Abstract
A 12-year old, castrated male domestic shorthair cat presented with a 2-year history of poor hair coat, seborrhea, generalized pruritus and otitis externa. Low circulating concentrations of total serum thyroxine (TT4) and free thyroxine (fT4) and an elevated thyroid stimulating hormone concentration supported a diagnosis of primary hypothyroidism. Thyroid scintigraphy did not show uptake of radioactive technetium in the thyroid area. Treatment with levothyroxine resulted in clinical improvement. Recurrence of dermatitis 8 months after onset of treatment resulted in euthanasia of the cat. On post-mortem examination, thyroid tissue was not identified on gross or histological examination. Pituitary immunohistochemistry identified hyperplasia of chromophobe cells.
A 12-year old, neutered male domestic shorthair cat was referred to the Ontario Veterinary College Teaching Hospital (OVC-TH) for evaluation of suspected hypothyroidism. Poor hair coat, alopecia, seborrhea and pruritus had been reported over the preceding 2 years, and did not improve with treatment using bimonthly methylprednisone acetate (Depomedrol; Upjohn) injections or with a hypoallergenic diet trial (Hill's d/d venison and green pea diet; Hill's Pet Nutrition). Intermittent otitis externa had also occurred in the previous 2 years, and temporary resolution was gained after treatment of each episode, initially with miconazole/polymyxin B/prednisolone acetate ear drops (Otomax; Schering-Plough), and then with gentamicin/betamethasone/clotrimazole ear drops (Surolan; Merial Canada). Progressively lethargy had developed 4 months prior to referral. The cat had access to the outdoors, and was routinely vaccinated for feline leukemia virus and rabies.
A complete blood count (Advia 120, Bayer Diagnostics) performed 4 months prior to referral, and 1 month following the last methylprednisone injection, documented moderate lymphopenia (0.5×103/ml; reference interval (RI) 1.5–7.0×103/l) and mild eosinophilia (1.8×103/ml; RI 0–1.5×103/ml). A serum biochemical profile (Hitachi 911 Automatic Analyzer, Roche Laboratories) was unremarkable. The total serum thyroxine (TT4) level (Immulite, Diagnostic Products Corporation) was low (7.6 nmol/l; RI 13.0–52.0 nmol/l [0.59 mg/dl; RI 1.0–4.1 mg/dl]). The serum TT4 concentration was subsequently evaluated on three separate occasions over the following 4 months and remained consistently low (<5.0 nmol/l [<0.39 mg/dl]). Marked elevation of the thyroid stimulating hormone (TSH) level was detected, using a canine-specific (cTSH) chemiluminescent immunometric assay (Immulite; Diagnostic Products Corporation) (3.29 ng/ml; RI 0–0.6 ng/ml), and free T4 (fT4) measured by equilibrium dialysis assay (Nichols Institute Diagnostics) was low (<3.4 pmol/l; RI 16.0–55.0 pmol/l [<0.26 ng/dl; RI 1.2–4.3 ng/dl]). Based on the low serum TT4 and fT4 concentrations, as well as the elevated cTSH, primary hypothyroidism was suspected.
Upon evaluation at the OVC-TH, the cat was quiet but alert and responsive. Heart and respiratory rates were normal, and the cat was hypothermic (rectal temperature of 37.4°C [99.3°F]). A poor hair coat with diffuse seborrhea was present, and the hair was easily epilated. Bilateral otitis externa was evident. A feline leukemia/feline immunodeficiency virus enzyme-linked immunosorbent assay test (Snap FIV Antibody/FeLV Antigen Combo Test; Idexx) was negative.
