Abstract
Purpose of the review:
Pompe disease is a rare, inherited, devastating condition that causes progressive weakness, cardiomyopathy and neuromotor disease due to the accumulation of glycogen in striated and smooth muscle, as well as neurons. While enzyme replacement therapy has dramatically changed the outcome of patients with the disease, this strategy has several limitations. Gene therapy in Pompe disease constitutes an attractive approach due to the multisystem aspects of the disease and need to address the central nervous system manifestations. This review highlights the recent work in this field, including methods, progress, shortcomings, and future directions.
Recent findings:
Recombinant adeno-associated virus (rAAV) and lentiviral vectors (LV) are well studied platforms for gene therapy in Pompe disease. These products can be further adapted for safe and efficient administration with concomitant immunosuppression, with the modification of specific receptors or codon optimization. rAAV has been studied in multiple clinical trials demonstrating safety and tolerability.
Summary:
Gene therapy for the treatment of patients with Pompe disease is feasible and offers an opportunity to fully correct the principal pathology leading to cellular glycogen accumulation. Further work is needed to overcome the limitations related to vector production, immunologic reactions and redosing.
Keywords: Pompe disease, gene therapy, adeno-associated virus, lentivirus
INTRODUCTION
Pompe disease is an autosomal recessive condition secondary to mutations in the acid-α-glucosidase (GAA) gene, responsible for lysosomal glycogen degradation[1]. Pompe disease has a predicted genetic prevalence of ~1:10,000–30,000 based on newborn screening data but historically this ranged between 1:35,000 and 1:138,000, with a carrier frequency of 1:77[2, 3]. The disease results in pathologic accumulation of glycogen primarily in cardiac, skeletal and smooth muscle, and it was once considered a muscle disease, however, there is growing evidence of the impact in endothelial cells and motor neurons with glycogen deposition in the central nervous system (CNS), progressive neuro-degeneration, vasculopathy and cognitive impairment, highlighting its multisystemic impact[4–6]. Similarly, the commonly seen respiratory dysfunction is caused by disruption of the proximal and distal airways structure and function, in addition to the well described muscular weakness[4, 7, 8]. Pompe disease is classified as infantile onset (IOPD) and late onset (LOPD)[9]. Classical IOPD is characterized by cardiomegaly, hypotonia and cardiorespiratory failure during the first year of life, and non-classical IOPD has a less severe phenotype[9, 10]. LOPD is subdivided into childhood or juvenile, and adult-onset, both characterized by myopathy that presents later in life and the lack of cardiomyopathy[9, 11].
Enzyme replacement therapy (ERT) was approved by the Food and Drug Administration (FDA) in 2006. The process of ERT involves intravenous administration of recombinant human alglucosidase alfa (rhGAA)[12, 13]. Early ERT impacts cardiomyopathy in IOPD patients but they continue to present skeletal and smooth muscle dysfunction with associated weakness[14]. rhGAA has limited efficiency due to preferential uptake of rhGAA by the liver, suboptimal binding of mannose-6-phosphate (M6P) to the cation-independent M6P receptor (CI-M6PR) and abnormal trafficking of M6P in the lysosomes[15]. Avalglucosidase-alpha, a synthetic oligosaccharide that includes M6P residues linked to rhGAA to enhance the affinity for CI-M6PR, was approved in 2021 for LOPD, with ongoing trials in IOPD[16–18]. Another approach is the use of small molecule chaperones to improve the bioavailability of rhGAA. Chaperones assist in the folding of rhGAA to prevent premature degradation, while retaining enzyme activity[19]. Cipaglucosidase alfa + miglustat uses this approach. A phase III clinical trial in LOPD patients concluded that the combination was not superior to standard ERT based on 6-minute-walk-test[20].
In addition to the aforementioned limitations, ERT requires biweekly infusions, does not cross the blood brain barrier (BBB) and can result in immunological reactions towards rhGAA[21–23]. Therefore, there is a need for novel interventions for patients with Pompe disease. Herein, we discuss the recent advancements in the field of gene therapy, as well as other recent strategies.
GENE THERAPY FOR POMPE DISEASE
A functional gene is introduced to substitute for the mutated gene, enabling endogenous production of GAA, which then undergoes natural posttranslational modifications for efficient trafficking to the lysosome[24]. It is crucial to target the right cells by selecting an appropriate vector capsid serotype, promoter, and route of administration[25].
1. Adeno-associated viral vectors in Pompe disease
Adequate selection of the gene promoter and adeno-associated virus (AAV) serotype is key in developing a gene therapy product. Some studies focus on the skeletal and cardiac symptoms of the disease using ubiquitous promoters, such as human cytomegalovirus (CMV) or the hybrid CMV enhancer/chicken β-actin (CBA). CMV and CBA promoters offer quick, strong, and long-lasting expression throughout the body[26, 27], however expression of GAA in other cell types, such as antigen presenting cells can cause adverse events. Conversely, a variety of muscle specific promoters are utilized to restrict expression to striated muscle, showing minimal expression in non-muscle tissues and potentially reducing the immune response to the transgene[28]. The desmin promoter is expressed in all cells with intermediate filaments and provides selective expression in motor neurons, skeletal and cardiac muscles. The synapsin promoter targets principally neurons and the liver-muscle promoter (LiMP) provides expression in non-dividing muscle cells and hepatic tissues[27, 29, 30].
1.1. Intravenous AAV-derived gene therapy
Several studies have tested AAV serotypes with increased muscle tropism to target and correct pathology in Pompe disease, either intravenously or directly into the muscle[29–49]. A single intravenous injection of an rAAV1-GAA vector restored GAA activity in the Pompe mouse model and exhibited a long-lasting corrective effect on cardiac and skeletal muscle resulting in improvement in force mechanics of the soleus and diaphragm[32]. In a separate study, an injection of rAAV-PHP.B, a rAAV9 variant, resulted in therapeutic levels of GAA activity while decreasing glycogen in skeletal and myocardial muscles resulting in improved gait and reduced peripheral neuropathy[50].
AAVB1-DES-hGAA, a vector with high affinity for muscle and CNS, and AAV9-DES-hGAA were utilized in Gaa−/− mice. AAVB1 treatment resulted in weight gain, forelimb strength, and higher levels of transduction in the diaphragm, tongue base, and thoracic spinal cord when compared to AAV9. Both groups displayed above-average GAA activity and reduced glycogen accumulation in the heart, diaphragm, tongue, gastrocnemius, and lung[45].
A study in mice and non-human primates using AT845, an AAV8 vector where GAA expression is driven by the murine muscle creatine kinase (MCK) promoter/enhancer and expressing a codon-optimized GAA, resulted in high enzyme levels, leading to dose dependent functional improvements and glycogen clearance. Unfortunately, higher doses in cynomolgus macaques led to immune responses and cardiac abnormalities requiring euthanasia. It was later determined that the immune responses were largely due to a xenogenic anti-GAA immune response[38**]. AT845, was used in a phase 1/2 trial in four LOPD patients, three subjects discontinued ERT and functional outcomes were stable 51 weeks later[51]. The study was recently on clinical hold due to neuropathy in one subject[52, 53].
A highly potent AAV variant, AAV.cc47 was recently tested in Gaa−/− mice. Mice received intravenous 1.3e14vg/kg with a single stranded genome encoding GAA driven by the CBh promoter. This resulted in GAA levels of 67% of wild type mice, compared to 26% in AAV9-GAA treated mice[46*].
