Abstract
This study examined the efficacy of doxorubicin-based chemotherapy used for rescue therapy in refractory feline lymphoma. Records of 23 cats with lymphoma treated with chemotherapy who received doxorubicin for the first time in a rescue setting were reviewed. Seventeen (74%) of the 23 cats had only one treatment of doxorubicin. Five (22%) of the 23 cats had a positive response to doxorubicin and were given additional doses. The response to therapy in 4/5 of these responders could be assessed objectively, of which, two cats (9%) achieved partial remission (PR) and two cats (9%) achieved complete remission (CR). The two cats that achieved CR had differing response durations (6 weeks and greater than 47 months). Three of these five (60%) responders had also received concurrent other chemotherapy in addition to doxorubicin. Cell type and the use of concurrent chemotherapy were significant predictors of response. Cats with small-medium cell lymphomas (P=0.001) and cats that received concurrent chemotherapy with doxorubicin rescue (P=0.007) were more likely to respond favorably. This study suggests that doxorubicin-based chemotherapy is not an effective rescue protocol for feline lymphoma.
Doxorubicin is one of the anthracycline antibiotics which attacks neoplastic cells via multiple mechanisms, including generation of reactive oxygen species, activating signal transduction pathways, stimulation of apoptosis, and inhibition of protein synthesis by intercalating with DNA and disrupting topoisomerase activity. In veterinary oncology doxorubicin has been used as part of various chemotherapeutic protocols for a broad range of tumor types including lymphoma, osteosarcoma, hemangiosarcoma, and carcinomas. It is one of the most commonly used drugs in canine lymphoma and has documented activity both as part of induction and maintenance therapies. 1–4 It has also shown to be effective as a rescue agent in dogs whose lymphomas have become resistant to standard COP-based (cyclophosphamide, vincristine, and prednisone) protocols and CHOP-based (cyclophosphamide, doxorubicin, vincristine, and prednisone) protocols. 5,6
Currently, chemotherapeutic treatments for feline lymphoma have not been as successful as their canine counterparts. While the median survival time of most canine protocols for lymphoma has been reported to be about 12 months, median survival times for cats with lymphoma lag behind those of dogs. 7,8 The reported median survival times for feline lymphoma ranges from about 2 to 8 months. 9–12
Rescue protocols are chemotherapy protocols use to treat refractory cancers. They are commonly used in the treatment of lymphoma after first-line therapy has failed in an attempt to induce a subsequent remission and prolong survival times. However, achieving a second remission in cats can be quite difficult and is often a source of frustration. Previous studies of feline lymphoma have evaluated the efficacy of doxorubicin as part of combination protocols and as a single agent in both induction and in maintenance protocols. 13–16 While doxorubicin is also used for rescue therapy of feline lymphoma, to the authors' knowledge, studies regarding the efficacy of doxorubicin in a rescue setting have not been published.
Studies investigating doxorubicin as a single agent for induction in cats have shown complete remission rates ranging from 26% to 32%. 15,16 Comparatively, complete response rates achieved with single-agent doxorubicin induction in dogs are much better, with a reported range from 50% to 76%. 1,2 Several studies have examined doxorubicin's role as part of combination therapy in cats. One study showed that cats switched to single-agent doxorubicin maintenance had substantially increased remission durations compared to those kept on COP protocols. 17 Another study showed complete remission rates of 80% when cats were treated with a combination protocol of l-asparaginase, vincristine, cyclophosphamide, doxorubicin and methotrexate. 13 However, a more recent study showed a similar response rate in cats treated with COP alone excluding doxorubicin, l-asparaginase, and methotrexate. 18 Vail and others investigated if the addition of doxorubicin to a standard COP-based protocol offered a survival advantage to cats with lymphoma. 19 While the addition was shown to result in longer, more durable remissions in univariate analysis, this did not maintain significance in multivariate analysis. 19
Doxorubicin's role in the therapy of feline lymphoma for induction and maintenance chemotherapy has been investigated, however, its role in rescue therapy has not been as well defined. While many veterinarians include doxorubicin as one of their first-line agents in treating feline lymphoma, there are some who prefer not to include doxorubicin in their initial protocol due to the drug's unique considerations in handling and administration. Veterinary oncologists are often referred cases at the time of relapse and it is these cases that are often being rescued with doxorubicin-based chemotherapy.
