Abstract
A young adult entire male domestic shorthair cat was presented with a known history of a road traffic accident. Survey radiographs suggested a congenital diaphragmatic hernia unrelated to the recent trauma. Positive contrast peritoneography was undertaken and findings were consistent with a pleuroperitoneal diaphragmatic hernia (or true hernia). This was repaired surgically and was thought to be an incidental finding. This case report demonstrates the use of positive contrast peritoneography as a simple and effective tool in the diagnosis of pleuroperitoneal diaphragmatic hernias.
Pleuroperitoneal or true diaphragmatic hernias are rare. They are caused by defects in the dome of the diaphragm when the ingrowth of collagenous tissue or musculature ceases prematurely. 1 They are often found incidentally and rarely show clinical signs. 2 This case report describes the radiographic technique and the radiological findings associated with this clinical entity.
A young adult entire male domestic shorthair cat was presented with a known history of a road traffic accident that had occurred days previously. The cat had no respiratory signs and cardiac auscultation was unremarkable. A 3/10th stifle lameness without joint effusion or instability was evident. Serum biochemistry, electrolytes and a full blood count were unremarkable. Premedication with acepromazine (ACP; Novartis Animal Health) at 0.02 mg/kg and buprenorphine (Vetergesic; Alstoe) at 0.01 mg/kg was administered by deep intramuscular injection. General anaesthesia was induced by propofol (Rapinovet; Schering Plough Animal Health) at 4 mg/kg and maintained on isoflurane (Isoflo Vet; Schering Plough Animal Health) in oxygen. Dorsoventral and right lateral thoracic radiographs, as well as caudocranial and lateral radiographs of the stifle, were obtained under general anaesthesia (Figs 1 and 2). Thoracic radiographs showed a 3 cm×4 cm well-circumscribed soft-tissue mass, causing slight displacement of the cardiac apex towards the left, in the right caudoventral thorax. The caudal margin of the mass effaced the cranial border of the right side of the diaphragm on the dorsoventral view. On the lateral view summation of the caudal border of the heart and dome of the diaphragm was evident. The caudal margin of the mass could not be defined on this projection. Differential diagnoses for such findings include acquired (eg, traumatic) or congenital (eg, pleuroperitoneal) diaphragmatic hernias, or a pulmonary, pleural, caudal mediastinal or diaphragmatic mass (eg, haematoma, neoplasia, abscess).
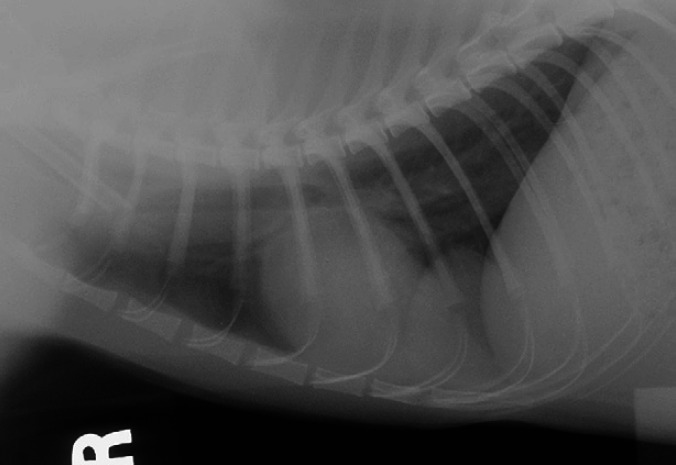
Fig 1.
Right lateral thoracic radiograph. Note the well circumscribed soft tissue mass in the right caudoventral thorax.
