Abstract
Dysautonomia is caused by degeneration of the autonomic ganglia. Failure of the autonomic system affecting the gastrointestinal and urinary tracts can cause oesophageal distension and/or dysfunction, gastric and bowel distension and hypomotility, and urinary bladder distension. The aim of this retrospective study was to describe diagnostic imaging findings in cats with dysautonomia. Common findings were megaoesophagus and/or oesophageal dysfunction, gastric distension and signs of intestinal ileus. Associated aspiration pneumonia and megacolon appeared less commonly. Although diagnostic imaging findings are not specific for this disease, if findings in multiple systems are detected, along with consistent clinical signs and neurological deficits, dysautonomia should be considered among the differential diagnosis.
Feline dysautonomia is a disease caused by failure of the autonomic nervous system due to autonomic ganglia degeneration. 1 Clinical signs are related to dysfunction of the sympathetic and parasympathetic nervous systems. Many cases of feline dysautonomia were described during the 80s and 90s after the condition's first description by Key and Gaskell in 1982 in Scotland. 2 The incidence apparently decreased after the many described cases during that period, with fewer cases reported since then. The aetiology of the disease is unclear, although association with Clostridium botulinum neurotoxin has been described in both horses and cats. 3,4 Canine dysautonomia was recognised shortly after the first report in cats 5 and has been described in other European countries and the United States. 6–8 No breed, gender or age predisposition has been described in cats, but dysautonomia is predominantly seen in young dogs. 8 Clinical signs are similar across species although some differences appear present between dogs and cats. Vomiting, diarrhoea, anorexia, lethargy, weight loss, dysuria, and inspiratory dyspnoea were the most frequent clinical signs described in a series of 65 dogs 8 while other signs were seen in less than 17% of the cases. In cats, dilated pupils, oesophageal dysfunction, dry nose, reduce lachrymal secretions, prolapse of the third eyelid, regurgitation and constipation were seen in over 75% of the 86 cases documented prior to 1984. 1,9
The diagnosis is suspected based on clinical signs. Pharmacological testing can be performed to provide supportive evidence for a diagnosis. Response to ocular instillation of dilute pilocarpine drops (0.05–0.1%) and subcutaneous injection of low doses of bethanecol chloride have been used to rule out the inability of the iris and detrusor muscles to contract and thus suggest denervation hypersensitivity, which would indicate a postganglionic lesion. 7,10,11 Although the diagnosis is confirmed by histopathological examination, recognition of the radiographic abnormalities, along with the clinical signs and neurological findings, and pharmacological and physiological autonomic function testing, might aid to support the suspected diagnosis and to assess the range of associated changes in the affected patients.
Radiographic findings of dysautonomia can include megaoesophagus, aspiration pneumonia, gaseous distension of the stomach and intestine, and distension of the urinary bladder. 9,12 Radiographic findings of canine dysautonomia have been described and expected to be analogous in other species, 13 but no studies discussing the radiographic findings in cats could be found. The aim of this study was to describe the radiographic findings in patients with feline dysautonomia and, where available, describe fluoroscopic and ultrasonographic findings.
Materials and Methods
Cats with a diagnosis of feline dysautonomia and concurrent imaging investigations between 2003 and 2007 in the Royal (Dick) School of Veterinary Studies and between 2001 and 2008 in the Glasgow University Veterinary School were included in this study. The cats were diagnosed by histopathological evaluation or clinically, by pharmacological tests and/or presence of typical clinical signs and neurological deficits supportive of a diagnosis of dysautonomia. When histopathological diagnosis was not available only patients with a strong suspicion of a diagnosis of dysautonomia, after excluding other possible differential diagnosis, were included. For this, consistent clinical signs and neurological deficits and positive pharmacological tests or histopathologically confirmed diagnosis in animals of the same outbreak had to be present. For the pharmacological test, one to two drops of 0.1% pilocarpine were applied on one eye; the test was considered positive when miosis occurred within 45 min of instillation.
Clinical records, thoracic and abdominal radiographs, fluoroscopic examinations, and abdominal ultrasound images and/or reports were reviewed, as available.
