Abstract
Diffuse thyroid lipomatosis (DTL) is a rare entity of unknown etiology that can be associated with amyloidosis and rarely, thyrotoxicosis. Here, we present a case of DTL with amyloid deposits and concurrent thyrotoxicosis. A 64-year-old South-Asian woman with a several-year history of an enlarging goiter, unintentional weight loss, and work-up 10 months prior suggestive of thyroiditis presented with a viral syndrome in setting of several weeks of progressive fatigue. Her examination was notable for resting sinus tachycardia and massive painless goiter. Initial work-up revealed nephrotic range proteinuria with hypoalbuminemia, which progressed to end-stage-renal disease, elevated inflammatory markers, and elevated free thyroxine (FT4) with a suppressed thyrotropin. Hemodialysis was initiated. Further testing revealed a negative antithyroid antibody panel, an enlarged fatty thyroid per thyroid ultrasound and neck computed tomography, and normal 24-hour uptake on radioactive iodine uptake scan. Both renal and thyroid core biopsies showed amyloid deposits, with the latter confirming benign adipose tissue with entrapped thyroid follicles. Given her rising FT4 levels and persistent tachycardia, methimazole and atenolol were initiated. FT4 levels nearly normalized after uptitration of methimazole and dosing after dialysis. Although the etiopathogenesis and natural history of DTL remain unclear, we discuss the possible mechanisms of thyrotoxicosis in our patient.
Keywords: hyperthyroidism, thyroid lipomatosis, thyroid amyloidosis, methimazole, thyroiditis
Introduction
Diffuse thyroid lipomatosis (DTL) is an extremely rare entity of unknown etiology characterized by diffuse fatty infiltration of the thyroid gland. Fewer than 40 cases have been reported in the literature [1]. In most cases, patients present with compressive goiter and euthyroidism [1], but other cases with hypothyroidism [2] and thyrotoxicosis [3, 4] have also been reported. Additionally, DTL can be associated with amyloidosis [1]. To the best of our knowledge, no cases of DTL and concomitant thyroid amyloidosis presenting with thyrotoxicosis have been described in the literature.
Case Presentation
A 64-year-old South-Asian woman with a past medical history of uncontrolled hypertension and a long-standing nontender goiter presented with 1 week of nasal congestion, dry cough, and watery diarrhea in the context of several weeks of progressively worsening fatigue and weakness. The patient reported that her goiter had been progressively enlarging over the past 5 to 6 years and she had experienced a 15-pound (6.8-kg) involuntary weight loss over the past 1 to 2 years. She denied other symptoms of thyrotoxicosis, hypothyroidism, or mass effect due to the goiter. She had sought work-up in her country of origin several months prior to moving to the United States. She had laboratory reports indicating thyrotoxicosis and an imaging report of a Tc-99 m pertechnetate scan with low uptake. The patient was told that she had thyroiditis. Propranolol and acetaminophen were prescribed, but she did not take these medications. She had no known family history of thyroid or autoimmune disease. On physical examination, the patient had a regular resting pulse rate of 110 beats per minute. Her neck examination revealed an approximately 100-g asymmetric nontender goiter without associated lymphadenopathy (Fig. 1). Eye examination showed lid lag and thyroid stare, but no proptosis. On neurological examination she was found to have a resting tremor and hyperreflexia.
Figure 1.
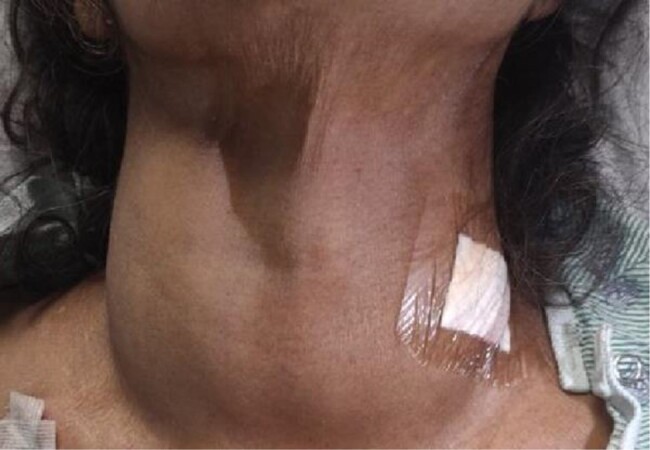
Painless, asymmetrical goiter.