Eleven rhesus macaques received AAVhu68 tagged with an insulin-like growth factor 2 variant (vIGF2) peptide to increase uptake (AAVhu68-vIGF2-hGAA). Five animals had immune responses, including dose independent T-cell-mediated myocarditis. Toxicity was associated with a major histocompatibility complex class I haplotype in three animals[47**].
Remarkably, the liver can secrete high levels of engineered proteins and can serve as a depot for secretion of rhGAA[54]. The liver can promote immune tolerance and help prevent immune reactions caused by transgene products, provided the gene is expressed only in liver cells[55]. AAV8 vectors in pre-clinical studies have shown GAA expression and secretion through hepatocytes, with evidence of reduced muscle glycogen content and improved functional tests in mice[29, 36, 56–59]. A study in a canine model demonstrated persistent correction of GAA activity two years after concurrent systemic- and liver-targeted vector delivery of rAAV9-DES-hGAA and rAAV9-LSPcoGAA in association with anti-thymocyte globulin and methylprednisolone. This strategy supports the use of dual vector to achieve GAA tolerance[60*].
Two current clinical trials are focused on liver-directed gene therapy by creating a liver depot for GAA production[61, 62]. The trial by SPARK therapeutics uses an AAVRh74 derived capsid in a phase 1/2 trial. (NCT04093349). Smith et al showed the preliminary results in three subjects receiving AAV8-LSPhGAA at 1.6×1012vg/kg. The authors demonstrated safety, however, the lack of glycogen lowering suggested that despite increased GAA activity in skeletal muscle, the efficacy was not sufficient to replace ERT[61**].
1.2. Central nervous system delivery of AAV-derived gene therapy
While intravenous administration of AAV leads to widespread gene transfer in the neonatal CNS, this approach is not easily translatable to LOPD since it would require high vector doses to treat the CNS manifestations[63, 64]. Intrathecal, intra-cisterna (ICM), and intracerebroventricular (ICV) routes are considered to treat these manifestations[65].
A single intrathecal dose of AAV9-CAG-hGAA or AAVrh10-CAG-hGAA to Gaa−/− mice led to low glycogen levels in the CNS and partial improvement of muscle strength but no changes in muscle glycogen. Furthermore, AAV9 treatment restored cardiac GAA levels and improved cardiomyopathy[66]. Intrathecal administration of AAV5-GAA at the C3-C4 level in Gaa−/− mice to target the phrenic nerve nucleus area decreased intraneuronal glycogen content and improved ventilation, even without enzymatic activity in the diaphragm[67].
1.3. Other AAV-derived gene therapy approaches
Intrapleural rAAV9-GAA to Gaa−/− mice resulted in improvement of ejection fraction in cardiac magnetic resonance, greater relative inspiratory burst amplitude during baseline conditions, and increased efferent phrenic burst amplitude. The effects were achieved by retrograde transport to motoneurons[42].
Intra-diaphragmatic administration of rAAV1-CMV-hGAA in nine IOPD subjects at two different doses (1.0×1012 and 5.0×1012vg) demonstrated no adverse events related to the product. Anti-capsid and anti-transgene antibody response was observed in all, except for those who received concomitant immunomodulation with sirolimus and rituximab[34]. Subjects from this cohort participated in inspiratory muscle conditioning demonstrating benefits to diaphragmatic function, particularly in subjects with higher neuromuscular function[68].
Recently, an AAV9 product encoding an excitatory Designer receptor exclusively activated by designer drugs (DREADD) (AAV9-hSyn-hM3D(gq)-mCherry) was injected to the posterior tongue of GAA−/− mice. Lingual electromyography (EMG) showed significant increases in tonic and phasic inspiratory activity after DREADD administration. mCherry expression was confirmed in hypoglossal motoneurons in all mice, confirming retrograde movement of AAV9. This approach could address dysphagia, dysarthria and sleep disordered breathing in patients with Pompe disease[69*].
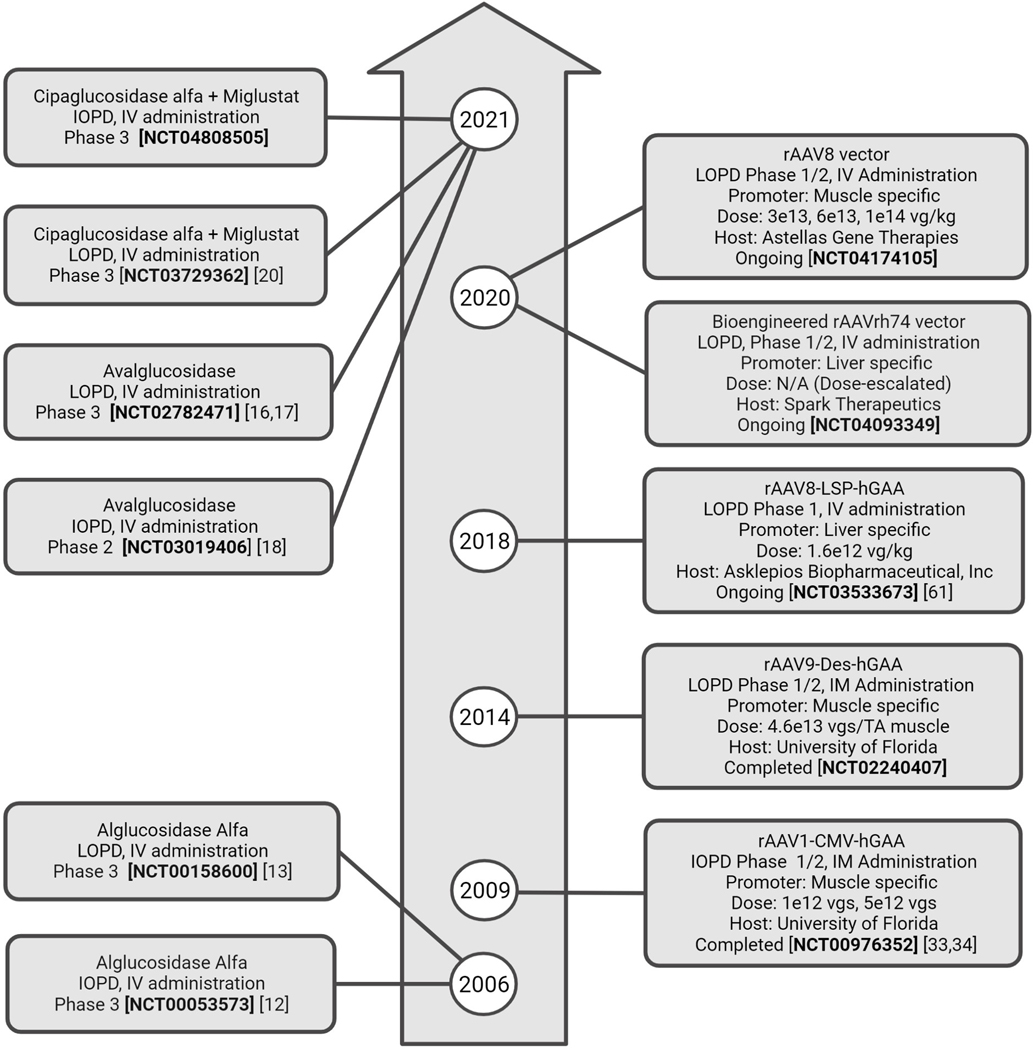
Figure 1 lists the most relevant ERT and AAV clinical trials to date.
