Abstract
Loss-of-function mutations of p16INK4a have been identified in a large number of human tumors. An established biochemical function of p16 is its ability to specifically inhibit cyclin D-dependent kinases in vitro, and this inhibition is believed to be the cause of the p16-mediated G1 cell cycle arrest after reintroduction of p16 into p16-deficient tumor cells. However, a mutant of Cdk4, Cdk4N158, designed to specifically inhibit cyclin D-dependent kinases through dominant negative interference, was unable to arrest the cell cycle of the same cells (S. van den Heuvel and E. Harlow, Science 262:2050–2054, 1993). In this study, we determined functional differences between p16 and Cdk4N158. We show that p16 and Cdk4N158 inhibit the kinase activity of cellular cyclin D1 complexes through different mechanisms. p16 dissociated cyclin D1-Cdk4 complexes with the release of bound p27KIP1, while Cdk4N158 formed complexes with cyclin D1 and p27. In cells induced to overexpress p16, a higher portion of cellular p27 formed complexes with cyclin E-Cdk2, and Cdk2-associated kinase activities were correspondingly inhibited. Cells engineered to express moderately elevated levels of cyclin E became resistant to p16-mediated growth suppression. These results demonstrate that inhibition of cyclin D-dependent kinase activity may not be sufficient to cause G1 arrest in actively proliferating tumor cells. Inhibition of cyclin E-dependent kinases is required in p16-mediated growth suppression.
Normal proliferation of eukaryotic cells must depend on a precisely controlled cell cycle engine, and deregulation of the cell cycle engine can contribute to tumorigenesis. Among the many cyclin-dependent kinases of the cell cycle engine, deregulation of cyclin D1-Cdk4 kinase may play a particularly important role in tumorigenesis (37). Cyclin D1 and Cdk4 genes are often amplified or overexpressed in many types of cancers (for a review, see reference 9). Forced overexpression of cyclin D1 in cultured cells can induce oncogenic transformation in cooperation with a defective E1A oncoprotein (14) or with activated Ha-ras (20). Transgenic mice with cyclin D1 overexpression in mammary glands develop mammary adenocarcinomas (43). Therefore, the cyclin D1 gene fulfills the criteria of an oncogene.
The importance of cyclin D1-Cdk4 deregulation in tumorigenesis is further demonstrated by the frequent deregulation of the downstream effectors and upstream regulators of cyclin D1-Cdk4 in human tumors. The primary target of cyclin D-dependent kinases is the retinoblastoma protein pRB, a classic tumor suppressor (44). Since phosphorylation of pRB inactivates its growth suppression activity, overexpression of cyclin D1-Cdk4 can result in the loss of pRB function equivalent to the effects of pRB mutations identified in retinoblastomas and many other types of cancers. The activities of cyclin-dependent kinases are regulated by a number of mechanisms. At least one regulator of cyclin D1-Cdk4, the cyclin-dependent kinase inhibitor p16INK4a, is also a known tumor suppressor gene product (17, 28) whose function is frequently lost in melanoma and in a variety of other cancers (for a review, see reference 9). Unlike the p21-p27 family of cyclin-dependent kinase inhibitors, p16 specifically inhibits cyclin D-dependent kinases in vitro (36). Thus, functional loss of p16 will lead to the same consequences as overexpression of cyclin D1-Cdk4.
An established biological function of p16 is its ability to arrest the cell cycle in G1 (18, 23, 25). Since p16 is a specific inhibitor of cyclin D-dependent kinases in vitro, p16-mediated growth suppression is believed to be caused by the inhibition of cellular cyclin D-dependent kinases with the accumulation of un- or hypophosphorylated pRB, which in turn acts to arrest the cell cycle in G1. Indeed, cells lacking functional pRB are resistant to p16-mediated growth suppression (18, 23, 25).
Results obtained from experiments with dominant negative mutants of Cdk4, however, seem to argue against a simple role of p16 as a specific inhibitor of cyclin D1-Cdk4 in mediating growth suppression. Dominant negative mutants provide a useful tool to study the functional roles of the wild-type counterpart in the cell (13). A dominant negative mutation abolishes the normal, usually enzymatic, function of the protein but does not affect the ability of this protein to physically interact with its regulators and/or effectors. When overexpressed in the cell, dominant negative mutants can functionally inactivate the cellular, wild-type protein by sequestering its regulators and/or effectors. Mutant Cdk4N158 contains an Asp-to-Asn mutation at amino acid residue 158, a position conserved in all cyclin-dependent kinases, and is involved in the binding of Mg2+-ATP, a necessary step for the enzymatic activity of these kinases. While overexpression of identically designed mutants of Cdk2 and Cdc2 efficiently blocked U2OS cell cycle progression in G1 and G2-M phases, respectively, Cdk4N158 overexpression at the same high levels did not have any effects on the cell cycle in the same assay (42). This result raises an interesting question: if p16 specifically inhibits cyclin D-Cdk4 activity to arrest the cell cycle, does Cdk4N158 do the same in the same cells?