Diagnostic Assessment
Initial laboratory evaluation showed that the thyrotropin was undetectable (<0.01 µIU/mL [<0.01 mIU/L], normal range [NR] = 0.45-5.33 µIU/mL [0.45-5.33 mIU/L]), free thyroxine (FT4) was elevated (2.17 ng/dL [27.92 pmol/L], NR = 0.64-1.42 ng/dL [8.23-18.27 pmol/L]), and inflammatory markers were markedly elevated erythrocyte sedimentation rate > 130 mm/h, NR = 0-30 mm/h; C-reactive protein 18.7 mg/dL [187 mg/L], NR = <10 mg/dL [<100 mg/L]). Additionally, the patient was newly diagnosed with end-stage renal disease (ESRD) with an estimated glomerular filtration rate of 4 mL/min/1.73 m2. She was admitted to the hospital for hemodialysis and further work-up.
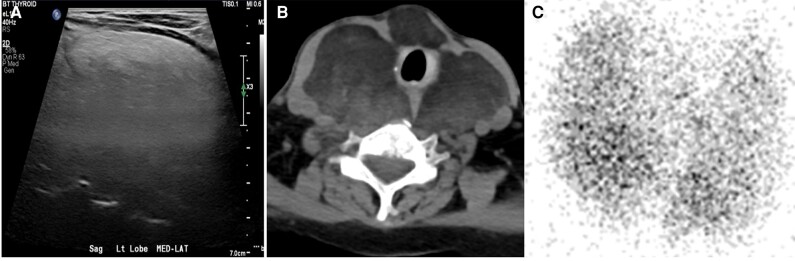
To further characterize her thyroid hormone dysfunction in the setting of ESRD and hypoalbuminemia (albumin 1.8 g/dL [18 g/L], NR = 3.7-5.3 g/dL [37-53 g/L]), total 3,5,3′-triiodothyronine (TT3) and total T4 levels were also measured, and were 42 ng/dL [0.65 nmol/L] (NR = 87-178 ng/dL; 1.34-2.74 nmol/L) and 9.3 mcg/dL [119.69 nmol/L] (NR = 6.09-12.23 ng/dL; 78.37-157.40 nmol/L), respectively. Thyroid-stimulating immunoglobulin, thyrotropin receptor antibodies, and thyroid peroxidase antibodies were negative, but thyroglobulin levels were elevated (625.9 ng/mL [625.9 µg/L], NR = 1.5-38.5 ng/mL [NR = 1.5-38.5 µg/L]). A 24-hour urine protein revealed nephrotic-range proteinuria (8294 mg/24 h [8.294 g/d], NR = <150 mg/24 h [<0.15 g/d]). Thyroid ultrasound and computed tomography of the neck revealed an enlarged fatty thyroid consistent with DTL. A radioactive iodine uptake (RAIU) scan was obtained that showed a normal 24-hour uptake (14.1%, NR = 7%-32%) in a slightly heterogeneous thyroid with no aberrant functioning thyroid tissue (Fig. 2A-2C). The patient underwent a renal biopsy as well as a core thyroid biopsy, both showing evidence of AA amyloid deposits. The thyroid biopsy also showed benign adipose tissue with entrapped thyroid follicles (Fig. 3). The patient underwent an extensive work-up for potential causes of amyloidosis including testing for tuberculosis, and bone marrow biopsy and flow cytometry revealed no evidence of hematologic malignancy as well as screening for rheumatological diseases, all of which was negative. Ultimately, although she was found to have a mini-clonal band in the gamma region identified by immunotyping as immunoglobulin G lambda on serum protein electrophoresis, as well as elevated serum lambda chains (386.8 mg/L, NR = 5.7-26.3 mg/L) and β-2 microglobulin (18.5 mg/L, NR = 0.1-1.8), these were deemed nonspecific and attributed to systemic inflammation and decreased renal clearance.
Figure 2.
A, Thyroid ultrasound showing diffuse hyperechogenicity. B, Neck computed tomography showing an enlarged fatty thyroid. C, Radioactive iodine uptake scan showing a normal tracer uptake.
