Abstract
Pain management in dogs and cats has undergone a dramatic evolution in the past decade. Current approaches focus on anticipation and prevention of pain, as well as both pharmacologic and non-pharmacologic management techniques. The veterinary team plays an essential role in educating pet owners about recognizing and managing pain in their pets. Journal of the American Animal Hospital Association 2007; 43:235–248.
Historically it was thought that animals did not feel pain or that they perceived pain differently than humans. As a corollary to this concept, it was suggested that pain following surgery or injury was beneficial to animals because it limited movement and thus prevented further injury.
Today there is a better understanding of how pain develops and is perpetuated. It is now well established that animals and humans have similar neural pathways for the development, conduction, and modulation of pain. According to the principle of analogy, because cats and dogs have neural pathways and neurotransmitters that are similar, if not identical, to those of humans, it is highly likely that animals experience pain similarly (Table 1).
Table 1.
Definitions associated with pain and pain management
| Type of pain | Definition |
|---|---|
| Adaptive pain—inflammatory * | Spontaneous pain and hypersensitivity to pain in response to tissue damage and inflammation. Occurs with tissue trauma, injury, surgery. Causes suffering. Responds to treatment. |
| Adaptive pain—nociceptive * | Transient pain in response to a noxious stimulus. Small aches and pains that are relatively innocuous and that protect the body from the environment. |
| Allodynia † | Pain caused by a stimulus that does not normally result in pain. |
| Analgesia † | Absence of pain in response to stimulation that would normally be painful. |
| Anesthesia ‡ | Medically induced insensitivity to pain. The procedure may render the patient unconscious (general anesthesia) or merely numb a body part (local anesthesia). |
| Distress § | Acute anxiety or pain. |
| Dysphoria § | A state of anxiety or restlessness, often accompanied by vocalization. |
| Hospice † | A facility or program designed to provide a caring environment for meeting the physical and emotional needs of the terminally ill. |
| Hyperalgesia † | An increased response to a stimulus that is normally painful. |
| Maladaptive pain—neuropathic * | Spontaneous pain and hypersensitivity to pain in association with damage to or a lesion of the nervous system. |
| Maladaptive pain—functional * | Hypersensitivity to pain resulting from abnormal processing of normal input. |
| Maladaptive pain—central neuropathic pain * | Pain initiated or caused by a primary lesion or dysfunction in the central nervous system. Often called ‘central pain.’ * |
| Modulation § | Altering or adaptation according to circumstances. |
| Multimodal analgesia ‖ | Use of more than one drug with different actions to produce optimal analgesia. |
| Neurogenic pain ‡ | Pain initiated or caused by a primary lesion, dysfunction, or transitory perturbation in the peripheral or central nervous system. |
| Nociception ¶ | Physiologic component of pain consisting of the processes of transduction, transmission, and modulation of neural signals generated in response to an external noxious stimulus. |
| Pain † | An unpleasant sensory and emotional experience associated with actual or potential tissue damage. |
| Palliative care † | Care that relieves or alleviates a problem (often pain) without dealing with the cause. |
| Peripheral neuropathic pain † | Pain initiated or caused by a primary lesion or dysfunction in the peripheral nervous system. |
| Pre-emptive analgesia ‖ | Administration of an analgesic before painful stimulation. |
| Principle of analogy § # | A similarity of forms having a separate evolutionary origin. Similar structures may have evolved through different pathways, a process known as convergent evolution, or may be homologous. |
| Wind-up pain ** †† | Heightened sensitivity that results in altered pain thresholds—both peripherally and centrally. |
Woolf CJ (2004) Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Annals of Internal Medicine 140, 1441–1451.
IASP (International Association for the Study of Pain): www.iasp-pain.org.
MSN Encarta Dictionary: www.encarta.msn.com.
Random House Webster's College Dictionary (1997) New York: Random House.
Gaynor J, Muir W (2002) Handbook of Veterinary Pain Management. St. Louis, MO: Elsevier Publishing.
Veterinary Clinics of North America Small Animal Practice 2000, 30(4):704.
Wikipedia: http://en.wikipedia.org/wiki/Main_Page.
Hansen B (2005) Managing pain in emergency and critical care patients. In: Proc Atlantic Coast Veterinary Conf.
Muir WW, Hubbell JA (2006) Handbook of Veterinary Anesthesia (4th edn). St. Louis, MO: Elsevier Publishing.
Veterinary practitioners also have more insight into how most drugs work to modulate pain and how and why a combination of therapies can benefit patients. Untreated pain decreases quality of life in all patients, and prolongs recovery from surgery, injury, or illness. Today's analgesic strategies allow people—and now animals—to live more comfortable lives. Preventing and managing pain has become a fundamental part of quality and compassionate patient care in veterinary medicine.
As advocates for their patients, the veterinary team has the responsibility to recognize, assess, prevent, and treat pain. Pain should be thought of as the fourth vital sign—after temperature, pulse, and respiration—and integrated into all patient evaluations.
Many health conditions and medical procedures cause pain in cats and dogs. Attention to pain is fundamental to every aspect of patient care, regardless of the patient's condition or reason for presentation to the veterinarian. Managing pain effectively requires looking for its signs and asking the right questions. Because many animals may not show obvious indications of pain, identifying the degree of pain and the amount of suffering associated with it can be a challenge. The most common sign of pain is change in behavior.
Incorporating pain management into the veterinary practice helps everyone. It benefits the patient through improved quality of life and reduced complications. It benefits clients through enhancement of the human–animal bond. It benefits the health care team through increased safety; improved morale, pride, and job satisfaction; and a less stressful environment. Contemporary approaches to pain management enable veterinarians to more effectively fulfill their responsibility to relieve animal suffering as pledged in the veterinarian's oath.
This document was developed by the American Animal Hospital Association (AAHA) and the American Association of Feline Practitioners (AAFP) through a collaborative effort between Task Force members. By their very nature as a consensus-built set of guidelines, these recommendations reflect a combination of expert opinion, personal experience, and scientific studies. They are intended to educate and inform members of the veterinary profession and should not be identified as standards of care, AAHA accreditation standards, or considered minimum guidelines, but rather as recommendations from AAHA and AAFP. These guidelines should not be construed as exclusive protocols, courses of treatment, or procedures. The Task Force recognizes that through continuing developments in research, technology, and experience, the information contained herein will be subject to change. Procedures and techniques other than those described in these guidelines may be deemed necessary based on the specific needs of the patient, available resources, and constraints due to the environmental conditions.
Types of pain
All tissue injury, including that from elective surgery, may cause pain. Pain-induced stress responses, mediated by the endocrine system, are one of the negative consequences of pain. Increased cortisol, catecholamines, and inflammatory mediators cause tachycardia, vasoconstriction, decreased gastrointestinal motility, delayed healing, and sleep deprivation. In addition, trauma causes unseen changes in the central nervous system. Inadequate pain prevention or management can lead to magnification of pain perception and a prolonged pain state.
