Abstract
Cats with inflammatory bronchial disease are usually treated with glucocorticoid (GC) drugs to reduce airway inflammation. Inhalant GC delivery can preserve airway effects while systemic effects are minimized. An appropriate dosage regimen for inhaled GC in cats has not been investigated. A blinded, randomized, cross-over study design was used to investigate the ability of three different dosages of the inhalant GC fluticasone propionate delivered by metered dose inhaler to ameliorate eosinophilic airway inflammation in cats with experimentally induced allergic airway inflammation. Further, suppression of the hypothalamic–pituitary–adrenal axis (HPAA) at each dose was assessed. Fluticasone administered at dosages of 44, 110, or 220 μg q 12 h reduced airway eosinophilia by 74%, 82%, or 81%, respectively (no difference). None of the dose regimens tested caused HPAA suppression. We conclude that a twice daily dosage of 44 μg fluticasone should be evaluated for the management of cats with naturally occurring inflammatory bronchial disease.
Feline inflammatory bronchial diseases (eg, asthma and chronic bronchitis) are common lower respiratory diseases in the cat, estimated to affect at least 1% of the population. The mainstay of therapy for inflammatory airway disease is the administration of glucocorticoids (GCs). 1,2 Although GC use is clearly indicated for the treatment of asthma and chronic bronchitis, GCs themselves are associated with the potential for adverse effects. 3 In fact, systemic administration of GC is relatively contraindicated in cats with common diseases such as diabetes mellitus, cardiac disease (eg, hypertrophic cardiomyopathy), or active infection. Topical application of GC to the airways via inhalational delivery allows for local anti-inflammatory activity while minimizing systemic absorption and resultant adverse effects. 4
Although inhalational delivery of GC results in reduced systemic absorption as compared to oral or injectable GC administration, systemic absorption still occurs to some extent. Adverse systemic effects of inhalant GC in humans include osteoporosis, cataracts, glaucoma, skin and vascular changes, iatrogenic Cushing's syndrome and suppression of the hypothalamic–pituitary–adrenal axis (HPAA). 4–7 Additionally, inhalational administration of GC can be associated with local adverse effects such as oral candidiasis in people or facial demodecosis in cats 4,8,9 (personal observation). Adverse GC events become more likely as the dose administered is increased. As with GC delivered by other routes, the dose of inhalant GC used should be sufficient to accomplish therapeutic goals but no greater. Studies in people with asthma demonstrate that either over-dosing or under-dosing of inhalant GC can result in substantial morbidity. 7,10–12
Propellant-driven pressurized metered dose inhalers (pMDI) containing GC have been adapted for use in cats with inflammatory bronchial disease. 1,2,13 Many GCs designed for inhalational use in humans are now available only in breath-actuated formulations (eg, disc inhalers) which are not adaptable for use in cats. However, fluticasone propionate continues to be manufactured in pMDI formulation which can be administered to conscious cats by attachment to a fitted spacer device and face mask. The pMDI canisters available in the USA are designed to deliver 44, 110, or 220 μg of fluticasone per actuation.
To our knowledge, there has been no previous evaluation of efficacy or adverse effects associated with the administration of different dosages of inhalant GC to cats. Using laboratory raised cats with mild bronchitis, a dose of 250 μg fluticasone delivered by pMDI once daily was demonstrated to be effective in reducing evidence of airway inflammation and airflow limitation. 14 A 250 μg twice daily pMDI dose of a similar GC compound, flunisolide, resulted in the amelioration of airway eosinophilia in an experimental model of feline allergic asthma. 15 Our study sought to compare the efficacy of three different doses (44, 110, or 220 μg twice daily) of fluticasone propionate delivered by pMDI for the reduction of eosinophilic airway inflammation in an experimental model of feline allergic asthma. Further, the extent of suppression of the HPAA was compared for each GC dose.