In this study, we determined and compared the functional mechanisms of p16 and Cdk4N158 in U2OS cells in an attempt to learn the reasons for their different effects on the cell cycle. We report here that the reason for the phenotypic differences between p16 and Cdk4N158 is that p16 and Cdk4N158 inhibited cyclin D1-Cdk4 through different mechanisms. Our results demonstrate that inhibition of cyclin D1-Cdk4 kinase activity may not be sufficient to arrest the cell cycle in G1; inhibition of cyclin E-Cdk2 is required for the growth-inhibitory effects of p16.
MATERIALS AND METHODS
Cells.
To establish inducible U2OS cell lines, pUHD15-1 (6) was first transfected into U2OS together with pSVneo for G418 selection. A derivative clone, U24, was found to be able to support the tetracycline-controlled expression of p16 from pUHD10-3p16, which was constructed by inserting a p16 cDNA (18) into the BamHI site of pUHD10-3. pUHD10-3p16 was transfected into U24 cells with pBabePuro (27) for puromycin selection. Clonal cell lines were screened for their ability to express p16 protein in the tetracycline-controlled manner. p16P114L-inducible cell lines were established in parallel. cDNA for Cdk4N158-hemagglutinin (HA) as a BamHI fragment was also cloned into pUHD10-3 that with pBabePuro, was used to transfect U24 cells to established Cdk4N158-inducible U2OS cell lines. For cyclin E-expressing cell lines, U2OS cells were transfected with pCMVcyclin E (15), selected with G418, and screened for expression levels of cyclin E. Multiple clones were obtained for each of these cell lines, and at least two independent clones were used in experiments described here.
Protein expression in insect cells.
Recombinant Cdk4 and Cdk4N158 baculoviruses were generated by inserting a Cdk4 or Cdk4N158 (42) coding sequence together with a glutathione S-transferase (GST)-encoding fragment of pGEX2T into transfer vector pVL1392 and were then cotransfected into Sf9 insect cells with BaculoGold DNA (Pharmingen). Recombinant cyclin D1 baculovirus was described previously (26). Hi Five insect cells (Invitrogen) were infected with the indicated recombinant baculoviruses and harvested 48 h postinfection. Wild-type and mutant Cdk4 kinases were purified through GST tags with glutathione-agarose beads. An in vitro kinase assay was carried out with purified GST-pRB-C fragment (47).
Immunoprecipitation, immunoblotting, kinase assay, and flow cytometry analysis.
These experiments were performed as previously described (47). The following antibodies used in this experiment were from Santa Cruz Biotechnology: H295 (cyclin D1), H22 (Cdk4), M2 (Cdk2), C19 (p27), H432 (cyclin A), and C19 (E). Anti-p16 antibody ZJ11 was obtained from NeoMarkers. Anti-p16 (JC6), anti-cyclin E (HE12), and anti-cyclin A (BF683) antibodies were gifts from Ed Harlow.
RESULTS
Cdk4N158 as a dominant negative mutant.
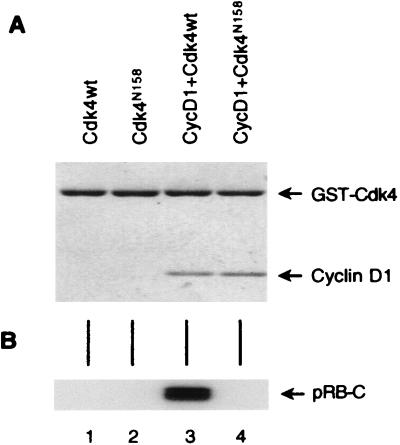
To determine whether Cdk4N158 has the expected properties of a dominant negative mutant, we expressed wild-type Cdk4 and Cdk4N158 with or without cyclin D1 in insect cells. Cdk4 proteins were purified through a GST tag fused to their amino termini and, after coinfection with cyclin D1, were subsequently tested for the presence of cyclin D1 protein and kinase activity. The results clearly show that Cdk4N158 bound to cyclin D1 as efficiently as the wild-type Cdk4 (Fig. 1A, lanes 3 and 4). However, while the kinase activity of wild-type Cdk4 was activated by cyclin D1 (Fig. 1B, lanes 1 and 3), the cyclin D1-Cdk4N158 complex did not have any detectable kinase activity (lane 4). Therefore, Cdk4N158 has the properties of a dominant negative mutant.
FIG. 1.
Dominant negative properties of Cdk4N158. (A) Purification of cyclin D1-Cdk4 and cyclin D1-Cdk4N158 complexes from insect cells infected with the indicated recombinant baculoviruses. wt, wild type. The gel was stained with Coomassie blue. (B) In vitro kinase assay on a GST-pRB-C-terminal fragment with the purified Cdk4 or cyclin D1-Cdk4 complexes shown in panel A.
Different cell cycle effects of p16 and Cdk4N158.