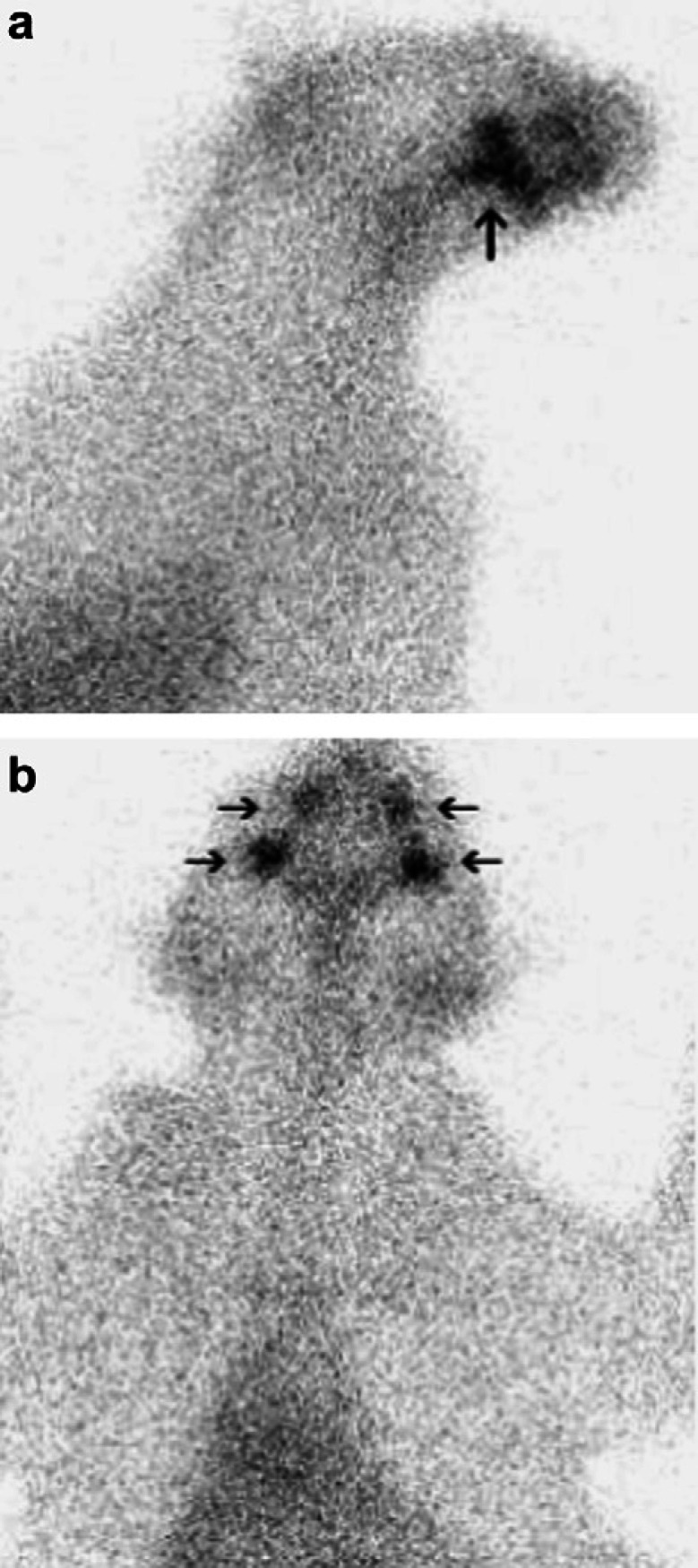
The cat was sedated with acepromazine (0.02 mg/kg IV) (Atravet; Ayerst) and butorphanol (0.2 mg/kg IV) (Torbugesic; Ayerst). Then, 135 MBq of sodium 99mTc-pertechnetate (99mTcO4-) was given intravenously. Images of the head, neck and cranial thorax were obtained 1 h post injection with a gamma camera (Technicare Omega 500 gamma camera) and processed using a dedicated nuclear scintigraphy computer and software (Mirage). Normal radiopharmaceutical uptake was evident in the zygomatic salivary glands, but radioactivity was not evident in the thyroid region (Fig 1, normal shown in Fig 2). A diagnosis of hypothyroidism was confirmed on the basis of the nuclear scintigraphy study. Levothyroxine (0.1 mg PO q24 h) (Synthroid; Abbott Laboratories) was administered.
Fig 1.
