Abstract
Renal dysplasia is defined as a condition of disorganised development of renal parenchyma due to abnormal differentiation. The case of a 5-month-old intact male Norwegian Forest Cat with a history of polyuria and polydipsia is reported. Ultrasonographic examination showed a slight enlargement of kidneys. Biochemical parameters, haematological examinations and clinical signs were compatible with chronic renal failure (CRF). Histological examination was correlated with a primary tubular disorganisation and modification of glomerular compartment. The clinical history together with the histological lesions is consistent with bilateral juvenile renal dysplasia in this cat. To our knowledge, feline renal dysplasia has been reported in fetal infections with panleukopenia virus; no reports indicate the idiopathic origin in feline dysplastic lesions.
Renal dysplasia is a kidney malformation leading to chronic renal failure (CRF) in young animals. Renal dysplastic lesions have been reported in dog, cow, horse, lamb and human. 1–7 The malformed kidney is usually smaller than normal or diffusely or partly cystic. The extent of the abnormality varies from a grossly disorganised multi-cystic dysplasia involving the whole kidney to a less severe segmental change, in which part of the kidney is unaffected. Renal dysplasia can be diagnosed only by histological examination. 1,8
This report describes the clinical and pathological findings of a vaccinated 5-month-old intact male Norwegian Forest Cat presented to a local veterinary surgery for polydipsia and anorexia. The cat tested negative for feline immunodeficiency virus (antibody-IDDEX) and feline leukaemia virus (antigen-IDDEX). The referring veterinarian diagnosed renal disease upon findings of increased serum creatinine and blood urea nitrogen (BUN), hypercalcemia, hyperphosphataemia and low urinary density, as reported in Table 1 (T0). An abdominal ultrasound examination was performed and demonstrated a slight enlargement of kidneys (left: 4.8 cm in length, right: 5.1 cm in length). No further abdominal abnormalities were identified. Therapy was limited to a renal diet (Hill's K/d).
Table 1.
Laboratory clinical course in a Norwegian Forest Cat with renal dysplasia
| T0 | T1 | T2 | NV | |
|---|---|---|---|---|
| RBC (106 cell/μl) | 5.48 | 2.76 | 5.42 | 6–10.1 |
| Ht (%) | 25.6 | 12.4 | 19.3 | 27.2–46.8 |
| Hb (g/dl) | 8.3 | 4.5 | 6.9 | 8.1–14.2 |
| MCV (fl) | 47 | 45 | 35.6 | 41.3–52.6 |
| MCHC (pg) | 32.3 | 33.1 | 31.9 | 27.0–32.6 |
| Retic (103 cell/μl) | nd | nd | 21.4 | 15–81 |
| TP (g/dl) | 7.1 | 7.4 | 7.6 | 5.4–7.5 |
| Alb (g/dl) | 3.0 | nd | 2.9 | 2.9–3.5 |
| BUN (mg/dl) | 117 | nd | 132 | 20–30 |
| Creatinine (mg/dl) | 5.6 | 4.9 | 6.1 | 0.5–1.5 |
| Phopshorus (mg/dl) | 8.3 | 7.9 | 7.3 | 2.6–6.2 |
| Calcium (mg/dl) | 16 | 15.4 | 14.5 | 9–11.3 |
| SG urine | 1016 | nd | 1010 | 1015–1045 |
| UPC | nd | nd | 0.7 | <0.6 |
RBC=red blood cells, Ht=haematocrit, Hb=haemoglobin, MCV=mean corpuscular volume, UPC = urinary protein/creatinine, MCHC=mean corpuscular haemoglobin concentration, Retic = reticolocytes, TP=total proteins, Alb=albumin, BUN=blood urea nitrogen, SG=urine specific gravity, T0, T1, T2 = time of visits, and NV=normal values.
Three months later, the cat was revisited by the same veterinarian due to worsened condition (ie, dysorexia and vomiting). Haematological and biochemical examinations showed a severe normochromic/normocytic non-regenerative anaemia, and confirmed the first diagnosis of renal failure (Table 1; T1). A target therapy with human recombinant erythropoietin (EPO: Eprex 1000; Janssen Cilag) 100 UI/kg was administered subcutaneously two times weekly, associated with fluid therapy (lactated Ringer's 40 ml/kg/SC in a single daily dose). Due to worsening of clinical conditions: polyuria/polydipsia, dysorexia, weight loss and vomiting, the owner was directed to the Teaching Veterinary Hospital for further diagnostic examinations.
