Abstract
An evaluation of histological findings in full-thickness biopsies from the gastrointestinal tract (GIT) and extraintestinal samples of 43 cats with chronic GIT disease signs was performed. In the majority of cases (46.5%) inflammatory bowel disease, ie, lymphocytic-plasmacytic enteritis/colitis (32.6%), eosinophilic gastroenterocolitis (11.6%) and mixed inflammatory infiltration (2.3%), was diagnosed. Furthermore, in four animals non-inflammatory mucosal band-shaped fibrosis (9.3%), and in 10 cats (23.3%) a diffuse lymphoma, was found. Six cats displayed only a gastritis (7.0%) or lymphangiectasia (7.0%), respectively. In two cats a mast cell tumour (4.7%) was diagnosed. In one cat no histopathological lesions were found. The availability of transmural biopsies from all segments of the intestine and the collection of extraintestinal samples, especially mesenteric lymph nodes, is especially helpful for diagnosing intestinal tumours such as lymphomas and tumours of mast cell origin.
The most important differential diagnoses of persisting or intermittent chronic gastrointestinal signs in cats are inflammatory bowel disease (IBD), food allergy, neoplasm or hyperthyroidism. 1–3
An important tool for diagnosing chronic gastrointestinal diseases is the histopathological evaluation of biopsies from the stomach and intestines. 3 IBD is a diagnosis of exclusion and histopathological analysis of gastrointestinal biopsy specimens is obligatory. 3–6 Biopsies can be obtained under endoscopic control or by exploratory laparotomy. 4,6–8 Endoscopy is non-invasive but has its limitations because, in general, only the mucosa of the gastrointestinal tract (GIT) can be sampled. By performing exploratory laparotomy, however, full-thickness (transmural) biopsies can be taken from the GIT and/or from extraintestinal organs (eg, mesenteric lymph node, pancreas), which are normally not accessible to endoscopy. 4,6,7,9–12 Furthermore, full-thickness biopsies have the advantage that all layers of the gastrointestinal walls including mucosa, submucosa and muscularis can be sampled and examined histologically. 4,7,8,13 Reports describing the histopathological appearance of gastrointestinal biopsies from cats suffering from chronic gastrointestinal signs focus on cases with IBD, ie, lymphocytic-plasmacytic enteritis (LPE) or colitis (LPC), or on cases with alimentary lymphoma and are mainly limited to findings in samples which were taken under endoscopic control. 2,8,14–18
The aim of this retrospective study was (1) to analyse the spectrum of histopathological lesions present in full-thickness biopsies obtained from 43 cats with chronic gastrointestinal diseases, and (2) to evaluate the possible advantages of examining full-thickness biopsy samples and extraintestinal tissue samples.
Materials and Methods
Gastrointestinal samples were obtained from 43 cats admitted to the Small Animal Clinic, University of Veterinary Medicine Hannover, during the years 1998–2005. The age of these animals ranged from 7 months to 15 years (mean 8 years). In all cats, chronic gastrointestinal signs, eg, vomiting, diarrhoea and anorexia, had been present for at least 3 weeks up to 1 year. The main clinical signs in decreasing frequencies were vomiting (n=15), vomiting and diarrhoea (n=11), diarrhoea (n=10) and anorexia (n=5). For all animals, except for two patients, anamnestic information, clinical signs on presentation, data of haematological and biochemical abnormalities and results of faecal examinations for parasites were completely available but were unspecific for making a definitive diagnosis. All cats underwent abdominal radiographic and ultrasonographic examinations. Cats in which discrete abdominal masses were recognised by radiography, ultrasonography or laparotomy, were excluded from this study. Transmural biopsy samples from diseased cats were collected during laparotomy under deep general anaesthesia. Normally, one biopsy each was obtained from the fundus region of the stomach, the duodenum descendens, the mid-jejunum and the ileum by using a 4 mm biopsy punch (GE Healthcare). Furthermore, transmural biopsies were also collected from the colon descendens in this study, although it is recommended that full-thickness biopsy of the colon should be avoided for safety reasons. 13 The following extraintestinal samples were also collected: liver (n=13), pancreas (n=9), mesenteric lymph node (n=28), mesenterium (n=2) and ovary (n=1). All tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections (2–4 μm) were cut and stained with haematoxylin and eosin (HE) and, in cases with suspected mast cell tumour, with toluidine blue. In cases with lymphoma, immunohistochemistry was performed as described by Lemburg et al. 19 These protocols were modified for feline tissues in relation to the antibodies against human CD3 (DakoCytomation; polyclonal; dilution 1:800) and human CD79α molecule (DakoCytomation; clone HM57; 1:60) as described by Carreras et al. 20 The mesenteric lymph node of a cat that contains T, B and plasma cells served as a positive control.