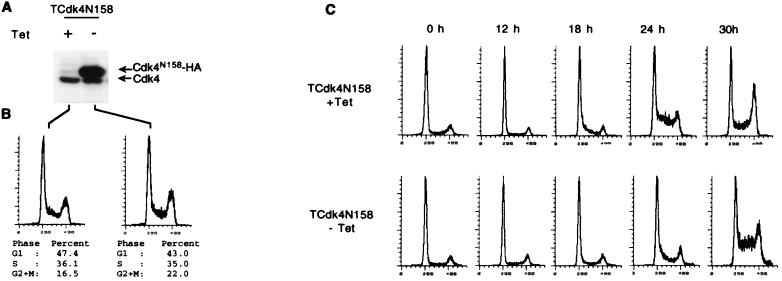
To study the effects of p16 and Cdk4N158 on cellular cyclin D-dependent kinases, we established U2OS cell lines overexpressing either p16 or Cdk4N158. U2OS cells lack functional p16 and have been used as a model to study the growth suppression function of p16 in transient transfection studies (18, 23, 25). U2OS cells were also used in a study of Cdk4N158 by transient transfection (42). Here, the tetracycline-regulated expression system (6) was used to establish U2OS cell lines inducible for p16 and Cdk4N158-HA expression. As a control for p16 function, inducible cell lines for a nonfunctional mutant of p16, p16P114L (18, 23), were also established. Induction of p16 and Cdk4N158 expression in representative cell lines is shown in Fig. 2A and 3A, respectively. Protein expression first became detectable 18 h after the withdrawal of tetracycline from the media, and the peak level of expression was observed 40 h after induction (data not shown). With the addition of an HA tag, the exogenously expressed Cdk4N158 could be easily distinguished from the endogenous Cdk4 in Western blots with an anti-Cdk4 antibody (Fig. 3A). One day after induction, the level of Cdk4N158 was 9.5-fold higher than the level of the endogenous Cdk4 in this experiment as quantified by densitometry (Fig. 3A).
FIG. 2.
U2OS cell lines with inducible expression of p16. (A) Expression of p16 in the presence (+) or absence (−) of tetracycline (Tet) or at different times after the withdrawal of tetracycline was determined by Western blotting of the total cell extracts with an anti-p16 antibody (JC-6). Shown are cell lines Tp16wt-17 and Tp16P114L-11 that are representatives of multiple similar cell lines established in this study. (B) Effects of p16 on cell cycle profiles of asynchronously growing U2OS cells were determined by flow cytometry analysis after propidium iodide staining (15,000 cells were routinely counted for each sample). The scale on the x axes indicates relative propidium iodide staining intensity.
FIG. 3.
U2OS cell lines with inducible expression of Cdk4N158. (A) Expression of Cdk4N158 in the presence (+) or absence (−) of tetracycline (Tet) was determined by Western blotting of the total cell extracts with an anti-Cdk4 antibody (H22). (B) Effects of Cdk4N158 overexpression on cell cycle profiles of asynchronously growing cells were determined by flow cytometry analysis. Scale on x axes indicates relative propidium iodide staining intensity. (C) Cdk4N158 inducible cells were serum starved for 5 days and released into media containing 10% serum in the presence and absence of tetracycline. At the indicated time points after release, an aliquot of cells was harvested for flow cytometry analysis to determine the cell cycle profiles.
Effects of p16 and Cdk4N158 on the cell cycle were then determined by fluorescence-activated cell sorter analysis. Induction of wild-type p16, but not mutant p16P114L, led to efficient cell cycle blocking in G1, with arrest profiles similar to those obtained in previous studies with transient transfection (Fig. 2B). In contrast, induction of Cdk4N158 expression did not change the cell cycle profile (Fig. 3B). To determine whether overexpression of Cdk4N158 had any effects on cell cycle progression emerging from quiescence, Cdk4N158 inducible cells were serum starved in the presence or absence of tetracycline for 5 days and released into serum-containing media with or without tetracycline to allow synchronous progression from G0 to S phase. As shown in Fig. 3C, cell cycle progression from quiescence to S phase was delayed for about 6 h by the induction of Cdk4N158. Thus, overexpression of Cdk4N158 was not without any phenotypes. Expression of Cdk4N158 delayed G0-to-S progression, while the expression of p16 arrested actively cycling cells in G1.
Inhibition of cellular cyclin-dependent kinases by p16 and Cdk4N158.
We used the p16 and Cdk4N158 inducible cell lines described above to determine the effects of p16 and Cdk4N158 on the activities of cellular cyclin-dependent kinases. In U2OS cells, cyclin D1-Cdk4 is the predominant cyclin D-dependent kinase, as cyclins D2, D3, and Cdk6 are not readily detectable (data not shown). Cdk2-associated kinase activities, which become activated by cyclins E and A later in G1 phase, were also examined. Total cellular extracts from cells before and after induction of p16 or Cdk4N158 expression were immunoprecipitated with an anti-cyclin D1 monoclonal antibody, and the associated kinase activity was determined according to established protocols (24). Induction of wild-type p16 and Cdk4N158 significantly reduced cyclin D1-associated kinase activities, while expression of mutant p16P114L did not have inhibitory effects (Fig. 4A). The Cdk4N158-mediated inhibition was as efficient as the p16-mediated inhibition in multiple tests with two independent cell lines, as quantified in Fig. 4A. Thus, Cdk4N158 functioned as a dominant negative mutant in vivo. Indeed, 24 h after induction, Cdk4N158 constituted about 90% of the total Cdk4 in cellular cyclin D1-Cdk4 complexes (see Fig. 6A below).