Right lateral (a) and ventral (b) views of the thyroid scan. Note the radioisotope uptake in the salivary glands (arrows), while there is no uptake visible in the cervical (thyroid) region.
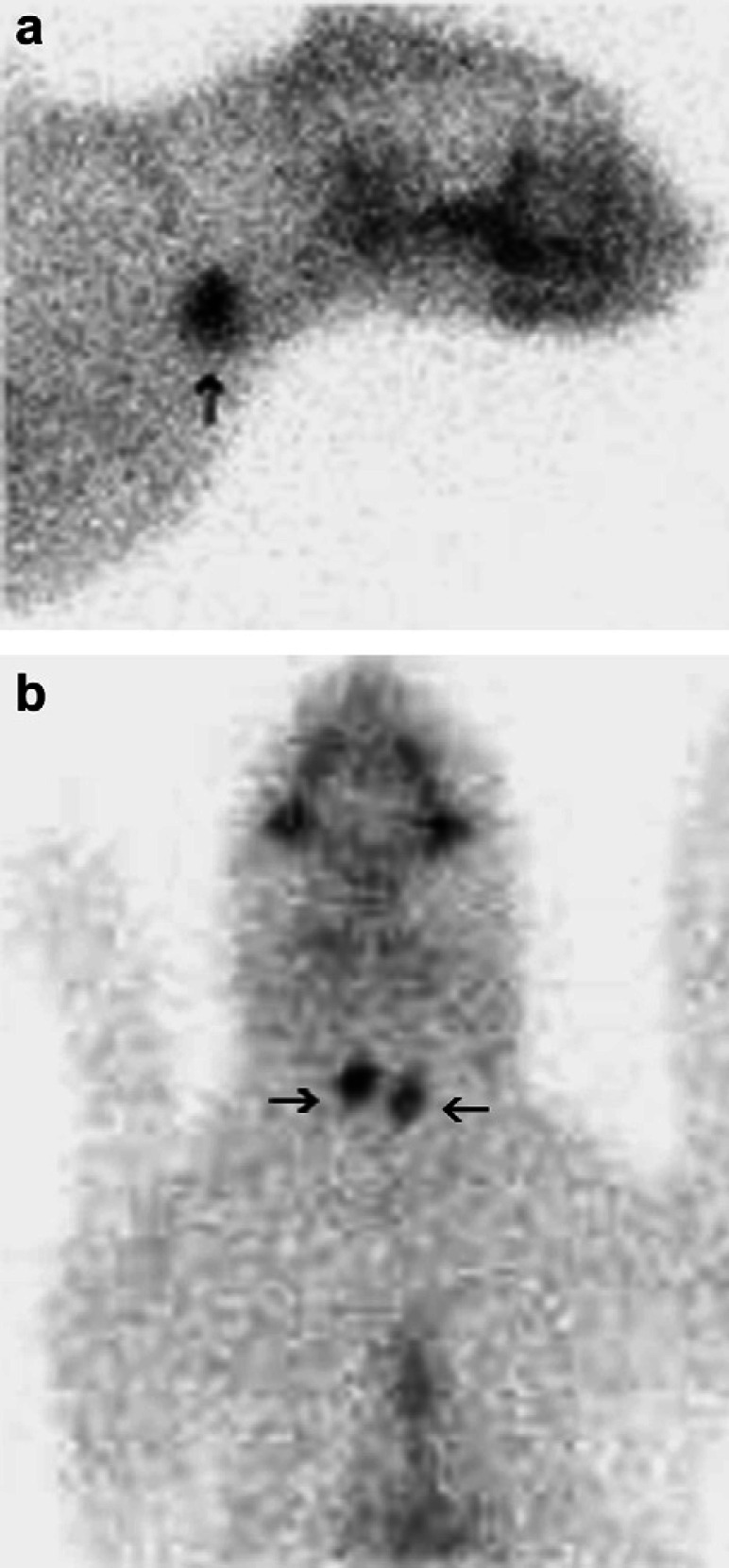
Fig 2.