For cases with IBD, ie, LPE or LPC, a histological grading system was used. 21
Results
Histopathological diagnoses
For all 43 cats histopathological diagnoses are summarised in Table 1. In 14 cats (32.6%) (mean age 7.7 years), mild or moderate lymphocytic-plasmacytic enterocolitis was diagnosed. In all cases, two or more biopsies showed a mild or moderate increase of lymphocytic-plasmacytic infiltration of the lamina propria mucosae. In total, 85.7% of duodenal samples were affected followed, in descending order, by the jejunum (84.6%), the ileum (62.5%), the colon (61.5%) and the stomach (15.4%). Lymphocytic-plasmacytic infiltration was limited to the lamina propria mucosae of the villi and/or crypt region except for 18/38 affected GIT samples in which a mild lymphocytic-plasmacytic infiltration was also seen in the submucosal layer. In 20/29 affected small intestinal samples mild to severe atrophy or fusion of villi, crypt abscessation and/or architectural distortion of crypts was present. Architectural changes as described above were also recognised in most of all colonic biopsies. Eight out of 14 animals with LPE also displayed a moderate mixed hepatitis and/or moderate to severe chronic pancreatitis or follicular hyperplasia of mesenteric lymph nodes. The mucosa of the ileum of four cats revealed mild to moderate fibrotic bands that were mainly located at the border between crypts and villi. In addition to fibrosis a slight increased inflammatory lymphocytic-plasmacytic infiltrate was present in the mucosa of these cases. In these cases, laparotomy would not have had any advantage over endoscopic techniques.
Table 1.
Histopathological diagnoses and number of affected samples of 43 cats suffering from chronic gastrointestinal disease signs * .
| Diagnosis | Nos. † | Stomach | Duodenum | Jejunum | Ileum | Colon | Extraintestinal organs ‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymph node | Liver | Pancreas | Mesenteric tissue | Ovary | |||||||
| LPE/LPC | 14 | 2/13 | 12/14 | 11/13 | 5/8 | 8/13 | 7/8 | 3/3 | 4/4 | – § | – |
| EGEC | 5 | 1/4 | 4/5 | 4/4 | 2/2 | 4/5 | 2/3 | – | 0/3 | 1/1 | – |
| Mixed | 1 | 1/1 | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 | – | – | – | – |
| Fibrosis | 4 | 2/4 | 0/4 | 2/4 | 4/4 | 0/4 | 1/1 | 2/2 | – | – | – |
| Gastritis | 3 | 3/3 | 0/3 | 0/1 | 0/1 | 0/3 | 1/2 | 2/2 | 0/1 | – | – |
| Intestinal LA | 3 | 0/3 | 3/3 | 3/3 | 2/2 | 1/1 | 2/2 | 2/2 | – | – | – |
| Lymphoma | 10 | 2/9 | 2/9 | 5/10 | 4/4 | 2/8 | 6/8 | 3/6 | 0/2 | 1/1 | 1/1 |
| MC tumour | 2 | 0/2 | 0/2 | 0/2 | 0/1 | 1/2 | 2/2 | – | – | – | – |
| No lesions | 1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | – | – | – | – | – |
LPE/LPC=lymphocytic-plasmacytic enteritis and/or colitis; EGEC=eosinophilic gastroenterocolitis; Mixed=mixed inflammatory infiltration; Fibrosis=intestinal fibrosis; Intestinal LA=intestinal lymphangiectasia; MC tumour=mast cell tumour; No lesions=no histopathological lesions.
Number of affected samples/number of samples examined.
Nos.=number of cases per diagnosis.
Note: for exact histopathological diagnosis see text; Lymph node=mesenteric lymph node.
–=No extraintestinal organs sampled.