Materials and Methods
Animals and treatments
Six healthy purpose-bred specific pathogen-free cats (one male, five females) were used. Cats were cared for according to the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals, and all experimental procedures were approved by the animal care and use committee of the University of Missouri – Columbia. At 8 weeks of age, all cats were sensitized as previously described to Bermuda grass allergen (BGA) to induce an asthmatic phenotype. 16 Briefly, cats were administered 12 μg BGA in 40 mg alum subcutaneously and 105 organisms Bordetella pertussis intramuscularly on day 0. On day 14 BGA (0.17 mg) was administered intranasally, and subcutaneous BGA and alum injection was repeated on day 21. Induction of an asthmatic phenotype was confirmed by demonstrating >15% eosinophils in bronchoalveolar lavage fluid (BALF) and by intradermal skin testing on day 28 post sensitization. Histamine and saline were used as positive and negative controls for intradermal testing, respectively. Aerosolization of BGA (150 μg in phosphate buffered saline) was performed in a sealed chamber twice weekly for seven treatments, and, thereafter, on a weekly basis to maintain the asthmatic phenotype.
The study was conducted using a randomized, cross-over design administered in a blinded fashion. Once the asthmatic phenotype was confirmed at 12 weeks of age, cats were divided into three treatment groups using a table of random numbers. Cats received twice daily treatment with either 44 μg/actuation, 110 μg/actuation, or 220 μg/actuation fluticasone propionate (Flovent HFA aerosol; GlaxoSmithKline, Research Triangle Park, NC) delivered by pMDI for 3 weeks. Metered dose inhalers were shaken and then attached to a valved spacer device (OptiChamber Advantage valved holding chamber; Respironics, Murrysville, PA) which was in turn attached to a feline anesthetic face mask. After placing the mask over the cat's face, a single actuation of the pMDI was administered and the cat was allowed to breathe into the device 10 times. Just before (baseline) and after completion of each 3 week treatment, adrenocorticotrophic hormone (ACTH) stimulation tests and BALF collection were completed. Samples were collected 48 h after BGA aerosol challenge. A 4 week washout period was allowed after completion of the 3 week treatment, and the cats were randomly assigned to a different fluticasone dose. Identical procedures were completed before and after 3 weeks of treatment at the second fluticasone dose. After a final 4 week washout each cat was treated with the remaining fluticasone dose, again undergoing both baseline and post treatment evaluation.
ACTH stimulation
ACTH stimulation tests were conducted in an identical fashion prior to treatment with each dose of fluticasone (baseline assays on day 0 of each dose treatment regimen) and after completion of each of the three, 3 week treatment regimens (day 21 of each dose treatment). ACTH stimulation tests were conducted in the early morning prior to any other manipulation. Blood was collected by jugular venepuncture just prior to intravenous injection of 5 μg/kg synthetic ACTH (Cortrosyn: Amphastar Pharmaceuticals, Rancho Cucamonga, CA) and again 30 and 60 min after injection. Serum was separated from collected blood and stored frozen at −20°C until analysis. Cortisol was assayed using a chemiluminescent assay (DCP Immulite chemiluminescent assay; Siemens AG, Tarrytown, NY) suitable for feline serum. 17 Pre-ACTH cortisol was subtracted from the highest post-ACTH cortisol (30 or 60 min) for determination of ACTH stimulation.
BALF collection
BALF was collected on day 0 and day 21 of each of the three dose treatments after all samples were collected for ACTH stimulation. Cats were anesthetized with ketamine HCl (5–10 mg/kg IV; Fort Dodge Animal Health, Fort Dodge, IA) to allow endotracheal intubation. Either a 7 French polypropylene catheter or an 8 French red rubber feeding catheter with the closed tip amputated was passed through the endotracheal tube and gently wedged into an airway. Fifteen milliters of room temperature sterile saline was instilled through the catheter and immediately retrieved by gentle aspiration. The sample was placed on ice until evaluation was completed within 1–2 h. A total nucleated cell count was performed on each BALF sample using a coulter counter (Z1 particle counter, Beckman Coulter, Fullerton, CA) or by manual count using a hemocytometer. Cytological evaluation and differential cell counts were performed on samples prepared by cytocentrifugation (Shandon Cytospin 4, ThermoElectron Corporation, Waltham, MA). After modified Wright stain was applied differential cells counts were performed on 200 nucleated cells/slide and the number of eosinophils were expressed as a percentage.