Although traditionally pain has been categorized as acute or chronic based on duration, a more contemporary approach considers pain as adaptive or maladaptive (Woolf 2004) (Table 1). Adaptive pain is a normal response to tissue damage. Adaptive pain includes inflammatory pain. Inflammation is a major component of many pain states (including acute pain following surgery or trauma) and some chronic pain states such as osteoarthritis. Inflammatory mediators sensitize neural pathways, increasing the perception of pain.
If adaptive pain is not appropriately managed, physical changes occur in the spinal cord and brain, leading to pain that is termed maladaptive. Examples of maladaptive pain are neuropathic and central pain. For instance, the thalamus typically serves as a relay station sending nerve impulses from the periphery to the cortex but may become a spontaneous pain generator if adaptive pain becomes maladaptive, central pain.
An awareness that acute and chronic pain can convert from adaptive to maladaptive pain helps veterinarians understand why pain is so difficult to control in some patients. The pain-induced changes in the nervous system cause it to become more sensitive, rather than less sensitive. The longer pain is unmanaged, the more likely the neuro-physiologic processes involved will cause a switch from adaptive to maladaptive pain, which is more serious and difficult to control. When that happens, some patients require specific drug therapy—such as N-methyl-d-aspartate (NMDA) receptor antagonists (amantadine) and gabapentin—aimed at restoring normal central transmission. Patients may also require multiple therapies (pharmacologic as well as nonpharmacologic) to manage their pain.
‘Wind-up pain’ is a heightened sensitivity that results in altered pain thresholds, both peripherally and centrally, such that pain is experienced in areas unrelated to the original source. Wind-up causes a worsening of acute pain and has been used to describe the processes that result in maladaptive pain.
Allodynia is pain caused by a stimulus that does not normally result in pain and can be a component of maladaptive pain. For instance, a cat with long-standing, untreated vertebral osteoarthritis may not tolerate even light stroking across its back.
Patients may experience both adaptive and maladaptive pain. As an example, a cat undergoing onychectomy (declawing) experiences inflammatory pain with the potential to develop long-term neuropathic or central pain if the pain is inadequately managed during the perioperative and healing periods. Effective, early, multimodal perioperative pain management is essential to minimize the development of these events. a
Anticipation and early intervention
An understanding of the underlying conditions that can result in pain better equips the practitioner to anticipate and potentially intervene or modulate pain development. Preventing risk factors early in life reduces the development of pain later in life. For example, providing lifelong dental care reduces the development of oral pain, and preventing obesity reduces the incidence and severity of osteoarthritis (Smith et al 2006).
Overlooked or unrecognized source of pain
Unintentional pain or discomfort associated with veterinary procedures is easily overlooked. Procedures that might cause discomfort or pain include IV catheterization, ear cleaning, manual stool evacuation, and anal sac expression (especially in cats). Evaluate in-hospital procedures where unintentional pain can occur, and reduce or eliminate those causes. If necessary, use an opioid to reduce the pain or discomfort of some procedures. If an animal must be restrained or handled excessively because of its fear, aggression, or pre-existing pain, use anxiolytics, sedation, and/or anesthesia as needed to prevent struggling, subsequent pain or injury, and aversion.
Conditions in which it is unclear how much pain the animal experiences include some visceral, gastrointestinal, and urogenital diseases; central nervous system disorders; and dermatologic disease. Table 2 lists conditions and procedures that may be overlooked or underestimated as causes of pain. Specific management for these conditions and procedures may include pharmacologic and/or non-pharmacologic management or simply a different or more careful approach to handling the animal.
Table 2.
Frequently overlooked causes of pain
| Type of pain | Cause |
| Cardiopulmonary | Congestive heart failure (pulmonary edema and pleural effusion); pleuritis, cerebral vascular accident, thromboembolism (clot). |
| Oncologic | Any and all cancer. |
| Dermatologic | Otitis, severe pruritus, burns, chronic wounds; abscess, cellulitis, clipper burns, urine scalding, severe chin acne. |
| Dental | Oral tumors, feline oral resorptive lesions (‘neck’ lesions), fractures (no matter how small), tooth abscess, ulcers, stomatitis. |
| Gastrointestinal | Constipation, obstipation, obstruction, megacolon; anal sac impaction; hemorrhagic gastroenteritis, pancreatitis, gastric dilatation–volvulus (GDV), foreign body. |
| Musculoskeletal | Most often overlooked in cats. Muscular soreness, arthritis, degenerative joint disease, tendon or ligament injury, intervertebral disc disease, facet pain of spondylosis, osteodystrophy, dislocations. |
| Ocular | Corneal disease and ulcers, glaucoma, uveitis. |
| Urogenital | Uroliths, ureteroliths, queening/whelping, feline lower urinary tract disease/interstitial cystitis, acute renal failure, enlarged kidneys (capsular swelling), lower urinary tract infections, urinary obstruction, vaginitis (especially in obese cats). |
| Hospital procedures | Restraint (examination, obtaining blood and urine samples, radiographs, and ultrasound; even gentle handling and hard surfaces can increase pain in an already painful animal). Urinary/IV catheterization, bandaging, surgery, thoracocentesis, chest tube placement and drainage procedures, abdominocentesis. Manual extraction of stool and anal sac expression (especially in cats). |
| Surgical procedures | Ovariohysterectomy, castration, onychectomy * , growth removal, and all other surgical procedures. |
| Neurologic | Diabetic neuropathy. |
Regardless of method used, onychectomy causes a higher level of pain than spays and neuters.
Some procedures and conditions that are commonly recognized as causing pain in dogs may be overlooked in cats. For example, osteoarthritis, intervertebral disc disease, and spondylosis are common in older cats, and yet many of the behavioral changes related to these diseases have been ascribed to ‘old age’ rather than pain.
Cats and dogs with behavior problems often have an underlying medical condition that may be painful. For example, the cat that urinates inappropriately may have painful lower urinary tract disease. In these kinds of cases, pain management plays an important part in the animal's treatment.
Anticipating and reducing surgical pain
The level of pain associated with surgery can be anticipated to some extent. In general, the more tissue trauma, the more pain, because pain is proportional to increasing levels of circulating cytokines (Kristiansson and Saraste 1999). Abdominal surgery generally produces more pain than do superficial soft-tissue procedures, and orthopedic procedures can cause severe and prolonged pain. Keep in mind, however, that even ‘routine’ procedures are painful. Repeat surgeries may be more painful than the original surgery due to the changes that occur in the spinal cord and brain with repetitive and prolonged stimulation (maladaptive pain). Good surgical technique and minimal tissue trauma may help alleviate pain.
