2000; 14: 184–189. [DOI] [PubMed] [Google Scholar]
- 3. Broughton SE, O’Neill DG, Syme HM, et al. Ionized hypercalcemia in 238 cats from a referral hospital population. J Vet Intern Med 2023; 37: 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang PK, Geddes RF, Chang YM, et al. Risk factors associated with disturbances of calcium homeostasis after initiation of a phosphate-restricted diet in cats with chronic kidney disease. J Vet Intern Med 2021; 35: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Broek DHN, Geddes RF, Lötter NS, et al. Ionized hypercalcemia in cats with azotemic chronic kidney disease. J Vet Intern Med 2022; 36: 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finch NC. Hypercalcaemia in cats: the complexities of calcium regulation and associated clinical challenges. J Feline Med Surg 2016; 18: 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zafalon RVA, Risolia LW, Pedrinelli V, et al. Vitamin D metabolism in dogs and cats and its relation to diseases not associated with bone metabolism. J Anim Physiol Anim Nutr (Berl) 2020; 104: 322–342. [DOI] [PubMed] [Google Scholar]
- 8. Christakos S, Dhawan P, Porta A, et al. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol 2011; 347: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mack JK, Alexander LG, Morris PJ, et al. Demonstration of uniformity of calcium absorption in adult dogs and cats. J Anim Physiol Anim Nutr (Berl) 2015; 99: 801–809. [DOI] [PubMed] [Google Scholar]
- 10. Coltherd JC, Staunton R, Colyer A, et al. Dietary calcium to phosphorus ratio affects postprandial phosphorus concentrations in feline plasma. Br J Nutr 2022; 128: 1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masayuma R, Nayaka Y, Katsumata A, et al. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J Bone Min Res 2003; 18: 1217–1226. [DOI] [PubMed] [Google Scholar]
- 12. van den Broek DHN, Chang YM, Elliott J, et al. Chronic kidney disease in cats and the risk of total hypercalcemia. J Vet Intern Med 2017; 31: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geddes RF, van den Broek DHN, Chang YM, et al. The effect of attenuating dietary phosphate restriction on blood ionized calcium concentrations in cats with chronic kidney disease and ionized hypercalcemia. J Vet Intern Med 2021; 35: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Brito Galvão JF, Parker V, Schenck PA, et al. Update on feline ionized hypercalcemia. Vet Clin North Am Small Anim Pract 2017; 47: 273–292. [DOI] [PubMed] [Google Scholar]
- 15. McClain HM, Barsanti JA, Bartges JW. Hypercalcemia and calcium oxalate urolithiasis in cats: a report of five cases. J Am Anim Hosp Assoc 1999; 35: 297–301. [DOI] [PubMed] [Google Scholar]
- 16. Fantinati M, Priymenko N. Managing feline idiopathic hypercalcemia with chia seeds (Salvia hispanica L.): a case series. Front Vet Sci 2020; 7. DOI: 10.3389/fvets.2020.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Association of American Feed Control Officials. Model bill and regulations. In: Association of American Feed Control Officials Official Publication. Champaign, IL: AAFCO, 2020. [Google Scholar]
- 18. Alexander J, Stockman J, Atwal J, et al. Effects of the long-term feeding of diets enriched with inorganic phosphorus on the adult feline kidney and phosphorus metabolism. Br J Nutr 2018; 121: 249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dobenecker B, Webel A, Reese S, et al. Effect of a high phosphorus diet on indicators of renal health in cats. J Feline Med Surg 2018; 20: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Summers SC, Stockman J, Larsen JA, et al. Evaluation of nutrient content and caloric density in commercially available foods formulated for senior cats. J Vet Intern Med 2020; 34: 2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Summers SC, Stockman J, Larsen JA, et al. Evaluation of phosphorus, calcium, and magnesium content in commercially available foods formulated for healthy cats. J Vet Intern Med 2020; 34: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geddes RF, Finch NC, Syme HM, et al. The role of phosphorus in the pathophysiology of chronic kidney disease. J Vet Emerg Crit Care 2013; 23: 122–133. [DOI] [PubMed] [Google Scholar]
- 23. Barber PJ, Rawlings JM, Markweu PJ, et al. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract 1999; 40: 62–70. [DOI] [PubMed] [Google Scholar]
- 24. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000; 41: 235–242. [DOI] [PubMed] [Google Scholar]
- 25. Geddes RF, Elliott J, Syme HM. The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med 2013; 27: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 26. Parker VJ. Nutritional management for dogs and cats with chronic kidney disease. Vet Clin North Am Small Anim Pract 2021; 51: 685–710. [DOI] [PubMed] [Google Scholar]
- 27. Hardy BT, de Brito Galvao JF, Green TA, et al. Treatment of ionized hypercalcemia in 12 cats (2006–2008) using PO-administered alendronate. J Vet Intern Med 2015; 29: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurtz M, Desquilbet L, Maire J, et al. Alendronate treatment in cats with persistent ionized hypercalcemia: a retrospective cohort study of 20 cases. J Vet Intern Med 2022; 36: 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Council N, Dyce J, Drost WT, et al. Bilateral patellar fractures and increased cortical bone thickness associated with long-term oral alendronate treatment in a cat. JFMS Open Rep 2017; 3. DOI: 10.1177/2055116917727137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larson MJ, Oakes AB, Epperson E, et al. Medication-related osteonecrosis of the jaw after long-term bisphosphonate treatment in a cat. J Vet Intern Med 2019; 33: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rogers-Smith E, Whitley N, Elwood C, et al. Suspected bisphosphate-related osteonecrosis of the jaw in a cat being treated with alendronate for idiopathic hypercalcaemia. Vet Rec Case Rep 2019; 7. DOI: 10.1136/vetreccr-2018-000798. [DOI] [Google Scholar]
- 32. Meneghetti LM, Perry KL. Management of insufficiency fractures associated with long-term bisphosphonate therapy in a cat. JFMS Open Rep 2023; 9. DOI: 10.1177/20551169231183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open 2015; 1. DOI: 10.1136/rmdopen-2014-000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nerhagen S, Moberg HL, Boge GS, et al. Prednisolone-induced diabetes mellitus in the cat: a historical cohort. J Feline Med Surg 2021; 23: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specific diets and nutrient profiles fed to cats at baseline.
Specific diets and nutrient profiles fed to cats during study.