Statistical analysis
Statistic analysis was conducted using standard software (SAS v9, SAS Institute, Cary, NC) and results were expressed as mean±standard deviation (SD). For all statistical analysis, P-value<0.05 was considered significant. Only samples from cats with at least 15% airway eosinophilia prior to initiation of each dose regimen were eligible for inclusion in analysis of BALF cell counts and determination of BALF eosinophilia. Data from all GC treated cats, regardless of the presence or absence of airway eosinophilia, were included in the analysis of the endocrine test results.
ACTH stimulation (peak cortisol minus pre-cortrosyn cortisol) prior to each dosage regimen (baseline) was compared by analysis of variance (ANOVA) with repeated measures. For each dosage group, ACTH stimulation was compared before and after the 3 week treatment via a paired t test. Morning cortisol concentrations at baseline were compared by ANOVA on ranks. Morning cortisol before and after the 44 or 110 μg fluticasone q 12 h dose treatments were compared by the paired t test. Due to failed normality testing, morning cortisol concentration before and after 3 weeks of treatment with 220 μg fluticasone q 12 h were compared using the Mann–Whitney rank sum test. Data points which were ≥2 SD from the mean were evaluated by Chauvenet's and Peirce's criterion to determine their status as statistical outliers.
The percentage of total cells accounted for by eosinophils in the BALF was compared at each baseline by ANOVA with repeated measures. After passing normality tests, the percentage of total cells accounted for by eosinophils in the BALF before and after each 3 week treatment were compared by means of paired t test.
Results
ACTH stimulation
No differences were observed in baseline ACTH stimulation in cats prior to 3 week treatments with 44, 110, or 220 μg q 12 h (P=0.859; 7.86±1.47 μg/dl; 8.31±1.38 μg/dl; 8.35±2.15 μg/dl, respectively). In all but one instance, peak cortisol after cosyntropin administration occurred at 60 min. No differences were noted between ACTH stimulation in cats before or after 3 weeks of therapy with any of the three dose regimens (Fig 1).
Fig 1.
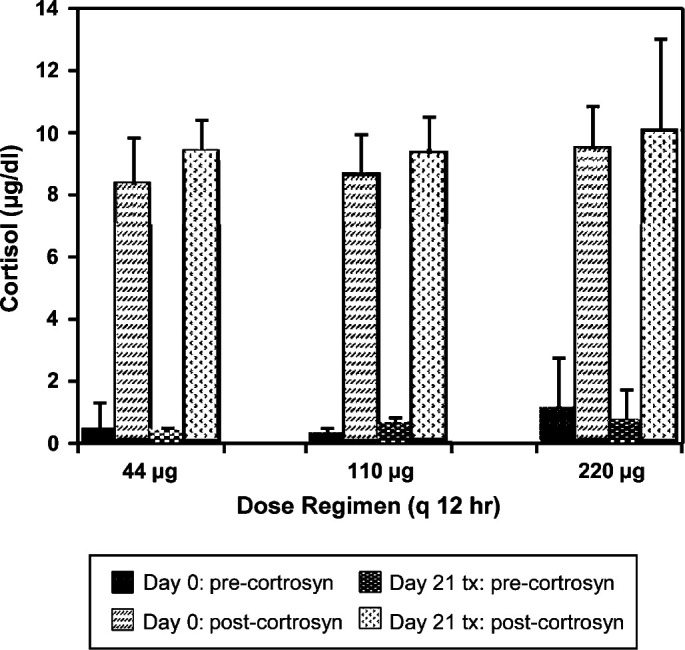
ACTH stimulation test results before and after 3 weeks of treatment with three, twice daily dose regimens of fluticasone propionate delivered by pMDI. There were no significant differences in stimulated (peak minus pre-cortrosyn) cortisol concentrations prior to any of the 3 week courses of therapy (44, 110, or 220 μg fluticasone q 12 h). Additionally, stimulated cortisol concentrations did not change significantly after the 3 week course of therapy at any dosage (44 μg, P=0.144; 110 μg, P=0.592; 220 μg, P=0.534).