Figure 1.
Timeline of the most relevant clinical trials using enzyme replacement therapy (left) and Adeno-associated viral vectors (right). IOPD: Infantile-onset Pompe disease. LOPD: Late-onset Pompe disease. IV: intravenous. IM: Intramuscular. TA: tibialis anterior. vgs: vector genomes
2. Lentiviral vectors in Pompe disease
Hematopoietic stem and progenitor cell (HSPC) mediated lentiviral gene therapy (HSPC-LVGT) is an attractive approach for the treatment of Pompe disease. The method involves transplantation of ex-vivo gene-modified autologous HSPCs to overexpress the needed transgene[70–73]. HSPC-LVGT has been used in other disorders, including B-thalassemia, Wiskott-Aldrich syndrome, and adrenoleukodystrophy[74–76].
HSPC-LVGT demonstrated long-term engraftment and continuous supply of GAA after one intervention in Gaa−/− mice with improvement of cardiac and motor function. However, it did not achieve glycogen reduction to normal levels and required a high vector copy number (VCN), which can increase the genotoxicity risk as previously seen with early design gammaretroviral vectors[77, 78]. Currently, third generation self-inactivating LV vectors are used to decrease these risks[79]. Attention has been drawn to the modification of the LV vector to improve receptor affinity, like in the case of IGF2[80]. Liang et al. created a vector that contains a codon-optimized GAA sequence fused to codon-optimized human IGF2 (LV-IGF2.GAAco) leading to correction of glycogen accumulation, autophagy, motor function and brain glycogen content at a much lower VCN[80*]. A similar approach has been used in other preclinical studies to create engineered GAA coding sequences, distinct peptide tags and codon optimizations. The use of LVGT with glycosylation-independent lysosomal targeting tags increased secretion and reduced glycogen, myofiber and CNS vacuolation in tissues, but maintained low GAA enzyme activity[81**].
Moreover, HSPC-LVGT can limit immunoglobin G (IgG) responses through tolerance induction against the transgene product—one of the key benefits of this technique[71]. It can also allow for complementary ERT, resulting in enhanced glycogen elimination from skeletal and cardiac muscles. In this approach, the existence of GAA-expressing HSPC-derived cells in the thymus indicated the establishment of central immune tolerance[82].
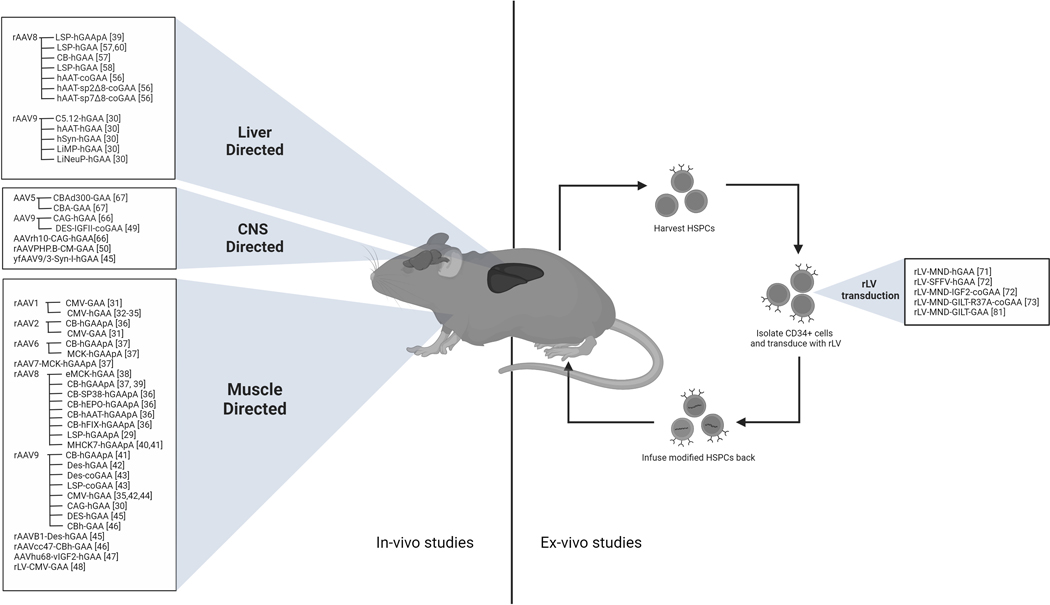
Figure 2 summarizes the AAV and LV-derived products based on tissue tropism.
Figure 2.
Summary of all in-vivo and ex-vivo vectors and promoters reported based on tissue tropism. CNS: Central nervous system. HSPC: Hematopoietic stem and progenitor cell
Challenges and potential solutions in gene therapy
1. Vector production
rAAV production methods are distinguished by cell line type, substrate for cell growth and precursor materials, however, these are often not suitable for large scale production. Chemical transient transfection is popular due to simplicity and availability of raw materials but has limited yield per cell and requires good manufacturing process (GMP) grade plasmids[83–85]. New technology has emerged to scale transient transfections, including packed bed reactors[86–88], conversion to suspension HEK293 lines[89], AAV process intensification[90*, 91*] and the use of novel chemical additives as transfection reagents [84, 89, 92-96*]. Non-plasmid DNA sources such as doggybone DNA and novel transfection reagents could facilitate manufacturing significantly, along with the use of certain chemical additives to boost rAAV production[95, 97–99].
Viral infection platforms, using insect baculovirus (rBV) or Human Herpes Simplex Type I virus (rHSV1) offer high yields per cell and are used in clinical trials[100, 101]. rHSV1 coinfection modifies AAV genome-end recombination, emphasizing the need for improvement of rHSV-1 production[102*]. The rBV system’s limitations include loss of AAV particle infectivity, insect virus contamination of cell lines, differences in post-translational modifications of the final product, which produce a negative impact on potency [101, 103] Meanwhile, the rHSV system appears more versatile in producing highly infectious AAV regardless of serotype[92, 93, 104].
Packaging/producer cell lines (PCL) are unique systems that stably incorporate viral and cargo genes into the cell lines prior to GMP manufacturing. Advantages of PCLs include scalability to 2,000L, compatibility with existing biologics production infrastructure, and consistent batch-to-batch performance[105–107]. However, PCLs require an established cell line development effort, and require wild-type Ad propagation as a process-related impurity[105–107].
rAAV manufacturing related impurities can result in significant immunogenicity. Research is needed to ensure effective development of rAAV vectors, while minimizing process and product derived impurities and ensuring safety[91, 93].
2. Immune responses to AAV, and vector re-administration
One major obstacle for successful gene therapy is the immunological response to the vector capsid and transgene product affecting safety and duration of effect[108]. These reactions can involve innate, cellular and humoral responses[109, 110*].
AAV re-administration has been an important concern especially for IOPD since transgene expression is expected to decrease due to somatic growth and vector dilution[111]. Several strategies to prevent anti-AAV antibody production and to allow for redosing have been proposed including plasmapheresis or antibody cleavage[85, 112]. Only one approach has been tested clinically and consisted in the use of sirolimus and rituximab to demonstrate that two consecutive intramuscular administrations of AAV vectors is possible (NCT02240407)[62].