Resources are available to assist in estimating the degree of pain associated with various conditions and procedures (see later). However, the practitioner must be aware that each individual animal will have a unique response to painful stimuli (Hunton et al 2005).
To optimize pain management and improve the safety of anesthesia, use a perioperative approach to pain management. The timing of administration of medications to prevent pain is critical. For example, giving an opioid prior to a surgical procedure is much more beneficial than administering the same dose afterward. An opioid administered as an anesthetic premedication not only helps to dampen the pain response but also decreases the doses of anesthetics required for anesthetic induction and maintenance. Providing adequate intraoperative and postoperative analgesia increases patient comfort and facilitates a smoother recovery from anesthesia. It may also prevent the development of maladaptive pain.
Some opioids have a very short duration of action and have a ceiling effect (eg, butorphanol), whereas others (eg, buprenorphine) have a longer duration of action but a delayed onset and time to peak effect. Understanding these pharmacologic differences helps to ensure that medications are used appropriately and with the most efficacy.
Anticipating changes in pain management
Initial pain management should be followed by ongoing reassessment and revision of pain management, titrating treatments up or down to meet patient needs. Anticipate that increased signs of pain may occur following discharge from the hospital, as (for example) when residual sedation or a local anesthetic wears off after the patient has returned home. Furthermore, some animals may mask behavioral signs of pain while hospitalized, and signs of pain become evident only at home. The client should be counseled about this contingency, and pain medications should be made available. (A later section provides more information on educating clients regarding pain management in their pets.)
Analgesic drugs and/or dosages may need to be modified with the patient's changing status. If a dog with osteoarthritis is on a non-steroidal anti-inflammatory drug (NSAID) and then undergoes intestinal surgery, a different type of drug must be prescribed if the animal becomes hypovolemic.
The practitioner should expect escalating pain management needs in animals with progressive diseases, such as osteoarthritis, cancer, intervetebral disc disease, or spondylosis, as well as in some end-of-life or hospice patients.
Recognition and assessment
Variations in pain response
Although all animals experience pain, expression of pain varies with age and species, as well as among individuals. Neonates have intact neural pathways for pain transmission, but both neonates and senior animals may not express their pain as plainly as other animals. Cats and dogs also tend to hide pain as a protective mechanism. However, a lack of expression or outward evidence does not necessarily indicate that these patients are not experiencing the negative consequences of pain.
Some animals experience residual pain following a surgery or injury, whereas other animals seem to return quickly to normal function with no obvious residual pain. Responses to surgery and injury or to therapy are unique to each individual, and the differences reflect genetic variation in such factors as the number, distribution, and morphology of opioid receptors (Janicki et al 2006, Kim et al 2006, Landau 2006).
Anecdotal evidence suggests that certain breeds appear more sensitive to painful stimuli and are more easily aroused than others. Whether this reflects different communication styles, arousal patterns, or actual pain perception is not known.
Individual animals undergoing the same procedure may experience or express their pain differently. In addition, an individual animal can experience more than one type of pain at any given time. For example, the senior patient with osteoarthritis that undergoes surgery to remove a mass may experience musculoskeletal pain due to positioning during the procedure, in addition to the pain associated with the surgery itself.
Differentiating pain from other conditions
Assessing behavior is an integral part of the history-taking and physical examination of any animal (Table 3). Understanding normal behavior is essential to identifying pain and selecting an appropriate intervention (American Association of Feline Practitioners 2004, American Association of Feline Practitioners 2006). The input of the owner is invaluable in determining abnormal behavior that may be linked to pain (Hunton et al 2005).
Table 3.
Signs of pain
| General signs | Specific signs |
| Loss of normal behavior | Decreased ambulation or activity, lethargic attitude, decreased appetite, decreased grooming (cats). Harder to assess in the hospital. |
| Expression of abnormal behaviors | Inappropriate elimination, vocalization, aggression or decreased interaction with other pets or family members, altered facial expression, altered posture, restlessness, hiding (especially in cats). |
| Reaction to touch | Increased body tension or flinching in response to gentle palpation of injured area and palpation of regions likely to be painful, eg, neck, back, hips, elbows (cats). |
| Physiologic parameters | Elevations in heart rate, respiratory rate, body temperature, and blood pressure; pupil dilation. |
Behavioral signs of pain, including both loss of normal behavior and development of new and abnormal behaviors, may be subtle and easily overlooked by both the owners and the veterinary health care team. A systematic and holistic approach that considers the animal as well as its environment is essential to recognizing changes in behavior and physiologic parameters. Physiologic signs such as increased respiration and heart rate, increased blood pressure, or dilated pupils may be manifestations of pain or stress but should not be relied on as the sole indicators of pain.
The line between ‘discomfort’ and ‘pain’ is imprecise. The practitioner must assess the conditions and procedures that cause sensations ranging from skin irritation to severe discomfort and then determine whether pain management is indicated. Tables 2 and 3 summarize often overlooked causes of pain and signs of pain, respectively.
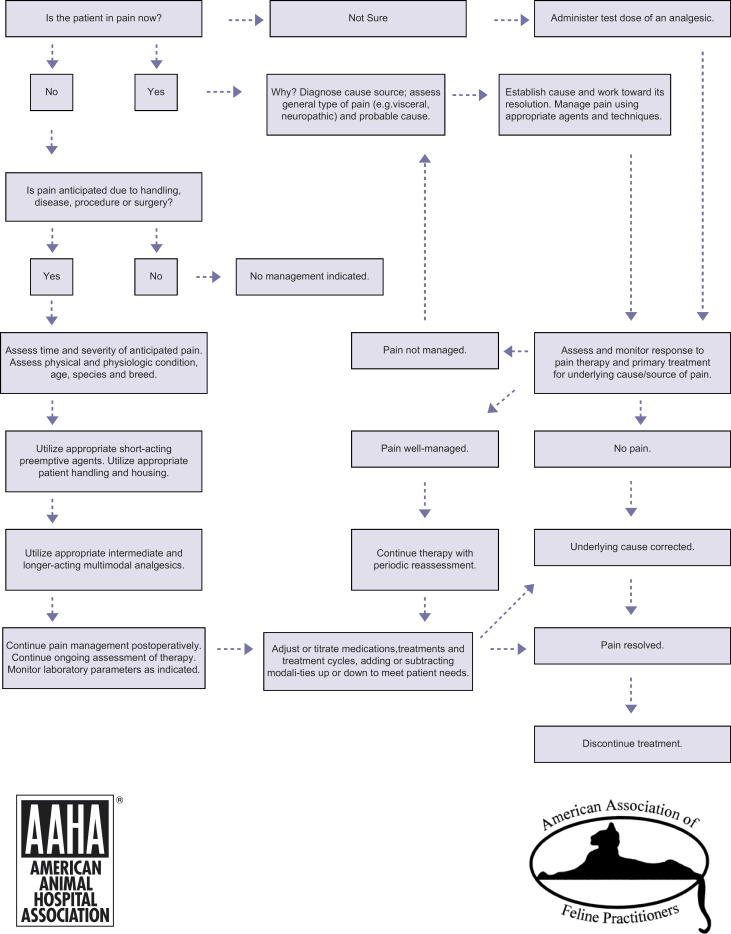
The decision-making process can be facilitated by the pain management algorithm shown in Fig 1, which offers a step-by-step approach to determining whether or what intervention is indicated. If a question persists regarding the presence of pain, administer an analgesic and assess the patient's response. Response to therapy is an appropriate and important tool in pain assessment. When pain management is needed, formulate a plan, note it in the medical record (and make a copy for the client), and update the plan as needed.