The purpose of the present study was to evaluate the efficacy of doxorubicin-based chemotherapy as rescue for refractory feline lymphoma and identify factors correlating with response. The hypothesis was that doxorubicin-based rescue would be well tolerated and would induce a remission in refractory feline lymphoma, and that it would be more successful in cats with lymphoblastic lymphomas compared to other cell types. Lymphoblastic lymphomas may have a higher growth fraction which could make them more responsive to a chemotherapeutic agent like doxorubicin.
Materials and Methods
Medical records were searched to identify cats with lymphoma treated with chemotherapy at the Matthew J Ryan Hospital of Veterinary Medicine of the University of Pennsylvania (MJR-VHUP) from the years 1997–2003 and that received doxorubicin or doxorubicin-based chemotherapy as part of treatment. Cats were only included in this retrospective study if lymphoma was their only tumor, they had failed standard feline lymphoma induction protocols, and they had no prior exposure to doxorubicin. All types of lymphomas were included in the search. Cats were determined to have failed the induction protocol if the attending clinician concluded that the cat had experienced a relapse of their disease. This determination was based on palpation, abdominal ultrasound, radiographs, or bloodwork.
Information regarding signalment, body weight, diagnosis, stage, treatment, and response to treatment was collected. Staging tests included physical examination findings, complete blood count, urinalysis, serum biochemical analysis, thoracic radiography, abdominal ultrasonography, bone marrow aspiration, retroviral testing, and lymph node aspiration cytology and or biopsy. Cats were grouped both by anatomic location and by the staging system suggested by Mooney and Hayes. 20 (See Table 1).
Table 1.
Clinical stage assignment as suggested by Mooney and Hayes 20
Stage 1
|
Stage 2
|
Stage 3
|
Stage 4
|
Stage 5
|
Response to treatment was evaluated objectively whenever possible. Such objective tumor response measurements included: physical examination findings, complete blood count, urinalysis, serum biochemical analysis, thoracic radiography, abdominal ultrasonography, bone marrow aspiration cytology, and lymph node aspiration cytology or biopsy. The objective responses of these cats were categorized into four groups as follows: Progressive disease (PD) or ≥25% increase in the size of the measurable tumor or lymph node or appearance of a new disease site, stable disease (SD) or a decrease of <50% or an increase of <25% of measurable disease or lymph nodes, partial remission (PR) or >50% reduction in their measurable disease, and complete remission (CR) or disappearance of all clinical evidence of tumor. When objective criteria could not be used, the response to therapy was determined subjectively by data found in the medical record indicating improved or resolving clinical signs or improved performance status.
Classification according to cell type
Cell type was determined either by an anatomic (EM) or a clinical pathologist (PM) who were both blinded to the clinical response of the cats. All cats were diagnosed either by cytology or biopsy. Twelve of the 14 original biopsy slides and all of the original cytology slides were available for review by the pathologists. All cats were placed into one of four groups by cell size: small-medium (which were slightly larger than the typical small cell or lymphocytic lymphomas), intermediate, large, and granular cells.
Statistical analysis
Remission duration was calculated from the date of initiation of therapy to date disease was noted to have progressed. Survival was calculated as time from date of initiation of therapy to date of death or last follow-up. Cats lost to follow-up or that were alive at time of last contact were censored at that time. The Kaplan-Meier product limit method was used to estimate disease free interval or survival for responders and non-responders to doxorubicin. To assess differences in survival and disease free interval between cats who responded to doxorubicin and those who did not, the log rank test was used. 21 The χ2 or Fisher's exact test was used to determine predictors of response to doxorubicin. Possible predictive parameters analyzed included sex, stage, breed, body weight, cell type, use of concurrent chemotherapy, and dosing method. Statistical significance was defined as P<.05. All analyses were performed with SAS statistical software (Version 9.1, SAS Software, SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Twenty-three cats met inclusion criteria for this study. Ten (43%) were castrated males. Thirteen (57%) were spayed females. The mean age at diagnosis was 8.7 years, with a range of 1–16 years. The mean weight at presentation was 3.8 kg. Most (n=19 or 83%) cats were domestic shorthairs. There were two (9%) Siamese cats, one (4.5%) Abyssinian, and one (4.5%) Maine Coon cat. The feline leukemia virus status was known in 17 cats, with three (18%) of those having a positive status. All cats were initially treated with COP-based protocols for their first-line therapy, which included prednisone, l-asparaginase, cyclophosphamide, and vincristine. (See Table 2 for the standard COP-based protocol used at MJR-VHUP). The median first remission duration was 8 weeks (range 1–30 weeks).