Thoracic radiographs were assessed for the presence or absence of megaoesophagus and aspiration pneumonia. The oesophagus was evaluated for gas or fluid distension indicative of megaoesophagus. When present, the severity of megaoesophagus was subjectively graded as mild, moderate, or severe. Maximum oesophageal diameter was recorded and thoracic inlet height (from the cranioventral aspect of first thoracic vertebral body to the sternum) was measured and the ratio between the two measurements calculated. Abdominal radiographs were reviewed for the presence of gastric, small intestinal or colonic distension, presence of granular mineral contents within the small intestine, signs of constipation, and urinary bladder distension. Severity of gastric distension was evaluated subjectively as mild, moderate, or severe and the number of intercostal spaces occupied by the distended stomach was also recorded. Distension of the intestinal loops (>1.2 cm in diameter), approximate percentage of gas content, and presence of granular mineral contents were evaluated for the small intestine. Distension of the colon was also assessed; the maximum diameter was recorded. The length of the seventh lumbar vertebra (L7) was also determined and the ratio of the maximum colonic diameter to the length of L7 was calculated: ratios>1.5 were considered indicative of megacolon. 14 Distension of the urinary bladder was recorded and graded subjectively as mild, moderate or severe.
When available, thoracic fluoroscopic studies, ultrasonographic images and/or written reports were reviewed. Thoracic fluoroscopic studies where assessed for oesophageal function. Ultrasonographic images and/or written reports were reviewed for the presence of gastric distension, distended small intestine, decreased intestinal motility, and distension of the urinary bladder. Decreased intestinal motility was assessed by imaging a region of intestine over a period of time and considered reduced when contractions were less than 4–5 per min for the duodenum and less than 1–3 per min for the rest of the small intestine.15
Results
During the study period, 11 cats were diagnosed with dysautonomia and had imaging investigations. Two other cats were excluded because imaging studies had not been performed. There were nine neutered males and two entire females, and the mean age was 6.27 years (range 1–11). There were four Birman, three domestic shorthair, one domestic longhair, one Siamese, one Colourpoint, and one Abyssinian cat. Reported clinical signs and their frequency of occurrence are listed in Table 1. Onset of clinical signs prior to the imaging examinations varied from 5 days to 12 weeks. A diagnosis of dysautonomia was reached in six cats by post-mortem histopathological examination of autonomic ganglia, in one by histopathological examination of autonomic ganglia and small intestinal enteric plexus in intestinal biopsies, and in one by a positive pilocarpine 0.1% test. The remaining three cats were part of the same closed colony of pet cats as two animals that had confirmed dysautonomia by post-mortem examination and they developed clinical signs between 4 and 6 days after the histopathologically confirmed cases. Five of the cases had been reported in a previous article.16
Table 1.
Range and frequency of the presented clinical signs.
| Clinical sign | Number of animals (percentage) |
|---|---|
| Vomiting | 11 (100%) |
| Anorexia | 9 (82%) |
| Midriasis | 9 (82%) |
| Regurgitation | 8 (73%) |
| Decreased pupillary reflex | 8 (73%) |
| Decreased tear production | 8 (73%) |
| Lethargy | 7 (64%) |
| Third eyelid prolapse | 7 (64%) |
| Constipation | 7 (64%) |
| Dehydration | 5 (45%) |
| Dysphagia | 5 (45%) |
| Dysuria/urinary retention | 5 (45%) |
| Dry mucous membranes | 5 (45%) |
| Weight loss | 5 (45%) |
| Bradycardia | 4 (36%) |
| Coughing | 2 (18%) |
| Dyspnoea | 1 (9%) |
The imaging studies that were available for each of the cats are summarised in Table 2. Lateral views of the thorax were available for all the animals. Seven animals had lateral views of the abdomen and in the remaining four the cranial abdomen including the stomach, part of the small intestine and ascending, transverse and cranial aspect of descending colon could be evaluated from the thoracic views. Fluoroscopic examinations with barium swallows to evaluate the oesophagus were available for five patients and abdominal ultrasound was available for three of the cats. Three cats had repeated thoracic radiographs and two of them also had repeated abdominal radiographs taken 1 day, 3 days, and 3 months after the first studies. During radiographic examination three of the cats were sedated with midazolam, one was sedated with acepromazine and butorphanol combination, one with medetomidine and butorphanol, and one was under general anaesthesia with isoflurane. The remaining five cats had no sedative or anaesthetic drugs administered during the imaging studies.