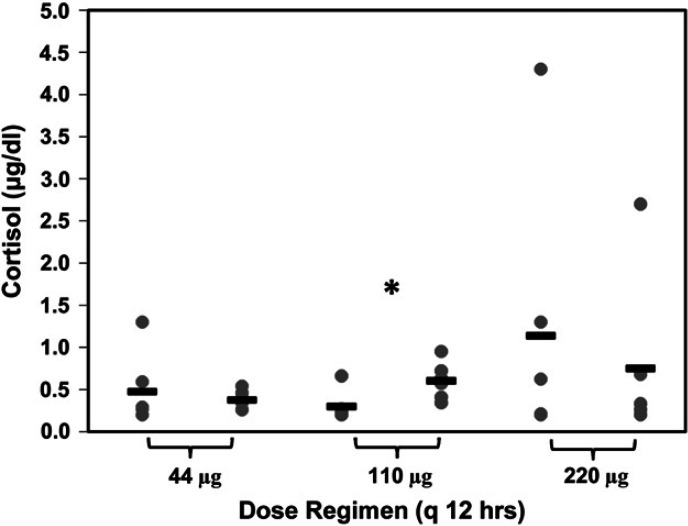
Morning cortisol
No differences were observed in baseline morning cortisol measurements prior to treatments with 44, 110, or 220 μg q 12 h (P=0.882; 0.48±0.43 μg/dl; 0.3±0.18 μg/dl; 1.14±1.60 μg/dl, respectively). Morning cortisol before and after the 3 week drug treatment did not differ for cats receiving either 44 μg or 220 μg dosages (Fig 2). For one cat, morning cortisol at both initiation and completion of the 220 μg dosage was twice the SD of the mean. These data were considered outliers using Chauvenet's criterion but not Peirce's criterion. When the 220 μg dosage was re-evaluated via a paired t test after removing the data from this cat, results were unchanged (P=0.543). Morning cortisol concentration was significantly higher after 3 weeks of treatment with 110 μg fluticasone q 12 h (Fig 2).
Fig 2.
Morning cortisol before and after 3 weeks of treatment with three, twice daily dose regimens of fluticasone propionate delivered by pMDI. No difference was detected between groups in morning cortisol prior to GC treatment. When morning cortisol after the 3 week treatment period was compared to morning cortisol prior to treatment, there were no significant differences in cats receiving either 44 μg or 220 μg fluticasone q 12 h (P=0.599 and 0.937, respectively). Morning cortisol was significantly increased after 3 weeks of treatment with 110 μg fluticasone q 12 h (*P=0.025).
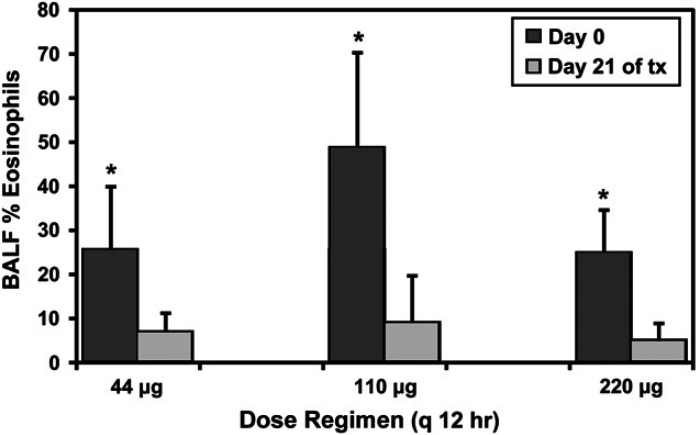
Airway eosinophilia
Data were excluded from analysis of airway eosinophilia in one cat during the 44 μg dose regimen (inadequate sample) and from another cat during the 110 μg dose regimen (baseline airway eosinophils <15%). Therefore, for measures of airway eosinophilia, analysis was based on either five (44 μg and 110 μg) or six (220 μg) cats.
Airway eosinophilia was not equivalent prior to treatments with 44, 110, or 220 μg fluticasone q 12 h (P=0.039; 25.5±14.4% (range 15–44); 48.8±21.5% (range 15–72); 24.7±9.9% (range 15–41)). Specifically, the percentage of airway eosinophils was greater in cats prior to the 110 μg q 12 h dose of fluticasone than the 220 μg dose (P=0.035).