Fig 1.
Pain management algorithm.
Differentiating dysphoria from pain can be challenging, especially in traumatic or surgical cases. Pain and dysphoria can occur simultaneously, complicating the picture. However, the observant practitioner can discern clues to differentiate between dysphoria and pain. Dysphoric animals are difficult to distract or calm by interaction or handling. Administering more opioids does not help the situation, and a source of pain is not readily identifiable. In contrast, animals in pain typically can be temporarily distracted and calmed by interaction or handling. Increased or repeated doses of opioids seem to help, and a source of pain can be identified.
Frequency of pain assessment depends on the presenting problem. In patients with surgical or traumatic pain, pain assessment is recommended at least every 2 h. In patients with chronic pain, assessment is recommended a minimum of every 3 months or whenever control seems to be waning. All patients should be assessed for pain during their regularly scheduled wellness exams.
Scoring tools such as identification and recording of behavioral changes and responses to therapy are useful for standardizing pain assessment. No single accepted pain scale has been developed for use in dogs or cats, and scales currently in use range from simple to complex (University of Glasgow 2005, Hellyer et al 2006). Whatever pain scale is adopted, it is essential that it fits the practice; otherwise, the scale will go unused. The pain scoring system should be incorporated into overall patient assessment, treatment, and reassessment protocols, as well as staff and client educational materials.
Pharmacologic intervention
The use and choice of pharmacologic agents are based on a thorough patient assessment that includes a physical exam; an evaluation of the patient's history, underlying or pre-existing conditions, and presenting complaint; and a laboratory evaluation of an appropriate database. When choosing medications to send home with patients, client compliance can be enhanced by taking into account such factors as the duration of analgesic action and ease of administration.
Classes of analgesic drugs are listed in Table 4. An exhaustive list of drugs and their indications, contraindications, side effects, and doses is referenced and may be found in other sources (Flecknell and Waterman-Pearson 2000, Gaynor and Muir 2002, Tranquilli et al 2004). A multimodal approach to pain management takes advantage of the different modes and sites of action of various analgesic agents (Table 4). Lower doses of each analgesic often can be used, reducing the potential for side effects and providing superior analgesia. This approach also utilizes the timing of administration of different agents (Lamont et al 2000, Tranquilli et al 2004).
Table 4.
| Drug class | Comments |
| Alpha-2 agonists | Analgesic, sedative, muscle relaxant; dose-related duration, reversible. |
| Anxiolytics | Antianxiety (anxiety enhances pain); preappointment or preanesthetic. |
| Corticosteroids | Analgesic; anti-inflammatory. |
| Local anesthetics | Analgesic; anesthetic-sparing; blocks pain recognition. |
| NMDA ‡ receptor antagonists | |
| Amantadine | Reduces ‘wind-up;’ good for chronic pain management in dogs; typically not used for adaptive pain. |
| Ketamine | Somatic analgesia; reduces ‘wind-up.’ |
| NSAIDs § | Analgesic; anti-inflammatory; long duration of action. |
| Opioids | Analgesic; anesthetic-sparing; reversible; short duration of action. |
| Topical anesthetics | Dermatologic conditions, anal/genital procedures, hospital procedures (eg, catheter placement). |
| Tricyclic antidepressants | Antidepressant and anxiolytic, with analgesic properties. Used as adjunctive analgesic; enhances opioid analgesia. Used in humans to treat chronic and neuropathic pain at lower doses than those used to treat depression. |
| Miscellaneous drugs | Comments |
| Gabapentin | Reduces ‘wind-up;’ good for chronic pain management in dogs and cats; typically not used for adaptive pain. |
| Tramadol | Analgesic; good for chronic pain management in dogs and some cats. |
Gaynor J, Muir W (2002) Handbook of Veterinary Pain Management. St Louis, MO: Elsevier Publishing.
Mayo Clinic Tools Online: www.mayoclinic.com/health/.
NMDA=N-methyl-d-aspartate.
NSAID=nonsteroidal anti-inflammatory drug.
Perioperative analgesia
Before an elective surgery, use an opioid that also reduces the anesthetic requirement. Before or during surgery, use a local anesthetic at the incision to block the transmission of noxious stimuli. During anesthetic recovery, use an NSAID to decrease the inflammation from the surgical trauma. This combination of pharmacologic-agents may help prevent the evolution of maladaptive pain. Regardless of the procedure performed, opioids are anesthetic-sparing and should be used for this effect.
Pharmacologic pain management must also be addressed by organizations with a high volume of surgical cases (ie, shelters, ovariohysterectomy–neuter programs, and feral cat programs) or in situations with little to no opportunity for follow-up. Economic considerations may play a role in these circumstances. The question may be, ‘What is the minimally acceptable degree of analgesia that should be offered for routine surgical procedures, and how is this most economically achieved?’ The answer will change as new information becomes available.
For instance, even when opioids are unavailable, other options exist. In a recent study, a single dose of carprofen given to shelter dogs undergoing ovariohysterectomy demonstrated good analgesia for up to 24 h in most dogs (Shih et al in press).
Analgesia for other procedures and conditions
Local or topical anesthetics are useful for managing pain or discomfort associated with a variety of conditions such as clipper burns or urine scalding or procedures such as IV catheter placement. Opioid administration for procedures such as manual extraction of stool and anal sac expression, especially in cats, decreases pain and helps prevent fear and anxiety associated with veterinary visits. Since pain is individual for each patient, if the patient is still in pain, additional analgesia and/or anesthesia are indicated.
Because anxiety and fear can amplify pain, and physical restraint may contribute to pain, anxiolytics should be used for anxious or fearful animals undergoing hospital procedures. Alprazolam is an excellent antianxiety medication. Tricyclic antidepressants may be indicated when the practitioner suspects persistent, aberrant pain that is refractory to traditional analgesics (eg, neuropathic pain). Recall that acepromazine is not an anxiolytic but rather a tranquilizer, causing sedation without decreasing anxiety. Acepromazine may disinhibit aggression, making the patient not only more fearful but also more dangerous. Although acepromazine is useful as part of an anesthetic protocol, it is not indicated for use to control fear or anxiety (Bowen and Heath 2005).