Right lateral (a) and ventral (b) views of normal thyroid radioisotope uptake in a euthyroid cat (arrows).
Four weeks after initiation of levothyroxine therapy, the cat was more active, and TT4 levels were normal (21.9 nmol/l [1.7 mg/dl]). TSH levels were not measured at this time. The hair coat and skin were markedly improved, with only mild residual seborrhea and otitis externa. The owner reported a decrease in the excessive grooming, interpreted as a decrease in the pruritus. The alopecic areas were still present but had not increased in area, and the hair was no longer easily epilated. The cat was reportedly becoming more active. The cat was lost to follow-up until 8 months after diagnosis of hypothyroidism. At that time, the cat presented to the referring veterinarian with a 2 month history of sporadic vomiting and diarrhea. The owner noted the presence of multifocal skin excoriations and excessive grooming behavior in the previous 1 month. The owner declined further diagnostic evaluation and elected euthanasia.
On post-mortem examination, the entire cervical region was sectioned in attempt to identify thyroid tissue histologically. Additionally, potential sites for ectopic thyroid tissue were examined, from the base of the tongue to the base of the heart. However, no identifiable thyroid tissue was found on either gross or histologic examination. Pituitary hyperplasia, characterized by proliferation of chromophobe cells commonly forming follicular structures that contained eosinophilic globoid material as well as an increase of acidophilic cells characterized by abundant homogenous pale eosinophilic cytoplasm, was present (Fig 3). Using immunohistochemistry, the chromophobe cells were strongly positive for TSH (Fig 4) whereas the acidophilic cells stained with oxytocin (Fig 5). Both cell types were negative for adrenocorticotropic hormone, follicle-stimulating hormone, melanocyte-stimulating hormone and endorphin. Normal feline pituitary tissue was used as a control for the immunohistochemistry. The control pituitary tissue identified thyrotroph cells present only in the anteromedial portion of the pars distalis and oxytocin-expressing cells were identified only in the neurohypophysis. Random patchy areas of alopecia and crusting were noted grossly over the lateral hind limb and in the inguinal areas bilaterally. Histologically, mild inflammation was noted in the dermis of these areas. The inflammation consisted mostly of eosinophils, with occasional mast cells noted. Mild epithelial hyperplasia was noted in the affected areas. Mild hyperkeratosis, mostly orthokeratotic with occasional parakeratotic areas, was overlying the epithelium. Rare cellular crusts, comprised of degenerate granulocytes, were present within the areas of hyperkeratosis. There were no lesions in the gastrointestinal tract or other organ systems to explain the intermittent vomiting and diarrhea. Several multifocal areas of nodular hyperplasia were present in the pancreas, but there was no evidence of inflammation or fibrosis indicative of acute or chronic pancreatitis. Post-mortem diagnoses of primary hypothyroidism and allergic dermatitis were made.
Fig 3.
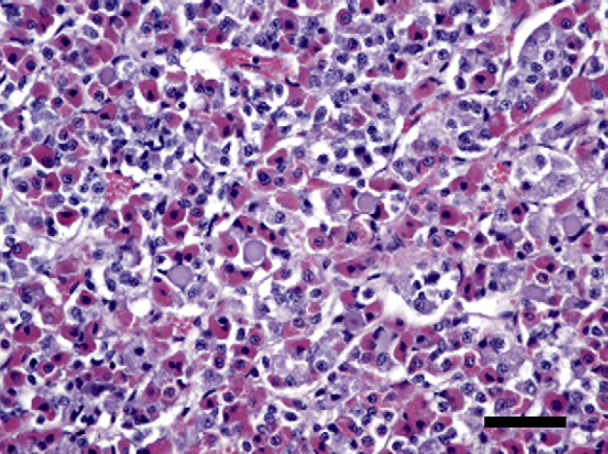
Pituitary cell hyperplasia, with proliferation of chromatophobe cells and an increase of acidophilic cells. Hematoxylin and eosin stain, bar=100 μm.
Fig 4.