Table 2.
Diagnostic imaging studies available for each cat.
| Cat | Thoracic X-rays | Abdominal X-rays | Fluoroscopy | Abdominal ultrasound |
| 1 | R lateral, DV | R lateral, DV | × | × |
| 2 | R lateral | * | ✓ | × |
| 3 | R lateral | * | ✓ | × |
| 4 | R, L lateral | R, L lateral | ✓ | × |
| 5 | R lateral | R lateral | ✓ | × |
| 6 | R lateral | R lateral, VD | ✓ | × |
| 7 | R lateral | R lateral, VD | × | × |
| 8 | R lateral | R lateral | × | ✓ |
| 9 | R lateral, VD, DV | * | × | ✓ |
| 10 | R lateral | * | × | ✓ |
| 11 | R lateral, VD | R lateral, VD | × | × |
✓=performed, ×=not performed, R=right, L=left, DV=dorsoventral, VD=ventrodorsal.
*No abdominal radiographs, but cranial abdomen included in thoracic films.
Diagnostic imaging findings for each cat along with the diagnostic method and the outcome are summarised in Table 3.
Table 3.
Imaging findings, type of diagnosis, and outcome for each cat.
| Cat | Megaoesophagus | Aspiration pneumonia | Gastric distension | SI distension | Gas filling> 50% SI | Mineral opacity in SI | Megacolon | Constipation | Distended bladder | Oesophagus dysfunction | SI hypomotility | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Moderate | ✓ | Severe | Upper limit | ✓ | × | × | × | × | NA | NA | PM | Died |
| 2 | × | × | Severe | × | × | ✓ | × | × | NA | ✓ | NA | CS | Died |
| 3 | × | × | Severe | × | × | × | × | × | NA | ✓ | NA | PM | Euth |
| 4 | Moderate | × | Severe | ✓ | ✓ | × | × | × | × | ✓ | NA | CS | Survived |
| 5 | × | Moderate | ✓ | ✓ | ✓ | ✓ | × | × | ✓ | NA | CS | Survived | |
| 6 | Moderate | × | Moderate | × | × | × | × | × | × | ✓ | NA | PhT | Survived |
| 7 | Severe | × | × | Upper limit | × | × | × | ✓ | ✓ | NA | NA | PM | Euth |
| 8 | Severe | × | Moderate | × | ✓ | × | × | × | ✓ | NA | ✓ | PM | Euth |
| 9 | Moderate | × | Severe | × | ✓ | × | × | × | NA | NA | ✓ | PM | Euth |
| 10 | Moderate | × | Severe | ✓ | ✓ | × | × | × | NA | NA | ✓ | PM | Euth |
| 11 | × | × | Moderate | × | × | × | × | × | × | NA | NA | Biopsy | Survived |
SI=small intestine, ×=not present, ✓=present, NA=not assessed, PM=post-mortem examination, CS=clinical signs, PhT=pharmacological test, Euth=euthanased.
Radiography
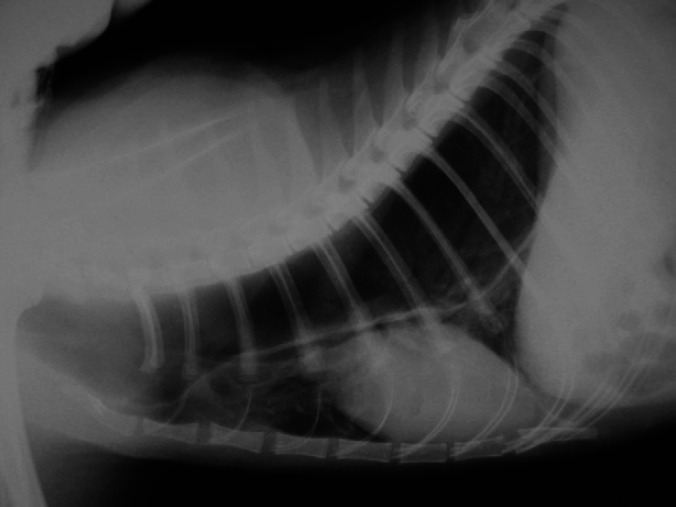
Megaoesophagus was detected in seven (67%) of the patients and it was graded as moderate in five and severe in two (Fig 1). In cats with moderate distension, the ratio between the maximum oesophageal diameter and the thoracic inlet ranged from 0.39 to 0.62 and the oesophagus was distended with gas in two of them, with fluid in two others and was distended with gas in one radiograph and with fluid in the repeated radiograph in the last cat. The cats with severe megaoesophagus had maximum oesophagus:thoracic inlet ratios of 1 and 1.25 with the gas distended oesophagus causing ventral displacement of the trachea and cardiac silhouette. Only one cat, which also had moderate megaoesophagus, presented with an alveolar pattern in the cranioventral lung field consistent with aspiration pneumonia. No additional abnormalities were present in the other thoracic structures of the rest of the cats with megaoesophagus. The thorax was considered unremarkable in four (36%) cats.