All three dosage regimens of fluticasone resulted in significant amelioration of airway eosinophilia (Fig 3). At dosages of 44, 110, or 220 μg q 12 h the percentage of total cells in BALF comprised of eosinophils decreased by 74%, 82%, or 81%, respectively. At each dose, the mean percentage of eosinophils in BALF after treatment was <9%. Similar results were obtained when absolute eosinophil counts were used in place of eosinophil percentage (data not shown). For all cats, the most common cell other than eosinophils within the BALF both before and after each treatment were macrophages, while neutrophils and lymphocytes were uncommon (Table 1). Total nucleated cell counts were collected using three different methodologies, hence absolute comparisons are difficult (interested readers may contact the authors directly if they wish to review these values).
Fig 3.
BALF eosinophilia in cats before and after 3 weeks of treatment with three, twice daily dose regimens of fluticasone propionate delivered by pMDI. The percentage of eosinophils among all cell types within the airway lavage fluid decreased significantly (*) after a 3 week course of treatment with twice daily fluticasone at 44 μg (P=0.034), 110 μg (P=0.018), and 220 μg (P=0.009) fluticasone q 12 h.
Table 1.
Differential cell counts from BALF of cats receiving varying dosages of inhaled fluticasone dipropionate.
| % Eosinophil | % Macrophage | % Neutrophil | % Lymphocyte | ||
|---|---|---|---|---|---|
| 44 μg bid | Baseline (day 0) | 25.5±14.4 | 65.8±13 | 10.2±12 | 2.2±0.8 |
| Post tx (day 21) | 6.6±4.5 | 84.0±9.8 | 8.6±10 | 0.8±0.8 | |
| 110 μg bid | Baseline (day 0) | 48.8±21.5 | 45.4±23.9 | 1.6±1.1 | 1.4±2.1 |
| Post tx (day 21) | 9.0±10.2 | 81.2±16.0 | 7.2±6.9 | 2.5±3.7 | |
| 220 μg bid | Baseline (day 0) | 24.7±9.9 | 66.2±12.1 | 6.5±8.7 | 1.8±1.2 |
| Post tx (day 21) | 4.8±4.1 | 84.8±9.7 | 8.3±7.0 | 1.7±1.4 |
Discussion
Inhalant delivery of GC preserves anti-inflammatory actions in the airways while minimizing unwanted systemic effects during the treatment of diseases such as asthma and chronic bronchitis. Although the efficacy of fluticasone and flunisolide has been documented in experimental settings at dosages of 250 μg once or twice daily, no other inhalant GC dosages have been evaluated in cats. As with any GC formulation, in order to minimize the risk of adverse effects the dose administered should be sufficient to achieve the desired clinical effect but no greater. Meta-analysis of dosage regimens used to treat adult humans with asthma reveals that the dose–response curve for fluticasone propionate delivered by pMDI begins to plateau at a dose of 100–200 μg/day and peaks at 500 μg/day. 11,18 Further, 80% of the benefit derived from a dosages of 1000 μg/day fluticasone in adults and adolescents was achieved at dosages from 70 to 170 μg/day and 90% of the benefit was achieved at dosages from 100 to 250 μg/day. 11 Although it is not valid to extrapolate dosages between species, it seems reasonable to speculate that a 5 kg cat may require less inhalant GC than a 70 kg human to achieve similar inhibition of airways inflammation.