Table 2.
The Matthew J Ryan Veterinary Hospital of the University of Pennsylvania feline weekly sequential lymphoma protocol
| Treatment week | Drug, dosage, and route |
|---|---|
| 1 | L-asparaginase, 400 IU/kg, SQ |
| 2 | Vincristine 0.5 mg/m2, IV |
| 3 | Cyclophosphamide 50 mg PO (25 mg PO day 1 and 25 mg PO on day 3) |
| 4 | Vincristine 0.5 mg/m2, IV |
| 5 | Methotrexate 2.5 mg PO * |
| 6–9 | Repeat week 2–5 |
The protocol is followed on a weekly basis for 4–6 months. If the patient is in a complete remission (CR) at 4–6 months, the treatments are spread out to alternating weeks for 6–8 additional months. If the patient is in a CR after a year of treatment, the treatments are spread out to every 3 weeks for an additional 6 months. If a patient is in a CR at 18 months, therapy is discontinued. A complete blood count (CBC) is performed initially before all chemotherapy treatments, then ultimately before each intravenous treatment. IV=intravenous, SQ=subcutaneous, PO=oral.
In cases of renal or central nervous system (CNS) lymphoma, substitute cytosine arabinoside (600 mg/m2, divided SQ 3–4 times daily for 4 days).
Staging
Cats were grouped using two different methods, both by anatomic location and by the staging system suggested by Mooney and Hayes. When grouped by anatomic location, the majority of cats (11 cats) had alimentary lymphoma. Two cats had mediastinal disease. One each had renal or nasal lymphoma. Eight remaining cats fell into a miscellaneous category, which included cats with extranodal lesions, ocular tumors, or combinations of the above sites that didn't clearly fit into a single category.
When divided into the stages proposed by Mooney and Hayes, most cats (n=12) were stage III of the clinical stage system. This stage includes lymphadenopathy on both sides of the diaphragm and unresectable abdominal disease. Many of the gastrointestinal lymphomas were grouped into this stage. Five cats had stage IV disease. Two cats each had stage V, stage I, and stage II disease.
Histopathological and cytological cell types
To determine the grade of the lymphoma, cytology and histopathology were performed. Fourteen specimens were diagnosed via biopsy and nine via cytology. No case had both cytology and biopsy performed. All diagnostic specimens except two (both biopsy samples) were located and reviewed by either a clinical (PM) or anatomic pathologist (EM). Large cell or lymphoblastic lymphomas were most common cell type, comprising 66% of the cats (n=14). Three were small-medium cell lymphomas. Two cats each had intermediate and granular cell lymphomas. None of the cats in this study had typical small cell or lymphocytic lymphoma. Of the nine cases diagnosed via cytology, eight were lymphoblastic lymphomas and one was a granular. The rest were diagnosed by histopathology.
Doxorubicin administration
Cats received either 1 mg/kg or 25 mg per square meter of doxorubicin. Dosing method was chosen by clinician preference, with the majority of cats (15 cats) dosed at 1 mg/kg. Doxorubicin was given intravenously diluted in saline over 10 to 20 min at 3-week intervals. Complete blood counts were routinely drawn 7–10 days following administration. All cats were maintained on oral prednisone therapy at 5 mg per day during therapy. Because of the potential gastrointestinal side effects, cats were routinely given oral metoclopramide or oral ondansetron following doxorubicin administration for nausea.