In five cats (11.6%) (mean age 10.2 years), an eosinophilic inflammation of the GIT was diagnosed. Evaluation of these five cases revealed that in four of them the colon together with the stomach and/or small intestine was affected. In all cases, increased numbers of eosinophils were found in the mucosa. In 7/20 biopsies eosinophilic infiltration was present in the submucosa and in three further samples also in the tunica muscularis. In addition, in one cat suffering from eosinophilic jejunitis and colitis, the duodenal sample had eosinophilic infiltration at the myenteric ganglia but the overlying mucosal and submucosal layers were without histopathological changes. Extraintestinal biopsies revealed in 2/3 mesenteric lymph nodes an eosinophilic lymphadenitis and in one case an eosinophilic infiltration of the mesenteric tissue. In three cases, the pancreas was without histological abnormalities. The advantages of full-thickness biopsy samples were the observation of eosinophilic transmural infiltrations, infiltration of ganglia without mucosal affection and involvement of extraintestinal tissue.
In one cat (2.3%) (aged 10 years), a mixed inflammatory infiltration with mainly neutrophilic granulocytes and few lymphocytes and plasma cells was observed (Fig 1). This mixed inflammatory lesion was not restricted to the mucosa but extended into the tunica muscularis. In this cat in all biopsy samples, excluding the duodenum, a mixed inflammation was present. The duodenum displayed mild LPE. The mixed inflammation was accompanied by architectural distortion, eg, focal epithelial necrosis and atrophy or dilation of crypts. The collected mesenteric lymph node was also affected by a mild mixed inflammation. These GIT lesions would have been detectable by endoscopic techniques as well.
Fig 1.

A mixed inflammatory infiltration with mainly neutrophilic granulocytes and few lymphocytes and plasma cells was observed in the colon of one cat. The mixed inflammation was accompanied by architectural distortion (indicated by arrows), eg, focal epithelial necrosis and atrophy of crypts. HE. Bar=200 μm.
In four cats (9.3%) (mean age 7.9 years) the only histopathological change was a marked band-shaped intestinal fibrosis of the lamina propria mucosae without increased inflammatory infiltrate or other architectural changes (Fig 2). These fibrotic bands were mainly located at the border between crypts and villi. In all four cases the ileum was affected. In one of these cats the stomach and jejunum were also affected and in two other cats the stomach or jejunum displayed fibrotic bands. Extraintestinal biopsies in one of these cats revealed a hydropic degeneration of hepatocytes accompanied by a moderate lymphocytic-plasmacytic hepatitis. In another cat a mild suppurative mesenteric lymphadenitis and hepatitis were recognised. In these four cases laparotomy had no advantages over endoscopy.
Fig 2.
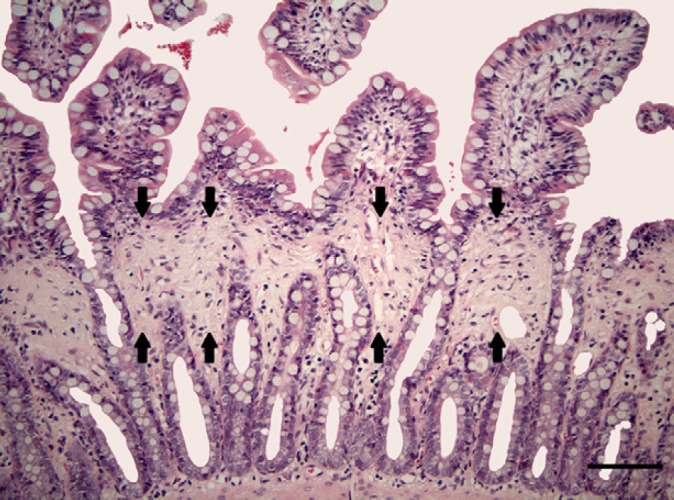
Band-shaped intestinal fibrosis of the ileal lamina propria mucosae without increased inflammatory infiltrate or other architectural changes. Fibrotic bands were mainly located at the border between crypts and villi (indicated by arrows). HE. Bar=100 μm.
In three cats (7.0%) (mean age 3.3 years), only the stomach was affected. In two of them a mixed gastritis was present, accompanied by loss of foveolae gastricae and fibrosis. The other case showed a mild lymphocytic-plasmacytic inflammation of the mucosa. Extraintestinal samples in one case revealed a mild hydropic degeneration of hepatocytes, in the second cat a mild purulent mesenteric lymphadenitis and in the third case a mild suppurative hepatitis.