FIG. 4.
Effects of p16 and Cdk4N158 on the activities of cellular cyclin-dependent kinases. (A) Kinase activity in the presence (+) or absence (−) of tetracycline (Tet) determined by immunoprecipitation and in vitro phosphorylation assay. Total cell extracts of Tp16wt, Tp16P114L, and TCdk4N158 cells before and 24 h after induction were immunoprecipitated with either anti-cyclin D1 (DCS11) or anti-Cdk2 (M2) antibodies, and kinase reactions were carried out with purified GST-pRB-C-terminal fragment. Reaction products were separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized either by autoradiography or on a StormImager. Quantitation was performed on StormImager with the ImagerQuant software with results obtained from four independent experiments. (B) Histone H1 kinase activity determined by immunoprecipitation of Cdk2 and in vitro kinase assay. Lanes are as marked at the top of panel A. (C) In vivo phosphorylation status of cellular pRB. Total cell extracts from the indicated cells, as marked at the top of panel A, were separated on an SDS–6% PAGE and blotted with anti-pRB monoclonal antibody (XZ77). pRBphos, phosphorylated pRB.
FIG. 6.
Effects of p16 (Tp16wt and T16P114L) and Cdk4N158 (TCdk4N158) overexpression in the presence (+) or absence (−) of tetracycline (Tet) on the composition of cellular cyclin-dependent kinases. The status of cyclin D1, Cdk4, Cdk2, and p27 complexes were determined by immunoprecipitation (IP) followed by Western blotting, as indicated. See Materials and Methods for a description of the antibodies used. Cyc, cyclin; αp27IP, anti-p27 IP.
In contrast, the Cdk2-associated kinase was significantly inhibited only after induction of wild-type p16; induction of p16P114L or Cdk4N158 did not have inhibitory effects (Fig. 4B). Phosphorylation of cellular pRB, a known substrate of cyclin-dependent kinases, was also inhibited only after induction of wild-type p16 (Fig. 4C). These results show that whereas both p16 and Cdk4N158 inhibited cyclin D-dependent kinase activities in cells, only p16 expression led to inhibition of Cdk2-associated kinase activity. The differential effects of p16 and Cdk4N158 on Cdk2-associated kinase activity and on the phosphorylation of cellular pRB may explain their different effects on the cell cycle.
Different effects of p16 and Cdk4N158 on cellular complexes containing cyclin D1, Cdk4, and p27.
The effects of p16 and Cdk4N158 expression may be mediated through altered endogenous levels of cyclins, cyclin-dependent kinases, or cyclin-dependent kinase inhibitors or through changes in the formation of cyclin-cyclin-dependent kinase complexes. Western blot analysis showed that the cellular levels of cyclin D1, cyclin E, cyclin A, Cdk4, Cdk2, and p27 remained unchanged 1 day after the induction of p16 or Cdk4N158 when the cell cycle effects of p16 were already evident (Fig. 5). Thus, altered expression levels of these proteins cannot be the causes of the different effects of p16 and Cdk4N158. We next determined the status of cyclin D1-Cdk4 complexes by coimmunoprecipitation (Fig. 6). The stable interaction between cyclin D1 and Cdk4 was largely disrupted after the induction of wild-type p16 but not mutant p16P114L (Fig. 6). The dissociated Cdk4 was bound to wild-type p16. In the same blot, cyclin D1 was not detected in p16 immunoprecipitation (negative data not shown), indicating that p16 was not able to bind monomeric cyclin D1 or cyclin D1-Cdk4 complexes. Consistent with the specificity of p16, Cdk2 was also not detected in p16 immunoprecipitation (negative data not shown). p16P114L did not bind to Cdk4. In Cdk4N158-expressing cells, Cdk4N158 formed stable complexes with cellular cyclin D1, largely replacing the endogenous Cdk4. Densitometry analysis indicated that the amount of Cdk4N158 in association with cyclin D1 was 9.1-fold higher than that of endogenous Cdk4 and the amount of endogenous Cdk4 in cyclin D1 complexes was reduced to 18% of that seen before the induction of Cdk4N158. These results clearly demonstrate that p16 inhibited cyclin D1-Cdk4 activity in vivo by preventing the formation of cyclin D1-Cdk4 complexes, while Cdk4N158 inhibited cyclin D1-Cdk4 by forming inactive cyclin D1-Cdk4N158 complexes.
FIG. 5.
Effects of p16 (Tp16wt and Tp16P114L) and Cdk4N158 overexpression on protein levels of cyclin D1 (Cyc D1), cyclin E (Cyc E), cyclin A (Cyc A), Cdk4, Cdk2, and p27 in the presence (+) or absence (−) of tetracycline (Tet). Protein levels were determined by immunoblotting with the indicated antibodies after SDS-PAGE of total cell extracts as indicated. Equal amounts of protein were loaded. In some cases, nonspecific bands are shown as loading controls, and molecular mass markers (in kilodaltons) are included for cyclin E and cyclin A.