Efficacy for each of the three dosage regimens in this study was determined by amelioration of airway eosinophilia. All three dosages were able to significantly reduce the proportion of eosinophils in airway lavage fluid. In fact, each dose resulted in BALF eosinophil concentrations which would be considered normal for healthy cats. 19 Eosinophilic airways infiltration is a prominent feature of allergic asthma and there is a strong correlation between eosinophils or eosinophil products in the lungs of humans with asthma disease pathology even when clinical signs of asthma are mild. 20–22 Eosinophils and their toxic products may result in fibrosis and permanent airway damage. 23 Reduction of airway eosinophilia and eosinophilic granule content is considered among the most reliable indicators of successful treatment of allergen-induced asthma exacerbation in people. 24
The model of feline allergic airway disease used in this study mimics many of the immunologic and inflammatory features of naturally occurring feline asthma. However, experimentally sensitized cats do not display easily quantifiable clinical signs of asthma except during aerosol challenge exposure. 16 Therefore, we were unable to evaluate efficacy of the various dose regimens based on clinical signs of asthma. Asthma is characterized by airways hyperreactivity and reversible bronchoconstriction in addition to airways eosinophilia. Although the experimental model used in these studies does recreate some of these features, 16 it is difficult to reliably quantify changes in airways resistance in conscious cats. 25 Repeated anesthetic episodes which would permit intubation and assessment of pulmonary function would likely influence HPAA testing. 26
Inhalational delivery allows for minimization of systemic GC effect with preservation of local anti-inflammatory effect. However, systemic GC effects are not completely eliminated by inhalational administration; both adverse local and systemic effects may be associated with the use of inhaled GC. 4–9 Suppression of the HPAA has been documented as a systemic effect of inhalant GC in humans, horses, dogs, and cats. 27–32 Suppression of the HPAA after treatment with inhaled GC may not only be relevant to clinical conditions such as iatrogenic Cushing's syndrome or adrenal crisis on discontinuation of therapy but may also serve as a quantifiable surrogate marker for potential systemic adverse effects of inhalant GC. 5,7,33 Although the ideal test to document HPAA suppression has not been definitively identified, both morning cortisol and ACTH stimulation tests are commonly used. 32,34,35 We were unable to document significant suppression of the HPAA in cats with experimentally induced allergic airway disease at any of the fluticasone dosages tested.
Suppression of the HPAA has been documented in healthy cats receiving 250 μg flunisolide delivered by pMDI q 12 h. 32 In that study, cats administered inhalant GC were found to have lower morning cortisol than placebo treated cats, lower ACTH-stimulated peak cortisol concentrations after 2 weeks of GC treatment than before treatment, and higher urine cortisol/creatinine ratios after 2 weeks of GC treatment. 32 Flunisolide is dosed similarly to fluticasone propionate although fluticasone has a longer half-life, greater HPAA suppression, and less effective potency than flunisolide in humans. 36–38 The reason we were unable to document HPAA suppression at a dose of 220 μg fluticasone q 12 h while suppression was documented with a dose of 250 μg flunisolide q 12 h in the prior study, is unclear. Surprisingly, in the same study in which suppression of the HPAA was documented in cats receiving flunioslide, HPAA suppression was not observed in cats receiving oral prednisone at a dose of 10 mg/day. 32
Although we identified statistically higher morning cortisol concentrations in cats after completing 3 weeks of treatment with 110 μg fluticasone q 12 h, this result is unlikely to have any biological significance. Treatment with GC should theoretically lead to suppression of the HPAA and diminished morning cortisol rather than to increased morning cortisol.
Results of this study suggest that fluticasone propionate delivered by pMDI is likely to be efficacious in the amelioration of airway inflammation in cats at dosages lower than those commonly suggested or those used in previous experimental studies. 1,13–15 Additionally, we determined that minimal suppression of the HPAA is likely in cats following treatment with clinically relevant dosages of fluticasone. In our experimental model overt clinical signs of airflow limitation occur only during aerosol exposure to the sensitizing allergen. The model provided a mechanism for assessment of GC dose effects on a readily quantifiable and consistent feature of allergic airway disease, namely airway inflammation. Similar assessment would be difficult in client-owned cats both due to the need for repeated anesthesia and collection of BALF and due to the waxing and waning nature of the disease. Now that we have documented that 44μg and 110 μg fluticasone delivered by pMDI q 12 h is able to ameliorate eosinophilic airway inflammation in an experimental model of asthma, these dosages should be evaluated in a prospective manner in cats with naturally occurring asthma.
Acknowledgements
The authors wish to thank Dr Linda Berent and Mr Mathew Haight for their technical assistance. This work was funded by the Committee on Research Clinician Scientist Award Program at the University of Missouri – College of Veterinary Medicine.