In three cats (7.0%) (mean age 5 years) varying degrees of lymphangiectasia of the lamina propria of the small and large intestinal mucosa, without increased inflammatory cell infiltrate, was the major histopathological feature (Fig 3). Two out of three cats displayed transmural lymphangiectasia accompanied by slight or moderate mucosal oedema. The severity of lymphangiectasia was not as dramatic as in dogs with protein-losing enteropathy. A moderate deep fibrosis of the stomach accompanied by a mild lymphocytic-plasmacytic superficial gastritis was also observed in one cat. In addition, a few neutrophilic granulocytes were scattered throughout the gastric mucosa in a second cat. Laboratory results of these three cats did not reveal a protein-losing enteropathy and dilated lacteals were visible on intestinal serosal surfaces at laparotomy in only one cat. In two cats, histopathological changes were seen in biopsies from the liver and mesenteric lymph node, ie, mild to moderate multifocal to coalescing hydropic degeneration of hepatocytes with moderate to severe portal fibrosis or mild lymphocytic-plasmacytic hepatitis in combination with mild nodular hyperplasia of lymph nodes in both cases.
Fig 3.

Transmural lymphangiectasia of the duodenal lamina propria and submucosa (arrows) without increased inflammatory cell infiltrate. HE. Bar=200 μm.
In 10 cats (23.3%) (mean age 9.3 years) a diffuse lymphoma was diagnosed. Except for four patients, one, two or all three biopsies from the small intestine were affected. In two cats the stomach or the colon, respectively, was also affected. In 8/15 affected biopsies, infiltration of the intestine with neoplastic lymphocytes was not only present in the lamina propria mucosae but also extended into the submucosa. In 5/15 affected biopsies, not only submucosal but also transmural infiltration was observed. In eight cases tumour cells were small sized and in two cases of large cell type. In three cats LPE or LPC was present in adjacent intestinal samples. One of these cats had no neoplastic cells within their gastrointestinal biopsies but a malignant lymphoma was diagnosed in the mesenteric tissue. In addition, the mesenteric lymph nodes of six cats, the liver of three cats and the ovary of one cat showed an infiltration with lymphoma cells. The neoplasia of six cats with small sized tumour cell population was of T cell origin (CD3 positive) whereas in the remaining four cases neoplastic cells were of B cell origin (CD79α positive). A thickening of the intestinal wall was noted in one cat at laparotomy. The ability to sample extraintestinal biopsies was the main advantage of laparotomy in contrast to endoscopy.
In biopsies from two cats (4.7%) (mean age 9.0 years) a mast cell tumour was diagnosed in the submucosa of the colon and mesenteric lymph node or in the mesenteric lymph node alone. The tumour was densely cellular, well demarcated and grew in a band-like manner or showed filiform growth in the lymph node of the second cat. The cells were loosely arranged or closely packed in the lymph node of the second cat growing in sheets with a fine fibrovascular stroma. They were round, measuring 12×18 μm with distinct cell borders and had a scant or in the lymph node a high amount of pale eosinophilic, slightly granular cytoplasm. The nucleus was round, centrally located with a coarsely stippled hyperchromatic chromatin pattern and one prominent basophilic nucleolus. In all locations the mitotic rate was low. Granula of the neoplastic cells showed metachromatic staining with toluidine blue. The tumour in the large intestinal samples of the first cat was infiltrated by numerous eosinophilic granulocytes. The small intestinal samples of this cat showed architectural distortion, ie, loss of crypts, shortening of villi and fibrosis, accompanied by a high amount of eosinophilic granulocytes. In addition, the stomach of this cat displayed a moderate chronic active gastritis. The duodenal biopsies of the second cat revealed only slightly elevated lymphocytic-plasmacytic infiltration of the mucosa. In both cases, clinical evaluation revealed a leukocytosis and peripheral eosinophilia whereas no morphological changes were obvious at laparotomy.
In biopsies from a male-neutered, 3-year-old European shorthair cat no histopathological lesions were found except for a mild aggregation of lymphoid cells in the lamina propria mucosae of the stomach.
Discussion
In the present study, 42/43 cats (97.7%) with chronic gastrointestinal disease signs revealed different histopathological gastrointestinal alterations in full-thickness biopsies from the stomach, small intestine and colon.