To understand how Cdk2-associated kinase activity was inhibited after induction of p16, we examined the status of cellular p21 and p27. These two proteins have a broad range of targets including cyclin D-, cyclin E-, and cyclin A-dependent kinases and have been found to switch among various cyclin-dependent kinases (31, 33, 35). In cycling cells, p21 and/or p27 are present and are believed to serve as a threshold mechanism to control cell cycle advance (38). In U2OS cells, the p21 level was difficult to detect without treatment with DNA-damaging agents (47), while p27 protein was easily detectable (Fig. 5). We therefore concentrated on p27. Cellular p27 was found to associate with Cdk4 and Cdk2 (Fig. 6A). After induction of wild-type p16, p27-associated Cdk4 decreased significantly while p27-associated Cdk2 increased by a similar extent. Reprobing the same blot with anti-cyclin E and anti-cyclin A antibodies revealed that the increase in p27-bound Cdk2 was accompanied primarily by an increase in bound cyclin E but not cyclin A (Fig. 6). After induction of Cdk4N158, p27 was bound to Cdk4N158, and the amount of p27-bound Cdk2 actually decreased somewhat.
We next performed an immunodepletion experiment to learn the extent of changes of non-p27-bound Cdk2 to p27-bound Cdk2. A single round of immunoprecipitation with the anti-p27 antibody efficiently depleted p27 protein in the extract, as evidenced by the disappearance of p27 in the supernatant after immunoprecipitation (Fig. 6B). In the absence of p16 induction, a certain amount of Cdk4 and Cdk2 proteins was associated with p27 in anti-p27 immunoprecipitates. However, immunodepletion with anti-p27 antibody did not detectably change the amount of either Cdk4 or Cdk2 in the extract, indicating that only a small fraction of total cellular Cdk4 and Cdk2 was in complexes with p27 in this cell type. This situation was clearly changed after the induction of p16. Although the amount of Cdk4 in the extract after p27 immunodepletion did not increase significantly, the amount of Cdk2 in the supernatant was now reduced to 63% as determined by densitometry analysis (Fig. 6B). As in the experiments described above, p27-associated Cdk2 increased significantly while p27-associated Cdk4 decreased significantly. Thus, p27 rearrangement after p16 induction caused a significant change in the amount of Cdk2 that was associated with p27. In a tetracycline-controlled expression system for p27 (35), it was shown that a modest increase in p27 levels above a threshold could cause a 90% inhibition of Cdk2-associated kinase activity. Together, these results suggest that the redistribution of p27 from Cdk4 to Cdk2 after induction of wild-type p16 may account for the inhibition of Cdk2-associated kinase activity and the cell cycle arrest in G1. The lack of this effect by Cdk4N158 may explain its inability to arrest the cell cycle.
Requirement for cyclin E-Cdk2 inhibition in p16-mediated G1 arrest.
To determine whether the inhibition of cyclin E-Cdk2 by p27 released from cyclin D1-Cdk4 was required for the growth suppression effects of p16, we sought to moderately increase the level of cyclin E-Cdk2 in the cell to sequester the released p27. Stable U2OS cell lines that contained three- to fivefold-higher amounts of cyclin E protein were generated. The expression levels of cyclin E in one of the cell lines (UE13) is shown in Fig. 7A. The cell cycle characteristics of UE13 cells were not different from those of U2OS cells as determined by fluorescence-activated cell sorter analysis (data not shown). We then compared the growth suppression effects of p16 and p27 in UE13 and U2OS cells. As expected, transient transfection of wild-type p16 or p27 efficiently blocked U2OS cells in G1, shown in Fig. 7B as the net increase in the percentages of G1 phase cells. In UE13 cells, however, p16 lost its growth suppression activity while p27, which directly inhibits cyclin E-Cdk2, still efficiently arrested the transfected cells in G1. These results suggest that although p16 specifically inhibits cyclin D1-Cdk4 activity, the indirect inhibition of cyclin E-Cdk2 by p27 is required for growth suppression.
FIG. 7.
Effects of cellular cyclin E (Cyc E) levels on growth suppression activities of p16 and p27. (A) Cyclin E protein levels in U2OS cells and a derivative cyclin E-transfected cell line (UE13) were determined by Western blotting of the total cell extracts with an anti-cyclin E antibody (HE12). (B) Growth suppression activities of p16 and p27 on U2OS and the cyclin E-transfected cell line were determined by transient transfection with pCMVp16 or pCMVp27 together with the transfection marker CD20. Empty vector was used as a baseline control. Transfected cells were analyzed by flow cytometry, and the cell cycle profiles of CD20-positive cells were determined. Differences in G1 percentage cells (Δ G1 %) between vector-transfected cells and cells transfected with p16 or p27 are presented.
DISCUSSION
p16 prevents formation of cyclin D1-Cdk4 complexes in vivo.