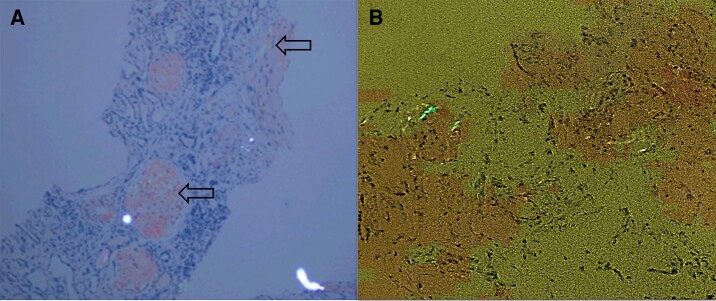
Figure 3.
A, Renal biopsy showing apple-green birefringence on Congo red staining within glomeruli, vasculature, and tubulointerstitium (arrows). B, Core thyroid biopsy showing benign adipose tissue with entrapped thyroid follicles, and perivascular amyloid deposits evidenced by apple-green birefringence.
Treatment
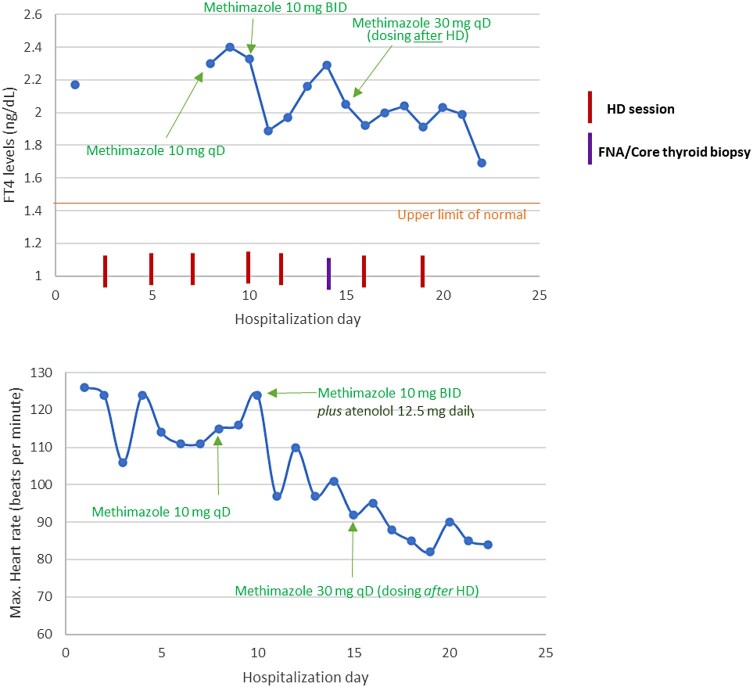
Despite gradual improvement in her respiratory and gastrointestinal symptoms, the patient remained tachycardic to 110 to 120 beats per minute and her FT4 continued to increase. Therefore, she was initiated on methimazole 10 mg once daily and atenolol 12.5 mg at bedtime. Although treatment with methimazole initially resulted in lowering of FT4 levels, they began to rise again. Methimazole was gradually uptitrated to 30 mg daily and its timing of administration was changed to after her dialysis sessions, resulting in near normalization of FT4 levels and resolution of the tachycardia. Changes in the patient’s FT4 levels and heart rate and their relationship with antithyroid medication and other interventions are depicted in Fig. 4.
Figure 4.
Thyroid function tests and heart rate with respect to antithyroid medication dosing and other potentially influencing interventions.
Outcome and Follow-up
The patient was monitored for efficacy, adverse effects, and toxicities while on methimazole, and responded well to treatment. At discharge, she was offered an outpatient appointment to further optimize her antithyroid regimen and a referral for possible total thyroidectomy, but unfortunately, the patient was lost to follow up.
Discussion
There are several case reports of thyroid amyloidosis in euthyroid and hypothyroid patients with DTL [1] as well as reported thyrotoxicosis in patients with DTL without amyloidosis [3, 4]. To our knowledge, there are no documented cases in the existing literature of DTL with evidence of amyloid deposits leading to thyrotoxicosis. This case contributes to the limited pool of reported cases in which DTL is associated with thyrotoxicosis, expanding our understanding of this exceptionally rare occurrence.