On initial physical examination, the cat revealed poor coat condition and weight loss. Normal mucous membranes were noted. A blood sample for complete blood count (CBC) and biochemical analysis was taken (Table 1; T2). On CBC, only a slight normochromic/normocytic non-regenerative anaemia was found. A urine sample was obtained by cystocentesis; a complete examination (chemical–physical and sediment) showed isostenuric urine, with pH=6.0, 2+ of proteins, absence of glucose, bilirubin, blood and ketonic acid. The sediment showed only <5 epithelial cell/hpf (normal values). Urine culture was negative. Analysis of proteinuria was undertaken by quantitative methods (colorimetric method red pirogallol; Sentinel Diagnostics) to evaluate the urinary protein/creatinine (UPC) ratio, and by qualitative method: sodium dodecyl-sulphate-agar gel electrophoresis (SDS-age). The UPC ratio was slightly increased, and proteinuria was classified as mixed, due to the presence of low molecular weight (LMW) proteins (<66–68 kDa) indicative of tubular origin and high molecular weight proteins, indicative of glomerular origin. Due to the severity of the clinical signs and pathological findings, the owner elected for euthanasia. A complete necropsy was performed. Post-mortem examination revealed no gross abnormalities, apart from the kidneys. Both kidneys were involved in the pathology and were markedly firm, irregular, pale with a very nodular surface (Fig 1). On cut section, the entire cortex contained a moderate and uniform distribution of small (0.5–1 mm) cystic structures. No abnormalities were present in the inner zone of the medulla or the pelvis.
Fig 1.
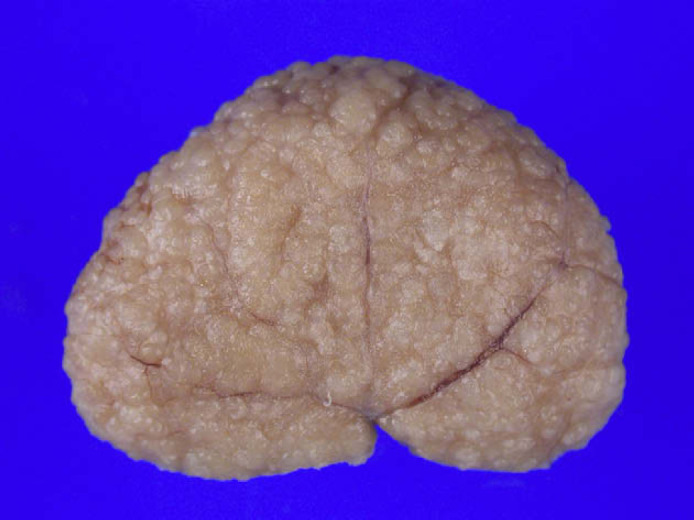
Renal dysplasia. External surface of right kidney.
Samples from each organ were fixed in phosphate-buffered 10% formalin solution and then routinely processed for histology. Tissue sections were stained with haematoxylin and eosin. Supplemental kidney sections were stained with periodic acid-Schiff (PAS), Jones' methenamine silver, Masson's trichrome, and Miller elastin. Immunoperoxidase methods for cytokeratin were performed using a standard streptavidin–biotin procedure on renal sections.
Apart from calcification of the space between gastric mucosa and submucosa and on the leaflet of mitral valve, histopathological examination of all other organs did not reveal any significant abnormalities. Renal histological examination showed diffuse cortical and medullary interstitial fibrosis that surrounded all tubular structures. Cystic glomerular atrophy and mature glomerular tufts were observed in cortex. Some glomeruli had markedly dilated capsular spaces. Mature glomeruli were diffusely enlarged, due to hyperplasia of mesangial cells. Some cells with slightly foamy and pigmented cytoplasm were present. Sclerotic glomeruli were distributed within the cortical region. No immature glomeruli were observed. Mineralisation of Bowman's capsule and tubular basement membrane was diffuse in the cortical zone (Fig 2). Primitive ducts and cysts were present and distributed throughout the parenchyma with no predilection to the cortical or medullary region. These structures were lined by atypical columnar epithelium and surrounded by concentric mantles of cellular mesenchyme (Fig 3). They were aggregated, forming nodular formations. Immature mesenchyme was seen and comprised spindle cells arranged circumferentially as a collar around the primitive ducts (Fig 4). Some tubules were filled with hypereosinophilic granular debris and necrotic tubular epithelial cells. Within scattered tubules, there were occasional clusters of calcium crystals. Minimal pyelonephritis was present, predominantly lymphocytes. Miller elastin showed signs of fibroelastosis in renal arterioles and, on PAS staining, hyaline deposits were seen within the arterial walls. The progression of the damage was extremely marked to have a clear image of the vascular system. Immunohistochemistry for cytokeratin showed a variable expression of tubular epithelial cells, especially in proximal tubules (Fig 5).