Response
Seventeen cats had either PD or continued clinical decline after doxorubicin therapy. All of these cats were only given one dose of doxorubicin. Six of the 17 cats received subsequent rescue attempts with other chemotherapeutic agents after failing doxorubicin, including lomustine, mechlorethamine, procarbazine, cytarabine, and chlorambucil. Two of the six cats responded to a subsequent chemotherapy. One responded to lomustine and the other responded to cytosar. Both of these cats had lymphoblastic lymphomas.
One cat achieved SD on doxorubicin. This cat had a palpable abdominal mass, liver involvement of his lymphoma, and elevated liver enzymes. The liver enzymes decreased after administration of doxorubicin but the abdominal mass was palpably unchanged. Because the cat was still enjoying a good quality of life, therapy was continued for an additional dose. This cat was given a dose of l'asparginase between the first and second dose of doxorubicin. The cat subsequently declined and chemotherapy was discontinued. This cat had a granular cell lymphoma.
Five cats had a positive response (either PR or CR) to doxorubicin-based rescue. Three out of these five did receive concurrent chemotherapy consisting of doses of cyclophosphamide or cytarabine interspersed between doses of doxorubicin. Each of these cats received multiple doses of doxorubicin, ranging from two to six cycles. The responses of 4/5 cats could be evaluated objectively using either abdominal ultrasound, palpation of the tumor, and/or bloodwork. These four cats were further classified based on their response; two had PRs and two achieved CRs. The remaining cat could not be evaluated objectively and was evaluated by clinical signs. This cat did have cessation of vomiting and diarrhea as well as an increased appetite and weight gain. This cat ultimately received six doses of doxorubicin and had a response duration of 5.6 months. The overall response rate (including the cat evaluated by clinical signs) was 22%. Only 9% (2/23 total cats) had CRs. The remission durations of the two cats achieving PR were each about 2 months (65 days and 67 days). The remission durations of the two cats achieving CR differed widely. One was 1.5 months and the other is still alive at the time of writing (47 months). Both of these cats received additional chemotherapy after doxorubicin.
Factors predictive for response
Statistical analysis was performed to assess factors predictive of response to doxorubicin-based rescue. Cell type and the use of concurrent chemotherapy were statistically significant for response.
The cats that responded to doxorubicin-based rescue were more likely to have small-medium cell lymphomas (P=0.001). All three of the small-medium cell lymphomas responded positively to the rescue. One achieved CR and the other two achieved PR. None of the cats with large cell lymphomas responded to doxorubicin-based rescue. Of the two cats with granular cell lymphomas, one achieved PR and the other SD. Of the two cats with intermediate cell lymphomas, one achieved CR while the other experienced PD.
The cats that responded to doxorubicin-based rescue were also more likely to have received concurrent chemotherapy interspersed between doses of doxorubicin than to have received doxorubicin alone (P=0.007). Three of five of the responders received concurrent chemotherapy consisting of either cyclophosphamide or cytarabine given between doses of doxorubicin. Both cats with CRs received concurrent chemotherapy. One cat with CR received four total doses of doxorubicin interspersed with cytarabine, vincristine, and l'asparaginase. The other cat with CR received three total doses of doxorubicin interspersed with lomustine.
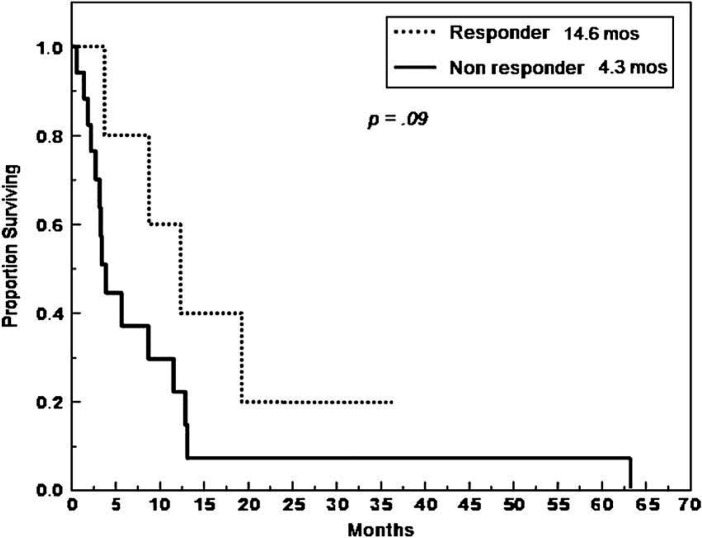
The median overall survival duration of the cats that responded to doxorubicin-based rescue was 14.6 months, while the median survival duration of those that did not was 4.3 months (P=0.09) (Fig 1).