Inflammatory lesions represented the most frequent alteration. In 20 cats (46.5%), an increase of inflammatory cells in the lamina propria mucosae comprising lymphocytes, plasma cells, eosinophilic or neutrophilic granulocytes was observed. In 14/43 cats (32.6%) LPE or LPC was present. In these cats, histopathological findings largely resembled those described by other authors. 2–4,14,18,22 Almost 50% of intestinal samples from cats with LPE or LPC had mononuclear infiltrates in the submucosa. It is important that these deeper infiltrates not be confused with lymphoma.
Histopathological changes were accompanied by lymphatic hyperplasia or pancreatitis with interstitial fibrosis. In three cats an inflammation of the small intestine was accompanied by hepatitis and pancreatitis. This constellation was described as ‘triaditis’ by Weiss et al. 23
In this study, eosinophilic inflammatory changes were found in five cats (11.6%). The cause of chronic eosinophilic inflammatory gastrointestinal disease of cats is not known. Food allergy has been proposed but not proven as a cause of eosinophilic gastroenteritis in cats. As trials to evaluate the clinical response to exclusion diets were not undertaken in this study, the underlying possible causes of eosinophilic inflammatory changes seen in gastrointestinal biopsy samples of our cases remain unknown. For cats in the present study endoparasitism as a possible cause of eosinophilic inflammatory infiltration of stomach, small intestine or colon could be ruled out because of negative results from faecal examinations for parasitic organisms. As seen in 50% of affected GIT samples of this study eosinophilic granulocytes had the tendency to infiltrate beyond the lamina propria mucosae, sometimes with transmural involvement. Eosinophilic infiltrates were also noted at myenteric ganglia in samples without histopathological changes of the mucosa. A possible interaction between eosinophilic granulocytes and the enteric nervous system may have contributed to clinical enteric dysfunction. These observations would not have been made if endoscopically obtained biopsy samples were examined.
Our results, like those of another report, suggest that in feline eosinophilic conditions the stomach is less frequently affected. 24 In contrast, mesenteric lymph nodes are frequently involved in eosinophilic conditions similar to those described by other authors and collection of these lymph nodes is very helpful for histopathological diagnostics. 4,7,24
In one cat (2.3%) the increased inflammatory infiltrate consisted of lymphocytes, plasma cells and mainly neutrophilic granulocytes. This mixed inflammation was not limited to the colon as described for feline chronic suppurative colitis. 25 Except from the duodenum, all other gastrointestinal samples were affected. As reported by Lecoindre and Chevallier, a suppurative colitis is a rare subcategory of feline IBD. 17 The collected mesenteric lymph node of this cat also showed an infiltration with neutrophilic granulocytes. In this case a bacteriological aetiology cannot be ruled out. Due to this fact and the widespread distribution in this case, it is questionable whether this condition should be summarised under the term IBD, but IBD cannot be ruled out because of chronic clinical signs and the unknown cause of the disease.
In four cats (9.3%) marked band-shaped fibrosis of the intestinal mucosa was the main histopathological finding. Fibrotic lesions were not accompanied by an increased inflammatory cell infiltrate in any of the investigated biopsies. In all four cases the ileum was affected showing varying degrees of fibrosis. Intestinal mucosal fibrosis has been described for feline and canine IBD. Four cases of this study with LPE/LPC showed fibrotic bands with an inflammatory infiltrate also in the ileum. The course for the high incidence of fibrosis in the ileum is unknown. As reported, fibrosis can be a histopathological feature of LPE/LPC with strictures of gut in severe cases. 2,14,18 So far, marked band-shaped mucosal fibrosis without inflammatory cell infiltrate in cats with chronic idiopathic gastrointestinal disease had not been described. This is the first study describing marked band-shaped fibrosis without inflammatory cell infiltration and is different from the recently published entity of eosinophilic sclerosing fibroplasia. 26 Other authors have described fibrosis in feline IBD but it is unclear if these cases were with or without inflammatory infiltrates. 18 The pathogenesis of fibrotic lesions in these four cats is unknown. It has been reported that continued exposure of the immune system to offending antigens may result in persistent inflammation and eventual fibrosis. 3,14 The fibrotic lesions in those cases may reflect a possible end-stage lesion of feline IBD.
In three cats (7.0%), a gastritis consisting of a mixed or lymphocytic-plasmacytic inflammation was present. The main clinical sign in these cats was vomiting and was presumably the consequence of the inflammatory lesions of the stomach because no other chemical or histopathological abnormalities were present. One of these three cats, however, had also diarrhoea. This raises the question whether inflammatory changes were present in intestinal segments which had not been sampled. Otherwise, it cannot be excluded that the gastritis influenced the neuromotoric intestinal activity, leading to hypermotility-related diarrhoea. Changes in intestinal motor activity are described for acute colitis in dogs and experimental colitis in cats. 27,28 Furthermore, it could also be possible that the hepatic changes present in these two cats were responsible for clinical signs.