A synthetic feline facial pheromone (FFP) helps reduce anxiety in unfamiliar surroundings, including the veterinary hospital. FFP diffusers can be plugged into each room and sprayed on tables, towels, and hands. Although this approach is beneficial with many cats, occasionally an individual animal may become more agitated (Pageat and Gaultier 2003, Kronen et al 2006).
Evaluating and monitoring drug metabolism and effects
It is important to treat all pain for an adequate period of time. Patients receiving pain management must be monitored via re-examination and laboratory testing at prescribed intervals to assess for efficacy and adverse events.
Drug metabolism varies among species, breed, and individuals. Specific contraindications of drug classes are noted by their manufacturers (for those drugs that are approved for use in dogs and cats) as well as in many texts.
Selection of pain medication for cats requires special attention. Some opioids—such as buprenorphine, meperidine, and methadone—cause fewer behavioral side effects in cats (eg, less excitement) than do other opioids. With respect to buprenorphine, the oral (transmucosal) route can be as effective as the intravenous route and is more convenient. Hydromorphone has been associated with hyperthermia and agitation in cats at the doses required to provide analgesia (Niedfeldt and Robertson 2006).
There is a misunderstanding regarding the degree and duration of analgesia provided by butorphanol. Butorphanol is not as effective or long-lasting as other opioids (although there are individual variations in response to drugs that target different opioid receptors, such as the mu- and kappa receptors, which has been demonstrated in cats) (Lascelles and Robertson 2004a, b). Limited uses of butorphanol include anesthetic premedication and prior to minimally invasive procedures.
Pets with transdermal fentanyl patches may be easier for owners to manage, but the absorption of the drug is highly variable and may not reach analgesic levels. If the decision is made to send a cat home with transdermal fentanyl, the client should be educated about how to identify whether the cat is still in pain, such that the use of other opioids becomes necessary (Egger et al 2003).
Because vomiting increases intracranial and intraocular pressure in animals with an eye injury or intracranial mass, opioids such as morphine or hydromorphone should be avoided in these patients. Exercise caution with their use in animals that are obtunded and have an increased risk of aspiration (eg, brachycephalics).
Non-pharmacologic intervention
Appropriate use of nonpharmacologic therapy as an adjunct to pharmacologic agents can enhance pain prevention, management, and treatment. These methods of pain control typically are used for chronic pain but may also be appropriate adjuncts to treatment of acute pain.
Many basic lifestyle changes can reduce pain. For example, controlled exercise and weight management are used to decrease joint stress and improve muscular support of the joints (Impellizeri et al 2000, Mlacnik et al 2006). Although home care varies with the condition, even simple environmental accommodations can benefit the pet and prevent or reduce discomfort. These include easy access to litter boxes (no hood, ramp, or stairs and a low-entry side); soft bedding; raised food and water dishes; non-slip floor surfaces, especially in food and litter areas; baby gates to prevent access to stairs; modified access to outdoors, especially in hot or cold weather; and appropriate warm-up prior to exercise (American Association of Feline Practitioners 2004). And lastly, positive, consistent interaction with the pet can improve the animal's demeanor.
Quality in-hospital care may include the use of soft padded bedding during illness or surgery to enhance comfort, warm water or air blankets to decrease pain and facilitate recovery from anesthesia, reduction of patient anxiety by minimizing the length of a hospital stay, and gentle and respectful patient handling. It is also beneficial to decrease visual and auditory stimulation and separate dogs and cats within the hospital setting. Shy or anxious cats should have a box or similar structure in their cage to provide a hiding place.
An array of medical approaches can be grouped under the umbrella of ‘complementary and alternative medicine.’ Use of some of these methods is controversial, in part because of the lack of scientific study and published evidence about them. Additional research is needed to elucidate both the benefits and the uses of complementary and alternative medicine. Of all the complementary procedures used for pain management, acupuncture is most supported by evidence; its use in humans is endorsed by the National Institutes of Health (National Institutes of Health 1997).
If alternative medical approaches are used, it is essential that the procedure or therapy be performed with the full and informed consent of the client and under applicable state laws. Where training or certification is available, the modality must be administered by individuals trained or certified in its use and limitations. b As with administration of pharmacologic agents, patients receiving alternative modalities should be periodically reassessed to determine the efficacy of treatment.
Nutrition is one of the most popular ‘alternative’ modalities. Nutraceuticals, such as glucosamine and chondroitin, may decrease joint inflammation and assist in cartilage repair, although a large meta-analysis conducted in human medicine indicated the need for further study (Hardie 1997, Towheed 2002, Richy et al 2003, Beale 2004, McCarthy et al 2006). There is evidence that omega-3 fatty acids decrease inflammation in cartilage of dogs with osteoarthritis, and dietary intervention can improve clinical signs in osteoarthritic dogs (Wander et al 1997, Neill et al 2005, Budsberg and Bartges 2006). Chondroprotective agents such as polysulfated glycosaminoglycans have been demonstrated to modify the progression of osteoarthritis by maintaining chondrocyte viability via the inhibition of cartilage degradation pathways (McNamara et al 1997, Sevalla et al 2000). At this time, most of the research that has been conducted to assess the roles of nutraceuticals and chondroprotective agents has been conducted in dogs. However, there are anecdotal reports of improved function in cats receiving chondroprotective agents.
Rehabilitation therapy (ie, the application of physical therapy techniques to animals) may be used to return a patient to normal function following surgery or trauma or as a part of a long-term strategy to manage pain. Rehabilitation includes techniques such as cryotherapy, heat therapy, massage, stretching, passive range-of-motion exercise, hydrotherapy, therapeutic exercise, use of dryland or underwater treadmill, and strength-building.
Additional therapies that fall under the rehabilitation umbrella include low-level laser, ultrasound, and transcutaneous electrical nerve stimulation (TENS). Some veterinary practices have incorporated these therapies on the basis of extrapolation from human medicine and anecdotal reports of their success. Currently there is insufficient published evidence of efficacy in dogs and cats to make specific recommendations about the use of these therapies.
Although chiropractic intervention occasionally has been used to treat chronic pain, chiropractic methods potentially can cause injury through the use of inappropriate technique or excessive force. Currently there are no clear standards for when chiropractic intervention should be applied or who is qualified to use chiropractic manipulations. Practitioners who have received formal training in animal chiropractic manipulation (typically 100–140 contact hours) report positive results in their patients experiencing chronic pain. There is currently insufficient published evidence of efficacy in dogs and cats to make specific recommendations about the use of chiropractic intervention.