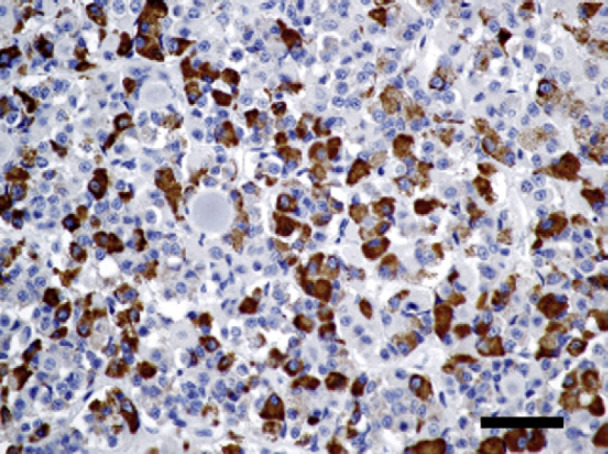
Immunohistochemistry staining with TSH. The pituitary chromatophobe cells are strongly positive for TSH. TSH stain, bar=100 μm.
Fig 5.
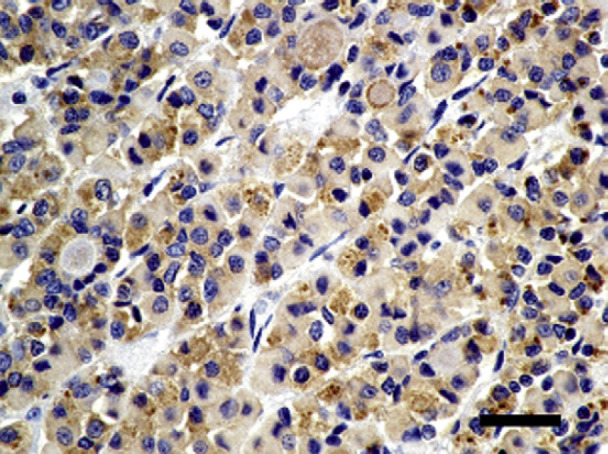
Immunohistochemistry staining with oxytocin. The pituitary acidophilic cells are strongly positive for oxytocin. Oxytocin stain, bar=100 μm.
While spontaneous hypothyroidism is a common endocrinopathy in dogs, it is rarely reported in cats. Many commercial laboratories offer measurement of TT4 concentrations as part of a geriatric feline profile to screen for hyperthyroidism. The most common cause of low serum TT4 concentrations in cats is concurrent non-thyroidal illness. 1,2
Iatrogenic hypothyroidism is a well-recognized potential complication after treatment of cats for hyperthyroidism, and can occur after bilateral thyroidectomy, 1,3,4 radioactive iodine treatment, 5–7 or overdose of anti-thyroidal drugs. 1 Up to 30% of hyperthyroid cats treated with radioactive iodine will develop hypothyroidism after treatment, although this is often transient. 5–8 Treatment of iatrogenic hypothyroidism in cats is rarely required, as only a small number of these cats develop clinical signs of hypothyroidism. 1,6
Clinical signs of adult-onset feline hypothyroidism are similar to those reported for dogs. The most common clinical signs of hypothyroid adult cats include lethargy, weight gain, depression, hypothermia and bradycardia. 9 Dermatological abnormalities reported with hypothyroid cats include tail head alopecia, hyperkeratosis, hyperpigmentation, seborrhea and pyoderma. 9,10 Cardiovascular abnormalities, including bradycardia and decreased cardiac contractility, can develop secondary to hypothyroidism. Neuropathies associated with hypothyroidism have been reported in dogs, but not in cats. 1
Diagnosis of hypothyroidism can be challenging in both dogs and cats. Low or low-normal serum TT4 levels are often caused by non-thyroidal illness (ie, euthyroid sick syndrome) or administration of drugs such as corticosteroids and phenobarbital. 1,2 Free T4 measured by equilibrium dialysis is less likely to be influenced by non-thyroidal illness and drug administration. 1 Similar to dogs, this assay may have increased sensitivity and specificity when diagnosing hypothyroidism in cats. 1,6,11 However, it has been shown that 6–12% of cats with non-thyroidal illness have elevated fT4 levels, while 3–17% of cats with non-thyroidal illness have fT4 levels below the reference range. 2,12 Serum tri-iodothyronine (T3) levels are usually below the reference range in hypothyroid cats. However, this test is unreliable in the identification of dogs with hypothyroidism and its utility in cats is questionable. In some clinician's experience, a normal serum T3 concentration does not rule out hypothyroidism in a cat with low serum TT4. 1 The canine TSH immunoradiometric assay has recently been validated for feline use. 13,14 Similar to dogs, elevation of TSH is thought to be consistent with a diagnosis of primary hypothyroidism. 1
The cat presented in this report had low TT4 and fT4 concentrations, with elevated serum TSH levels, consistent with primary hypothyroidism. Thyroid nuclear scintigraphy was used as a non-invasive technique to confirm the diagnosis of primary hypothyroidism. Normal thyroid scintigraphy (Fig 2) in a cat is characterized by uniform radiopharmaceutical uptake throughout both thyroid lobes, which have smooth margins. The radiopharmaceutical uptake is usually quantified by comparison with the zygomatic salivary gland uptake in the form of a ratio. 15 The normal feline thyroid to salivary ratio is 0.48–1.66. 16