Mechanisms of inhibition of cyclin D-Cdk4 and/or cyclin D-Cdk6 (cyclin D-Cdk4/Cdk6) kinase activity by p16 have been previously studied in vitro. Using in vitro-synthesized cyclin D1 and Cdk4 or Cdk6 (Cdk4/Cdk6), Parry et al. showed that bacterially expressed histidine-tagged p16 bound to Cdk4-Cdk6 and prevented the association of Cdk4/Cdk6 with cyclin D1 (30). However, enzymatically active cyclin D2-Cdk4 complexes generated in baculovirus-infected insect cells were inactivated by the addition of purified GST-p16 and a p16 homolog, p19, through the formation of cyclin D2-Cdk4-p16 ternary complexes (16).
p16 functions have also been inferred from studies with pRB mutant tumor cells or cells transformed by viral oncoproteins that inactivate pRB family proteins. In these cells, p16 levels are usually elevated, and p16 proteins can be found in complexes with Cdk4/Cdk6 but not cyclin D1 (4, 30, 36, 46). However, protein levels of D-type cyclins are usually very low in pRB mutant cells. These and other observations have led to the hypotheses that the lack of pRB functioning leads to deregulated expression of p16, that p16 binds to Cdk4 and Cdk6 to prevent their association with cyclin D, and that monomeric cyclin D is degraded faster than Cdk-bound cyclin D. The fact that this situation is not reproduced in fibroblasts derived from pRB knockout mice (21) suggests that additional changes may have occurred in these oncogenically transformed cells.
The p16-inducible cell lines established in our study offer a powerful means to examine the immediate effects of p16 in vivo. Our results clearly demonstrate that p16 binds to Cdk4 and prevents the formation of cyclin D-Cdk4 complex. The steady-state level of cyclin D1 remains unchanged after the induction of p16, indicating that the low levels of cyclin D proteins in pRB mutant tumor cells may not simply result from the dissociation from Cdk4/Cdk6. Recently, the functional mechanisms of a p16 homolog, p15, were studied by the inducible-expression approach (34). In contrast to p16, p15 induction in Mv1Lu cells led to the formation of inactive cyclin D-Cdk4/Cdk6-p15 complexes. Whether this difference is due to the different cell types used or to the intrinsic functional differences between these two homologs remains to be determined.
It is important to note that while the levels of p15 are acutely induced in many cell lines after transforming growth factor β treatment, acute induction of p16 has not been observed in physiologically relevant processes. Although loss of p16 is a frequent event in tumorigenesis, reintroduction of functional p16 through ectopic expression may not create a situation equivalent to that in a cell with normal p16 functions. The exact mechanism of p16-mediated growth suppression in the prevention of tumorigenesis therefore must be further investigated. However, chronic induction of p16 has been demonstrated in cultured senescent cells (1, 10, 45). In these cells, p16 binds to Cdk4 and Cdk6 without cyclin D1, and cyclin D1 levels remain high (1). These phenotypes are not unlike what was observed in this study, and chronic induction of p16 with our inducible expression system is also able to induce a senescent-like phenotype in tumor cells (our unpublished data). In this respect, the functional mechanisms of p16 demonstrated in our current study may be used in physiologically relevant settings as well.
The role of cyclin D-dependent kinases in the cell cycle.
Our analysis of Cdk4N158 provides a unique probe of the functional roles of cyclin D-dependent kinases. Since Cdk4N158 forms stable complexes with cyclin D in the cells, it inhibits the kinase activity of cyclin D-dependent kinases through a mechanism different from the binding mechanism of p16. Our experiments differed from other methods previously used to study the role of cyclin D-dependent kinases. Inhibition of cyclin D1-dependent kinases by anti-cyclin D1 antibodies or cyclin D1 antisense constructs as reported in previous experiments (3, 21) all led to the disruption of cyclin D1-Cdk4 complexes. Of the four anti-cyclin D1 antibodies used in previous microinjection experiments, only those antibodies that dissociated the cyclin D-Cdk4 complex in vitro were able to efficiently block the cell cycle (21). These results left open the possibility that the formation of cyclin D1-Cdk4 complex may be a key determinant of the observed effects.
Cdk4N158 overexpression delayed cell cycle entry after serum starvation yet did not affect the cell cycle of actively proliferating cells. p16 overexpression in cells with elevated levels of cyclin E-Cdk2 also could not arrest the cells in G1. These data suggest that the kinase activity of cyclin D-Cdk4/Cdk6 is important in G0-to-S transition but may not be required in actively cycling cells. A more important function of cyclin D-Cdk4/Cdk6 in actively cycling cells may be to bind to p27 or related molecules to regulate their distribution among various cyclin-dependent kinases in the cell. This hypothesis is consistent with results from previous studies in which wild-type Cdk4 and Cdk4 dominant negative mutants were compared. Like the wild-type Cdk4, Cdk4N158 was also able to reverse the cell cycle block imposed by p16 (18). Both wild-type Cdk4 and Cdk4N158 were able to reverse growth suppression mediated by the tumor suppressor p53 (19). Like cyclin D1-Cdk4, cyclin D1-Cdk4N158 could also lead to efficient phosphorylation of pRB in cotransfection experiments (our unpublished results). Apparently, the kinase activity of Cdk4 was not needed in these functional assays. In the future, introduction of kinase-inactivating mutations into cellular Cdk4/Cdk6 genes will provide specific information as to the roles of the kinase activities of these proteins.