OTHER TREATMENT APPROACHES
Multiple additional approaches have been reported to date[16, 20, 113–119] Recently, progress has been made in the following:
1. In-utero ERT:
A fetus with CRIM-negative IOPD received in-utero ERT starting at 24 weeks of gestation, postnatal immune tolerance was started and ERT continued. The child had normal cardiac and motor function at 13 months of age[120**].
2. Glycogen-synthase-1 inhibitors:
Glycogen production is regulated by glycogen synthase (GYS1), which can be inhibited by the phosphorylation of S641 and S645 through the mTORC1 pathway[121, 122]. A recent phase I/II clinical trial (NCT05249621) evaluated an oral GYS-inhibitor in healthy subjects and showed good tolerance and reduced glycogen in peripheral blood mononuclear cells[123].
3. Fusion proteins for targeted delivery of GAA:
These retain enzymatic function and bind to effector proteins that traffic to the lysosome. This approach was done using CD63 and Integrin-subunit-alpha 7 (ITGA7). α-hCD631IgG:GAA and α-ITGA7IgG:GAA internalized in a CI-M6PR independent mechanism with the former being more effective. Similar findings happened when using a single-chain fragment variable (scFv) instead of IgG. In a second step, the same study used AAV2/8 viruses encoding α-hCD631scFv:GAA driven by a liver-specific promoter and showed higher GAA levels compared to AAV-GAA treated mice[124*]. A similar strategy was used to create VAL-1221, a fusion protein comprising the Fab portion of a cell penetrating-antibody and rhGAA, tested in a 3-month phase I/II study in 12 adults with LOPD. The study showed dose dependent improvements but no significant changes in PD markers[125].
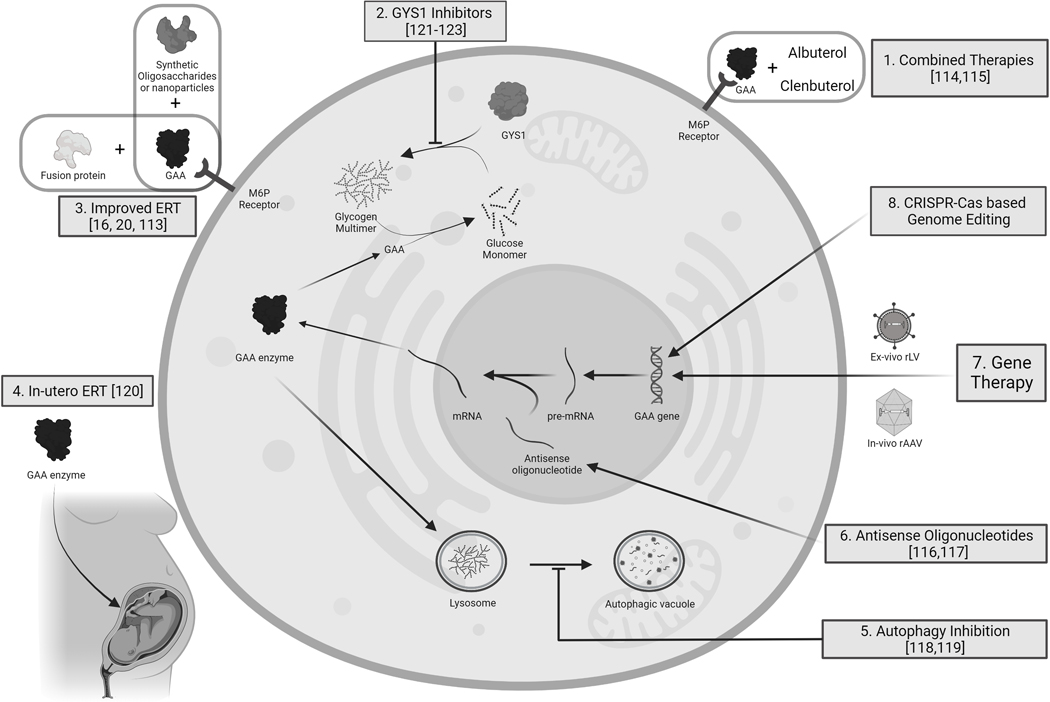
Figure 3 summarizes all possible strategies in PD treatment
Figure 3.
Preclinical and clinical strategies to treat Pompe disease. ERT: enzyme replacement therapy, GAA: acid alfa glucosidase. M6P: Mannose-6-Phosphate. GYS 1: Glycogen synthase 1
CONCLUSIONS
ERT significantly improves the outcomes of patients with Pompe disease. ERT targets cardiomyopathy and skeletal muscle pathology but has limited effect in other tissues. Gene therapy strategies have the potential to treat multiple systems, including the CNS. Newer viral vectors allow for enhanced expression, specificity and increased enzyme activity based on preclinical and clinical studies. However, gene therapy continues to face major challenges related to vector production, immunological response and redosing.
Key Points.
Gene therapy for Pompe Disease uses primarily Adeno-associated viral vectors and lentiviral vectors
Recombinant viral vector production has multiple challenges
Redosing of gene therapy in Pompe disease is limited by immunological responses
Financial support and sponsorship
This work was supported by the following grants: R01 HL139708–02 and U01-NS116752–01A1(MC, BJB). R35 NS116824 (MSG) and P01 NS097197 (MSG). NIH grant 1R01AG066653, 1R01AG078702, 1R01CA266004, V-Scholar Grant (RCS).
Footnotes
Conflicts of interest
RCS has received research support and received consultancy fees from Maze Therapeutics and is a member of the Medical Advisory Board for Little Warrior Foundation. MSG has research support, research compounds, or consultancy fees from Maze Therapeutics, Valerion Therapeutics, Ionis Pharmaceuticals, PTC Therapeutics, and the Glut1-Deficiency Syndrome Foundation. RCS and MSG are co-founders of Attrogen LLC. MC has received research support from Sanofi, Friedreich Ataxia Research Alliance (FARA), Amicus, AavantiBio, Lacerta, Provention Bio, Sarepta, Duchenne Research Fund, Muscular Dystrophy Association (MDA), GoFAR, Cydan, Audentes. MC has received consulting fees from AavantiBio, Reata, Lilly, Avexis and Gilbert foundation, SwanBio and PCT Therapeutics. BJB has received research support from SolidBio, ProventionBio, Barth Syndrome Foundation. BJB has received consulting fees from AavantiBio, Amicus Therapeutics, Rocket Pharma, Pfizer, Sanofi, and Sarepta Therapeutics. MC and BB are co-founders of Ventura, LLC.