Fig 1.
Lateral radiograph of the thorax of cat 7 with severe megaoesophagus. The oesophagus is markedly distended with gas, occupying most of the height of the thoracic inlet and causing ventral displacement of the trachea and heart.
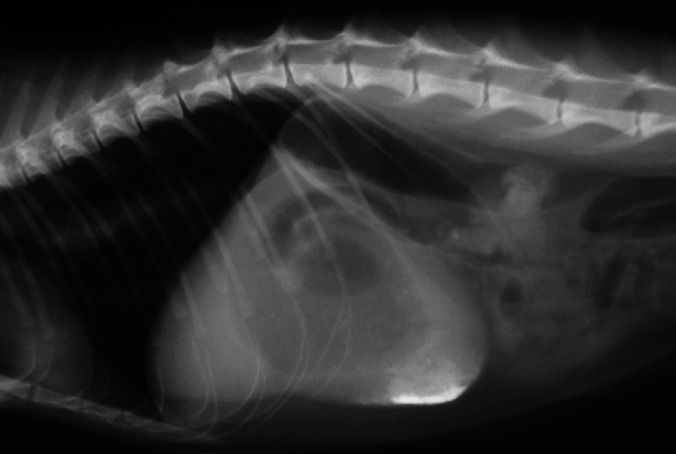
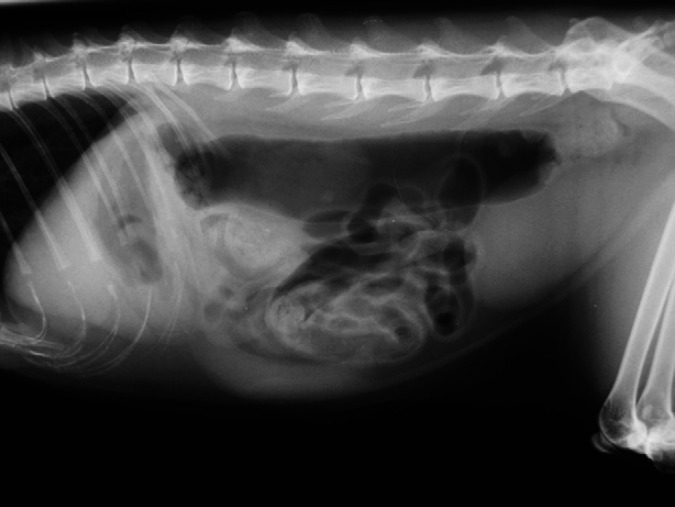
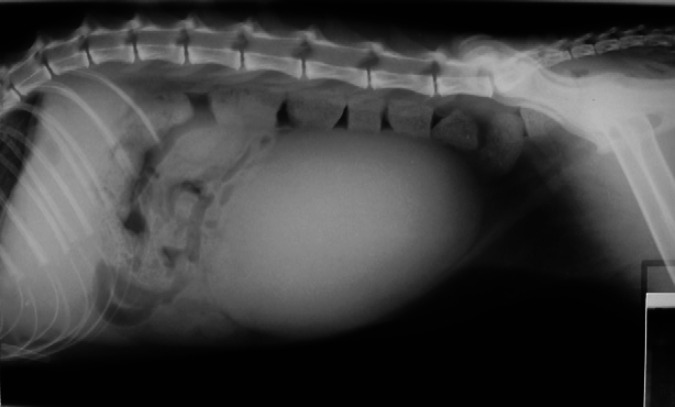
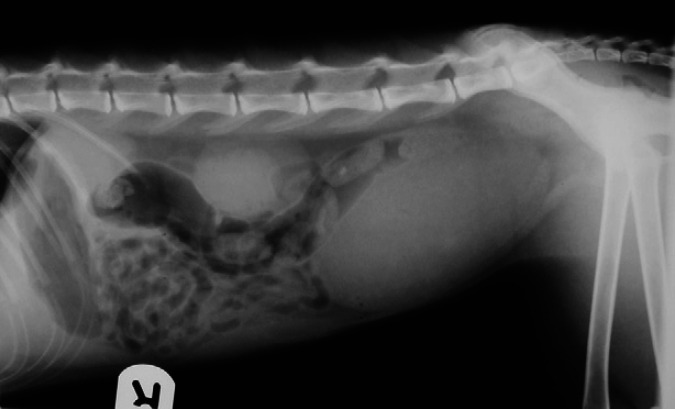
Abdominal radiographs revealed gastric gas distension in 10/11 patients (91%); in six this was considered severe (Fig 2), with the stomach occupying 3.5–4 intercostal spaces, and moderate in four, with the stomach occupying 1.5–2 intercostal spaces. Retention of barium in the stomach after being completely eliminated from the rest of the bowel was observed in one patient. Small intestine distension (>1.2 cm diameter) was present in three of the patients (Fig 3, with measurements of 1.5–1.6 cm; in two other patients the small intestine diameter was just at the upper normal limit (1.2 cm). Additionally, an abnormal amount of gas was found in the small intestine in six of the patients, including those with distension, with more than 50% of the small intestine being filled with gas. A small amount of granular mineral opacity was present within the small intestine in two of the patients. Megacolon was found in one patient, in which the ratio between the maximum colon diameter and L7 length was 1.86. Radiographic signs of constipation were seen in another patient, with well-formed radiopaque faeces throughout the length of the descending colon (Fig 4). Abnormal urinary bladder distension was seen in 2/7 cats for which full abdominal radiographs were available, causing cranial displacement of the small intestinal loops and dorsal displacement of the colon (Fig 5). Bladder size was considered normal for the rest of the cats. No additional findings were found in abdominal radiographs.
Fig 2.
Lateral radiograph of the cranial abdomen of cat 1, showing severe gastric distension. The stomach is distended with both gas and fluid opacity and retention of barium contrast medium is seen in the pyloric region. Moderately gassy distension of the duodenum and colon is also visible.
Fig 3.