Fig 2.
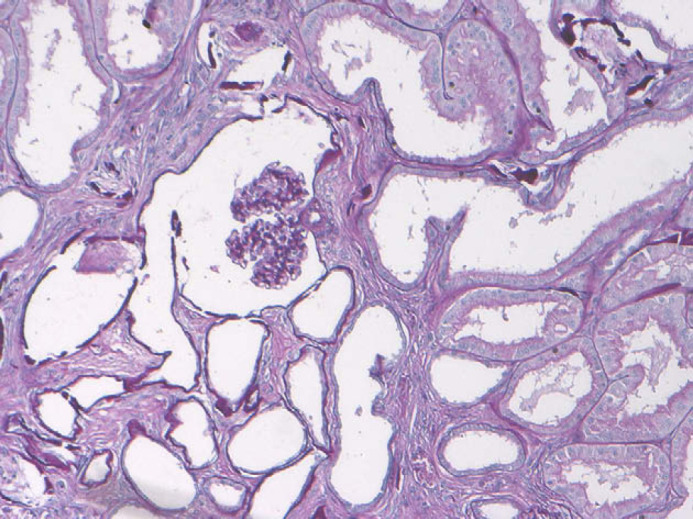
Renal dysplasia. Cystic glomerular atrophy and mineralisation of tubular basement membrane (PAS, ×200).
Fig 3.
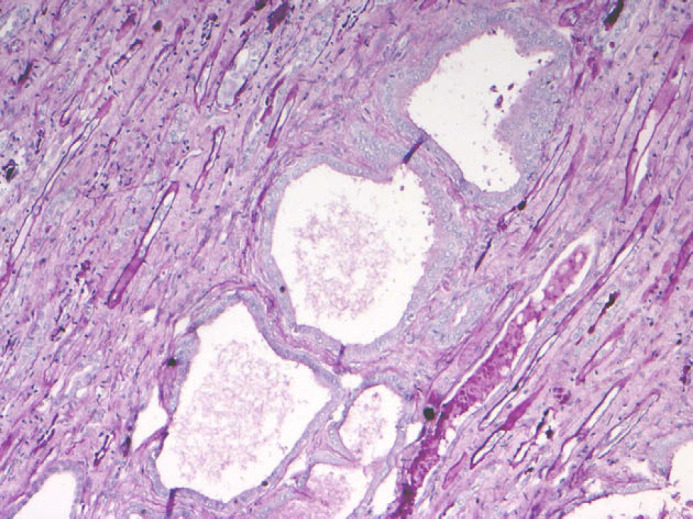
Renal dysplasia. Metanephric ducts in medulla surrounded by undifferentiated mesenchymal stroma, characterised by tall pseudostratified columnar epithelium (PAS, ×200).
Fig 4.
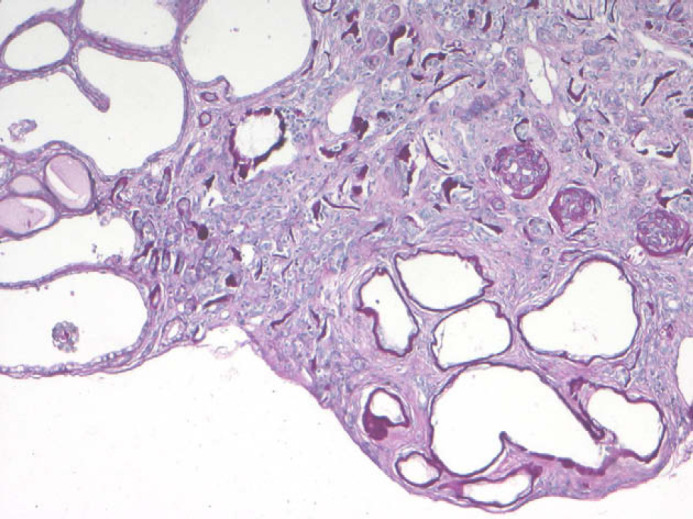
Renal dysplasia. Complete disorganisation of the renal parenchyma and sub-capsular cystic formations (PAS, ×200).