REFERENCES
- 1.Kohler L, Puertollano R, Raben N. Pompe Disease: From Basic Science to Therapy. Neurotherapeutics, 2018. 15(4): p. 928–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zern SC, Marshall WJ, Shewokis PA, Vest MT. Use of simulation as a needs assessment to develop a focused team leader training curriculum for resuscitation teams. Adv Simul (Lond), 2020. 5: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fatehi F, Ashrafi MR, Babaee M, et al. Recommendations for Infantile-Onset and Late-Onset Pompe Disease: An Iranian Consensus. Front Neurol, 2021. 12: p. 739931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuller DD, Trejo-Lopez JA, Yachnis AT, et al. Case Studies in Neuroscience: Neuropathology and diaphragm dysfunction in ventilatory failure from late-onset Pompe disease. J Neurophysiol, 2021. 126(2): p. 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne BJ, Fuller DD, Smith BK, et al. Pompe disease gene therapy: neural manifestations require consideration of CNS directed therapy. Ann Transl Med, 2019. 7(13): p. 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korlimarla A, Lim JA, Kishnani PS, Sun B. An emerging phenotype of central nervous system involvement in Pompe disease: from bench to bedside and beyond. Ann Transl Med, 2019. 7(13): p. 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith BK, Allen S, Mays S, et al. , Dynamic respiratory muscle function in late-onset Pompe disease. Sci Rep, 2019. 9(1): p. 19006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller DD, ElMallah MK, Smith BK, et al. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol, 2013. 189(2): p. 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishnani PS, Steiner RD, Bali D, et al. Pompe disease diagnosis and management guideline. Genet Med, 2006. 8(5): p. 267–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien YH, Lee NC, Thurberg BL, et al. Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics, 2009. 124(6): p. e1116–25. [DOI] [PubMed] [Google Scholar]
- 11.Teener W Late-onset Pompe’s disease. Semin Neurol, 2012. 32(5): p. 506–11. [DOI] [PubMed] [Google Scholar]
- 12.Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology, 2007. 68(2): p. 99–109. [DOI] [PubMed] [Google Scholar]
- 13.van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med, 2010. 362(15): p. 1396–406. [DOI] [PubMed] [Google Scholar]
- 14.Scheffers LE, Kok R, van den Berg LE, et al. Effects of enzyme replacement therapy on cardiac function in classic infantile Pompe disease. Int J Cardiol, 2023. [DOI] [PubMed] [Google Scholar]
- 15.Cardone M, Porto C, Tarallo A, et al. Abnormal mannose-6-phosphate receptor trafficking impairs recombinant alpha-glucosidase uptake in Pompe disease fibroblasts. Pathogenetics, 2008. 1(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz-Manera J, Kishnani PS, Kushlaf H, et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): a phase 3, randomised, multicentre trial. The Lancet Neurology, 2021. 20(12): p. 1012–1026. [DOI] [PubMed] [Google Scholar]
- 17.Kishnani PS, Diaz-Manera J, Toscano A, et al. Efficacy and Safety of Avalglucosidase Alfa in Patients With Late-Onset Pompe Disease After 97 Weeks: A Phase 3 Randomized Clinical Trial. JAMA Neurol, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishnani PS, Kronn D, Brassier A, et al. Safety and efficacy of avalglucosidase alfa in individuals with infantile-onset Pompe disease enrolled in the phase 2, open-label Mini-COMET study: The 6-month primary analysis report. Genet Med, 2023. 25(2): p. 100328. [DOI] [PubMed] [Google Scholar]
- 19.Parenti G, Fecarotta S, la Marca G, et al. A chaperone enhances blood alpha-glucosidase activity in Pompe disease patients treated with enzyme replacement therapy. Mol Ther, 2014. 22(11): p. 2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoser B, Roberts M, Byrne BJ, et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): an international, randomised, double-blind, parallel-group, phase 3 trial. Lancet Neurol, 2021. 20(12): p. 1027–1037. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi T, Yang HW, Pennybacker M, et al. Clinical and metabolic correction of pompe disease by enzyme therapy in acid maltase-deficient quail. The Journal of Clinical Investigation, 1998. 101(4): p. 827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elder ME, Nayak S, Collins SW, et al. B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr, 2013. 163(3): p. 847–54 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messinger YH, Mendelsohn NJ, Rhead W, et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med, 2012. 14(1): p. 135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoser B, Laforet P. Therapeutic thoroughfares for adults living with Pompe disease. Curr Opin Neurol, 2022. 35(5): p. 645–650. [DOI] [PubMed] [Google Scholar]
- 25.Sun B, Brooks ED, Koeberl DD. Preclinical Development of New Therapy for Glycogen Storage Diseases. Curr Gene Ther, 2015. 15(4): p. 338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray SJ, Foti SB, Schwartz JW, et al. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Gene Ther, 2011. 22(9): p. 1143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacak CA, Sakai Y, Thattaliyath BD, et al. Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet Vaccines Ther, 2008. 6: p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salva MZ, Himeda CL, Tai PW, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther, 2007. 15(2): p. 320–9. [DOI] [PubMed] [Google Scholar]
- 29.Sun B, Bird A, Young SP, et al. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet, 2007. 81(5): p. 1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colella P, Sellier P, Costa Verdera H, et al. AAV Gene Transfer with Tandem Promoter Design Prevents Anti-transgene Immunity and Provides Persistent Efficacy in Neonate Pompe Mice. Mol Ther Methods Clin Dev, 2019. 12: p. 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraites TJ Jr, Schleissing MR, Shanely RA, et al. Correction of the enzymatic and functional deficits in a model of Pompe disease using adeno-associated virus vectors. Mol Ther, 2002. 5(5 Pt 1): p. 571–8. [DOI] [PubMed] [Google Scholar]
- 32.Mah C, Pacak CA, Cresawn KO, et al. Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors. Mol Ther, 2007. 15(3): p. 501–7. [DOI] [PubMed] [Google Scholar]
- 33.Smith BK, Collins SW, Conlon TJ, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther, 2013. 24(6): p. 630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corti M, Liberati C, Smith BK, et al. Safety of Intradiaphragmatic Delivery of Adeno-Associated Virus-Mediated Alpha-Glucosidase (rAAV1-CMV-hGAA) Gene Therapy in Children Affected by Pompe Disease. Hum Gene Ther Clin Dev, 2017. 28(4): p. 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmallah MK, Falk DJ, Nayak S, et al. Sustained Correction of Motoneuron Histopathology Following Intramuscular Delivery of AAV in Pompe Mice. Molecular Therapy, 2014. 22(4): p. 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun B, Zhang H, Benjamin DK Jr, et al. Enhanced Efficacy of an AAV Vector Encoding Chimeric, Highly Secreted Acid α-Glucosidase in Glycogen Storage Disease Type II. Molecular Therapy, 2006. 14(6): p. 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun B, Zhang H, Franco LM, et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther, 2005. 11(6): p. 889–98. [DOI] [PubMed] [Google Scholar]
- 38. Eggers M, Vannoy CH, Huang J, et al. Muscle-directed gene therapy corrects Pompe disease and uncovers species-specific GAA immunogenicity. EMBO Molecular Medicine, 2022. 14(1): p. e13968. ** A preclinical study using AT845, an AAV vector that expresses GAA in skeletal muscle and heart. It showed dose dependent increase in GAA and glycogen clearance in mice. High doses in macaques caused anti GAA immune response and cardiac abnormalities.