Pharmaceutical and non-pharmaceutical intervention can be combined for pain management. For example, a cat with osteoarthritis is given both drug therapy and a reduced-calorie diet for weight loss. The cat also may benefit from acupuncture, massage, physical rehabilitation techniques, and care in handling. In the home, the pet's quality of life can be enhanced by such environmental modifications as soft bedding, ramps or steps that allow the cat to use its favorite places, non-slip floor surfaces, and food dishes raised to the height of the cat's elbow.
When pain persists: referrals, hospice, and palliative care
Due to the complex nature of many pain conditions, consultation or referral may be appropriate in some cases. Situations that warrant consultation or referral include a lack of anticipated response or an unsatisfactory response to treatments despite the use of multiple modalities. Patients may also be referred if their condition requires surgical intervention by a specialist or a procedure not provided by the primary practice, eg, acupuncture, radiotherapy, or advanced diagnostic workups such as computed tomography (CT) or magnetic resonance imaging (MRI). Practitioners may want to seek consultation for diagnostic confirmation or help in fine-tuning pain management protocols and procedures. Referrals are also recommended for patients requiring more aggressive or complex pain management beyond the scope of most practices.
Many pet owners welcome the possibility of being able to provide hospice or palliative care for their pets when it is made available. Hospice is defined as ‘a system which provides compassionate comfort care to patients at the end of their lives and also supports their families in the bereavement process. Hospice care for terminally ill patients is characterized by recognition that the life expectancy is less than 6 months.’ (American Animal Hospital Association, American Association of Human–Animal Bond Veterinarians 2002)
Palliative care is defined as ‘the active total care of patients whose disease is not responsive to curative treatment. Control of pain is paramount. The goal of palliative care is achievement of the best quality of life for patients and their families.’(World Health Organization 2007) Palliative care maximizes the pet's quality of life while managing pain and discomfort. Providing palliative care treats the patient but not necessarily the disease. Examples of palliative care include palliative radiation (eg, to decrease pain related to osteosarcoma), appropriate analgesics and other pharmacologic agents, and nutritional support (eg, esophagostomy or gastrostomy tube).
With both hospice and palliative care, regular client communication is essential. Create client support systems within the practice, using the veterinary team to assist with client education and the most appropriate means of delivery of medications or treatment modalities at home.
It is important to create reasonable expectations for the client. Clients can be helped by the use of decision-making trees and explanations of probable outcomes and by being given choices. Euthanasia can be a gift to relieve pain and suffering and should be included as a reasonable and humane option at some point. A client may not apprehend the level of suffering the pet is experiencing or have any way to gauge quality of life. Quality of life indices are being developed to assist pet owners in making these kinds of difficult decisions (Wiseman-Orr et al 2004).
At the onset of an animal's terminal condition, it is beneficial to ask the client to remember what activities the pet enjoyed and have the client compare these to the pet's current status. This process helps to clarify the client's understanding of quality of life. When possible, these issues should be discussed with the client while the pet is still healthy, before the animal is ill or in pain.
Client and staff education
A team approach that involves the client, veterinary team, and patient reinforces the doctor–client–patient relationship and strengthens the human–animal bond. The veterinary team serves as the client's source of reliable information regarding identification and management of pain for the pet. The client plays an essential role in the ongoing assessment and success of treatment.
Education is an important component of a comprehensive veterinary practice. Both staff members and clients should be instructed on how to best handle both cats and dogs respectfully and the additional support and respect needed when animals are fearful. It is important to educate owners early in their experiences with their pets, at the first kitten or puppy visit, discuss handling, dental care, weight management, ovariohysterectomy and neuter procedures, semiannual wellness exams, and planned procedures such as ongoing dental prophylaxis, all of which can impact the future onset of pain.
Clients generally need help to recognize the subtle signs of pain and should be advised that methods for the alleviation of pain are available, effective, and generally safe. Even subtle changes in behavior are reasons to contact the veterinary clinic because these are the first signs of illness and pain.
If a pain management plan is required, the client should be given a copy of the plan as well as written, verbal, and hands-on instruction in how to administer medications. To reinforce verbal information about pain assessment, provide the client with handouts with general information about pain in pets as well as any side effects of medication. Owner compliance will be increased if the administration of the medication is tailored not only to the patient but also to the owner's abilities, with regard to both schedule and method of administration.
Many of the medications used for a variety of canine and feline conditions, including pain, are not approved for such use. For example, on the basis of its clinical performance, gabapentin is becoming more widely used to interrupt the cycle of chronic pain in arthritic dogs and cats, yet the drug is not approved by the Food and Drug Administration (FDA) for such use. Nonetheless, veterinarians should not avoid provision of pain management simply because approved drugs are not available. Legal experts advise that informed consent be obtained in these circumstances and that copies of the signed consent form be kept in the medical record. As treatments for alleviating pain continue to evolve, veterinarians can monitor the peer-reviewed literature, veterinary conferences, and appropriate associations for new information.
Team members who are involved in client interactions must be trained to effectively practice active listening. They should be willing and able to address clients' concerns and answer questions via periodic appointments, email, and/or phone support from the practice. Any discussions with clients about specific patients should be documented in the medical record. In shelters, ovariohysterectomy–neuter programs, and feral cat programs, educating veterinarians and staff members about pain management should be a part of the group's long-term strategy to raise the standard of care.
Education needs to address the various learning styles of clients and team members. Veterinarians, veterinary teams, and clients can find useful information from a variety of sources and in a variety of forms, including written, online (text and video), and video or audio recordings. Written and audio sources include journals and veterinary conference proceedings. Other educational sources include commercial companies, information-sharing groups, and government agencies. Team training resources are available for use both in the veterinary practice and the home setting.
Veterinarians should be prepared to direct their clients and staff to websites that provide accurate and current information. When possible, videos, CDs, and/or DVDs should be available in the hospital for either viewing on site or loan to pet owners. Industry and FDA-approved information about medications and their adverse effects is available from each drug manufacturer and at the Center for Veterinary Medicine website at www.fda.gov/cvm/default.html. Table 5 offers a list of useful websites and the kind of information they provide.
Table 5.
Useful Internet links
| Name of organization | Web address | Type of information available |
| American Animal Hospital Association | www.aahanet.org | Pain management standards; analgesic position statement. |
| American Association of Feline Practitioners | www.catvets.com | Feline Behavior Guidelines, Appendix 1: behavioral assessment; feeding tips to prevent obesity in your cat; how to help your cat have pleasant veterinary visits; environmental enrichment enhances quality of life. |
| Center for Veterinary Medicine, Food and Drug Administration | www.fda.gov/cvm | Information about specific approved drugs. |
| Cornell University College of Veterinary Medicine, Feline Health Center | www.felinevideos.vet.cornell.edu | Giving your cat medication, other cat care. |
| International Academy of Veterinary Pain Management | www.cvmbs.colostate.edu/ivapm | Information for professionals and pet owners. |
| International Association for the Study of Pain | www.iasp-pain.org | Terminology, definitions. |
| United States Pharmacopoeia | www.usp.org | Drug information including modes of action and potential adverse effects. |
In addition to providing extensive information about pain management, the Internet is a resource for materials related to ancillary procedures. Veterinarians can download legal forms for informed consent and refusal of recommendation, client information forms, pain management handouts, discharge instructions, and instructions on administration of medications—all useful tools for implementing a successful pain management plan (Lifelearn 2004/2005, American Animal Hospital Association 2005, Wilson 2006, Cornell University 2007, Brock 2007).