In contrast to samples from dogs with chronic gastrointestinal signs, which were taken under the same conditions, in only three cases of this study (7.0%) was the predominating histopathological finding intestinal lymphangiectasia. 29 All biopsy samples from the small and large intestine displayed lymphangiectasia at least in one layer of the gut wall and these dilatations of lacteals were accompanied by an oedema of the lamina propria mucosa. The possible cause of intestinal lymphangiectasia and oedema in these animals is not clear. Oedema of the intestinal lamina propria may be a possible artefact occurring in biopsies obtained by endoscopy from the intestinal tract of dogs and cats. 30 As discussed for dogs the possibility of being a biopsy artefact is highly unlikely. 29 In general, intestinal lymphangiectasia in cats occurs less often than in dogs. 31 It can be a primary disorder, ie, congenital or idiopathic, or acquired as a result of lymphatic blockage by neoplastic lesions or inflammatory infiltrates, eg, IBD, or elevated venous pressure, eg, congestive heart failure or portal hypertension. 31,32 It cannot be excluded, however, that lymphangiectatic and oedematous changes without increase in the cellularity of the lamina propria mucosae or other lesions may be due to an increased capillary permeability induced by the effects of certain mediators originating from inflammatory cells, eg, mast cells, located in the lamina propria mucosae. Further, it cannot be ruled out that the histopathological changes of extraintestinal samples contributed to the lymphangiectasia because in one case portal fibrosis of the liver was noted leading eventually to portal hypertension.
In 23.3% of the cases of this study a diffuse lymphoma was diagnosed. One of the most important differential diagnoses of diffuse intestinal lymphoma is LPE and in many cases the demonstration of transmural spread of the neoplastic lymphoid cells is a very helpful method of diagnosing a neoplastic infiltration of the intestinal wall. 7,8 The results of the present study clearly demonstrate the advantage of sampling transmural and extraintestinal biopsies for diagnosing intestinal lymphoma, and are consistent with results of Evans et al. 8 In 13/15 affected intestinal samples an extension of the neoplastic process into deeper layers of the intestinal walls was found, with lymphoid tumour cells infiltrating the submucosa or in five biopsies both submucosa and tunica muscularis. Submucosal spread of inflammatory cells also occurred in cases with LPE/LPC in this study. But transmural infiltration, affecting all layers of the gut wall including tunica muscularis, was not noted in any cases with IBD in contrast to malignant lymphoma (34%) in this study. As mentioned by Tams and seen in this study, mesenteric lymph nodes and the liver are frequently involved in cases of diffuse intestinal lymphoma and should be sampled whilst performing laparotomy. 4,7 Without sampling extraintestinal biopsies one case would have been misdiagnosed as LPE because neoplastic cells were only located in the mesenteric tissue. It is reported that malignant lymphoma of the GIT mainly arises in the jejunum and ileum, as observed in this study. 7,8,12 If only stomach, duodenum and/or colon had been available for histological investigations, LPE would have been diagnosed in three cats on our study. In two cats no histopathological lesions would have been detected because neoplastic cells were limited to jejunum and/or ileum and/or extraintestinal biopsies. In general, the ileum seems to be the most consistently affected organ and, therefore, should always be biopsied when lymphoma is a consideration. Findings of lymphocytes outside the GIT can be helpful in diagnosing malignant lymphoma. It has been reported that intestinal lymphoma in cats arises on the basis of LPE. 3,6,30 In three cats with lymphoma we found a mild to severe LPE or LPC, respectively, in samples obtained from adjacent intestinal segments, indicating progression of IBD to intestinal lymphoma in these three cases.
In two cats (4.7%) a mast cell tumour was diagnosed. One of these cats showed an eosinophilic enteritis in 3/5 five intestinal biopsies, whereas the lamina propria of the colon displayed a diffuse infiltration with neoplastic mast cells. It is reported that infiltrating eosinophilic granulocytes can be so numerous in mast cell tumours mimicing an eosinophilic enteritis. In the second cat a mast cell neoplasm was only detected in the additional extraintestinal sample, namely the mesenteric lymph node, and would have been misdiagnosed as LPE in endoscopically sampled biopsies.