DTL's origins remain obscure, but 3 hypotheses have been postulated [1, 5, 6]. The first hypothesis suggests that DTL originates early in embryogenesis due to fat accumulation before the development of the thyroid capsule [1, 5]. However, this explanation seems less plausible in our case, considering the onset of goiter in middle age. A second hypothesis posits that the loss of somatic deletion and expression of mitochondrial succinate dehydrogenase subunit B, a crucial component in the tricarboxylic acid cycle and oxidative phosphorylation pathways, in follicular cells and adipocytes—demonstrated by Lau et al [5]—may lead to excess adipose accumulation. This interference with lipid metabolism causes defective oxidative phosphorylation, resulting in an imbalance between fatty acid synthesis, fatty acid oxidation, and lipolysis, ultimately favoring adipocyte growth and adipose tissue accumulation [5]. Finally, specifically for DTL associated with amyloidosis, it is hypothesized that chronic tissue hypoxia associated with amyloid-induced capillary damage contributes to stromal fibroblast-to-adipocyte metaplasia [6], leading to progressive fatty infiltration of the thyroid. The mechanisms contributing to thyrotoxicosis in DTL remain unclear, potentially involving the leakage of preformed thyroid hormone, increased thyroid hormone synthesis, or a combination of both.
Our hypothesis regarding the cause of thyrotoxicosis in our patient centers on the chronic or intermittent leakage of preformed thyroid hormone in DTL, speculatively due to a thyroiditis-like picture. This is supported by her markedly elevated thyroglobulin levels and a Tc-99 m pertechnetate scan with low uptake conducted 10 months before her presentation. This leakage could be triggered by a stress response to amyloid-associated damage to follicular cells and capillaries, or profound local follicular hypoxia. In regard to the latter, the extent of local thyroid hypoxia might be a crucial factor, resulting from increased blood vessel-to-follicle distance and reduced oxygen tension induced by large ectopic adipocytes in DTL, leading to a stress response akin to what has been postulated in obese adipose tissue [7]. This could hypothetically result in critical follicular cell hypoxia leading to the release of thyroid hormone independent of blood oxygen saturation. This is supported by an in vitro experiment in which release of thyroid hormones by human thyroid cell lines was observed after brief exposure to 3% oxygen [8]. But interestingly, this would not explain the possible increased thyroid hormone synthesis component seen in our patient on presentation (as evidenced by her second RAIU scan performed on admission, which was inappropriately normal, and the apparent response to methimazole). It is plausible that our patient might have had underlying seronegative Graves disease or could have been in the early recovery phase of a recurrent bout of thyroiditis, resulting in an inappropriately normal RAIU scan. In the latter scenario, the observed decline in FT4 with methimazole could be a part of the natural history of thyroiditis. Unfortunately, the patient was lost to follow-up, preventing further clarification of this conundrum in subsequent outpatient visits.
In our patient, recent history of a viral infection, and biochemical and imaging results initially pointed toward thyrotoxicosis secondary to painless thyroiditis. However, considering the work-up already performed in her home country and the reported continued weight loss during the 10-month interim in which she presented to our hospital, we thought it unusual for thyroiditis to present with thyrotoxicosis for more than 10 months, which prompted us to consider increased endogenous thyroid hormone production and treat with methimazole as described earlier. TT3 levels were low, but this was most likely in the setting of hypoalbuminemia from underlying nephrotic syndrome, intrinsically altered thyroid hormone metabolism in ESRD, as well as decreased clearance of inflammatory cytokines in the setting of ESRD that inhibit peripheral conversion of T4 to T3 [9]. The elevated inflammatory cytokines could be attributed to her underlying chronic inflammatory state that triggered amyloidosis, as well as viral syndrome with high erythrocyte sedimentation rate and C-reactive protein at presentation. We hypothesized the presence of hyperfunctioning thyroid tissue was masked by surrounding adipose tissue, especially considering that her heart rate failed to normalize following resolution of respiratory and gastrointestinal symptoms and euvolemic status, and that FT4 continued to rise. Given that Pradeep et al [3] previously reported that euthyroidism was achieved with carbimazole and propranolol in their patient with DTL and reported hyperthyroidism, we started methimazole with subsequent gradual uptitration as well as atenolol. Despite the initial lowering of FT4 levels, these began to rise again. Because methimazole is not protein-bound, we hypothesized the drug was being cleared by the dialysate during her dialysis sessions. Thus, the decision to dose methimazole after hemodialysis was made, as previously described for its prodrug carbimazole [10]. As shown in Fig. 4, this eventually resulted in near normalization of FT4 levels on day of discharge, which correlated with the gradual decrease in heart rate. Nonetheless, as discussed earlier, a caveat to consider is that recurrence of thyroiditis and early phase of thyroid recovery cannot be ruled out, as the continued weight loss between presentations could also be explained by the underlying cause of the systemic amyloidosis, which remained elusive.