Inhibition of cyclin E-Cdk2 by redistributed p27 is required for p16-mediated cell cycle blocking.
The redistribution of p27 from cyclin D1-Cdk4 to cyclin E-Cdk2 after the induction of p16 is reminiscent of similar changes observed in several experimental systems. In transforming growth factor β-treated Mv1Lu cells, p27 was found to transfer from Cdk4/Cdk6 to Cdk2 (35), which was later confirmed to be the effect of p15 with p15-inducible expression (34). Whether p27 is redistributed to cyclin E-Cdk2, cyclin A-Cdk2, or both, and whether p27 redistribution is required for growth suppression were questions not addressed in these studies. In Swiss 3T3 fibroblasts, p27 was redistributed from cyclin D-Cdk4 to cyclin A-Cdk2 as cells moved from G1 into S phase (33). When the same cells were arrested in G1 by lovastatin or UV irradiation, cyclin D1 became degraded and p27 was redistributed to cyclin A-Cdk2 (33). More recently, it was reported that p21, a cyclin-dependent kinase inhibitor related to p27, was redistributed from cyclin E-Cdk2 to cyclin D1-Cdk4 when MCF-7 human mammary carcinoma cells were released from tamoxifen-induced G0-G1 arrest by estradiol (31). p21 was also shown to be able to replace other molecules that associate with cyclin-dependent kinases through similar LFG-related sequence motifs such as p107 (47) and p130 (39). It has been further demonstrated that the effects of p27 on cyclin D-Cdk4/Cdk6 and cyclin E-Cdk2 complexes are different. While p27 efficiently inhibits the activity of cyclin E-Cdk2, the p27-cyclin D-Cdk4/Cdk6 complexes are rather active (5, 40). Therefore, p27 is not only in constant dynamic equilibrium with its binding partners but also regulates differently the activities of different partners.
In addition to documenting that p16 is capable of preventing association between cyclin D1 and Cdk4 to cause p27 redistribution to cyclin E-Cdk2 in vivo, our results provide two lines of evidence to suggest that the redistribution of p27 to cyclin E-Cdk2 is required for the p16-mediated growth suppression. First, by investigating the reason for the inability of Cdk4N158 to arrest the cell cycle compared with p16, we show that p16 and Cdk4N158 inhibited cyclin D1-Cdk4 activity through clearly different mechanisms. p16 caused cyclin D1-Cdk4 dissociation leading to p27 redistribution to cyclin E-Cdk2, while Cdk4N158 formed stable complexes with cyclin D1 and p27 resulting in less p27 on Cdk2. Thus, inhibition of cyclin D-dependent kinase activity by itself may not be sufficient to arrest the actively cycling tumor cells. The mechanism of inhibition seems to be an important factor. Second, to test whether the p27 released from cyclin D1-Cdk4 complexes is involved in growth suppression, we established cell lines containing moderately elevated levels of cyclin E. These cells, while still efficiently arrested by high levels of p27 after transient transfection, became resistant to the growth suppression effects of p16. This result is consistent with recently published studies showing that overexpression of cyclin E can override p16-mediated growth suppression (2, 22).
A number of cell lines have also been found to be resistant to p16-mediated growth suppression (18, 23, 25). These cells usually do not contain functional pRB, which was believed to be the cause of resistance to p16-mediated growth suppression. Since our results in this study suggest that indirect inhibition of cyclin E-Cdk2 by p27 is required for p16-mediated growth suppression, it can be predicted that the inability of p16 to cause inhibition of cyclin E-Cdk2 may also be the reason for resistance to p16. This prediction indeed appears correct. Saos-2 and C33A cells are resistant to p16 (18, 23, 25) and to the p16 homolog p18 (8). These cells have been shown to contain no cyclin D-Cdk4/Cdk6 complexes (4). Hence, no p27 redistribution results after p16 expression. Mouse embryo fibroblasts with pRB+/+ genotypes are sensitive to p16, but matched pRB−/− cells are not. pRB−/− mouse embryo fibroblasts, however, have been shown to contain 10 times more cyclin E protein (12). There are also tumor cell lines that contain functional pRB but that can proliferate in the presence of p16 overexpression (e.g., the breast cancer cell line MDA-MB-157 [7]). These cells also contain high levels of cyclin E. Therefore, the statuses of cyclin D-dependent kinase complexes, p27 complexes, cyclin E, and pRB all influence the cellular response to p16.
Since p16 specifically inhibits cyclin D-dependent kinase for which pRB is the primary substrate and since cells without functional pRB are resistant to p16, it has been proposed that inhibition of pRB phosphorylation may be the primary effect of p16 functioning in a linear pathway. If inhibition of cyclin E-Cdk2 were also required in the p16-mediated growth suppression, the cause of growth suppression by p16 may be more complex. Although able to phosphorylate pRB (15), cyclin E-Cdk2 clearly has other important targets. The functions of cyclin D1 become dispensable in pRB-deficient cells (21), but cyclin E is still required (29). p27 can suppress proliferation of cells lacking functional pRB (11, 32, 41). Thus, functions other than those of pRB can be regulated by cyclin E, and their inhibition may be involved in p16-mediated growth suppression.