- 39.Franco LM, Sun B, Yang X, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther, 2005. 12(5): p. 876–84. [DOI] [PubMed] [Google Scholar]
- 40.Sun B, Li S, Bird A, et al. Antibody formation and mannose-6-phosphate receptor expression impact the efficacy of muscle-specific transgene expression in murine Pompe disease. J Gene Med, 2010. 12(11): p. 881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han SO, Li S, Brooks ED, et al. Enhanced efficacy from gene therapy in Pompe disease using coreceptor blockade. Hum Gene Ther, 2015. 26(1): p. 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falk DJ, Mah CS, Soustek MS, et al. Intrapleural Administration of AAV9 Improves Neural and Cardiorespiratory Function in Pompe Disease. Molecular Therapy, 2013. 21(9): p. 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doerfler PA, Todd AG, Clément N, et al. Copackaged AAV9 Vectors Promote Simultaneous Immune Tolerance and Phenotypic Correction of Pompe Disease. Hum Gene Ther, 2016. 27(1): p. 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todd AG, McElroy JA, Grange RW, et al. Correcting Neuromuscular Deficits With Gene Therapy in Pompe Disease. Ann Neurol, 2015. 78(2): p. 222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keeler AM, Zieger M, Todeasa SH, et al. Systemic Delivery of AAVB1-GAA Clears Glycogen and Prolongs Survival in a Mouse Model of Pompe Disease. Hum Gene Ther, 2019. 30(1): p. 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gonzalez TJ, Simon KE, Blondel LO, et al. Cross-species evolution of a highly potent AAV variant for therapeutic gene transfer and genome editing. Nat Commun, 2022. 13(1): p. 5947. * The article describes the discovery of a cross species compatible variant of AAV (AAV.cc47). Transduction demonstrated in macaques, pigs and mice. Considered better than AAV9. The study also describes improved genome editing packaging CRISPR/Cas9 and improved dystrophin restoration in the mdx mouse model
- 47. Hordeaux J, Ramezani A, Tuske S, et al. Immune transgene-dependent myocarditis in macaques after systemic administration of adeno-associated virus expressing human acid alpha-glucosidase. Front Immunol, 2023. 14: p. 1094279. ** Rhesus macaques received AAVhu68, a clade F AAV related to AAV9, that expresses rhGAA, tagged with an insulin-like growth factor 2 variant (vIGF2) peptide for enhanced cell uptake. Some animals presented a severe immune response to GAA, including T cell mediated myocarditis. Toxicity appeared to be linked o a major histocompatibility complex class I haplotype, highilighting a possible genetic component in the response to AAV
- 48.Kyosen SO, Iizuka S, Kobayashi H, et al. Neonatal gene transfer using lentiviral vector for murine Pompe disease: long-term expression and glycogen reduction. Gene Ther, 2010. 17(4): p. 521–30. [DOI] [PubMed] [Google Scholar]
- 49.Doyle BM, Turner SMF, Sunshine MD, et al. AAV Gene Therapy Utilizing Glycosylation-Independent Lysosomal Targeting Tagged GAA in the Hypoglossal Motor System of Pompe Mice. Mol Ther Methods Clin Dev, 2019. 15: p. 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim JA, Yi H, Gao F, et al. Intravenous Injection of an AAV-PHP.B Vector Encoding Human Acid α-Glucosidase Rescues Both Muscle and CNS Defects in Murine Pompe Disease. Mol Ther Methods Clin Dev, 2019. 12: p. 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Astellas Announces Update on Preliminary Safety and Efficacy Data from FORTIS Study of Investigational AT845 in Adults with Late-Onset Pompe Disease. Available from: https://www.prnewswire.com/news-releases/astellas-announces-update-on-preliminary-safety-and-efficacy-data-from-fortis-study-of-investigational-at845-in-adults-with-late-onset-pompe-disease-301750884.html.
- 52.Astellas Announces FDA Update on the FORTIS Clinical Trial of AT845 in Adults with Late-Onset Pompe Disease. 2022; Available from: https://www.astellas.com/en/news/25956.
- 53.Astellas Announces Hold Lifted by FDA on FORTIS Clinical Trial of AT845 Investigational Treatment for Adult Patients with Late-Onset Pompe Disease. 2023; Available from: https://www.astellas.com/en/news/26931.
- 54.Mingozzi F, Liu YL, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest, 2003. 111(9): p. 1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiegs G, Lohse AW. Immune tolerance: What is unique about the liver. Journal of Autoimmunity, 2010. 34(1): p. 1–6. [DOI] [PubMed] [Google Scholar]
- 56.Ziegler RJ, Bercury SD, Fidler J, et al. Ability of Adeno-Associated Virus Serotype 8-Mediated Hepatic Expression of Acid α-Glucosidase to Correct the Biochemical and Motor Function Deficits of Presymptomatic and Symptomatic Pompe Mice. Human Gene Therapy, 2008. 19(6): p. 609–621. [DOI] [PubMed] [Google Scholar]
- 57.Puzzo F, Colella P, Biferi MG, et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid a-glucosidase. Science Translational Medicine, 2017. 9(418): p. eaam6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang P, Sun B, Osada T, et al. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine pompe disease. Hum Gene Ther, 2012. 23(5): p. 460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han SO, Ronzitti G, Arnson B, et al. Low-Dose Liver-Targeted Gene Therapy for Pompe Disease Enhances Therapeutic Efficacy of ERT via Immune Tolerance Induction. Mol Ther Methods Clin Dev, 2017. 4: p. 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pope MK, Coleman K, Wichman M, et al. Assessment of Gene Therapy Treatment in the Pompe Disease Canine Model. Molecular Therapy Vol 30 No 4S1, April 2022, 2022. *Abstract publication of treatment of a canine model with PD with dual vector administration, along with immunosuppression, achieving GAA tolerance and adequate functional outcomes.
- 61. Smith EC, Hopkins S, Case LE, et al. Phase I study of liver depot gene therapy in late-onset Pompe disease. Mol Ther, 2023. Feb 18:S1525–0016(23)00077. ** Results of a phase 1 clinical trial in four subjects with LOPD that received AAV8-LSPhGAA. Subjects were able to stop ERT, although glycogen content was unchanged in 2 of 3 subjects.
- 62.A gene transfer study for Late onset Pompe disease (RESOLUTE) Available from: https://clinicaltrials.gov/ct2/show/NCT02240407.
- 63.Gombash SE, Cowley CJ, Fitzgerald JA, et al. Intravenous AAV9 efficiently transduces myenteric neurons in neonate and juvenile mice. Front Mol Neurosci, 2014. 7: p. 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foust KD, Nurre E, Montgomery CL, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol, 2009. 27(1): p. 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salabarria SM, Nair J, Clement N, et al. Advancements in AAV-mediated Gene Therapy for Pompe Disease. Journal of Neuromuscular Diseases, 2020. 7: p. 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hordeaux J, Dubreil L, Robveille C, et al. Long-term neurologic and cardiac correction by intrathecal gene therapy in Pompe disease. Acta Neuropathologica Communications, 2017. 5(1): p. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu K, Falk DJ, Reier PJ, et al. Spinal Delivery of AAV Vector Restores Enzyme Activity and Increases Ventilation in Pompe Mice. Molecular Therapy, 2012. 20(1): p. 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith BK, Martin AD, Lawson LA, et al. Inspiratory muscle conditioning exercise and diaphragm gene therapy in Pompe disease: Clinical evidence of respiratory plasticity. Exp Neurol, 2017. 287(Pt 2): p. 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Singer ML, Rana S, Benevides ES, et al. Chemogenetic activation of hypoglossal motoneurons in a mouse model of Pompe disease. J Neurophysiol, 2022. 128(5): p. 1133–1142 *Preclinical study in Gaa−/− mice. Mice received AAV9 encoding an excitatory designer receptors exclusively activated by designer drugs (DREADDs), administered to the posterior tongue of mice. Transgene expression was confirmed in hypoglossal motoneurons. This can address dysphagia, dysarthria, sleep disordered breathing.