Lateral radiograph of the abdomen of cat 5 showing gassy distension of the small intestine and colon. There is generalised gas distension of the small intestine and some loops measure more than 1.2 cm. A moderate amount of granular mineral opacity is seen in some loops of small intestine.
Fig 4.
Lateral radiograph of the abdomen of cat 7. Well-formed faeces of increased radiopacity suggestive of constipation are seen in the descending colon. The urinary bladder is severely distended, causing dorsal displacement of the colon and cranial displacement of small intestinal loops.
Fig 5.
Lateral radiograph of the abdomen of cat 8. The urinary bladder is distended, causing dorsal displacement of the colon and cranial displacement of the small intestine. Generalised increased gas content is seen in the intestine and stomach, although the stomach is not abnormally distended.
Fluoroscopy
Fluoroscopic examinations were available for five of the patients. In all of them oesophageal dysfunction was evident. Oesophageal hypomotility, contrast retention, retrograde peristalsis, gastro-oesophageal reflux, contrast pooling at the thoracic inlet, contrast pooling caudal to the base of the heart and delayed transit time were observed. In three of these patients thoracic radiographs had been unremarkable.
Abdominal ultrasound
Abdominal scans had been performed in three of the cats. The stomach appeared much distended with ingesta and/or gas in two of the patients. Small intestinal hypomotility was recorded for the three of them. No images or records were found to suggest urinary bladder distension.
Two cats died 6 and 8 days after presentation, four of the cats were euthanased within 1 week of presentation, and one cat was euthanased 3 weeks after presentation due to the severity of its clinical condition and probable poor outcome; four (36%) cats survived. All the cats that died showed abnormalities in their imaging studies, with all of them showing at least two different abnormalities, with at least one of those being rated as severe in all of them. The cats that survived had between 0 and 5 radiographic abnormalities, and in only one of them one of the abnormalities was rated as severe.