References
- 1.Byers C.G., Dhupa N. Feline bronchial asthma: Treatment, Compend Contin Educ Pract Vet 27, 2005, 426–432. [Google Scholar]
- 2.Padrid P. Feline asthma. Diagnosis and treatment, Vet Clin North Am Small Anim Pract 30, 2000, 1279–1293. [DOI] [PubMed] [Google Scholar]
- 3.Cohn L.A. Glucocorticoids. Ettinger S.J., Feldman E.C. Textbook of veterinary internal medicine, 6th edn, 2005, WB Saunders: Philadelphia, 503–508. [Google Scholar]
- 4.Allen D.B., Bielory L., Derendorf H., Dluhy R., Colice G.L., Szefler S.J. Inhaled corticosteroids: Past lessons and future issues, J Allergy Clin Immunol 112, 2003, S1–S40. [DOI] [PubMed] [Google Scholar]
- 5.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma, Respir Med 100, 2006, 1307–1317. [DOI] [PubMed] [Google Scholar]
- 6.Iles R., Williams R.W., Deeb A., Ross-Russell R., Acerini C.L. A longitudinal assessment of the effect of inhaled fluticasone propionate therapy on adrenal function and growth in young children with asthma, Pediatr Pulmonol 43, 2008, 354–359. [DOI] [PubMed] [Google Scholar]
- 7.Todd G.R., Acerini C.L., Buck J.J., et al. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate, Eur Respir J 19, 2002, 1207–1209. [DOI] [PubMed] [Google Scholar]
- 8.Ellepola A.N., Samaranayake L.P. Inhalational and topical steroids, and oral candidosis: A mini review, Oral Dis 7, 2001, 211–216. [PubMed] [Google Scholar]
- 9.Kelly H. Potential adverse effects of the inhaled corticosteroids, J Allergy Clin Immunol 112, 2003, 469–478. [PubMed] [Google Scholar]
- 10.Graft D.F. Finding the correct inhaled corticosteroid dose in asthma, Postgrad Med 117, 2005, 21–24. [DOI] [PubMed] [Google Scholar]
- 11.Holt S., Suder A., Weatherall M., Cheng S., Shirtcliffe P., Beasley R. Dose–response relation of inhaled fluticasone propionate in adolescents and adults with asthma: Meta-analysis, Br Med J 323, 2001, 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masoli M., Holt S., Weatherall M., Beasley R. The dose–response relationship of inhaled corticosteroids in asthma, Curr Allergy Asthma Rep 4, 2004, 144–148. [DOI] [PubMed] [Google Scholar]
- 13.Dowling P.M. Options for treating feline asthma, Vet Med 96, 2001, 353–356. [Google Scholar]
- 14.Kirschvink N., Leemans J., Delvaux F., et al. Inhaled fluticasone reduces bronchial responsiveness and airway inflammation in cats with mild chronic bronchitis, J Feline Med Surg 8, 2006, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinero C.R., Decile K.C., Byerly J.R., et al. Effects of drug treatment on inflammation and hyperreactivity of airways and on immune variables in cats with experimentally induced asthma, Am J Vet Res 66, 2005, 1121–1127. [DOI] [PubMed] [Google Scholar]
- 16.Reinero C.R. Norris, Decile K.C., Berghaus R.D., et al. An experimental model of allergic asthma in cats sensitized to house dust mite or bermuda grass allergen, Int Arch Allergy Immunol 135, 2004, 117–131. [DOI] [PubMed] [Google Scholar]
- 17.Singh A.K., Jiang Y., White T., Spassova D. Validation of nonradioactive chemiluminescent immunoassay methods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses, J Vet Diagn Invest 9, 1997, 261–268. [DOI] [PubMed] [Google Scholar]
- 18.Masoli M., Weatherall M., Beasley R. Fluticasone given once versus twice a day: Meta-analysis, Respirol 10, 2005, 183–188. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins E., Kennedy-Stoskopf S., Levy J., et al. Cytologic characterization of bronchoalveolar lavage fluid collected through an endotracheal tube in cats, Am J Vet Res 55, 1994, 795–802. [PubMed] [Google Scholar]
- 20.Jacobsen E.A., Ochkur S.I., Lee N.A., Lee J.J. Eosinophils and asthma, Curr Allergy Asthma Rep 7, 2007, 18–26. [DOI] [PubMed] [Google Scholar]
- 21.Stelmach I., Majak P., Grzelewski T., et al. The ECP/Eo count ratio in children with asthma, J Asthma 41, 2004, 539–546. [DOI] [PubMed] [Google Scholar]