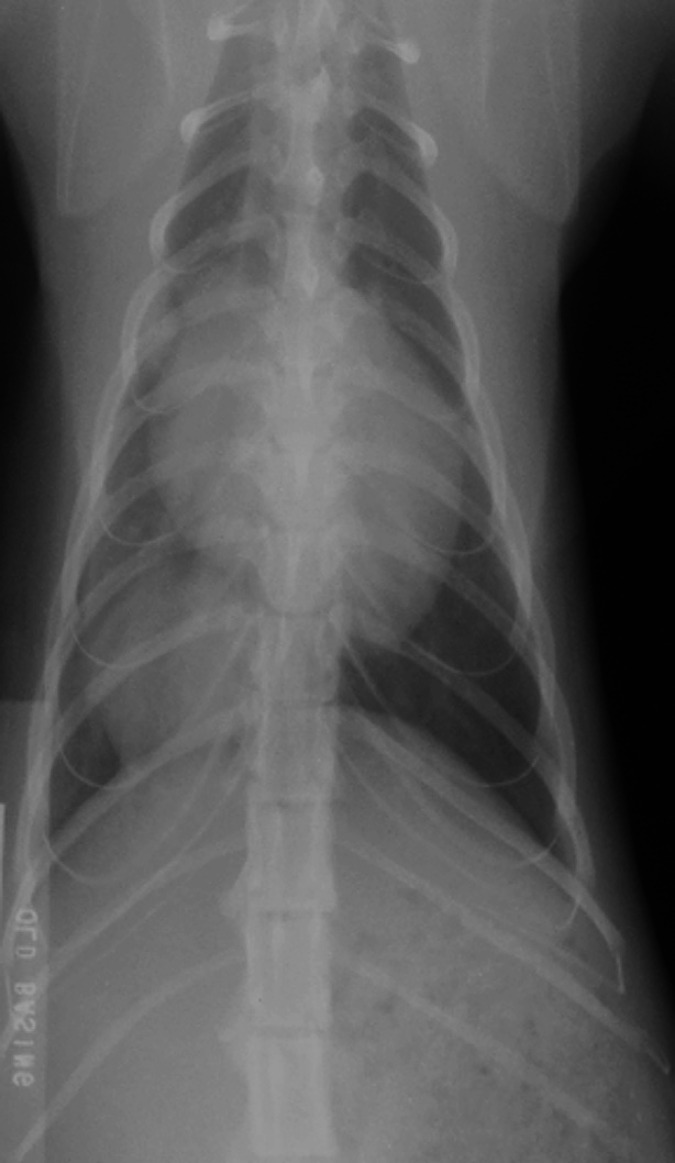
Fig 2.
Dorsoventral thoracic radiograph. Note the well circumscribed soft tissue mass in the right caudoventral thorax.
Positive contrast peritoneography was undertaken to differentiate the presence of herniation from primary thoracic pathology. The patient was positioned in dorsal recumbency and an area of the abdomen 1 cm cranial to the umbilicus was prepared aseptically. A 21 gauge 16 mm needle was used and 2 ml/kg body weight of iohexol (Omnipaque 300 mgI/ml, Nycomed Amersham) was administered into the peritoneal cavity. The abdomen was then gently ballotted and the hind quarters were elevated for 1 min. Orthogonal thoracic films were obtained (Figs 3 and 4). Accumulation of the contrast medium was evident caudal to the diaphragm and within the thorax. It outlined and was limited to a circumscribed sac continuous with the right crus of the diaphragm. These radiographic findings were consistent with a pleuroperitoneal diaphragmatic hernia.
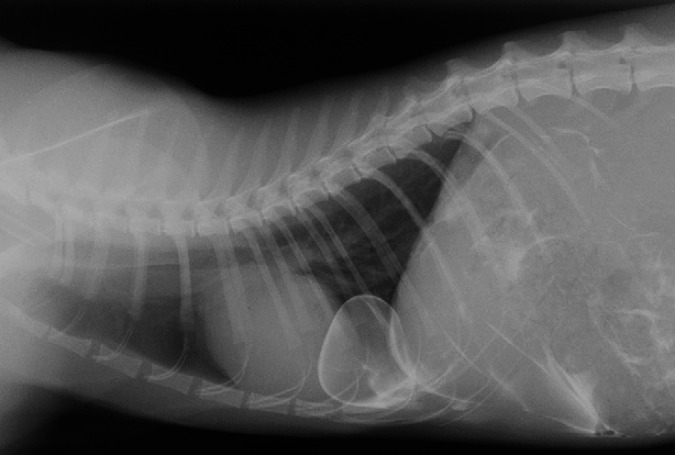
Fig 3.
Right lateral thoracic radiograph after positive contrast peritoneography. Accumulation of the contrast medium is evident caudal to the diaphragm and within the thorax. It outlines and is limited to a circumscribed sac continuous with the right crus of the diaphragm.