Fig 5.
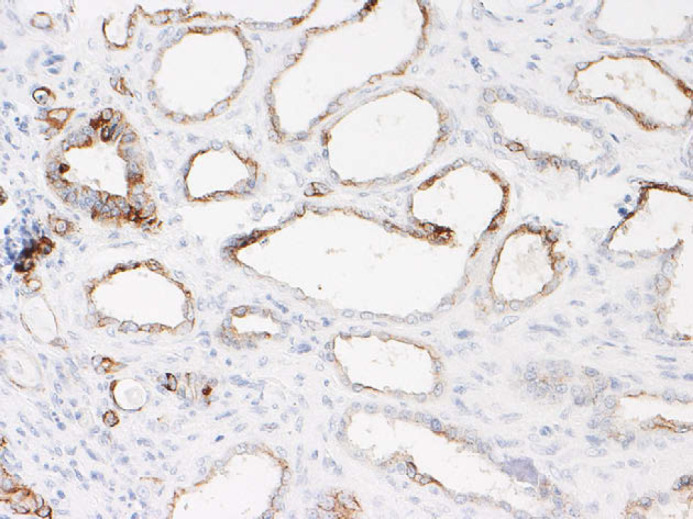
Renal dysplasia. Scattered expression of cytokeratin antibody in epithelial tubular cells (immunohistochemistry (IHC), ×400).
The diagnosis of renal dysplasia depends primarily upon disorganisation of the renal parenchyma, and the presence of ‘primitive’ ducts. Lesions are characterised by dysplastic tubules connected to cysts and surrounding undifferentiated tissue. Renal dysplasia is thought to be a developmental aberration of mesenchymal–epithelial transformation. 1 The term renal dysplasia is used to define a condition of disorganised development of renal parenchyma due to abnormal differentiation. Renal dysplasia can be unilateral and function if the opposite kidney is normal. When the disease is bilateral, renal failure is the ultimate result, with an image of end-stage kidney. 9
In this cat, the clinical history taken with the histological lesions is consistent with bilateral juvenile renal dysplasia. Clinical and haematological alterations in young animals are similar to those of old animals with CRF. Puppy and kittens with renal dysplasia are often clinically normal for extended periods of time before signs of CRF ensue. Age of onset of clinical signs ranges from 4 weeks to 5 years; however, as in this case, most of clinical signs are seen before 2 years of age. 10 In the Shih Tzu 4,11 two different clinical evolutions are described: one characterised by rapid clinical manifestations and evolution, the other related with gradually developing azotaemia over several years. In the present case, at first presentation clinical signs were minimal, but laboratory analysis were indicative of CRF. Subsequent examination (Table 1; T2) showed slight worsening of renal parameters (BUN, creatinine), but this is not strictly correlated with progression of renal damage. In this case, SDS-age examination was helpful for qualitative evaluation of proteinuria and to identify the localisation of the lesion (ie, tubulointerstitial and glomerular damage). This method has a good sensitivity in disclosure of both lesions, glomerular and tubulointerstitial, but a moderate specificity. 12 Histopathology, confirmed the localisation of the lesions supposed by the SDS-age. Furthermore, the presence of a very LMW proteins (14 kDa) indicates a severe tubulointerstitial damage, as described by another veterinarian study 12 and in human medicine, 13 where the presence of very LMW proteins (evaluated by SDS-page, similar method) is associated with severe damage and poor prognosis in patients with glomerulonephritis. SDS-age is not able to identify the type of lesions. Clinical data on non-regenerative anaemia, associated with worsening of clinical conditions, are indicative of a chronic progression of renal disease. Response to EPO target therapy confirms that medullary erythropoiesis was preserved. Congenital renal diseases are hardly recovered with any treatments, but feeding reduced-protein diets in growing dogs and cats is discouraged while integration with dietary phosphorus binder is suggested. 10,14
The criteria of identification of renal dysplasia are variable. In this cat, the most striking lesions were correlated with primary tubular disorganisation. The scattered expression of cytokeratin in primitive tubules suggests the incomplete mechanism of differentiation of tubular cells. Diffuse interstitial fibrosis, sclerotic glomeruli and a reduction in the number of nephrons are considered secondary changes, while mesangial cells hyperplasia represents a compensatory mechanism of kidney. The lesion in this cat could be ascribed more strictly as a tubular dysplasia. To our knowledge, feline renal dysplasia has been reported in fetal infections with panleukopenia virus. 10,15 No reports indicate the familial origin in feline dysplastic lesions.