- 22.Bousquet J., Chanez P., Lacoste J.Y., et al. Eosinophilic inflammation in asthma, N Engl J Med 323, 1990, 1033–1039. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen E.A., Taranova A.G., Lee N.A., Lee J.J. Eosinophils: Singularly destructive effector cells or purveyors of immunoregulation?, J Allergy Clin Immunol 119, 2007, 1313–1320. [DOI] [PubMed] [Google Scholar]
- 24.Venge P. Monitoring the allergic inflammation, Allergy 59, 2004, 26–32. [DOI] [PubMed] [Google Scholar]
- 25.van den Hoven R., van den Hoven R. A jack-in-the-box of respiratory research: Is the technique of barometric whole body plethysmography a disappointing surprise?, Vet J 173, 2007, 250–251. [DOI] [PubMed] [Google Scholar]
- 26.Moon P.F. Cortisol suppression in cats after induction of anesthesia with etomidate, compared with ketamine– diazepam combination, Am J Vet Res 58, 1997, 868–871. [PubMed] [Google Scholar]
- 27.Sim D., Griffiths A., Armstrong D., Clarke C., Rodda C., Freezer N. Adrenal suppression from high-dose inhaled fluticasone propionate in children with asthma, Eur Respir J 21, 2003, 633–636. [DOI] [PubMed] [Google Scholar]
- 28.Masoli M., Weatherall M., Holt S., Shirtcliffe P., Beasley R. Inhaled fluticasone propionate and adrenal effects in adult asthma: Systematic review and meta-analysis, Eur Respir J 28, 2006, 960–967. [DOI] [PubMed] [Google Scholar]
- 29.Kaliner M.A. Pharmacologic characteristics and adrenal suppression with newer inhaled corticosteroids: A comparison of ciclesonide and fluticasone propionate, Clin Ther 28, 2006, 319–331. [DOI] [PubMed] [Google Scholar]
- 30.Rush B.R., Raub E.S., Thomsen M.M., Davis E.G., Matson C.J., Hakala J.E. Pulmonary function and adrenal gland suppression with incremental doses of aerosolized beclomethasone dipropionate in horses with recurrent airway obstruction, J Am Vet Med Assoc 217, 2000, 359–364. [DOI] [PubMed] [Google Scholar]
- 31.Cohn L.A., DeClue A.E., Reinero C.R. Endocrine and immunologic effects of inhaled fluticasone propionate in healthy dogs, J Vet Intern Med 22, 2008, 37–43. [DOI] [PubMed] [Google Scholar]
- 32.Reinero C.R., Brownlee L., Decile K.C., et al. Inhaled flunisolide suppresses the hypothalamic–pituitary–adrenocortical axis, but has minimal systemic immune effects in healthy cats, J Vet Intern Med 20, 2006, 57–64. [DOI] [PubMed] [Google Scholar]
- 33.Zollner E.W. Hypothalamic–pituitary–adrenal axis suppression in asthmatic children on inhaled corticosteroids (Part 2)—the risk as determined by gold standard adrenal function tests: A systematic review, Pediatr Allergy Immunol 18, 2007, 469–474. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein D.I., Allen D.B. Evaluation of tests of hypothalamic–pituitary–adrenal axis function used to measure effects of inhaled corticosteroids, Ann Allergy Asthma Immunol 98, 2007, 118–127. [DOI] [PubMed] [Google Scholar]
- 35.Zollner E.W. Hypothalamic–pituitary–adrenal axis suppression in asthmatic children on inhaled corticosteroids: Part 1. Which test should be used?, Pediatr Allergy Immunol 18, 2007, 401–409. [DOI] [PubMed] [Google Scholar]
- 36.Casale T.B., Nelson H.S., Stricker W.E., Raff H., Newman K.B. Suppression of hypothalamic–pituitary–adrenal axis activity with inhaled flunisolide and fluticasone propionate in adult asthma patients, Ann Allergy Asthma Immunol 87, 2001, 379–385. [DOI] [PubMed] [Google Scholar]
- 37.Derendorf H., Nave R., Drollmann A., Cerasoli F., Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma, Eur Respir J 28, 2006, 1042–1050. [DOI] [PubMed] [Google Scholar]
- 38.Abdullah A.K., Khan S. Relative oral corticosteroid-sparing effect of 7 inhaled corticosteroids in chronic asthma: A meta-analysis, Ann Allergy Asthma Immunol 101, 2008, 74–81. [DOI] [PubMed] [Google Scholar]