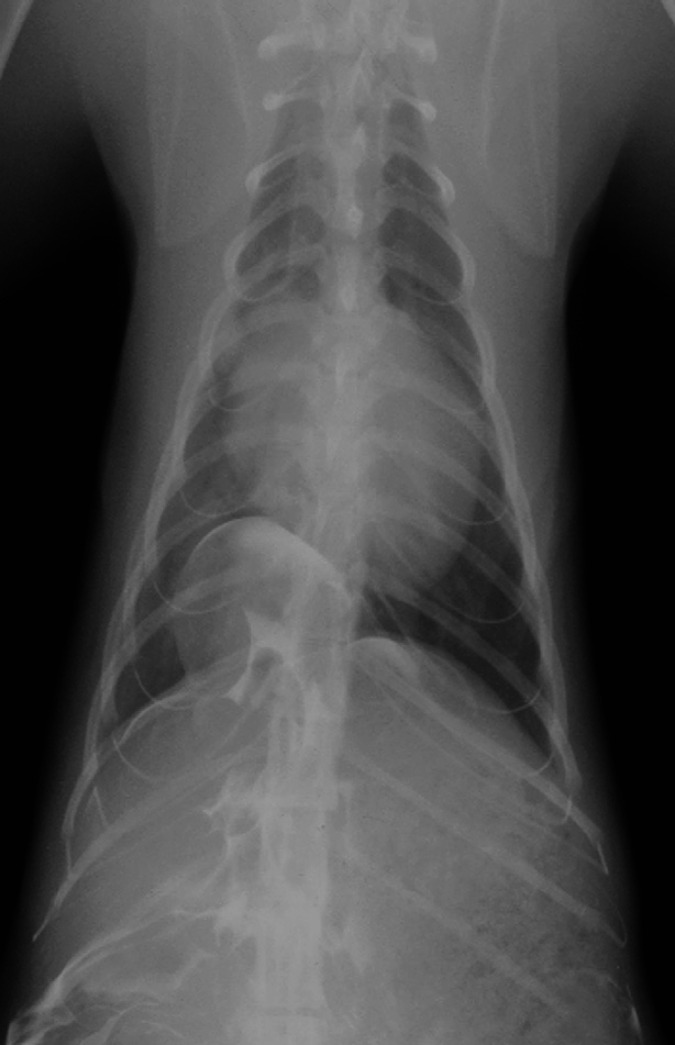
Fig 4.
Dorsoventral thoracic radiograph after positive contrast peritoneography. Accumulation of the contrast medium is evident caudal to the diaphragm and within the thorax. It outlines and is limited to a circumscribed sac continuous with the right crus of the diaphragm.
A cranial midline coeliotomy was performed. A herniated sac, containing part of the right medial lobe of the liver, was found extending through the right crus of the diaphragm, mid-way between the right body wall and the central tendon. There was no evidence of trauma. The hernia was reduced and the margins of the diaphragmatic defect were debrided. The defect was closed with 3/0 polydioxanone (PDS II, Ethicon) in a simple interrupted pattern. Air was evacuated from the pleural cavity by thoracocentesis through the diaphragm. The closure of the laparotomy wound was routine. The cat went on to make a full recovery.
The diaphragm separates the thoracic and abdominal cavities. It is dome-shaped and formed from a body and paired crura. It consists of a central tendon and a muscular periphery that is divided into lumbar, sternal and costal portions, according to their attachments. The left and right crura arise from the lumbar portion of the peripheral muscle. The right crus is considerably larger than the left. There are three openings in the diaphragm. The most dorsal is the aortic hiatus, transmitting the aorta, azygos vein and thoracic duct. The oesophageal hiatus lies more ventrally within the right crus and transmits the oesophagus, dorsal and ventral vagal trunks and associated vessels. The caval foramen is the most ventral of the openings and transmits the caudal vena cava. 3,4
The most common congenital diaphragmatic hernia is the peritoneopericardial diaphragmatic hernia, which involves a communication between the peritoneal and pericardial cavities. 2 Congenital pleuroperitoneal hernias are rare in small animals. They result from incomplete closure of the pleuroperitoneal canals (dorsal part of the diaphragm) or from failure of the pleuroperitoneal folds to incorporate muscular parts of the body wall. The former situation results in rapid death from respiratory insufficiency. In the latter category, the lumbar part of the diaphragm remains membranous instead of developing a strong muscular component. 5
Although there was a history of recent trauma in this case, the radiographic findings were consistent with a congenital diaphragmatic hernia. At surgery, the abdominal organs and diaphragm were assessed for evidence of trauma or adhesions. No such evidence was found.