References
- 1.Picut C.A., Lewis R.M. Microscopic features of canine renal dysplasia, Vet Pathol 24, 1987, 156–163. [DOI] [PubMed] [Google Scholar]
- 2.Jones T.O., Clegg F.G., Morgan G., Wijeratne W.V. A vertically transmitted cystic renal dysplasia of lambs, Vet Rec 127, 1990, 421–424. [PubMed] [Google Scholar]
- 3.Ronen N., van Amstel S.R., Nesbit J.W., van Rensburg I.B. Renal dysplasia in two adult horses: Clinical and pathological aspects, Vet Rec 132, 1993, 269–270. [DOI] [PubMed] [Google Scholar]
- 4.Ohara K., Kobayashi Y., Tsuchiya N., Furuoka H., Matsui T. Renal dysplasia in a Shih Tzu dog in Japan, J Vet Med Sci 63, 2001, 1127–1130. [DOI] [PubMed] [Google Scholar]
- 5.Ohba Y., Kitagawa H., Okura Y., Kitoh K., Sasaki Y. Clinical features of renal tubular dysplasia, a new hereditary disease in Japanese Black cattle, Vet Rec 149, 2001, 115–118. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki Y., Kitagawa H., Kitoh K., et al. Pathological changes of renal tubular dysplasia in Japanese black cattle, Vet Rec 150, 2002, 628–632. [DOI] [PubMed] [Google Scholar]
- 7.Plummer P.J. Congenital renal dysplasia in a 7-month-old quarter horse colt, Vet Clin North Am Equine Pract 22, 2006, e63–e69. [DOI] [PubMed] [Google Scholar]
- 8.Morita T., Michimae Y., Sawada M., et al. Renal dysplasia with unilateral renal agenesis in a dog, J Comp Pathol 133, 2005, 64–67. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto T., Wakizaka S., Matsuyama S., et al. A control of a golden retriever with renal dysplasia, J Vet Med Sci 59, 1996, 939–942. [DOI] [PubMed] [Google Scholar]
- 10.Greco D.S. Congenital and inherited renal disease of small animals, Vet Clin North Am Small Anim Pract 31, 2001, 393–399. [DOI] [PubMed] [Google Scholar]
- 11.Hoppe A., Swenson L., Jönsson L., Hedhammar A. Progressive nephropathy due to renal dysplasia in Shih Tzu dogs in Sweden: A clinical pathological and genetic study, J Small Anim Pract 31, 1990, 83–91. [Google Scholar]
- 12.Zini E., Bonfanti U., Zatelli A. Diagnostic relevance of qualitative proteinuria evaluated by use of sodium dodecyl sulfate-agarose gel electrophoresis and comparation with renal histologic findings in dogs, Am J Vet Res 65, 2004, 964–971. [DOI] [PubMed] [Google Scholar]
- 13.Bazzi C., Petrini C., Rizza V., Arrigo G., Beltrame A., D'Amico G. Characterization of proteinuria in primary glomerulonephritides. SDS-PAGE patterns: Clinical significance and prognostic value of low molecular weight (tubular) proteins, Am J Kidney Dis 29, 1997, 27–35. [DOI] [PubMed] [Google Scholar]
- 14.Landerville A.J., Seshadri R. Utilization of continuous renal replacement therapy in a case of feline acute renal failure, J Vet Emerg Crit Care 14, 2004, 278–286. [Google Scholar]
- 15.Lulich J.P., Osborne C.A., Lawler D.F., O'Brien T.D., Johnston G.R., O'Leary T.P. Urologic disorders of immature cats, Vet Clin North Am Small Anim Pract 17, 1987, 663–696. [DOI] [PubMed] [Google Scholar]