Fig 1.
Duration of survival for cats given doxorubicin treatment.
Discussion
The response rate of cats receiving doxorubicin-based rescue was low, at 22% overall with only 9% achieving CRs. This low response rate may be due in part to a selection bias. Information regarding cell type (ie, small cell vs large cell) is routinely available to clinicians at MJR-VHUP and incorporated into treatment decisions protocol choices. Cats with small cell lymphoma are typically treated with prednisone and chlorambucil, as a first-line therapy, whereas cats with intermediate-sized cells and blastic cells are often treated with COP or CHOP chemotherapy. In retrospect, doxorubicin was probably chosen as a rescue agent more often in cases of large cell lymphoma, which were precisely the type of lymphoma showing no positive response to the drug. The lymphoblastic cells that comprise large cell lymphoma are thought to be high grade lymphomas with higher growth fractions and therefore presumed increased sensitivity to chemotherapy. However, in rescue attempts, this phenomenon may work against the desired effect. The lymphoblastic cell types may also develop resistance earlier than smaller cell lymphomas. In this study, all cats were previously treated with COP-based protocols which contain drugs, such as prednisone and vincristine, known to induce the multi-drug resistance genes (Okai et al 2000). 22 This may have resulted in acquired resistance to doxorubicin in these cats. Doxorubicin may be more effective when used in induction protocols, when cells are at their most sensitive and have had less opportunity to generate resistance.
Additionally, as none of the cats with large cell lymphomas received more than one dose of the drug and some of these cats were only evaluated by clinical signs, doxorubicin may have been discontinued because of chemotherapy side effects and not necessarily because of drug failure. For instance, the clinical signs of gastrointestinal toxicity from doxorubicin (such as vomiting, diarrhea, and anorexia) may be difficult to distinguish from the clinical signs of a cat with gastrointestinal lymphoma with progressive disease. 23 The efficacy of doxorubicin in these cats might, therefore, have been underestimated in some of these cats. Nevertheless, if toxicity from doxorubicin decreased the quality of life of the cats, the value of doxorubicin as part of rescue in such patients may be questionable.
Unlike the hypothesis, the cats with large cell lymphomas were not ones who responded to doxorubicin-based rescue, but the cats with small-medium cell lymphomas were. Perhaps histologic and cytologic cell type is not always reflective of the clinical behavior of these tumors. At MJR-VHUP, cats with small cell lymphoma that have PD on prednisone and chlorambucil are often rescued with COP chemotherapy and often show success on these drugs even though the small cell lymphomas are considered to be more indolent in behavior. Furthermore, some of the differences in outcome observed in this study may have been confounded by innate differences in the disease progression of different cell types of feline lymphoma. 24
The cats that responded had much longer survival times. The cats that responded to doxorubicin-based rescue had a median overall survival of 14.6 months, where as those that did not respond had a median overall survival of 4.3 months. Doxorubicin-based rescue may have helped to provide a durable response in some of these cats. While not statistically significant (P=0.09) this might have shown significance in a study with larger numbers of cases. In this study, remission duration was calculated from the time of treatment to the time to progression. While using the date of the start of treatment may have overestimated the actual true duration of remission, this date was used for standardization purposes. In cats with visceral disease it is difficult to objectively pinpoint the exact time remission is achieved and the date of initiation of therapy is often used in this manner when analyzing feline lymphoma remission durations. 18 In many cats abdominal ultrasound would be the preferred and most sensitive measure to document the exact time of response, but this is not routinely performed in the clinical setting for practical purposes, especially if a cat is doing well.