Positive contrast peritoneography is a simple and safe procedure to perform. Ionic or non-ionic water-soluble iodine containing contrast agents can be used. Non-ionic or low osmolality iodinated contrast agents such as iohexol are considered safer as they exert only minimal osmotic potential. 6 In this study the contrast agent used was iohexol. Contraindications to the technique include peritonitis, hypovolaemia, renal impairment or hypersensitivity to the contrast agent. A negative study does not preclude a diagnosis of diaphragmatic herniation. False negatives may occur if viscera obstruct the diaphragmatic defect and prevent the flow of contrast. 7 A false positive result may occur with a paracostal hernia and intercostal tear allowing contrast to enter the pleural cavity without passing through the diaphragm. 8 Misdiagnosis may result if contrast is inadvertently injected into the thorax or if contamination of the patient's coat or X-ray table is present. 9 Although simple to perform, general anaesthesia is required and this is a disadvantage compared to non-invasive diagnostic procedures such as transhepatic ultrasonography. This consideration was not important in this case, but may be important in those cases deemed unsuitable for anaesthesia.
Transhepatic ultrasonography can also be used to assess the diaphragm for defects and for the presence of, and contents within, any herniation. 10 Although ultrasonography is non-invasive it is not always conclusive in cases of diaphragmatic hernia and may be limited by operator experience. This procedure was not performed in this case, as it was not available to the author.
This case has presented the value of positive contrast peritoneography in the demonstration of a pleuroperitoneal diaphragmatic hernia. It is simple to perform and requires only very basic equipment. The technique may be useful for differentiating those patients with true rupture of the diaphragm from those with a true hernia. Surgical reconstruction may not be necessary if clinical signs are not evident. In either case, reconstruction of normal anatomy may be deemed important as a prophylactic procedure, in order to prevent incarceration of hernia contents and to restore normal anatomy.
Acknowledgements
The author would like to thank Paul Mahoney and Livia Benigni for their assistance in the preparation of this manuscript.
References
- 1.Voges A.K., Bertrand S., Hill R.C., Neuwirth L., Schaer M. True diaphragmatic hernia in a cat, Vet Radiol Ultrasound 38, 1997, 116–119. [DOI] [PubMed] [Google Scholar]
- 2.Suter P.F. Abnormalities of the diaphragm, Thoracic radiography: a text atlas of thoracic disease of the dog and cat, 1984, Peter F. Suter: Switzerland, 179–204. [Google Scholar]
- 3.Dyce K.M., Sack W.O., Wensing C.J.G. The locomotor apparatus. Pedersen D. Textbook of veterinary anatomy, 1987, WB Saunders: Philadelphia, 49–51. [Google Scholar]
- 4.Valentine B.A., Cooper B.J., Dietze A.E., Noden D.M. Canine congenital diaphragmatic hernia, J Vet Intern Med 2, 1988, 109–112. [DOI] [PubMed] [Google Scholar]
- 5.Auger J.M., Riley S.M. Combined hiatal hernia and pleuroperitoneal hernia in a Sharpei, Can Vet J 38, 1997, 640–642. [PMC free article] [PubMed] [Google Scholar]
- 6.Selin K., Emanuelsson H., Renaa T. Iohexol in coronary angiography. A comparison of ionic and non-ionic contrast media, Acta Radiol Suppl 366, 1983, 115–120. [PubMed] [Google Scholar]
- 7.Evans S.M., Biery D.N. Congenital peritoneopericardial diaphragmatic hernia in the dog and cat: A literature review and 17 additional case histories, Vet Radiol 21, 1980, 108–116. [Google Scholar]
- 8.Rendano V.T. Positive contrast peritoneography: An aid in the radiographic diagnosis of diaphragmatic hernia, Vet Radiol 20, 1979, 67–72. [Google Scholar]
- 9.Stickle R.L. Positive contrast celiography (peritoneography) for the diagnosis of diaphragmatic hernia in dogs and cats, J Am Vet Med Assoc 185, 1984, 295–298. [PubMed] [Google Scholar]
- 10.Reichle J.K., Wisner E.R. Non-cardiac thoracic ultrasound in 75 feline and canine patients, Vet Radiol Ultrasound 41, 2000, 154–162. [DOI] [PubMed] [Google Scholar]