Primary hypothyroidism accounts for over 95% of canine cases of hypothyroidism, and is usually caused by lymphocytic thyroiditis (analogous to Hashimoto's thyroiditis in humans) or idiopathic atrophy of the thyroid gland. 1,17 In the only previously reported case of adult-onset feline hypothyroidism, thyroid biopsy identified marked interstitial lymphocytic infiltrate, similar to lymphocytic thyroiditis observed in dogs and people. 10 The cause of primary hypothyroidism was not determined in the case presented in this report. Marked thyroid atrophy noted at postmortem could explain the lack of identifiable thyroid tissue in the cat in this report. It has been suggested that atrophy of the thyroid gland may be the result of end-stage lymphocytic thyroiditis. 1,18 However, when dogs with biopsy-confirmed lymphocytic thyroiditis progressed to an atrophied stage, residual inflammatory infiltrate persisted in the thyroid region. 18 Given the lack of identifiable thyroid tissue, as well as lack of inflammatory infiltrate in the thyroid region, idiopathic thyroid atrophy is a possible etiology of hypothyroidism in this cat. Additionally, thyroid atrophy is a response to levothyroxine supplementation, and therefore treatment of the cat may be responsible for the lack of thyroid tissue on post-mortem examination. The lack of thyroid tissue identified on thyroid scintigraphy suggests marked atrophy of the thyroid gland was present even prior to levothyroxine therapy. However, the presence of a small number of thyroid follicles taking up 99mTcO4- below the threshold required for scintigraphic detection cannot be ruled out. There was no previous history of trauma or surgery in the cervical region. Lack of blood flow to the thyroid tissue could explain thyroid atrophy. The cat wore a collar and trauma to the cervical region affecting thyroidal tissue and/or its blood supply cannot be ruled out. While there were no signs of thromboembolic disease or atherosclerosis that could have led to a local lack of blood flow in this cat, the onset of clinical signs preceded the post-mortem examination by at least 8 months and, therefore, these changes may not have been apparent.
The proliferating chromophobe cells that secreted abundant homogenous pale eosinophilic globoid material in the pituitary gland were confirmed by immunohistochemistry to produce TSH. The presence of TSH in this cat's pituitary was unexpected, as adequate supplementation with levothyroxine should provide sufficient negative feedback to the pituitary resulting in low to undetectable levels of TSH. The expression of TSH in the pituitary may be due to undersupplementation of this cat's hypothyroid condition. The TT4 was within the normal reference range (21.9; RI 13–52 nmol/l) 1 month after onset of treatment. At this time the dose was not adjusted, and a recheck of the thyroid profile was recommended in another 1 month. Unfortunately, the cat did not return for this recheck and the case was lost to follow-up until the cat presented 8 months after diagnosis with skin lesions and gastrointestinal tract signs. During this time, the client had been giving the same dose of levothyroxine. Thyroxine absorption in dogs is highly variable, and it is possible that the initial dose prescribed was not sufficient to adequately supplement this cat.