Limitations of this study include the small sample size and the overlap in the clinical presentation of signs of drug toxicity with signs of disease progression. While using performance status as an indicator of disease response may confound interpretation, it has been used repeatedly in the veterinary literature studying feline lymphoma for practical purposes. 15,24 In the current study we attempted to delineate assessments of response into both subjective and objective categories to reduce confusion.
Staging classification of cats with lymphoma is difficult due to variations in presentations of this disease and remains a frustration in studying feline lymphoma. Despite applying two different staging systems to the cats in this study, stage was not prognostic for response to doxorubicin-based rescue.
Furthermore, the use of concurrent chemotherapy could have confounded the results making it difficult to pinpoint which drug was responsible for the response. Three out of five cats in the response group had concurrent chemotherapy. This suggests that some of the responses might not be due to doxorubicin but could have been a result of the other drugs received interspersed between the doses of doxorubicin. As discussed in the paper by Kristal et al, doxorubicin may be most useful when used in combination with other chemotherapy agents and it is commonly administered this way in practice. 16
The results of this study demonstrate that doxorubicin-based rescue is not an effective rescue protocol in feline lymphoma. The times that it was effective were in cases of small-medium cell lymphoma or when doxorubicin was given with concurrent chemotherapy. None of the 14 cats with large cell lymphoma in our study responded to treatment with doxorubicin. Especially in cases of large cell lymphomas, doxorubicin may be better used in induction protocols, when cells are at their most sensitive and have had less opportunity to generate resistance to the drug, than in a rescue setting. Further studies are needed to identify more successful chemotherapy agents to effectively treat refractory feline lymphomas.
Acknowledgement
The authors acknowledge Jennie Stevens for her help in preparing this manuscript.
References
- 1.Carter R.F., Harris C.K., Withrow S.J. Chemotherapy of canine lymphoma with histopathological correlation: Doxorubicin alone compared to COP as first treatment regimen, J Am Anim Hosp Assoc 23, 1987, 587–596. [Google Scholar]
- 2.Hahn K.A., Richardson R.C., Teclaw R.F., et al. Is maintenance chemotherapy appropriate for the management of canine malignant lymphoma?, J Vet Int Med 6, 1992, 3–10. [DOI] [PubMed] [Google Scholar]
- 3.Valerius K.D., Ogilvie G.K., Mallinckrodt C.H., Getzy D.M. Doxorubicin alone or in combination with asparaginase, followed by cyclophosphamide, vincristine, and prednisone for treatment of multicentric lymphoma in dogs: 121 cases (1987–1995), J Am Vet Med Assoc 210, 1997, 512–516. [PubMed] [Google Scholar]
- 4.Postorino N.C., Susaneck S.J., Withrow S.J., Macy D.W., Harris C. Single agent therapy with adriamycin for canine lymphosarcoma, J Am Anim Hosp Assoc 25, 1989, 221–225. [Google Scholar]
- 5.Calvert C.A., Leifer C.E. Doxorubicin for treatment of canine lymphosarcoma after development of resistance to combination chemotherapy, J Am Vet Med Assoc 179, 1981, 1011–1012. [PubMed] [Google Scholar]
- 6.Van Vechten M., Helfand S.C., Jeglum K.A. Treatment of relapsed canine lymphoma with doxorubicin and dacarbazine, J Vet Int Med 4, 1990, 187–191. [DOI] [PubMed] [Google Scholar]