ACKNOWLEDGMENTS
We thank Hermann Bujard, Manfred Gossen, Ed Harlow, Phil Hinds, Jim Koh, and Sander van den Heuvel for various reagents used in this study and Peter Guida and Anthony Karnezis for critical reading of the manuscript. We also thank David Gebhard of the Einstein Cancer Center Flow Cytometry Facility for assistance with flow cytometry analysis.
H.S.C. is a recipient of a Physician Postdoctoral Fellowship Award from the Howard Hughes Medical Institute. This work was supported by funds from the Albert Einstein College of Medicine and the American Cancer Society Research Project Grant 97-125-01. The Albert Einstein Cancer Center core support is also acknowledged.
REFERENCES
- 1.Alcorta D A, Xiong Y, Phelps D, Hannon G, Beach D, Barrett J C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alevizopoulos K, Vlach J, Hennecke S, Amati B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 1997;16:5322–5333. doi: 10.1093/emboj/16.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 4.Bates S, Parry D, Bonetta L, Vousden K, Dickson C, Peters G. Absence of cyclin D/cdk complexes in cells lacking functional retinoblastoma. Oncogene. 1994;9:1633–1640. [PubMed] [Google Scholar]
- 5.Blain S W, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1996;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- 6.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray-Bablin J, Zalvide J, Fox M P, Knickerbocker C J, DeCaprio J A, Keyomarsi K. Cyclin E, a redundant cyclin in breast cancer. Proc Natl Acad Sci USA. 1996;93:15215–15220. doi: 10.1073/pnas.93.26.15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan K-L, Jenkins C W, Li Y, Nichols M A, Wu X, O’Keefe C L, Matera A G, Xiong Y. Growth suppression by p18, a p16INK4/MTS1 and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 9.Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67–108. doi: 10.1016/s0065-230x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 10.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper J W, Elledge S J, Keyomarsi K, Dynlacht B, Tsai L-H, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, Fox M P, Wei N. Inhibition of cyclin-dependent kinase by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrera R E, Sah V P, Williams B O, Makela T P, Weinberg R A, Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996;16:2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987;329:219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- 14.Hinds P W, Dowdy S F, Eaton E N, Arnold A, Weinberg R A. Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci USA. 1994;91:709–713. doi: 10.1073/pnas.91.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 16.Hirai H, Roussel M F, Kato J-Y, Ashmun R A, Sherr C J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamb A, Gruis N A, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian S V X, Stockert E, Day R S R, Johnson B E, Skolnick M H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 18.Koh J, Enders G H, Dynlacht B D, Harlow E. Tumor-derived p16 alleles encoding proteins defective in cell cycle inhibition. Nature. 1995;375:506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- 19.Latham K M, Eastman S W, Wong A, Hinds P W. Inhibition of p53-mediated growth arrest by overexpression of cyclin-dependent kinases. Mol Cell Biol. 1996;16:4445–4455. doi: 10.1128/mcb.16.8.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovec H, Sewing A, Lucibello F C, Müller R, Möröy T. Oncogenic activity of cyclin D1 revealed through cooperation with Ha-ras: link between cell cycle control and malignant transformation. Oncogene. 1994;9:323–326. [PubMed] [Google Scholar]
- 21.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 23.Lukas J, Parry D, Aagaard L, Mann D J, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 24.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medema R H, Herrera R E, Lam F, Weinberg R A. Growth suppression by p16ink4 required functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobori T, Miura K, Wu D J, Lois A, Takabayashi K, Carson D A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 29.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parry D, Bates S, Mann D J, Peters G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumor suppressor gene product. EMBO J. 1995;14:503–511. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Planas-Silva M D, Weinberg R A. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 33.Poon R Y C, Toyoshima H, Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin-CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell. 1995;6:1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynisdóttir I, Massagué J. The subcellular locations of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 35.Reynisdóttir I, Polyak K, Iavarone A, Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 36.Serrano M, Hannon G J, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 37.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 38.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 39.Shiyanov P, Bagchi S, Adami G, Kokontis J, Hay N, Arroyo M, Morozov A, Raychaudhuri P. p21 disrupts the interaction between cdk2 and the E2F-p130 complex. Mol Cell Biol. 1996;16:737–744. doi: 10.1128/mcb.16.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soos T J, Kiyokawa H, Yan J S, Rubin M S, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7:135–146. [PubMed] [Google Scholar]
- 41.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 42.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 43.Wang T C, Cardiff R D, Zukerberg L, Lees E, Arnold A, Schmidt E V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 45.Wong H, Riabowol K. Differential CDK-inhibitor gene expression in aging human diploid fibroblasts. Exp Gerontol. 1996;31:311–325. doi: 10.1016/0531-5565(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 46.Xiong Y, Zhang H, Beach D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993;7:1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]
- 47.Zhu L, Harlow E, Dynlacht B D. p107 uses a p21CIP1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes Dev. 1995;9:1740–1752. doi: 10.1101/gad.9.14.1740. [DOI] [PubMed] [Google Scholar]