Patients with DTL may present with a wide spectrum of thyroid hormonogenesis including low, normal, or elevated thyroid levels. This could be related to the disease's natural history, which mirrors thyroiditis. Classic thyroiditis begins with thyrotoxicosis and is followed by hypothyroidism and finally euthyroidism. On the contrary in DTL, we speculate that euthyroidism precedes as tissue is replaced by fat, which is then followed by a stress response leading to thyrotoxicosis finally followed by “thyroid burnout” leading to hypothyroidism. More experimental evidence and prospective studies are needed to better understand DTL's pathobiology.
Learning Points
DTL is a rare etiology of goiter that can present with hypothyroidism, euthyroidism, or thyrotoxicosis. It is unclear whether these presentations represent different stages in DTL's natural history.
Treatment with antithyroid drugs should be considered in patients with DTL and persistent thyrotoxicosis. In patients with hyperthyroidism and ESRD on dialysis, it is best to dose methimazole after dialysis.
Acknowledgments
The authors would like to acknowledge Dr Hamza Quadri and all medical and ancillary staff involved in the care of this patient, as well as their valuable recommendations in the management of this patient.
Abbreviations
- DTL
diffuse thyroid lipomatosis
- ESRD
end-stage renal disease
- FT4
free thyroxine
- NR
normal range
- RAIU
radioactive iodine uptake
- TT3
total 3,5,3′-triiodothyronine
Contributor Information
Adrian M Gonzalez-Gil, Department of Medicine, Baylor College of Medicine, Houston, TX 77030, USA.
Marco A Ruiz-Santillan, Division of Endocrinology, Diabetes and Metabolism, Baylor College of Medicine, Houston, TX 77030, USA.
Bahar K Force, Division of Endocrinology, Diabetes and Metabolism, Baylor College of Medicine, Houston, TX 77030, USA.
Ruchi Gaba, Division of Endocrinology, Diabetes and Metabolism, Baylor College of Medicine, Houston, TX 77030, USA.
Contributors
A.M.G.G. and M.A.R.S. were involved in manuscript writing and submission; B.K.F., R.G., A.M.G.G., and M.A.R.S. were involved in the diagnosis and management of this patient; and B.K.F. and R.G. reviewed and edited manuscript.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent obtained directly from patient.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Cavaco DR, Alves Rafael A, Cabrera R, Vilar H, Leite V. Case report: a rare association of diffuse thyroid lipomatosis with amyloid deposition. Eur Thyroid J. 2021;10(6):528‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ge Y, Luna MA, Cowan DF, Truong LD, Ayala AG. Thyrolipoma and thyrolipomatosis: 5 case reports and historical review of the literature. Ann Diagn Pathol. 2009;13(6):384‐389. [DOI] [PubMed] [Google Scholar]
- 3. Pradeep P, Kumar R, Ragavan M, Ramakrishna B. Diffuse lipomatosis of thyroid with hyperthyroidism. J Postgrad Med. 2010;56(1):35‐36. [DOI] [PubMed] [Google Scholar]
- 4. Sanuvada RV, Chowhan AK, Rukmangadha N, Patnayak R, Yootla M, Amancharla LY. Thyrolipomatosis: an inquisitive rare entity. Gland Surg. 2014;4:E6‐E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lau E, Freitas P, Costa J, et al. Loss of mitochondrial SDHB expression: what is its role in diffuse thyroid lipomatosis? Horm Metab Res. 2015;47(3):165‐167. [DOI] [PubMed] [Google Scholar]
- 6. Schröder S, Böcker W. Lipomatous lesions of the thyroid gland: a review. Appl Pathol. 1985;3(3):140‐149. [PubMed] [Google Scholar]
- 7. Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633‐643. [DOI] [PubMed] [Google Scholar]
- 8. Tani N, Ishikawa M, Watanabe M, Ikeda T, Ishikawa T. Thyroid-related hormones as potential markers of hypoxia/ischemia. Hum Cell. 2020;33(3):545‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohamedali M, Reddy Maddika S, Vyas A, Iyer V, Cheriyath P. Thyroid disorders and chronic kidney disease. Int J Nephrol. 2014;2014:520281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varughese GI, Tahrani AA, Smith JL, Clayton RN, Hanna FW. Carbimazole therapy in the setting of end-stage renal disease and haemodialysis. Nephrol Dial Transplant. 2006;21(8):2318‐2319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.