Summary
Offering and providing adequate pain management enhances patient quality of life, improves the human–animal bond, encourages the team, and benefits the practice. For veterinarians who wish to improve their approach to pain management, the following strategies are a good place to start.
Use the pain management algorithm (Fig 1) to aid in pain identification, prevention, and management. Develop anesthetic protocols to include pain prevention, using appropriate agents at specific times. Involve and train the whole veterinary team in pain management protocols. Discuss common case examples. Develop and provide scoring and assessment tools. Teach team members to use open-ended questions during their history-taking with clients to maximize the team's understanding of each patient's situation.
Educate pet owners as described previously, starting when the animal is young. Develop written materials for client education. Begin developing the client's awareness of the importance of identifying and treating pain whenever it occurs.
The profession's understanding of pain management is evolving with new agents and techniques and the application of evidence-based medicine. The need to be open to these changes and challenges is essential. It is hoped that this document provides a framework to approaching and managing pain and to understanding the challenges that face the profession in the future.
Acknowledgements
The American Animal Hospital Association and the American Association of Feline Practitioners would like to acknowledge and thank the members of the Pain Management Guidelines Task Force for their time and commitment to this project. The Task Force members' dedication to relieving and, when able, eliminating animal suffering is evidenced by this work.
AAHA and AAFP gratefully acknowledge the following for their sponsorship of an educational grant for the AAHA/AAFP Pain Management Guidelines for Dogs and Cats: IDEXX Laboratories, Merial Ltd, Pfizer Animal Health, Schering-Plough Animal Health Corporation and Novartis Animal Health.
Footnotes
Declawing is a controversial procedure. However, if it is performed, the procedure should include effective multimodal pain therapy including opioids, an NSAID, and local analgesia, with individualized timing and assessment as described for all surgical patients.
For example, the oldest certifying program in canine rehabilitation is located at the University of Tennessee, Knoxville (www.canineequinerehab.com).
References
- American Animal Hospital Association. Hospice Care—Ending Life With Compassion—AAHA Pet Care Library.
- American Association of Human–Animal Bond Veterinarians What is Hospice or End-of-Life Care?, http://members.aol.com/guyh7/hospice.htm, 2002.
- American Association of Feline Practitioners AAFP Feline Behavior Guidelines, www.catvets.com, 2004.
- American Animal Hospital Association Pain management. Client information brochure, American Animal Hospital Association, 2005.
- American Association of Feline Practitioners Healthy Cats for Life—Subtle Signs of Illness, www.catvets.com, 2006.
- Brock N. Veterinary Anesthesia Update, 2nd edn, 2007, AAHA Press: Lakewood, CO. [Google Scholar]
- Beale B.S. Use of nutraceuticals and chondroprotectants in osteoarthritic dogs and cats, Veterinary Clinics of North America: Small Animal Practice 34 (1), 2004, 271–289, (viii) [DOI] [PubMed] [Google Scholar]
- Budsberg S.C., Bartges J.W. Nutrition and osteoarthritis in dogs—does it help?, Veterinary Clinics of North America: Small Animal Practice 36 (6), 2006, 1307–1323. [DOI] [PubMed] [Google Scholar]
- Bowen J., Heath S. Behavior Problems in Small Animals—Practical Advice for the Veterinary Team, 2005, Edinburgh Elsevier Saunders, p. 51, 81,, 87,, 89. [Google Scholar]
- Cornell University, College of Veterinary Medicine, Feline Health Center Videos, www.felinevideos.vet.cornell.edu, 2007.
- Egger C.M., Glerum L.E., Allen S.W., et al. Plasma fentanyl concentrations in awake cats and cats undergoing anesthesia and ovariohysterectomy using transdermal administration, Veterinary Anaesthesia and Analgesia 30 (4), 2003, 229–236. [DOI] [PubMed] [Google Scholar]
- Flecknell P., Waterman-Pearson A. Pain Management in Animals, 2000, WB Saunders: London. [Google Scholar]
- Gaynor J., Muir W. Handbook of Veterinary Pain Management, 2002, Elsevier Publishing: St. Louis, MO. [Google Scholar]
- Hellyer P.W., Uhrig S.R., Robinson N.G. Canine Acute Pain Scale and Feline Acute Pain Scale, 2006, Colorado State University Veterinary Medical Center: Fort Collins CO, www.cvmbs.colostate.edu/ivapm/professionals/members/drug_protocols/painscalecaninenobandagesPAH.pdf. [Google Scholar]
- Hunton E., Ascher A., Tokiwa M., et al. Animal welfare task force: guidelines for preventing, recognizing, and treating pain in the hospital setting and guidelines for pet owners for recognizing pain in their dogs and cats, New Jersey Veterinary Medical Association, 2005.