- 70.Biffi A Hematopoietic Stem Cell Gene Therapy for Storage Disease: Current and New Indications. Mol Ther, 2017. 25(5): p. 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Douillard-Guilloux G, Richard E, Batista L, Caillaud C. Partial phenotypic correction and immune tolerance induction to enzyme replacement therapy after hematopoietic stem cell gene transfer of alpha-glucosidase in Pompe disease. J Gene Med, 2009. 11(4): p. 279–87. [DOI] [PubMed] [Google Scholar]
- 72.van Til NP, Stok M, Aerts Kaya FS, et al. Lentiviral gene therapy of murine hematopoietic stem cells ameliorates the Pompe disease phenotype. Blood, 2010. 115(26): p. 5329–37. [DOI] [PubMed] [Google Scholar]
- 73.Kan SH, Aoyagi-Scharber M, Le SQ, et al. Delivery of an enzyme-IGFII fusion protein to the mouse brain is therapeutic for mucopolysaccharidosis type IIIB. Proc Natl Acad Sci U S A, 2014. 111(41): p. 14870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eichler F, Duncan C, Musolino PL, et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N Engl J Med, 2017. 377(17): p. 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson AA, Walters MC, Kwiatkowski J, et al. Gene Therapy in Patients with Transfusion-Dependent beta-Thalassemia. N Engl J Med, 2018. 378(16): p. 1479–1493. [DOI] [PubMed] [Google Scholar]
- 76.Ferrua F, Cicalese MP, Galimberti S, et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol, 2019. 6(5): p. e239–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stok M, de Boer H, Huston MW, et al. Lentiviral Hematopoietic Stem Cell Gene Therapy Corrects Murine Pompe Disease. Mol Ther Methods Clin Dev, 2020. 17: p. 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braun CJ, Witzel M, Paruzynski A, et al. Gene therapy for Wiskott-Aldrich Syndrome-Long-term reconstitution and clinical benefits, but increased risk for leukemogenesis. Rare Dis, 2014. 2(1): p. e947749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zufferey R, Dull T, Mandel RJ, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol, 1998. 72(12): p. 9873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liang Q, et al. , IGF2-tagging of GAA promotes full correction of murine Pompe disease at a clinically relevant dosage of lentiviral gene therapy. Mol Ther Methods Clin Dev, 2022. 27: p. 109–130. *Preclinical study in Gaa−/− mice using a LV vector. Insulin-like growth factor 2 (IGF2) was fused to a codon-optimized version of GAA (LV-IGF2.GAAco) to improve cellular uptake. The study demonstrates how this approach was superior to LV-GAAco at normalizing glycogen levels, pathology and autophagy
- 81. Dogan Y, et al. , Screening chimeric GAA variants in preclinical study results in hematopoietic stem cell gene therapy candidate vectors for Pompe disease. Mol Ther Methods Clin Dev, 2022. 27: p. 464–487 **Preclinical study in mice. 9 different engineered GAA coding sequences with distinct peptide tags and codon optimization were tested. LV Vectors with glycosylation-independent lysosomal targeting tags enhanced secretion and improved reduction of glycogen, myofiber, and CNS, but with lower GAA activity.
- 82.Liang Q, Catalano F, Vlaar EC, et al. Lentiviral gene therapy prevents anti-human acid α-glucosidase antibody formation in murine Pompe disease. Mol Ther Methods Clin Dev, 2022. 25: p. 520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lock M, Alvira M, Vandenberghe LH, et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Human gene therapy, 2010. 21(10): p. 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rashnonejad A, Chermahini GA, Li S, et al. Large-scale production of adeno-associated viral vector serotype-9 carrying the human survival motor neuron gene. Molecular biotechnology, 2016. 58: p. 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bertin B, Veron P, Leborgne C, et al. Capsid-specific removal of circulating antibodies to adeno-associated virus vectors. Sci Rep, 2020. 10(1): p. 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lesch HP, Heikkilä KM, Lipponen EM, et al. Process development of adenoviral vector production in fixed bed bioreactor: from bench to commercial scale. Human gene therapy, 2015. 26(8): p. 560–571. [DOI] [PubMed] [Google Scholar]
- 87.Powers AD, Piras BA, Clark RK, et al. Development and optimization of AAV hFIX particles by transient transfection in an iCELLis® fixed-bed bioreactor. Human gene therapy methods, 2016. 27(3): p. 112–121. [DOI] [PubMed] [Google Scholar]
- 88.Emmerling VV, Pegel A, Milian EG, et al. Rational plasmid design and bioprocess optimization to enhance recombinant adeno‐associated virus (AAV) productivity in mammalian cells. Biotechnology journal, 2016. 11(2): p. 290–297. [DOI] [PubMed] [Google Scholar]
- 89.Grieger JC, Soltys SM, Samulski RJ. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Molecular Therapy, 2016. 24(2): p. 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mendes JP, Fernandes B, Pineda E, et al. AAV process intensification by perfusion bioreaction and integrated clarification. Front Bioeng Biotechnol, 2022. 10: p. 1020174. *The study shows that alternating tangential flow and tangential flow depth filtration techniques can help in he production and harvest of AAV, simplifying the process and improving efficiency.
- 91. Guan JS, Chen K, Si Y, et al. Process improvement of adeno-associated virus (AAV) production. Front Chem Eng, 2022. 4:830421. * To support large scale preclinical animal studies in research laboratories, the study compared multiple production platforms and optimized a scalable suspension manufacturing process for AAV.
- 92.Yu C, Trivedi PD, Chaudhuri P, et al. NaCl and KCl mediate log increase in AAV vector particles and infectious titers in a specific/timely manner with the HSV platform. Mol Ther Methods Clin Dev, 2021. 21: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trivedi PD, Yu C, Chaudhuri P, et al. Comparison of highly pure rAAV9 vector stocks produced in suspension by PEI transfection or HSV infection reveals striking quantitative and qualitative differences. Mol Ther Methods Clin Dev, 2022. 24: p. 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Loo JC, Wright JF. Progress and challenges in viral vector manufacturing. Human molecular genetics, 2016. 25(R1): p. R42–R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Whiteley Z, Massaro G, Gkogkos G, et al. Microfluidic production of nanogels as alternative triple transfection reagents for the manufacture of adeno-associated virus vectors. Nanoscale, 2023. 15(12): p. 5865–5876 * The study describes a novel, cheaper and feasible alternative to most used transfection reagent, polyethylenimine. Researchers indicated that equivalent titers can be achieved with easy-to-implement microfluidic technology at comparably lower costs than traditional reagents. They found that polypropyleneimine was more sensitive to endosomal pH than polyethylenimine, and had a larger capacity for bacterial condensation of plasmid DNA. Therefore, polypropyleneimine could be adapted to replace the transfection reagent
- 96. Saeki R, Kobayashi S, Shimazui R, et al. Characterization of polypropyleneimine as an alternative transfection reagent. Anal Sci, 2023; 10.1007/s44211-023-00284-x. * The study compares polypropyleneimine and polyetylneimine for vector production, It considers responsiveness to endosomal pH, cytotoxycity, transfection efficacy.