In samples from one cat, in spite of the presence of chronic clinical gastrointestinal disease signs, no histopathological changes were found. It cannot be ruled out that the biopsy samples obtained did not include any lesions which may have been located adjacent to the areas sampled. As no haematological or biochemical abnormalities or other abnormal clinical findings were recorded the possible reason for the clinical signs in the animal remains unclear. In cases without histopathological findings, a patchy distribution of IBD or lymphoma could be the underlying cause of clinical signs and neither endoscopic nor transmural samples are able to solve this problem.
Our study revealed that the availability of transmural biopsies from all segments of the intestine including the submucosal tissue layer and the collection of extraintestinal samples are helpful for diagnosing intestinal tumours such as lymphomas and tumours of mast cell origin. The collection of mesenteric lymph node may be very helpful for diagnosing chronic gastrointestinal disease of unknown origin. IBD in the cat can be diagnosed with transmural or endoscopic samples, but in the latter misdiagnoses, especially of diffuse intestinal lymphoma, may occur. Furthermore, this investigation showed that the main histopathological diagnoses in feline chronic enteropathies are inflammatory lesions which can be mainly grouped under the term IBD.
Acknowledgements
We wish to thank Mr K-P Kuhlmann for his excellent technical assistance.
References
- 1.Jergens A.E., Moore F.M., Haynes J.S., Miles K.G. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987–1990), J Am Vet Med Assoc 201, 1992, 1603–1608. [PubMed] [Google Scholar]
- 2.Dennis J.S., Kruger J.M., Mullaney T.P. Lymphocytic/plasmacytic colitis in cats: 14 cases (1985–1990), J Am Vet Med Assoc 202, 1993, 313–318. [PubMed] [Google Scholar]
- 3.Guilford W.G. Idiopathic inflammatory bowel disease. Guilford W.G., Center S.A., Strombeck D.R., Williams D.A., Meyer D.J. Strombeck's small animal gastroenterology, 1996, WB Saunders: Philadelphia, 451–486. [Google Scholar]
- 4.Tams T.R. Chronic feline inflammatory bowel disorders. Part I. Idiopathic inflammatory bowel disease, Compend Contin Educ Pract Vet 8, 1986, 371–376. [Google Scholar]
- 5.Jergens A.E. Feline idiopathic inflammatory bowel disease, Compend Contin Educ Pract Vet 14, 1992, 509–518. [Google Scholar]
- 6.Tams T.R. Feline inflammatory bowel disease, Vet Clin North Am Small Anim Pract 23, 1993, 569–586. [DOI] [PubMed] [Google Scholar]
- 7.Tams T.R. Chronic feline inflammatory bowel disorders. Part II. Feline eosinophilic enteritis and lymphosarcoma, Compend Contin Educ Pract Vet 8, 1986, 464–471. [Google Scholar]
- 8.Evans S.E., Bonczynski J.J., Broussard J.D., Han E., Baer K.E. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats, J Am Vet Med Assoc 229, 2006, 1447–1450. [DOI] [PubMed] [Google Scholar]
- 9.Hall E.J., Simpson K.W. Diseases of the small intestine. Ettinger S.J., Feldman E.C. Testbook of veterinary internal medicine, 2000, WB Saunders: Philadelphia, 1182–1238. [Google Scholar]
- 10.Willard M.D., Lovering S.L., Cohen N.D., Weeks B.R. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats, J Am Vet Med Assoc 219, 2001, 474–479. [DOI] [PubMed] [Google Scholar]
- 11.Zoran D.L. Gastroduodenoscopy in the dog and cat, Vet Clin North Am Small Anim Pract 31, 2001, 631–656. [DOI] [PubMed] [Google Scholar]
- 12.Richter K.P. Feline gastrointestinal lymphoma, Vet Clin North Am Small Anim Pract 33, 2003, 1083–1098. [DOI] [PubMed] [Google Scholar]