Discussion
Diagnostic imaging findings in patients with dysautonomia include evidence of megaoesophagus, aspiration pneumonia, gastrointestinal ileus, and urinary bladder distension. 7,9,12,13 Although these changes are non-specific and can be found in many other conditions, their presence, especially if many of the changes are detected in a patient with consistent clinical signs, should promote the inclusion of dysautonomia in the differential diagnoses.
Thoracic radiographs showed evidence of megaoesophagus in 7/11 cases in the current study. Although the small number of cases in the present study are insufficient to make statements about incidence, the frequency appears similar to the incidence of megaoesophagus described for dogs with dysautonomia. 13 In another study including 40 cats, evidence of megaoesophagus was present in 89% of the cases and the presence of severe megaoesophagus appeared to be associated with poor prognosis. 9 In the current series both cats with severe megaoesophagus were euthanased and all cats that survived had either no signs of megaoesophagus or only moderate megaoesophagus. Signs of concurrent aspiration pneumonia were only found in one of our cases. This appears to differ from the incidence of associated aspiration pneumonia described for dogs with dysautonomia, where pneumonia was found in 71% of the patients with megaoesophagus, 13 but again the small number of animals makes this comparison equivocal. In another study, only 6/44 cats with oesophageal motility dysfunction presented patterns suggestive of aspiration pneumonia on radiographs. 17 This appears to suggest a lower incidence of associated aspiration pneumonia in cats with dysautonomia in comparison to dogs. Fluoroscopic examination showed oesophageal dysmotility in all the cases in which it was performed; in three of these cases no abnormalities had been detected in the thoracic radiographs. A previous report noted the detection of oesophageal dysmotility in cases without overt clinical signs of dysautonomia. 16 This would suggest that dysautonomia should be included as a differential diagnosis on any feline thoracic radiographic or fluoroscopic examination that demonstrates evidence of oesophageal dysfunction in the absence of other clinical signs suggestive of dysautonomia, or other imaging findings that might otherwise explain the oesophageal abnormality. Oesophageal dysmotility is also described in equine cases of grass sickness. 18 This suggests that, where available, fluoroscopy should be performed in these patients (even if no signs of megaoesophagus are observed in plain thoracic radiographs) as this will provide information about oesophageal motility that cannot be assessed radiographically.
On abdominal radiographs signs of ileus, with either gastric and/or small intestinal distension were seen in 9/11 cats in the present study. Normal cats usually present with no gas in the stomach or small intestine; for example, in one study only 7% of normal cats presented with a minimal amount of gas in the stomach or small intestine. 19 In the present study all but one cat presented with abnormal amounts of gas in the stomach and/or the small intestine. In two of the cats, a small amount of mineral opacity material was present within the gastrointestinal tract in several loops of intestine. This was thought to be associated with decreased intestinal motility, as opposed to being a classical ‘gravel sign’ where a focal collection of mineralised particles is suggestive of a chronic partial gastrointestinal obstruction. 20 Gastric distension was found in 65% and small bowel distension in 50% of dogs with dysautonomia. 13 In some of these cases, gastrointestinal distension was very severe and mimicked a mechanical obstruction. In one of our cases this was suspected by the referring veterinary surgeon and an exploratory laparotomy had been performed leading to the removal of a small hairball from the jejunum. The presence of other imaging findings and clinical signs consistent with dysautonomia might aid in differentiating gastrointestinal distension due to autonomous nervous system dysfunction from that caused by mechanical obstruction.
Abdominal ultrasound showed small intestinal hypomotility in all the animals in which it was performed. Decreased intestinal motility can be assessed by upper gastrointestinal studies after barium administration and it has been described in animals with dysautonomia. 9,18 However, ultrasound is less time consuming, requires no radiation exposure, and allows for the ruling out of other differential diagnoses such as mechanical obstruction.