- 97.Neri M AAV large scale manufacturing. in HUMAN GENE THERAPY. 2022. [Google Scholar]
- 98.Karbowniczek K, Extance J, Milsom S, et al. Doggybone DNA: an advanced platform for AAV production. Cell Gene Ther. Insights, 2017. 3: p. 731–738. [Google Scholar]
- 99.Scarrott JM, Johari YB, Pohle TH, et al. Increased recombinant adeno-associated virus production by HEK293 cells using small molecule chemical additives. Biotechnol J, 2023. 18(3): p. e2200450. [DOI] [PubMed] [Google Scholar]
- 100.Cecchini S, Virag T, Kotin RM. Reproducible high yields of recombinant adeno-associated virus produced using invertebrate cells in 0.02-to 200-liter cultures. Human gene therapy, 2011. 22(8): p. 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Y, Jiang L, Geng H, et al. A recombinant baculovirus efficiently generates recombinant adeno-associated virus vectors in cultured insect cells and larvae. Molecular Therapy-Methods & Clinical Development, 2018. 10: p. 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Meier AF, Tobler K, Michaelsen K, et al. Herpes Simplex Virus 1 Coinfection Modifies Adeno-associated Virus Genome End Recombination. J Virol, 2021. 95(13): p. e0048621 * This manuscript demonstrates how HSV coinfection significantly changed the type of AAV genome end recombination without significant changes in circular AAV genomes. Thus supporting that further improvements of the HSV-1 production platform may enhance packaging of the recombinant AAV particles..
- 103.Rumachik NG, Malaker SA, Poweleit N, et al. Methods matter: standard production platforms for recombinant AAV produce chemically and functionally distinct vectors. Molecular Therapy-Methods & Clinical Development, 2020. 18: p. 98–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clément N, Knop DR, Byrne BJ. Large-Scale Adeno-Associated Viral Vector Production Using a Herpesvirus-Based System Enables Manufacturing for Clinical Studies. Human Gene Therapy, 2009. 20(8): p. 796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Farson D, Harding TC, Tao L, et al. Development and characterization of a cell line for large‐scale, serum‐free production of recombinant adeno‐associated viral vectors. The Journal of Gene Medicine: A cross‐disciplinary journal for research on the science of gene transfer and its clinical applications, 2004. 6(12): p. 1369–1381. [DOI] [PubMed] [Google Scholar]
- 106.Thorne BA, Takeya RK, Peluso RW. Manufacturing recombinant adeno-associated viral vectors from producer cell clones. Human gene therapy, 2009. 20(7): p. 707–714. [DOI] [PubMed] [Google Scholar]
- 107.Martin J, Frederick A, Luo Y, et al. Generation and characterization of adeno-associated virus producer cell lines for research and preclinical vector production. Human gene therapy methods, 2013. 24(4): p. 253–269. [DOI] [PubMed] [Google Scholar]
- 108.Verdera HC, Kuranda K, Mingozzi F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol Ther, 2020. 28(3): p. 723–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salabarria SM, Norman S, Berthy J, et al. Systemic AAV Delivery Activates the Classical Complement Pathway Leading to Thrombotic Microangiopathy, in American Society of Gene and Cell Therapy. 2021. [Google Scholar]
- 110. Earley J, Piletska E, Ronzitti G, Piletsky S. Evading and overcoming AAV neutralization in gene therapy. Trends Biotechnol, 2022. * Review article. Comprehensive review of AAV neutralizing antibodies and methods to potentially overcome the immune response and suggestions for redosing
- 111.Corti M, Cleaver B, Clément N, et al. Evaluation of Readministration of a Recombinant Adeno-Associated Virus Vector Expressing Acid Alpha-Glucosidase in Pompe Disease: Preclinical to Clinical Planning. Hum Gene Ther Clin Dev, 2015. 26(3): p. 185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leborgne C, Barbon E, Alexander JM, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med, 2020. 26(7): p. 1096–1101. [DOI] [PubMed] [Google Scholar]
- 113.Zhu Y, Jiang JL, Gumlaw NK, et al. Glycoengineered acid alpha-glucosidase with improved efficacy at correcting the metabolic aberrations and motor function deficits in a mouse model of Pompe disease. Mol Ther, 2009. 17(6): p. 954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koeberl DD, Case LE, Smith EC, et al. Correction of Biochemical Abnormalities and Improved Muscle Function in a Phase I/II Clinical Trial of Clenbuterol in Pompe Disease. Mol Ther, 2018. 26(9): p. 2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koeberl DD, Case LE, Desai A, et al. Improved muscle function in a phase I/II clinical trial of albuterol in Pompe disease. Mol Genet Metab, 2020. 129(2): p. 67–72. [DOI] [PubMed] [Google Scholar]
- 116.v van der Wal E, Bergsma AJ, Pijnenburg JM, et al. Antisense Oligonucleotides Promote Exon Inclusion and Correct the Common c.−32–13T>G GAA Splicing Variant in Pompe Disease. Mol Ther Nucleic Acids, 2017. 7: p. 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bergsma AJ, In ‘t Groen SL, Verheijen FW, et al. From Cryptic Toward Canonical Pre-mRNA Splicing in Pompe Disease: a Pipeline for the Development of Antisense Oligonucleotides. Mol Ther Nucleic Acids, 2016. 5(9): p. e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim JA, Li L, Shirihai OS, et al. Modulation of mTOR signaling as a strategy for the treatment of Pompe disease. EMBO molecular medicine, 2017. 9(3): p. 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raben N, Schreiner C, Baum R, et al. Suppression of autophagy permits successful enzyme replacement therapy in a lysosomal storage disorder--murine Pompe disease. Autophagy, 2010. 6(8): p. 1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cohen JL, Chakraborty P, Fung-Kee-Fung K, et al. In Utero Enzyme-Replacement Therapy for Infantile-Onset Pompe’s Disease. N Engl J Med, 2022. 387(23): p. 2150–2158. ** Case report of a fetus with CRIM negative Pompe disease that received in-utero ERT. The manuscript shows a comprehensive summary of laboratory and imaging findings throughout treatment. The infant started immune modulation at birth and had age-appropriate motor function and biomarkers
- 121.Clayton NP, Nelson CA, Weeden T, et al. Antisense Oligonucleotide-mediated Suppression of Muscle Glycogen Synthase 1 Synthesis as an Approach for Substrate Reduction Therapy of Pompe Disease. Mol Ther Nucleic Acids, 2014. 3(10): p. e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fastman NM, Liu Y, Ramanan V, et al. The structural mechanism of human glycogen synthesis by the GYS1-GYG1 complex. Cell Rep, 2022. 40(1): p. 111041. [DOI] [PubMed] [Google Scholar]
- 123.Maze Therapeutics Announces New Clinical Data Supporting MZE001 as a Potential Treatment for Pompe Disease. 2023. 04/01/2023]; Available from: https://mazetx.com/maze-therapeutics-announces-new-clinical-data-supporting-mze001-as-a-potential-treatment-for-pompe-disease/.
- 124. Baik AD, Calafati P, Zhang X, et al. Cell type-selective targeted delivery of a recombinant lysosomal enzyme for enzyme therapies. Mol Ther, 2021. 29(12): p. 3512–3524. * Preclinical study demonstrating the use of different fusion proteins to improve uptake and processing of GAA as a novel strategy to improve ERT.
- 125.Kishnani P, Lachmann R, Mozaffar T, et al. Safety and efficacy of VAL-1221, a novel fusion protein targeting cytoplasmic glycogen, in patients with late-onset Pompe disease. Molecular Genetics and Metabolism, 2019. 126(Issue 2): p. S85–S86. [Google Scholar]