- 13.Willard M.D. Feline inflammatory bowel disease: A review, J Feline Med Surg 1, 1999, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson R.W., Dimperio M.E., Long G.G. Lymphocytic-plasmacytic colitis in the cat, J Am Vet Med Assoc 184, 1984, 1133–1135. [PubMed] [Google Scholar]
- 15.Wolf A.M. Feline lymphocytic-plasmacytic enterocolitis, Semin Vet Med Surg (Small Anim) 7, 1992, 128–133. [PubMed] [Google Scholar]
- 16.Mahony O.M., Moore A.S., Cotter S.M., Engler S.J., Brown D., Penninck D.G. Alimentary lymphoma in cats: 28 cases (1988–1993), J Am Vet Med Assoc 207, 1995, 1593–1598. [PubMed] [Google Scholar]
- 17.Lecoindre P., Chevallier M. Contribution to the study of feline inflammatory bowel disease: 51 cases (1991–1994), Rev Med Vet (Toulouse) 148, 1997, 893–902. [Google Scholar]
- 18.Baez J.L., Hendrick M.J., Walker L.M., Washabau R.J. Radiographic, ultrasonographic, and endoscopic findings in cats with inflammatory bowel disease of the stomach and small intestine: 33 cases (1990–1997), J Am Vet Med Assoc 215, 1999, 349–354. [PubMed] [Google Scholar]
- 19.Lemburg A.K., Meyer-Lindenberg A., Hewicker-Trautwein M. Immunohistochemical characterization of inflammatory cell populations and adhesion molecule expression in synovial membranes from dogs with spontaneous cranial cruciate ligament rupture, Vet Immunol Immunopathol 97, 2004, 231–240. [DOI] [PubMed] [Google Scholar]
- 20.Carreras J.K., Goldschmidt M., Lamb M., McLear R.C., Drobatz K.J., Sørenmo K.U. Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997–2000), J Vet Intern Med 17, 2003, 326–331. [DOI] [PubMed] [Google Scholar]
- 21.Day M.J., Bilzer T., Mansell J., et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group, J Comp Pathol 138 (suppl 1), 2008, 1–43. [DOI] [PubMed] [Google Scholar]
- 22.Willard M.D., Dalley J.B., Trapp A.L. Lymphocytic-plasmacytic enteritis in a cat, J Am Vet Med Assoc 186, 1985, 181–182. [PubMed] [Google Scholar]
- 23.Weiss D.J., Gagne J.M., Armstrong P.J. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis and nephritis in cats, J Am Vet Med Assoc 209, 1996, 1114–1116. [PubMed] [Google Scholar]
- 24.Hendrick M. A spectrum of hypereosinophilic syndromes exemplified by six cats with eosinophilic enteritis, Vet Pathol 18, 1981, 188–200. [DOI] [PubMed] [Google Scholar]
- 25.Leib M.S., Sponenberg D.P., Wilcke J.R., Loar A.S. Suppurative colitis in a cat, J Am Vet Med Assoc 188, 1986, 739–741. [PubMed] [Google Scholar]
- 26.Craig L.E., Hardam E.E., Hertzke D.M., Flatland B., Rohrbach B.W., Moore R.R. Feline gastrointestinal eosinophilic sclerosing fibroplasia, Vet Pathol 46, 2009, 63–70. [DOI] [PubMed] [Google Scholar]
- 27.Sethi A.K., Sarna S.K. Colonic motor activity in acute colitis in conscious dogs, Gastroenterol 100, 1991, 954–963. [DOI] [PubMed] [Google Scholar]
- 28.MacPherson B.R., Shearin N.L., Pfeiffer C.J. Experimental diffuse colitis in cats: Observations on motor changes, J Surg Res 25, 1978, 42–49. [DOI] [PubMed] [Google Scholar]
- 29.Kleinschmidt S., Meneses F., Nolte I., Hewicker-Trautwein M. Retrospective study on the diagnostic value of full-thickness biopsies from the stomach and intestines of dogs with chronic gastrointestinal disease symptoms, Vet Pathol 43, 2006, 1000–1003. [DOI] [PubMed] [Google Scholar]
- 30.Wilcock B. Endoscopic biopsy interpretation in canine or feline enterocolitis, Semin Vet Med Surg (Small Anim) 7, 1992, 162–171. [PubMed] [Google Scholar]
- 31.Peterson P.B., Willard M.D. Protein-losing enteropathies, Vet Clin North Am Small Anim Pract 33, 2003, 1061–1082. [DOI] [PubMed] [Google Scholar]
- 32.Tams T.R. Chronic diseases of the small intestine. Tams T.R. Handbook of small animal gastroenterology, 2nd edn, 2003, Elsevier: Philadelphia, 211–250. [Google Scholar]