Megacolon was found in one of the cats. The ratio between L7 and the maximum diameter of the colon was used to define megacolon in the present study. In this cat, the ratio was 1.86, and was therefore suggestive of megacolon. However, L7 in this patient appeared shorter than normal, as was the sacrum, suggesting the presence of a lumbosacral transitional vertebra; this would probably make the ratio between the colon and L7 less reliable in this case. Other measurements have been recently suggested to overcome this problem; for example, using L5 instead of L7 for the ratio. 21 Megacolon does not appear to be a common finding in either cats or dogs with dysautonomia, as was not present in any of the dogs with dysautonomia in a previous study.13
Urinary bladder distension was found in only 2/7 patients with abdominal radiographic studies. This appears less common than in dogs, where 45% of the animals in one series presented with urinary bladder distension in radiographs. 13 However, it should be taken into account that in some cases urinary retention might have not been detected, for example, if the bladder had been manually emptied prior to the examination as part of the cat's supportive care.
The prognosis for feline dysautonomia is poor. In one study, 7/9 cats were euthanased between 48 h and 5 days after presentation to the referral centre. 22 In another study, 1 70% of the cats had died or had been euthanased by 18 months. Cats showing mild clinical signs or signs limited to a particular organ system have the best chance for long-term survival. In the present study, the cats that showed both gastrointestinal and urinary bladder distension were euthanased on the day of presentation or the following day. All the cats that died or were euthanased had severe gastric distension and/or severe megaoesophagus and always had more than one imaging finding. One of the cats that survived had severe gastric distension but this was not evident in the follow-up radiographs 3 months later. Two other survivors had only moderate changes and the fourth had no imaging abnormalities. Although this might suggest that more severe and multiple system abnormalities were associated with poorer outcome, the small number of animals and differences in the studies available makes this interpretation uncertain.
Limitations of the present study are the small number of cases, lack of histopathological evaluation for four of them, and the inconsistent availability of the studies between the different animals. The diagnosis of dysautonomia was made in one of the cats based on a positive 0.1% pilocarpine test. Although some publications consider a positive test to be the best single test for confirming ante-mortem diagnosis, 12 other studies contradict this and question the reliability of the test. 8,23 In three other cats the diagnosis was made based on consistent clinical signs, neurological deficits and the fact these were seen as part of a same outbreak where two other cats had a histopathological confirmed diagnosis. Thoracic radiographs were available for all the patients, but only the cranial abdomen could be evaluated in four of the patients, preventing complete evaluation of the gastrointestinal and urinary tracts. Fluoroscopy and abdominal ultrasound were only available for five and three of the patients, respectively. The retrospective nature of the study also makes interpretation of ultrasound less accurate as limited information was available from stored images and written reports. In addition, five of the cats of the study were under sedation and one was under anaesthesia, situations that might effect oesophageal distension.
In conclusion, common imaging findings in cats with dysautonomia are megaoesophagus and/or oesophageal dysfunction, and gastric and/or small intestinal distension. Associated aspiration pneumonia and megacolon appear less common. Although diagnostic imaging findings are not specific for this disease, if multiple findings in multiple systems are detected, along with consistent clinical sings, dysautonomia should be considered among the differential diagnosis.
Acknowledgements
The authors would like to thank all members of staff in the Small Animal Hospitals of Glasgow and Edinburgh University Veterinary Schools who helped with these cases and the pathologists who confirmed the diagnoses.