The increase in oxytocin uptake by the acidophilic cells in the anterior pituitary gland was an unexpected finding as oxytocin is normally expressed in the posterior pituitary gland. Human pituitary adenomas can retain function and may have the ability to secrete new hormones including oxytocin. 19,20 However, a pituitary adenoma was not identified histologically in this case and, therefore, does not explain the presence of oxytocin expression in the pituitary of this cat. Non-tumorous pituitary cells can adapt to systemic demands requiring hypersecretion of a specific hormone, resulting in transdifferentiation whereby the cells of one line transform into another. 21,22 However, pituitary cell transdifferentiation in hypothyroid humans has only described the transdifferentiation of somatotrophs into thyrotrophs, and does not explain the presence of oxytocin-staining cells in the anterior pituitary of this hypothyroid cat. 21
Hypothyroid cats may be treated with thyroxine supplementation, at a dosage of 0.05–0.1 mg/cat orally once a day. Therapeutic monitoring and subsequent adjustment of the dosage can be performed as in dogs. Prognosis for adult-onset hypothyroidism in dogs is good, and most adult dogs with primary hypothyroidism receiving appropriate therapy will not have an altered lifespan. 1 In the previous report of a cat with spontaneous adult-onset hypothyroidism, rapid clinical response to thyroid hormone supplementation was observed. 10 Long-term prognosis has not been evaluated.
Spontaneous adult-onset hypothyroidism is rare in cats and has similar clinical features to primary hypothyroidism in adult dogs. Other systemic illness must be ruled out in any cat with a low thyroid hormone level. Additional diagnostics in a cat with suspected hypothyroidism include measurement of fT4 and TSH. Nuclear scintigraphy of the thyroid gland can identify primary hypothyroidism in a cat. However, the use of this diagnostic test for diagnosis of hypothyroidism is limited to specialized facilities. Similar to hypothyroid dogs, cats with adult-onset primary hypothyroidism appear to respond well to thyroid hormone supplementation.
References
- 1.Feldman E.C., Nelson R.W. Hypothyroidism. Feldman E.C., Nelson R.W. Canine and feline endocrinology and reproduction, 3rd edn, 2004, Saunders: St Louis, 86–151. [Google Scholar]
- 2.Mooney C.T., Little C.J., Macrae A.W. Effect of illness not associated with the thyroid gland on serum total and free thyroxine concentrations in cats, J Am Vet Med Assoc 208, 1996, 2004–2008. [PubMed] [Google Scholar]
- 3.Welches C.D., Scavelli T.D., Matthiesen D.T., Peterson M.E. Occurrence of problems after three techniques of bilateral thyroidectomy in cats, Vet Surg 18, 1989, 392–396. [DOI] [PubMed] [Google Scholar]
- 4.Birchard S.J. Thyroidectomy in the cat, Clin Tech Small Anim Pract 21, 2006, 29–33. [DOI] [PubMed] [Google Scholar]
- 5.Meric S.M., Rubin S.I. Serum thyroxine concentrations following fixed-dose radioactive iodine treatment in hyperthyroid cats: 62 cases (1986–1989), J Am Vet Med Assoc 197, 1990, 621–623. [PubMed] [Google Scholar]
- 6.Peterson M.E., Becker D.V. Radioiodine treatment of 524 cats with hyperthyroidism, J Am Vet Med Assoc 207, 1995, 1422–1428. [PubMed] [Google Scholar]