- Hardie E.M. Management of osteoarthritis in cats, Veterinary Clinics of North America: Small Animal Practice 27 (4), 1997, 945–953. [DOI] [PubMed] [Google Scholar]
- Impellizeri J.A., Lau R.E., Azzara I. Effect of weight reduction on clinical signs of lameness in dogs with hip osteoarthritis, Journal of the American Veterinary Medical Association 216, 2000, 1089–1091. [DOI] [PubMed] [Google Scholar]
- Janicki P.K., Schuler G., Francis D., et al. A genetic association study of the functional A118G polymorphism of the human mu-opioid receptor gene in patients with acute and chronic pain, Anesthesia and Analgesia 103 (4), 2006, 1011–1017. [DOI] [PubMed] [Google Scholar]
- Kronen P.W., Ludders J.W., Erb H.N., et al. A synthetic fraction of feline facial pheromones calms but does not reduce struggling in cats before venous catheterization, Veterinary Anaesthesia and Analgesia 33 (4), 2006, 258–265. [DOI] [PubMed] [Google Scholar]
- Kim H., Mittal D.P., Iadarola M.J., et al. Genetic predictors for acute experimental cold and heat pain sensitivity in humans, Journal of Medical Genetics 43 (8), 2006, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansson M., Saraste L., Soop M., et al. Diminished interleukin-6 and C-reactive protein responses to laparoscopic versus open cholecystectomy, Acta Anaesthesiologica Scandinavica 43 (2), 1999, 146–152. [DOI] [PubMed] [Google Scholar]
- Lamont L.A., Tranquilli W.J., Grimm K.A. Physiology of pain, Veterinary Clinics of North America: Small Animal Practice 30 (4), 2000, 704, 720,–723, 753. [DOI] [PubMed] [Google Scholar]
- Landau R. One size does not fit all: genetic variability of mu-opioid receptor and postoperative morphine consumption, Anesthesiology 105 (2), 2006, 235–237. [DOI] [PubMed] [Google Scholar]
- Lascelles B.D., Robertson S.A. Use of thermal threshold response to evaluate the antinociceptive effects of butorphanol in cats, American Journal of Veterinary Research 65 (8), 2004a, 1085–1089. [DOI] [PubMed] [Google Scholar]
- Lascelles B.D., Robertson S.A. Antinociceptive effects of hydromorphone, butorphanol, or the combination in cats, Journal of Veterinary Internal Medicine 18 (2), 2004b, 190–195. [DOI] [PubMed] [Google Scholar]
- Lifelearn Client Handouts on CD, Small Animal Series, 2004/2005, Lifelearn Ltd: Newmarket, England. [Google Scholar]
- Mlacnik E., Bockstahler B.A., Muller M., et al. Effects of caloric restriction and a moderate or intense physiotherapy program for treatment of lameness in overweight dogs with osteoarthritis, Journal of the American Veterinary Medical Association 229 (11), 2006, 1756–1760. [DOI] [PubMed] [Google Scholar]
- McCarthy G., O'Donovan J., Jones B., et al. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis, Veterinary Journal Apr 27, 2006. [DOI] [PubMed]
- McNamara P.S., Johnston S.A., Todhunter R.J. Slow-acting, disease-modifying osteoarthritic agents, Veterinary Clinics of North America: Small Animal Practice 27, 1997, 863–867. [DOI] [PubMed] [Google Scholar]
- Neill K.M., Caron J.P., Orth M.W. Role of glucosamine and chondroitin sulfate in treatment for and prevention of osteoarthritis in animals, Journal of American Veterinary Medical Association 226 (7), 2005, 1079–1088. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health NIH Panel Issues Consensus Statement on Acupuncture, www.acucouncil.org/reports/nih_consensus.htm, 1997.
- Niedfeldt R.L., Robertson S.A. Postanesthetic hyperthermia in cats: a retrospective comparison between hydromorphone and buprenorphine, Veterinary Anaesthesia and Analgesia 33 (6), 2006, 381–389. [DOI] [PubMed] [Google Scholar]
- Pageat P., Gaultier E. Current research in canine and feline pheromones, Veterinary Clinics of North America: Small Animal Practice 33 (2), 2003, 187–211. [DOI] [PubMed] [Google Scholar]
- Richy F., Bruyere O., Ethgen O., et al. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta-analysis, Archives of Internal Medicine 163 (13), 2003, 1514–1522. [DOI] [PubMed] [Google Scholar]
- Shih AC, Robertson S, Isaza N, et al. A comparison between buprenorphine or carprofen alone and in combination for analgesia after ovariohysterectomy in dogs. Veterinary Anaesthesia and Analgesia, in press. [DOI] [PubMed]
- Sevalla K., Todhunter R.J., Vernier-Singer M., et al. Effect of polysulfated glycosaminoglycan metabolism in normal and osteoarthritic canine articular cartilage explants, Veterinary Surgery 29, 2000, 407–414. [DOI] [PubMed] [Google Scholar]
- Smith G.K., Paster E.R., Powers M.Y., et al. Lifelong diet restriction and radiographic evidence of osteoarthritis of the hip joint in dogs, Journal of the American Veterinary Medical Association 229 (5), 2006, 690–693. [DOI] [PubMed] [Google Scholar]
- Towheed T.E. Published meta-analyses of pharmacological therapies for osteoarthritis, Osteoarthritis Cartilage 10 (11), 2002, 836–837. [DOI] [PubMed] [Google Scholar]
- Tranquilli W.J., Grimm K.A., Lamont L.A. Pain Management for the Small Animal Practitioner—A Made-Easy Series Book, 2nd edn, 2004, Teton NewMedia: Jackson, WY. [Google Scholar]
- Tranquilli W., Grimm K., Lamont L. Pain Management for the Small Animal Practitioner, 2004, Teton NewMedia: Jackson, WY. [Google Scholar]
- University of Glasgow, Faculty of Veterinary Medicine Glasgow Pain Scale, www.gla.ac.uk/faculties/vet/research/cascience/painandwelfare/cmps.htm, 2005.
- World Health Organization WHO Definition of Palliative Care, www.who.int/cancer/palliative/definition/en/, (accessed 2007)
- Wiseman-Orr M.L., Nolan A.M., Reid J., et al. Development of a questionnaire to measure the effects of chronic pain on health-related quality of life in dogs, American Journal of Veterinary Research 65 (8), 2004, 1077–1084. [DOI] [PubMed] [Google Scholar]
- Wilson J. Legal Consents for Veterinary Practices, 2006, Priority Press Ltd: Yardley, PA. [Google Scholar]
- Wander R.C., Hall J.A., Gradin J.L., et al. Ratio of dietary (n−6) to (n−3) fatty acids influences immune system function, eicosanoid metabolism, lipid peroxidation, and vitamin E status in aged dogs, Journal of Nutrition 127, 1997, 1198–1205. [DOI] [PubMed] [Google Scholar]
- Woolf C.J. Pain: moving from symptom control toward mechanism-specific pharmacologic management, Annals of Internal Medicine 140, 2004, 441–451. [DOI] [PubMed] [Google Scholar]
Resources for assessing pain associated with various procedures and conditions
- Firth A.M., Haldane S.L. Development of a scale to evaluate postoperative pain in dogs, Journal of American Veterinary Medical Association 214, 1999, 651–659. [PubMed] [Google Scholar]
- Hellyer P. Objective, categoric methods for assessing pain and analgesia. Gaynor J.S., Muir W.W. Handbook of Veterinary Pain Management, 2002, Mosby: St. Louis, Mo, 82–107. [Google Scholar]
- Holton L.L., et al. Comparison of three methods used for assessment of pain in dogs, Journal of American Veterinary Medical Association 212, 1998, 61–66. [PubMed] [Google Scholar]
- Holton L., Reid J., Scott E.M., Pawson P., Nolan A. Development of a behaviour-based scale to measure acute pain in dogs, The Veterinary Record 148 (17), 2001. Apr 28, 525–531. [DOI] [PubMed] [Google Scholar]
- Reid J., Scott M., Nolan A. Development of a short form of the Glasgow Composite Measure Pain Scale (CMPS) as a measure of acute pain in the dog, Veterinary Anaesthesia and Analgesia 32 (6), 2005, 7. [Google Scholar]