References
- 1.Sharp N.J.H., Nash A.S., Griffiths I.R. Feline dysautonomia (Key–Gaskell syndrome): a clinical and pathological study of forty cases, J Small Anim Pract 25, 1984, 599–615. [Google Scholar]
- 2.Key T.J.A., Gaskell C.J. Puzzling syndrome in cats associated with pupillary dilatation, Vet Rec 110, 1982, 160. [DOI] [PubMed] [Google Scholar]
- 3.Hunter L.C., Miller J.K., Poxton I.R. The association of Clostridium botulinum type C with equine grass sickness: a toxicoinfection?, Equine Vet J 31, 1999, 492–499. [DOI] [PubMed] [Google Scholar]
- 4.Nunn F., Cave T.A., Knottenbelt C., Poxton I.R. Association between Key–Gaskell syndrome and infection by Clostridium botulinum type C/D, Vet Rec 155, 2004, 111–115. [DOI] [PubMed] [Google Scholar]
- 5.Rocklitz I., Bennett A. Key–Gaskell syndrome in a bitch, Vet Rec 112, 1983, 614–615. [DOI] [PubMed] [Google Scholar]
- 6.Pollin M., Sullivan M. A canine dysautonomia resembling the Key–Gaskell syndrome, Vet Rec 118, 1986, 402–403. [DOI] [PubMed] [Google Scholar]
- 7.Longshore R.C., O'Brien D.P., Johnson G.C., Grooters A.M., Kroll R.A. Dysautonomia in dogs: a retrospective study, J Vet Intern Med 10, 1996, 103–109. [DOI] [PubMed] [Google Scholar]
- 8.Harkin K.R., Andrews G.A., Nietfeld J.C. Dysautonomia in dogs: 65 cases (1993–2000), J Am Vet Med Assoc 220, 2002, 633–639. [DOI] [PubMed] [Google Scholar]
- 9.Rochlitz I. Feline dysautonomia (the Key–Gaskell or dilated pupil syndrome): a preliminary review, J Small Anim Pract 25, 1984, 587–598. [Google Scholar]
- 10.Guiford W.G., O'Brien D.P., Allert A., Ermeling H.M. Diagnosis of dysautonomia in a cat by autonomic nervous system function testing, J Am Vet Med Assoc 193, 1988, 823–828. [PubMed] [Google Scholar]
- 11.Coates J.R. Tail, anal and bladder dysfunction. Platt S.R., Olby N.J. BSAVA manual of canine and feline neurology, 3rd edn, 2004, British Small Animal Veterinary Association: Glouchester, 302–319. [Google Scholar]
- 12.O'Brien D.P., Johnson G.C. Dysautonomia and autonomic neuropathies, Vet Clin North Am Small Anim Pract 32, 2002, 251–264. [DOI] [PubMed] [Google Scholar]
- 13.Detweiler D.A., Biller D.S., Hoskinson J.J., Harkin K.R. Radiographic findings of canine dysautonomia in twenty-four dogs, Vet Radiol Ultrasound 42, 2001, 108–112. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien T. Large intestine. O'Brien T. Radiographic diagnosis of abdominal disorders in the dog and cat, 1978, Covell Park Vet Company: Davis, CA, 352–395. [Google Scholar]
- 15.Bradley K. The small intestine. O'Brien R., Barr F. BSAVA manual of canine and feline abdominal imaging, 2009, British Small Animal Veterinary Association: Glouchester, 110–131. [Google Scholar]
- 16.Cave T.A., Knottenbelt C., Mellor D.J., Nunn F., Nart P., Reid S.W. Outbreak of dysautonomia (Key–Gaskell syndrome) in a closed colony of pet cats, Vet Rec 153, 2003, 387–392. [PubMed] [Google Scholar]
- 17.Moses L., Harpster N.K., Beck K.A., Hartzband L. Esophageal motility dysfunction in cats: a study of 44 cases, J Am Anim Hosp Assoc 36, 2000, 309–312. [DOI] [PubMed] [Google Scholar]
- 18.Greet T.R., Withwell K.E. Barium swallow as an aid to the diagnosis of grass sickness, Equine Vet J 18, 1986, 294–297. [DOI] [PubMed] [Google Scholar]
- 19.Morgan J.P. The upper gastrointestinal examination in the cat: normal radiographic appearance using positive contrast medium, Vet Radiol Ultrasound 22, 1981, 159–169. [Google Scholar]
- 20.Graham J.P., Berry C.R., Thrall D.E. Technical issues and interpretation principles relating to the canine and feline abdomen. Thrall D.E. Textbook of veterinary diagnostic radiology, 5th edn, 2007, Saunders Elsevier: St Louis, 626–644. [Google Scholar]
- 21.Trevail TJ, Carrera I., Courcier E., Sullivan M.. Radiographic diameter of the colon in normal, constipated and cats with megacolon. Proceedings of the 15th International Veterinary Radiology Association Congress; 2009 July 26–31; Buzios, Brazil, 2009: 48. [DOI] [PubMed]
- 22.Kidder A.C., Johannes C., O'Brien D.P., Harkin K.R., Schermerhorn T. Feline dysautonomia in the Midwestern United States: a retrospective study of nine cases, J Feline Med Surg 10, 2008, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niessen S.J.M., Eastwood J., Smyth J.B.A., Cherubini G.B. Five cases of canine dysautonomia in England (2004–2006), J Small Anim Pract 48, 2007, 346–352. [DOI] [PubMed] [Google Scholar]