- 7.Nykamp S.G., Dykes N.L., Zarfoss M.K., Scarlett J.M. Association of the risk of development of hypothyroidism after iodine 131 treatment with the pretreatment pattern of sodium pertechnetate tc 99m uptake in the thyroid gland in cats with hyperthyroidism: 165 cases (1990–2002), J Am Vet Med Assoc 226, 2005, 1671–1675. [DOI] [PubMed] [Google Scholar]
- 8.Theon A.P., Van Vechten M.K., Feldman E. Prospective randomized comparison of intravenous versus subcutaneous administration of radioiodine for treatment of hyperthyroidism in cats, Am J Vet Res 55, 1994, 1734–1738. [PubMed] [Google Scholar]
- 9.Greco D.S. Diagnosis of congenital and adult-onset hypothyroidism in cats, Clin Tech Small Anim Pract 21, 2006, 40–44. [DOI] [PubMed] [Google Scholar]
- 10.Rand J.S., Levine J., Best S.J., Parker W. Spontaneous adult-onset hypothyroidism in a cat, J Vet Intern Med 7, 1993, 272–276. [DOI] [PubMed] [Google Scholar]
- 11.Schachter S., Nelson R.W., Scott-Moncrieff C., et al. Comparison of serum-free thyroxine concentrations determined by standard equilibrium dialysis, modified equilibrium dialysis, and 5 radioimmunoassays in dogs, J Vet Intern Med 18, 2004, 259–264. [DOI] [PubMed] [Google Scholar]
- 12.Peterson M.E., Melian C., Nichols R. Measurement of serum concentrations of free thyroxine, total thyroxine, and total triiodothyronine in cats with hyperthyroidism and cats with nonthyroidal disease, J Am Vet Med Assoc 218, 2001, 529–536. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson D.C., Caffall Z., Hoenig M. Obesity increases free thyroxine proportionally to nonesterified fatty acid concentrations in adult neutered female cats, J Endocrinol 194, 2007, 267–273. [DOI] [PubMed] [Google Scholar]
- 14.Wakeling J., Moore K., Elliott J., Syme H. Diagnosis of hyperthyroidism in cats with mild chronic kidney disease, J Small Anim Pract 49, 2008, 287–294. [DOI] [PubMed] [Google Scholar]
- 15.Daniel G., Berry C. Textbook of veterinary nuclear medicine, 2nd edn, 2006, American College of Veterinary Radiology [Google Scholar]
- 16.Henrikson T.D., Armbrust L.J., Hoskinson J.J., et al. Thyroid to salivary ratios determined by technetium-99m pertechnetate imaging in thirty-two euthyroid cats, Vet Radiol Ultrasound 46, 2005, 521–523. [DOI] [PubMed] [Google Scholar]
- 17.La Perle K., Capen C. Endocrine organs. McGavin M., Zachary J. Pathologic basis of veterinary disease, 4th edn, 2007, Mosby Elsiver Science: St Louis, 693–741. [Google Scholar]
- 18.Conaway D.H., Padgett G.A., Bunton T.E., Nachreiner R., Hauptman J. Clinical and histological features of primary progressive, familial thyroiditis in a colony of borzoi dogs, Vet Pathol 22, 1985, 439–446. [DOI] [PubMed] [Google Scholar]
- 19.Levy A., Lightman S. Molecular defects in the pathogenesis of pituitary tumours, Front Neuroendocrinol 24, 2003, 94–127. [DOI] [PubMed] [Google Scholar]
- 20.Tan E.U., Ho M.S., Rajasoorya C.R. Metamorphosis of a non-functioning pituitary adenoma to cushing's disease, Pituitary 3, 2000, 117–122. [DOI] [PubMed] [Google Scholar]
- 21.Vidal S., Horvath E., Kovacs K., Cohen S.M., Lloyd R.V., Scheithauer B.W. Transdifferentiation of somatotrophs to thyrotrophs in the pituitary of patients with protracted primary hypothyroidism, Virchows Arch 436, 2000, 43–51. [DOI] [PubMed] [Google Scholar]
- 22.Vidal S., Horvath E., Kovacs K., Lloyd R.V., Smyth H.S. Reversible transdifferentiation: Interconversion of somatotrophs and lactotrophs in pituitary hyperplasia, Mod Pathol 14, 2001, 20–28. [DOI] [PubMed] [Google Scholar]