- 7.Garrett L.D., Thamm D.H., Chun R., Dudley R., Vail D.M. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma, J Vet Int Med 16, 2002, 704–709. [DOI] [PubMed] [Google Scholar]
- 8.Keller E.T., MacEwen E.G., Rosenthal R.C., Helfand S.C., Fox L.E. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma, J Vet Int Med 7, 1993, 289–295. [DOI] [PubMed] [Google Scholar]
- 9.Mooney S.C., Hayes A.A., MacEwen E.G., Matus R.E., Geary A., Shurgot B.A. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977–1981), J Am Vet Med Assoc 194, 1989, 696–699. [PubMed] [Google Scholar]
- 10.Milner R.J., Peyton J., Cooke K., et al. Response rates and survival times for cats with lymphoma treated with the University of Wisconsin-Madison chemotherapy protocol: 38 cases (1996–2003), J Am Vet Med Assoc 227, 2005, 1118–1122. [DOI] [PubMed] [Google Scholar]
- 11.Rassnick K.M., Mauldin G.N., Moroff S.D., Mauldin G.E., McEntee M.C., Mooney S.C. Prognostic value of argyrophilic nucleolar organizer region (AgNOR) staining in feline intestinal lymphoma, J Vet Int Med 13, 1999, 187–190. [DOI] [PubMed] [Google Scholar]
- 12.Jeglum K.A., Whereat A., Young K. Chemotherapy of lymphoma in 75 cats, J Am Vet Med Assoc 190, 1987, 174–178. [PubMed] [Google Scholar]
- 13.Malik R., Gabor L.J., Foster S.F., McCorkell B.E., Canfield P.J. Therapy for Australian cats with lymphosarcoma, Austral Vet J 79, 2001, 808–817. [DOI] [PubMed] [Google Scholar]
- 14.Mauldin GE, Mooney SC, Meleo KA. Chemotherapy in 132 cats with lymphoma: 1988–1994. 15th Annual Conference of the Veterinary Cancer Society, Tucson, AZ 1995.
- 15.Peaston A.E., Maddison J.E. Efficacy of doxorubicin as an induction agent for cats with lymphosarcoma, Austral Vet J 77, 1999, 442–444. [DOI] [PubMed] [Google Scholar]
- 16.Kristal O., Lana S.E., Ogilvie G.K., Rand W.M., Cotter S.M., Moore A.S. Single agent chemotherapy with doxorubicin for feline lymphoma: A retrospective study of 19 cases (1994–1997), J Vet Int Med 15, 2001, 125–130. [DOI] [PubMed] [Google Scholar]
- 17.Moore A.S., Cotter S.M., Frimberger A.E., Wood C.A., Rand W.M., L'Heureux D.A. A comparison of doxorubicin and COP for maintenance of remission in cats with lymphoma, J Vet Int Med 10, 1996, 372–375. [DOI] [PubMed] [Google Scholar]
- 18.Teske E., van Straten G., van Noort R., Rutteman G.R. Chemotherapy with cyclophosphamide, vincristine, and prenisolone (COP) in cats with malignant lymphoma: New results with an old protocol, J Vet Int Med 16, 2002, 179–186. [DOI] [PubMed] [Google Scholar]
- 19.Vail D.M., Moore A.S., Ogilvie G.K., Volk L.M. Feline lymphoma (145 cases): Proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats, J Vet Int Med 12, 1998, 349–354. [DOI] [PubMed] [Google Scholar]
- 20.Mooney S.C., Hayes A.A. Lymphoma in the cat: An approach to diagnosis and management, Sem Vet Med Surg (Small Animal) 1, 1986, 51–57. [PubMed] [Google Scholar]
- 21.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations, J Am Stat Assoc 53, 1958, 457–481. [Google Scholar]
- 22.Okai Y.O., Nakamura N., Matsushiro H., et al. Molecular analysis of multidrug resistance in feline lymphoma cells, Am J Vet Res 61, 2000, 1122–1127. [DOI] [PubMed] [Google Scholar]
- 23.O'Keefe D.A., Sisson D.D., Gelberg H.B., Schaeffer D.J., Krawiec D.R. Systemic toxicity associated with doxorubicin administration in cats, J Vet Int Med 7, 1993, 309–317. [DOI] [PubMed] [Google Scholar]
- 24.Fondacaro J.V., Richter K.P., Carpenter J.L., Hart J.R., Hill S.L., Fettman M.J. Feline gastrointestinal lymphoma: 67cases (1988–1996), Euro J Comp Gastroent 4, 1999, 5–11. [Google Scholar]